Open Access Article
Open Access ArticleStrengthening epoxy adhesives at elevated temperatures based on dynamic disulfide bonds†
Hsing-Ying
Tsai
ab,
Yasuyuki
Nakamura
a,
Takehiro
Fujita
a and
Masanobu
Naito
 *ab
*ab
aData-driven Polymer Design Group, Research and Services Division of Materials Data and Integrated System (MaDIS), National Institute for Materials Science (NIMS), 1-2-1, Sengen, Tsukuba, Ibaraki 305-0047, Japan. E-mail: NAITO.Masanobu@nims.go.jp
bProgram in Materials Science and Engineering, Graduate School of Pure and Applied Sciences, University of Tsukuba, 1-1-1, Tenodai, Tsukuba, Ibaraki 305-8571, Japan
First published on 21st November 2020
Abstract
Epoxy resins incorporating aromatic disulfide bonds demonstrated improved adhesive properties with increasing temperature below their glass transition points. This behaviour was explained in terms of the relatively low topology freezing transition temperature of these materials as compared with their glass transition temperatures.
Thermosetting structural adhesives have a wide range of applications, including in the aerospace, automobile and infrastructure industries, because they tend to add less weight than conventional mechanical fastening methods. The majority of such adhesives are composed of crosslinked network polymers such as epoxy resins, and provide excellent dimensional stability, good mechanical properties and superior creep and chemical resistance.1–6 However, although these substances are often required to be stable under harsh high temperature conditions, the adhesive strength of traditional thermosetting resins tends to be reduced with increases in temperature. The development of materials with greater adhesive strength at high temperatures would enable the reliable application of environmentally-friendly adhesive joints in lightweight automobiles or various infrastructure components.
To this end, our group has focused on the use of dynamic covalent chemistry (DCC) to develop adhesives that maintain their strength at higher temperatures. Network polymers with dynamic covalent bonds generally behave as conventional thermoset resins but can exhibit thermoplastic characteristics as a result of reversible bond exchange reactions in response to specific external stimuli, such as heating or exposure to ultraviolent (UV) radiation or ultrasound.7–11 To date, Diels–Alder reaction,12–14 olefin metathesis,15 imine/amine exchange,16 siloxane/silanol exchange,17 disulfide metathesis,18 transamination,19 and transesterification20,21 have all been utilized to devise exchangeable bond systems. The introduction of adaptable bonding into thermoset resins using these approaches provides a variety of unique properties, including self-healing and the ability to reprocess, remold and recycle these materials. However, the adhesive properties of such resins have not yet been fully exploited because these adaptable bonding systems (typically based on transesterification) require the use of insoluble catalysts that can damage metal substrates. In addition, the prolonged reaction times that are required may induce thermal degradation and poor mechanical strength, both of which can limit the applications of resins as structural adhesives.
Epoxy resins based on exchangeable aromatic disulfide exhibit exceptional thermal and mechanical stability and can be synthesized using catalyst-free reactions. These materials also provide rapid exchange rates and healing efficiency compared with other dynamic bonding systems. Even though the degradation, self-healing characteristics and electrical resistance properties of epoxy resins incorporating disulfide bonds have been examined,22–25 the adhesive characteristics of these substances, especially the effects of temperature on adhesion, have not been fully elucidated. The present study therefore performed a detailed examination of the adhesive characteristics of epoxy resins having aromatic disulfide bonds. The results of this work demonstrate unusual thermal strengthening of adhesive joints at elevated temperatures. These results are discussed herein with regard to the thermal properties of epoxy resins with adaptable bonding systems, especially with regard to glass transition temperature (Tg) and topology freezing transition temperature (Tv).
