DOI:
10.1039/C9NA00307J
(Review Article)
Nanoscale Adv., 2020,
2, 70-108
Progress in supercapacitors: roles of two dimensional nanotubular materials
Received
16th May 2019
, Accepted 28th October 2019
First published on 31st October 2019
Abstract
Overcoming the global energy crisis due to vast economic expansion with the advent of human reliance on energy-consuming labor-saving devices necessitates the demand for next-generation technologies in the form of cleaner energy storage devices. The technology accelerates with the pace of developing energy storage devices to meet the requirements wherever an unanticipated burst of power is indeed needed in a very short time. Supercapacitors are predicted to be future power vehicles because they promise faster charging times and do not rely on rare elements such as lithium. At the same time, they are key nanoscale device elements for high-frequency noise filtering with the capability of storing and releasing energy by electrostatic interactions between the ions in the electrolyte and the charge accumulated at the active electrode during the charge/discharge process. There have been several developments to increase the functionality of electrodes or finding a new electrolyte for higher energy density, but this field is still open to witness the developments in reliable materials-based energy technologies. Nanoscale materials have emerged as promising candidates for the electrode choice, especially in 2D sheet and folded tubular network forms. Due to their unique hierarchical architecture, excellent electrical and mechanical properties, and high specific surface area, nanotubular networks have been widely investigated as efficient electrode materials in supercapacitors, while maintaining their inherent characteristics of high power and long cycling life. In this review, we briefly present the evolution, classification, functionality, and application of supercapacitors from the viewpoint of nanostructured materials to apprehend the mechanism and construction of advanced supercapacitors for next-generation storage devices.
 Pritam Kumar Panda | Pritam Kumar Panda is pursuing a Ph.D. in the Department of Physics and Astronomy, Uppsala University, Sweden under the supervision of Prof. Rajeev Ahuja and Priv.-Doz. Dr habil. Yogendra Kumar Mishra from Mads Clausen Institute, NanoSYD, University of Southern Denmark, Sønderborg, Denmark. He has a double Master's in Bioinformatics from Utkal University & D.Y. Patil University in 2014 and 2016 respectively. He has worked as a junior research fellow in the Infection Biology lab, KIIT University, India and later joined as a graduate scientist in the Division of Pediatric Haematology and Oncology, University of Freiburg, Germany. He has published more than 30 papers including many in top ranked journals, such as Leukemia (Nature), Nanomedicine, Scientific Reports (Nature), Toxicological Sciences, Materials Science and Engineering C, Gut Pathogens (BMC), etc. and also serves as a referee for various reputed journals. His expertise includes bioinformatics, computational biology, biophysics, 2D material modelling of electron transport in nanoscale devices, and computational modelling of bio-nanoparticles. His primary topic of research is computational modelling of 2D materials for bio-sensing applications. |
 Anton Grigoriev | Dr Anton Grigoriev is a researcher and member of the Condensed Matter Theory group (CMT) and Uppsala University UniMolecular Electronics Centre (U3MEC). His primary research area focuses on surface physics, surface chemistry and electron transport properties of nanosystems. He uses computer simulations to study surface structures, for understanding molecular adsorption and intermolecular interactions. The major application area considered is molecular electronics. He is an expert in modelling of electron transport in metal–molecule–metal systems. |