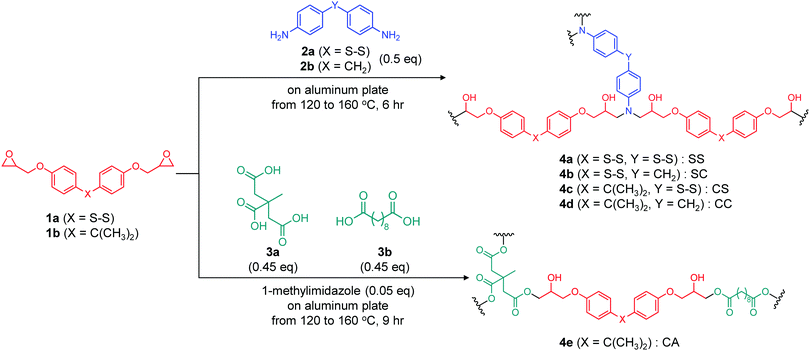
Ester and disulfide bonds were introduced into aromatic epoxy networks to obtain model epoxy adhesives with adaptable bonding (Scheme 1). The disulfide system comprised bis(4-glycidyloxyphenyl)disulfide (BGPDS, termed the 1a herein) and dithiodianiline (DTDA, 2a) as the epoxy monomer and diamine hardener, respectively. The diglycidyl ether of bisphenol A (DGEBA, 1b) and diaminodiphenyl methane (DDM, 2b) were employed as controls analogues of BGPDS and DTDA, respectively, without disulfide bonding. The chemical reactivities of the aromatic disulfide in the epoxy monomer and amine hardener were assumed to be almost identical. The epoxy adhesives were synthesized by combining the epoxy monomer (either 1a or 1b) and the diamine hardener (2a or 2b) followed by mixing at 90 °C for 30 min in stoichiometric ratios (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the ingredients. The mixture of the epoxy monomer and hardener was transferred onto the pre-treated aluminium substrates. This was followed by curing at 120 °C for 2 h, 140 °C for 2 h and 160 °C for 2 h. Aluminium substrates were employed because these are chemically inert with regard to thiols and because aluminium is an important lightweight structural material.26,27 To systematically elucidate the effects of adaptable bonding, the epoxy monomer and amine hardener with/without disulfide bonding were prepared in all combination, to give specimens referred to herein as SS (4a), SC (4b), CS (4c) and CC (4d) (Scheme 1). Note that the extent of disulfide bonding in the SS, SC and CS samples was in the ratio of 3
1) of the ingredients. The mixture of the epoxy monomer and hardener was transferred onto the pre-treated aluminium substrates. This was followed by curing at 120 °C for 2 h, 140 °C for 2 h and 160 °C for 2 h. Aluminium substrates were employed because these are chemically inert with regard to thiols and because aluminium is an important lightweight structural material.26,27 To systematically elucidate the effects of adaptable bonding, the epoxy monomer and amine hardener with/without disulfide bonding were prepared in all combination, to give specimens referred to herein as SS (4a), SC (4b), CS (4c) and CC (4d) (Scheme 1). Note that the extent of disulfide bonding in the SS, SC and CS samples was in the ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which meant the most content of disulfide bonding in the SS sample. For comparison purposes, a previously reported epoxy network with ester bonding (referred to as CA sample (4e) herein) was prepared by mixing citric acid and sebacic acid followed by the addition of DGEBA together with 1-methylimidazole as a catalyst. This mixture was subsequently precured at 120 °C for 3 h, followed by full curing at 160 °C for 6 h. The ratio of CA (4e) for DGEBA, citric acid, sebacic acid, and 1-methylimidazole was 1
1, which meant the most content of disulfide bonding in the SS sample. For comparison purposes, a previously reported epoxy network with ester bonding (referred to as CA sample (4e) herein) was prepared by mixing citric acid and sebacic acid followed by the addition of DGEBA together with 1-methylimidazole as a catalyst. This mixture was subsequently precured at 120 °C for 3 h, followed by full curing at 160 °C for 6 h. The ratio of CA (4e) for DGEBA, citric acid, sebacic acid, and 1-methylimidazole was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45
0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.45
0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05. An excess of the epoxy monomer was added to this composition so as to increase the crosslinking density.28 Details of the sample preparation conditions are provided in Table S1 (ESI†). Prior to the adhesive tests, the thermomechanical properties of these epoxy adhesives were evaluated. Specifically, Tg, Tv, activation energy of stress relaxation (Ea) and storage modulus (E′) were determined based on dynamic mechanical analysis (DMA) (Table 1).
0.05. An excess of the epoxy monomer was added to this composition so as to increase the crosslinking density.28 Details of the sample preparation conditions are provided in Table S1 (ESI†). Prior to the adhesive tests, the thermomechanical properties of these epoxy adhesives were evaluated. Specifically, Tg, Tv, activation energy of stress relaxation (Ea) and storage modulus (E′) were determined based on dynamic mechanical analysis (DMA) (Table 1).
| Item | T g (°C) | T v (°C) | E a (stress relaxation) (kJ mol−1) | Storage modulus (25 °C) (GPa) | Storage modulus (Tg + 30 °C) (MPa) |
|---|---|---|---|---|---|
| SS | 133.7 | −26 | 37.7 | 1.93 | 15 |
| SC | 144.0 | 67 | 65.5 | 2.22 | 30 |
| CS | 160.5 | 91 | 85.9 | 1.88 | 20 |
| CC | 177.9 | — | — | 1.88 | 200 |
| CA28 | 73 | 105 | 106 | 1.5–1.8 | 4–5 |
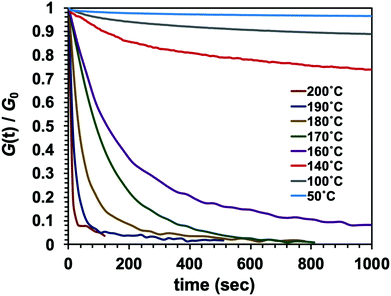
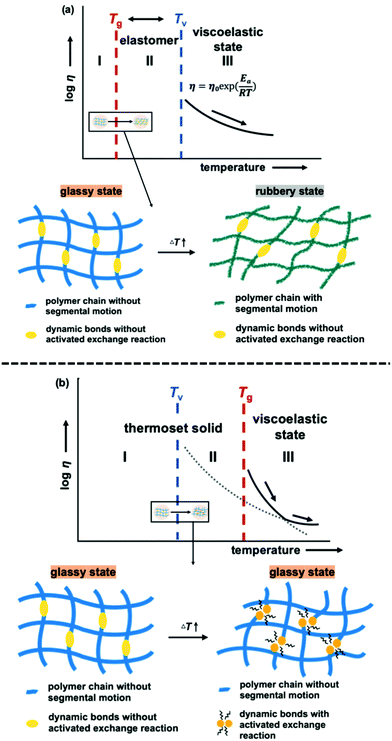
It is important to note that the viscoelastic behaviour of polymer networks having adaptable bonding systems can be characterized by two transition temperatures. One is associated with the transition between glassy and rubbery states, which is related to intramolecular chain motions. Segmental chain movements will occur above Tg, resulting in dramatic decrease of mechanical strength. On the other hand, rigid structure below Tg will limit the movement, so that the mechanical strength only slightly decreases with increasing temperature within this region. The other transition mode is associated with the topology freezing transition, which occurs at the point at which the material exhibits a viscosity of 1012 pa s. Tv is generally considered as the critical point at which the exchange rate becomes sufficiently rapid such that bond breaking and bond rearrangement can occur dynamically. This transition temperature is calculated by varying the sample temperature during a stress relaxation test, as shown in Fig. 1. In this process, the relaxation times at different temperatures are obtained based on the Maxwell model, defined as the time required for a 63% drop in the effective modulus, after which these times are plotted as a function of temperature in the manner of an Arrhenius plot (Fig. 2). The Tv values determined for the epoxy networks having disulfide and ester bonds in the present work are summarized in Table 1,28 while the Tv and Tg values for various adaptable bonding epoxies reported in the literature are presented in Table S2 (ESI†). It should be noted that the viscoelastic properties of the dynamic covalent systems could be classified into two categories based on the relationship between the Tv and Tg values of the materials.8,10 In the case of the Tv value was much greater than the Tg value, (that is, the CA sample), as shown in Fig. 3(a), the heating of the thermosetting epoxy to temperatures within the intermediate region II (between Tg and Tv) was expected to induce a transition from a glassy to rubbery state and so provide increased chain mobility. As a consequence, the material would acquire the properties of an elastomer, but would not present the exchange reaction such as transesterification in this region. Upon further heating above Tv (region III), the exchange reaction started so that the polymer would change from an elastomer to a viscoelastic liquid. In contrast, the epoxy samples with aromatic disulfide bonding (that is, the SS, SC and CS specimens) had Tg values higher than their Tv values, as can be seen from Fig. 3(b). Here, the intrinsically rapid exchange reaction associated with disulfide bonding was able to take place in the epoxy network even within