 Yogendra Kumar Mishra | Yogendra Kumar Mishra is a professor with special responsibilities in nanomaterials at Mads Clausen Institute, NanoSYD, University of Southern Denmark, Sønderborg, Denmark. Prior to moving Denmark, he was leading a scientifically independent group (2011–2019) and Alexander von Humboldt Fellow (2009–2011) at Functional Nanomaterials Chair, Institute for Material Science, Kiel University, Kiel, Germany. In Kiel, he introduced a new fabrication technique, the “Flame Transport Synthesis FTS Approach”, allowing versatile nanostructuring of metal oxides and their 3D interconnected networks as “Flexible Ceramics”. The developed tetrapodal 3D shaped ZnO nanostructures by him found many applications in engineering and biomedical fields. The sacrificial nature of ZnO tetrapods enables them to be used as templates for the growth of various new materials (inorganic, organic, polymer, ZIFs, MOFs, etc.) which offered a wide range of multifunctional applications. He has published more than 150 papers (total citations >6200; H index of 44) including many in top-ranked journals, such as Materials Today, Advanced Materials, Nature Communications, Advanced Functional Materials, Nanoscale, and many others. He is an editorial board member for several journals, e.g., Scientific Reports (Nature), Materials Today Chemistry, Nanomaterials, etc. and also serves as a referee for various prestigious journals, funding agencies, faculty promotions, etc. At NanoSYD, his main research focus is Hybrid and Smart 3D Materials for Sustainable Technologies. |
 Rajeev Ahuja | Rajeev Ahuja is a full time Professor in the Department of physics and astronomy at Uppsala University, Sweden, and heads a research group of 17 theoretical physicists. He is one of the most highly cited researchers in Sweden. In his research career, he has supervised 30 graduate students and 35 postdoctoral research associates. At present he has a group of 12 people, which include 5 PhD students and 5 post-doctoral fellows. He has published over 825 papers (citations more than 30000; H-index 80) in his research career in leading international journals. Ahuja has been awarded the Wallmark prize for 2011 by the Royal Swedish Academy of Sciences, Stockholm. This award is presented to young scientists by the King of Sweden. He has been awarded the Beller Lectureship for the APS March Meeting, 13–17 March 2017, in New Orleans, Louisiana. Ahuja is a member of the Royal Research Society, Uppsala and is on the executive board of the European High-Pressure Research Group (EHPRG). He is the editor in chief of Cogent Physics (Taylor & Francis Group) and an editorial board member at Nature Scientific Reports. |
Introduction
State-of-the-art technology has revolutionized the energy industry on a large scale and has led to many discoveries thereafter. A lot has been achieved in this direction, although achieving longer cycle life and high energy density is still a challenge. A primary challenge in this scientific era has been imposed by us (human population) due to high energy consumption and a high pollution generation rate to reduce the energy consumption that led many researchers to develop efficient supercapacitors. The key issues, e.g., high production cost, low energy density, and short cycle life, have been the major concerns in the supercapacitor industry to date and to overcome these aforementioned problems, various critical approaches have been explored as follows: (a) developing aqueous electrolytes to reduce high cost, (b) hybrid supercapacitor, solid-state supercapacitor and nonporous electrode materials production to overcome the low energy density problem, and (c) introduction of novel electrode materials (ionic liquids) to overcome the problem of short cycle life. Above all, the understanding of fundamental and basic science, e.g., surface chemistry, dielectric properties, and materials properties, may help overcome the major challenges and the vision of achieving low-cost production, high energy density, longer cycle life, and high efficiency can be fulfilled. Over the past three decades, intensive research has been carried out to meet the demand for next-generation technologies to overcome the global energy crisis with the advent of human reliance on energy-consuming labor-saving devices in which supercapacitors play a pivotal role. They have been known for many decades under colloquial terms, e.g., “supercapacitors”, “double-layer capacitors, “ultracapacitors”, “power capacitors”, “gold capacitors”, “power cache” etc. and it's still a very rapidly emerging topic with the hope to fulfill the criteria of energy storage and hence avert future energy crises.1 In the early 1950s, the design of fuel cells and rechargeable batteries came into action with the usage of carbon electrodes with a high specific surface area and thus the era of designing capacitors to achieve high energy storage was initiated. A low voltage electrolytic capacitor with porous carbon electrodes was developed by H. Becker in 1957, which led to the design of a capacitor with high capacitance.2 Extending the concept of Becker, in the period of 1966–1970, Standard Oil (Ohio) developed electrical