the intermediate temperature region II (above Tv and below Tg). In this scenario, although the exchange reaction involving the disulfide bonds was able to proceed at temperatures in excess of Tv, a lack of chain motion below Tg would be expected to limit the segmental movement of the epoxy network, resulting in rigid and glassy structure. Above Tg (region III), these samples also transitioned to viscoelastic liquids because the chain motions were able to proceed along with active exchange reactions associated with the disulfide bonds. The data show that a higher content of aromatic disulfide bonding in the network lowered the Tv value. This effect is attributed to the increased probability of exchange reactions with higher densities of disulfide bonds, which accelerated the bond cleavage and bond reorganization. This behaviour can also be explained by considering activation energy, and the activation energy value for the stress relaxation of each composition was obtained from the data in Fig. 2 based on the Arrhenius equation. The resulting values are presented in Table 1. Although the exchange reaction of dynamic bonds would result in relaxation, significant rearrangement of whole polymer network would also be required for stress release. In the present work, the SS specimen was found to have the lowest activation energy for stress relaxation among all the compositions. Both the Tv values and activation energies demonstrate that higher concentrations of aromatic disulfide bonds promoted the stress release because of the easier reorganization of the polymer network and the increased possibility of exchange occurring.
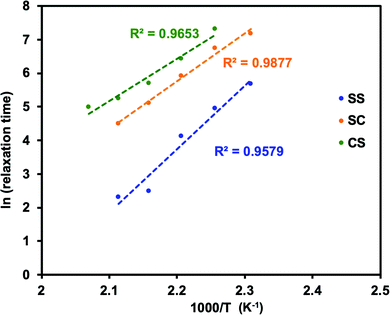 | ||
| Fig. 2 Fitting line of the relaxation time to Arrhenius equation for dynamic epoxy networks SS, SC, and CS (R-square = 0.9579, 0.9877, and 0.9653, separately). | ||
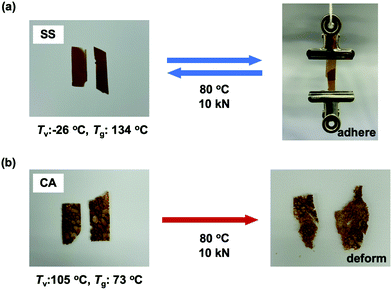
Most importantly, the viscoelastic properties derived from the Tg and Tv of these materials had a significant effect on their performance as adhesives. The single lap shear test is a standard means of assessing adhesive strength and so the room temperature lap shear strength of each epoxy sample was ascertained. In these trials, each epoxy was applied to a pretreated aluminium substrate followed by curing at temperatures that exceeded both the Tv and Tg of the epoxies with dynamic covalent bonding (see ESI,† for details). In addition, to evaluate recycling and rebonding ability, the used aluminium specimens were rebonded to their original configurations by hot-pressing. Each epoxy showed a relatively high initial shear strength in the range of 13 to 19 MPa (Fig. 4). Thus, introducing disulfide bonds into the epoxy adhesive did not significantly affect the adhesive strength, regardless of whether such bonding resulted from the phenolic epoxy or the aromatic amine hardener. After rebonding, the shear strength of the epoxy with adaptable bonding was found to be 83 to 95% of the initial shear strength, while the sample without adaptable bonding (that is, the CC specimen) did not exhibit any adhesion when rebonding was attempted. These results can be explained in terms of both the viscoelastic nature of the epoxy network and the exchange reaction associated with the adaptable bonding system. In the case that an epoxy adhesive with adaptable bonding was heated above both its Tg and Tv, the material behaved as a viscoelastic liquid within region III (see Fig. 3). Simultaneously, the bond exchange reaction proceeded among the adaptable bonds at a rapid rate to restore the polymer network, resulting in recovery of the shear strength. Evidence for these effects is provided by the correlation between the recovery of shear strength and the amount of disulfide bonding, such that the shear strength of the SS sample was 95% of the original value. This was higher than the recovery percentages showed by the other two compositions (the SC and CS). Moreover, the CC sample (without exchangeable dynamic bonding) could not adhere again once it was cleaved. These results confirm that a high degree of adaptable bonding is required to allow the efficient recovery of the polymer network, leading to restoration of the adhesive strength. Similarly, the CA sample also showed good recovery of shear strength when heated to a viscoelastic state because of the presence of dynamic ester bonds, although a longer recovery time was needed. As a result, there were no significant differences between the rebonding strengths of the epoxies with the disulfide and ester bonds when the specimens were repaired by heating above both Tg and Tv. However, there were obvious differences in adhesive behaviour after heating to within region II, intermediate between Tv and Tg. For this reason, the adhesive properties of the epoxy specimens with adaptable bonding were evaluated in this intermediate region. Although there have been several reports concerning the adhesive characteristics of adaptable bonding systems to date, the effect of temperature on these properties has not yet been fully established, especially in the range between Tv and Tg. Even so, this temperature range is important because it may coincide with temperatures applied during industrial bonding processes. Variations in adhesive properties in this temperature range were examined by preparing two SS and CA film samples and adhering these specimens at 80 °C using a compressive force of 10 kN. Here it is important to note again that the SS sample had a Tg (134 °C) higher than its Tv (−26 °C), whereas the CA sample had a Tg (73 °C) lower than its Tv (105 °C). The pressing temperature of 80 °C was chosen so as to be intermediate between the Tv and Tg of both materials (Fig. 3). The subsequent testing of these specimens indicated that the SS showed good adhesive properties (Fig. 5a) while the CA film pieces did not adhere but simply deformed from their original shapes during testing (Fig. 5b). When the SS film was heated to 80 °C, the bond exchange reaction was initiated but chain motions of the epoxy network could not proceed efficiently because the material was below its Tg. Therefore, the film shape could be maintained such that it exhibited solely good adhesion. At the same temperature, the transesterification process in this polymer is thought to have been dormant, while chain motions would have been able to occur. Therefore, only deformation of the film occurred without adhesion between the CA films.
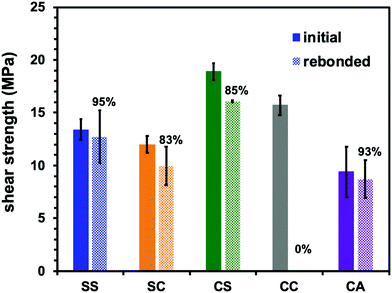 | ||
| Fig. 4 Single lap shear strength for initial and rebonded dynamic epoxy adhesive networks at room temperature. | ||
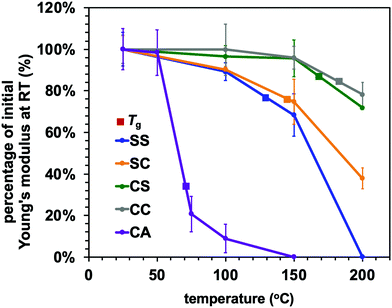
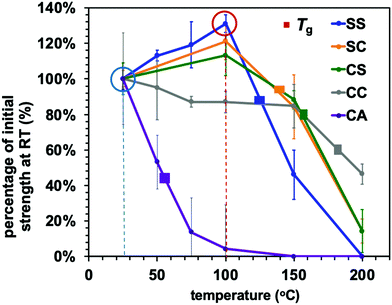