energy storage apparatus which was further documented by Donald L. Boos in 1970 as an electrolytic capacitor with activated carbon electrodes. In 1971, SOHIO did not commercialize their invention rather they licensed their technology to a Japanese multinational information technology and electronics company that finally marketed “Supercapacitors” with the purpose to provide backup power for computer memory. The actual term “supercapacitor” was introduced by Brian Evans Conway in 1999 illustrating fundamental experiments on ruthenium oxide electrochemical capacitors to explain the increased capacitance due to surface redox reactions with faradaic charge transfer between electrodes and ions ultimately leading to increased electrochemical energy storage.3,4 The evolution of supercapacitors caused a boost in power applications that led to the introduction of “ultracapacitors” in 1992 by Maxwell laboratories which further concentrated on the increase in the electrolyte's breakdown voltage. In 1994, an “Electrolytic-Hybrid Electrochemical Capacitor” was introduced by David A. Evans which combined a high dielectric strength anode with a high pseudo-capacitance metal oxide cathode yielding a hybrid capacitor. Eventually the previous developments led to the emergence of lithium-ion capacitors in 2007, which took the industry to the next level in terms of power storage and applications. The phase of 1957–2007 has marked the evolution of supercapacitors in a drastic manner but they have some limitations, for instance, high production cost, low energy density and short cycle life (Fig. 1). Several projects were initiated after the introduction of lithium-ion supercapacitors that offered the highest gravimetric specific energy (15 W h kg−1, 54 kJ kg−1). Their limitations were greater than their advantages up to 2013, but the focus has been on improving specific energy, reducing internal resistance, increasing lifetimes and reducing costs.5 Graphene6 based supercapacitors were introduced in 2010 by Belle Dumé; more specifically, curved graphene sheets that could store high energy with a specific charge density of 85.6 W h kg−1 at room temperature and 136 W h kg−1 at 80 °C.6 The technology marked the beginning of the developmental phase of supercapacitors that are comparable to nickel-metal hydride batteries . Researchers around the world have made strides in improving the specific capacitance of graphene-based supercapacitor electrodes but they have some major drawbacks in terms of capacitance which did not meet the criteria of the theoretical capacitance of 550 F g−1 despite the high specific surface area of single-layered graphene sheets (∼3000 m2 g−1).7
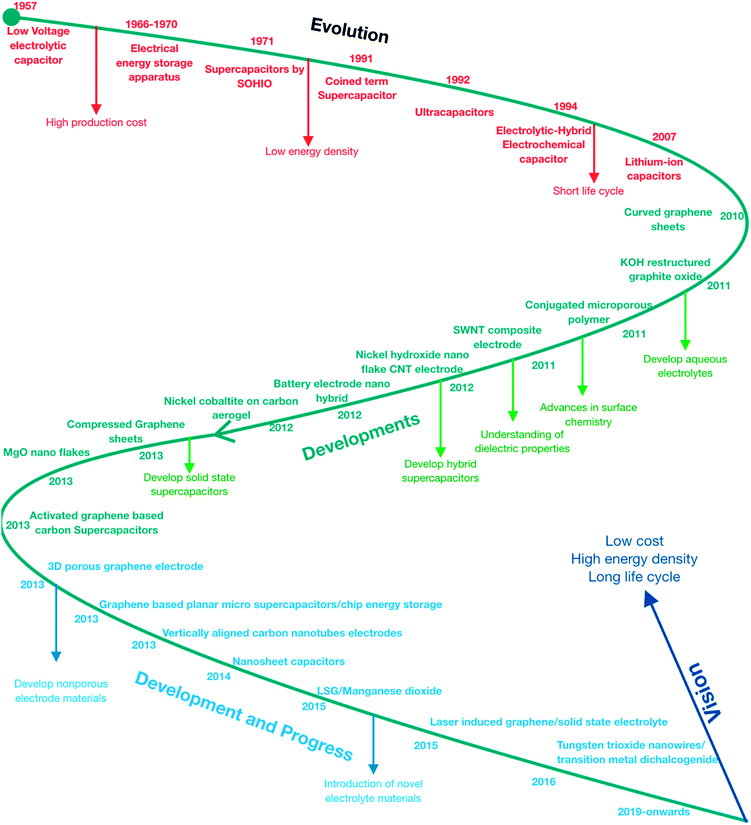 |
| | Fig. 1 Roadmap of the evolution, progress, and developments in supercapacitors. | |
Several modifications have been made towards improving the specific capacitance, longer cycle life, and particular materials used for electrodes and electrolytes. The discovery of carbon-based supercapacitors by the activation of graphene marked the progress of supercapacitors in energy technology and introduced new materials for use as electrode materials to achieve high energy density, high charge density, higher surface area, longer cycle life, and high capacitance.8Table 1 demonstrates the developments made thereafter by the introduction of carbon-based supercapacitors.
Table 1 Developmental progress of supercapacitors after 2010
| Year |
Material |
Development |
Specific energy |
Capacitance |
Cycles |
Specific power |
| 2011 |
Potassium hydroxide434 |
Restructured-graphite oxide |
85 W h kg−1 |
200 F g−1 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 2011 |
Aza-fused-π-conjugated microporous435 |
Conjugated microporous polymer |
53 W h kg−1 |
270 F g−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| 2011 |
Single-walled-carbon nanohorn/nanotube composite436 |
Composite electrode |
|
|
|
990 W kg−1 |
| 2012 |
Nickel hydroxide437 |
Nanoflake-CNT composite electrode |
50.6 W h kg−1 |
3300 F g−1 |
— |
— |
| 2012 |
Li4Ti5O12 (LTO)438 |
Battery-electrode nanohybrid |
40 W h l−1 |
|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.5 W l−1 |
| 2012 |
Nickel cobaltite439 |
Carbon-aerogels composites |
53 W h kg−1 |
1700 F g−1 |
|
2.25 W kg−1 |
| 2013 |
Graphene sheets440 |
Liquid-mediated transport network |
60 W h l−1 |
|
|
|
| 2013 |
Activated graphene210 |
Macro- and meso-porous electrodes |
74 W h kg−1 |
|
|
|
| 2013 |
NaxMnO2 (ref. 441) |
Nanoflakes |
110 W h kg−1 |
1000 F g−1 |
|
|
| 2013 |