The effect of temperature on the Young's modulus and adhesive strength values of the adaptable bonding specimens was further assessed by performing single lap shear strength tests at temperatures ranging from ambient to 200 °C (Fig. 6 and 7). As can be seen from Fig. 6, the Young's modulus values of all compositions were slightly decreased with increases in temperature below the glass transition point but dramatically declined upon heating above Tg. This was the case for both the dynamic systems and the conventional thermoset polymers. Consequently, the heat-induced change in the mechanical properties of the adhesive resins is primarily ascribed to the glass–rubber transition. However, when epoxy resins are applied as adhesives, their adhesive strength shows more complicated behaviour that can be placed into two categories. In the case of a standard thermosetting adhesive, the adhesive strength tends to gradually decrease with increases in temperature and then drastically drop at the Tg of the material, as is also observed for bulk resins (see Fig. 6).29–31 This effect originates from the transition from a glassy to rubbery state. Similar to these typical thermoset adhesives, the lap shear strength of the CC sample transitioned from moderate to very poor over the temperature range of 150 to 200 °C, which contains the Tg of the sample CC (178 °C).
Conversely, the epoxy samples with disulfide bonds (the SS, SC and CS) exhibited unusual increases in lap shear strength on heating from room temperature to 100 °C. In particular, the SS specimen showed a 30% increase in lap shear strength at 100 °C as compared with room temperature (Fig. 7). In general, epoxy adhesives accumulate internal stresses in their three-dimensional network structures as a result of volume shrinkage during the curing process and the cooling process after curing. When external forces are applied to these polymer networks at room temperature, these materials therefore tend to exhibit brittle fracture because of cracks caused by internal stress and/or decreases in the elongation of the three-dimensional network structure, resulting in the cleavage of chemical bonds. In addition, considering that thiols will not readily react with aluminium/aluminium oxide surfaces, the exchange reaction of the disulfide bonds is thought to be the primary means of relieving localized internal stress via rearrangement of the polymer network. To confirm the occurrence of efficient exchange reactions among the aromatic disulfide bonds, diphenyl disulfide and dithiodianiline were dissolved in dimethyl sulfoxide (DMSO) as low molecular weight model compounds. The nuclear magnetic resonance (NMR) spectra of these solutions (Fig. S5, ESI†) indicate that the exchange reactions of these compounds proceeded even at room temperature.32 Although it should be noted that the rapid exchange of disulfide bonds may be more limited in a rigid polymeric structure at room temperature, we assumed that the dissociation and association of disulfide bonds could still occur within such fixed networks. Increasing the temperature would be expected to enhance both the bond cleavage and recombination rates as well as the probability of exchange and rearrangement. As a result, epoxy networks containing disulfide bonds will become tougher at higher temperatures because of the rapid exchange and reorganization that occur above Tv. At the same time, if the sample is below its Tg, the mechanical strength of the adhesive resin will be maintained owing to its rigid structure. As a sample approaches its Tg, its adhesive strength will greatly decrease and adhesive joints will tend to break, as is observed with conventional polymeric materials. The present data establish that a high density of aromatic disulfide bonds strengthened the adhesive effect. However, when the CA sample was heated, the bond strength was drastically decreased. This effect can likely be attributed to the frequent chain motions in the sample (because of its relatively low Tg of 73 °C) but lack of exchange reaction, as transesterification did not occur.
The Tg of a polymer reflects its transition from a rigid brittle solid to a flexible rubbery state due to chain movement. In addition, the effect of temperature on polymers containing adaptable bonds is also often evaluated based on Tv. Both Tv and Tg are associated with changes in viscosity, but for entirely different reasons. The Tg transition originates from changes in intramolecular chain motion, while Tv is attributed to intermolecular bond exchange processes. This difference leads to dissimilar phenomena in terms of adhesive properties. Specifically, bonding strength is greatly decreased above Tg but adhesion may be strengthened while the occurrence of fractures is minimized as a result of bond exchange reactions above Tv. Compared with other dynamic covalent bonds, aromatic disulfide systems tend to have low Tv and high Tg values, which increase the rates of dissociation, diffusion and association of the disulfide bonds and widen the temperature range over which these materials can be applied as industrial adhesives. On the contrary, adhesives with transesterification bonds will show high Tv and low Tg values, and so undergo glass transition before toughening.19,23–25,28,33,34
In summary, this work represents the first observation of improved adhesion behaviour at elevated temperatures as a result of adaptable aromatic disulfide bonding. An improved understanding of disulfide-based networks may permit the development of new dynamic systems and broaden the potential range of material design.
Conclusions
This study demonstrated the application of epoxy polymers incorporating aromatic disulfide bonds as adhesives. The improved adhesive properties of these materials at elevated temperatures below the glass transition region (compared with other dynamic systems and traditional thermosets) is ascribed to their extremely low Tv and activation energy values. These factors resulted in tougher adhesion because the easier rearrangement of aromatic disulfide bonds allowed the release of internal stress. This study also established that a high density of disulfide bonds accelerated the exchange process by increasing the likelihood of bond cleavage and rearrangement, which in turn improved adhesion.With regard to industrial applications, epoxy-based adhesive systems with dynamic disulfide bonding represent an environmentally-friendly and economical option. These resins not only show good adhesion properties and permit simple rebonding procedures, but also demonstrate improved high-temperature adhesive performance as a result of the incorporation of exchangeable disulfide bonds that expand their usable temperature range. These factors could permit the development of next-generation adhesives based on dynamic covalent chemistry and should be further investigated in the future. The knowledge gained from the present study should also be applicable to other epoxy adhesive systems, especially because bisphenol A is often used in epoxy adhesives.
Notes
The Maxwell relationship viscosity = G·t was used to determine the relaxation times for Tv calculations. Here, G is the shear modulus, calculated as G = E′/2(1 + v) where v is Poisson's ratio for a rubber (with a value of 0.5) and E′ is the storage modulus for each composition in a rubbery state as determined using DMA. The E′ values for the SS, SC and CS compositions in the rubbery state were 15, 30 and 20 MPa, respectively.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This article is based on results obtained from a project (JPNP14014) commissioned by the New Energy and Industrial Technology Development Organization (NEDO).References
- E. M. Petrie, Handbook of Adhesives and Sealants, McGraw-Hill Education, New York, 2nd edn, 2007 Search PubMed.
- S. Ebnesajjad and A. H. Landrock, in Adhesives Technology Handbook, ed. S. Ebnesajjad and A. H. Landrock, William Andrew Publishing, Boston, 3rd edn, 2015, pp. 1–18 Search PubMed.
- J. Shields, in Adhesives Handbook, ed. J. Shields, Butterworth-Heinemann, 3rd edn, 1984, pp. 1–6 Search PubMed.
- S. Pruksawan, S. Samitsu, Y. Fujii, N. Torikai and M. Naito, ACS Appl. Polym. Mater., 2020, 2, 1234–1243 CrossRef CAS.
- S. Pruksawan, G. Lambard, S. Samitsu, K. Sodeyama and M. Naito, Sci. Technol. Adv. Mater., 2019, 20, 1010–1021 CrossRef CAS.
- S. Pruksawan, S. Samitsu, H. Yokoyama and M. Naito, Macromolecules, 2019, 52, 2464–2475 CrossRef CAS.
- C. J. Kloxin and C. N. Bowman, Chem. Soc. Rev., 2013, 42, 7161–7173 RSC.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- W. Zou, J. Dong, Y. Luo, Q. Zhao and T. Xie, Adv. Mater., 2017, 29, 1606100 CrossRef.
- G. M. Scheutz, J. J. Lessard, M. B. Sims and B. S. Sumerlin, J. Am. Chem. Soc., 2019, 141, 16181–16196 CrossRef CAS.
- J. M. Winne, L. Leibler and F. E. Du Prez, Polym. Chem., 2019, 10, 6091–6108 RSC.
- B. J. Adzima, H. A. Aguirre, C. J. Kloxin, T. F. Scott and C. N. Bowman, Macromolecules, 2008, 41, 9112–9117 CrossRef CAS.
- G. Zhang, Q. Zhao, L. Yang, W. Zou, X. Xi and T. Xie, ACS Macro Lett., 2016, 5, 805–808 CrossRef CAS.
- S. Das, S. Samitsu, Y. Nakamura, Y. Yamauchi, D. Payra, K. Kato and M. Naito, Polym. Chem., 2018, 9, 5559–5565 RSC.
- C. Liu, E. Park, Y. Jin, J. Liu, Y. Yu, W. Zhang, S. Lei and W. Hu, Angew. Chem., Int. Ed., 2018, 57, 1869–1873 CrossRef CAS.
- P. Taynton, K. Yu, R. K. Shoemaker, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS.
- P. Zheng and T. J. McCarthy, J. Am. Chem. Soc., 2012, 134, 2024–2027 CrossRef CAS.
- A. Rekondo, R. Martin, A. Ruiz De Luzuriaga, G. Cabañero, H. J. Grande and I. Odriozola, Mater. Horiz., 2014, 1, 237–240 RSC.
- A. Chao and D. Zhang, Macromolecules, 2019, 52, 495–503 CrossRef CAS.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef CAS.
- K. Yu, P. Taynton, W. Zhang, M. L. Dunn and H. J. Qi, RSC Adv., 2014, 4, 10108–10117 RSC.
- S. P. Black, J. K. M. Sanders and A. R. Stefankiewicz, Chem. Soc. Rev., 2014, 43, 1861–1872 RSC.
- A. Ruiz De Luzuriaga, R. Martin, N. Markaide, A. Rekondo, G. Cabañero, J. Rodríguez and I. Odriozola, Mater. Horiz., 2016, 3, 241–247 RSC.
- A. Takahashi, T. Ohishi, R. Goseki and H. Otsuka, Polymer, 2016, 82, 319–326 CrossRef CAS.
- F. Zhou, Z. Guo, W. Wang, X. Lei, B. Zhang, H. Zhang and Q. Zhang, Compos. Sci. Technol., 2018, 167, 79–85 CrossRef CAS.
- S. L. Nesbitt, J. A. Emerson and J. P. Bell, Int. J. Adhes. Adhes., 2000, 20, 429–436 CrossRef CAS.
- C. Vericat, M. E. Vela, G. Corthey, E. Pensa, E. Cortés, M. H. Fonticelli, F. Ibañez, G. E. Benitez, P. Carro and R. C. Salvarezza, RSC Adv., 2014, 4, 27730–27754 RSC.
- F. I. Altuna, C. E. Hoppe and R. J. J. Williams, RSC Adv., 2016, 6, 88647–88655 RSC.
- R. D. Adams, J. Coppendale, V. Mallick and H. Al-Hamdan, Int. J. Adhes. Adhes., 1992, 12, 185–190 CrossRef CAS.
- L. F. M. da Silva and R. D. Adams, Int. J. Adhes. Adhes., 2007, 27, 216–226 CrossRef CAS.
- L. D. R. Grant, R. D. Adams and L. F. M. da Silva, Int. J. Adhes. Adhes., 2009, 29, 535–542 CrossRef CAS.
- S. Nevejans, N. Ballard, J. I. Miranda, B. Reck and J. M. Asua, Phys. Chem. Chem. Phys., 2016, 18, 27577–27583 RSC.
- Z. Ding, L. Yuan, Q. Guan, A. Gu and G. Liang, Polymer, 2018, 147, 170–182 CrossRef CAS.
- X. Yang, L. Guo, X. Xu, S. Shang and H. Liu, Mater. Des., 2020, 186, 108248 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, experimental details and characterization (NMR, FTIR, DMA). See DOI: 10.1039/d0ma00714e |
| This journal is © The Royal Society of Chemistry 2020 |