Graphene electrodes442 |
3D porous graphene electrodes |
98 W h kg−1 |
231 F g−1 |
|
|
| 2013 |
Graphene443 |
Chip-based energy storage |
2.42 W h l−1 |
|
|
|
| 2013 |
Carbon nanotubes444 |
Vertically aligned CNTs |
13.50 W h kg−1 |
|
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
37.12 W g−1 |
| 2014 |
Ru0.95O20.2–/Ca2Nb3O10–/Ru0.95O20.2– nanosheet ultrathin capacitors445 |
Room temperature solution-based capacitors |
|
27.5 μF cm−2 |
|
|
| 2015 |
Laser-scribed graphene/MnO2 (ref. 446) |
Hybrid capacitor |
42 W h l−1 |
|
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
10 kW l−1 |
| 2015 |
Graphene447 |
Flexible and stackable laser-induced supercapacitors |
|
9 mF cm−2 |
|
0.02 mA cm−2 |
| 2016 |
WO3/2D WS2 layers448 |
Transition-metal dichalcogenides |
100 W h l−1 |
|
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1 kW l−1 |
Taxonomic classification
A typical Ragone plot9 (Fig. 3(a)) shows that supercapacitors play a pivotal role in bridging the gap between traditional capacitors and batteries and occupy a prominent position in developing energy storage devices. Despite intensive research in energy storage mechanisms, lithium battery technologies are still lagging behind the increasingly punitive performance stipulated by industries. To overcome this problem supercapacitors have been widely used extensively, ranging from portable devices to industrial power and energy applications to induce a long-overdue change towards a sustainable generation, management and consumption of energy without relying explicitly on renewable resources. The combination of high specific energy with high power density demands specific energy storage mechanisms in which supercapacitors are best in the aspects of performance and delivery from small scale energy consumption to industrial scale. The taxonomy of supercapacitors' broad classification based on electrolytes, materials, and configurations draws significant attention in terms of energy storage capacity and their application,10 as shown in Fig. 2.
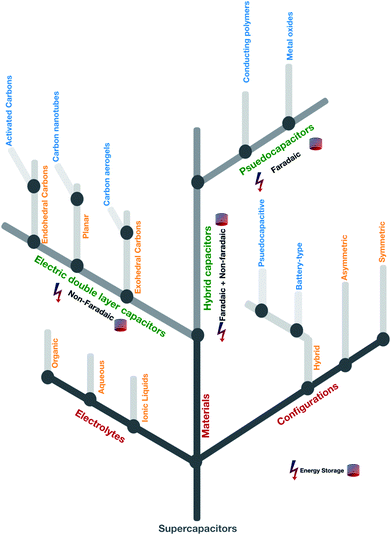 |
| | Fig. 2 An overview of the taxonomical classification of supercapacitors. The lightning bolt symbol represents energy and the cylindrical stack represents the storage. | |
Further divisions in electrolytes consist of organic, aqueous and ionic liquids in which a longer cycle life can be achieved in the order of ionic liquids ≫ aqueous ≫ organic in terms of high potential difference, high specific energy, and maximum power density.11–21 Materials-based supercapacitors are subdivided into electric double-layer capacitors (EDLCs), pseudocapacitors, and hybrid capacitors.22 The unique storage mechanism of the materials based classification involves two significant concepts mainly; one utilizes the charge transfer concept between electrodes and electrolytes (faradaic) and the other is without any chemical reaction/mechanism (non-faradaic).23 Material-based subdivisions and mechanisms are listed in Fig. 2 and Table 2. As far as the configurations are concerned, the classification is based on the type of symmetry, e.g., symmetric (two equal electrodes), asymmetric (two different electrodes with the same mechanism) and hybrid (two different electrodes and different mechanisms) among which to achieve better efficiency, hybrid-based supercapacitors are mainly used nowadays.24,25
Table 2 Taxonomical classification of material-based supercapacitorsa,b
| Types |
Mechanism |
Energy storage |
Performance |
Common materials |
|
Chemical transfer: Charge transfer between electrodes and electrolytes through electrosorption, redox reactions, and intercalation processes.8
Physical transfer: Charges are distributed on the surface without making or breaking chemical bonds.
|
| EDLCs |
Chemical transfer |
Non-faradaic |
0.335 kW kg−1 average specific power and 5 kW kg−1 maximum specific power in the potential range of 0.0–2.8 V (ref. 449) |
Carbon-based |
| Pseudocapacitors |
Chemical transfer (quick) |
Faradaic |
Average specific capacitance range of 200–500 μF cm−2 |
Metal oxide-based |
| 1–2 orders of magnitude higher than that of EDLCs8 |
| Hybrid |
Physical transfer |
Faradaic + non-faradaic |
Average specific power (0.5 kW kg−1) and maximum specific power (9 kW kg−1) at 5 mA cm−2 in the potential region of 1.0–3.0 V (ref. 449) |
Both carbon and conducting polymer or metal oxide-based composites1 |
They are preferred because of their improved performance in energy density without altering the power density , which delivers higher specific capacitance due to their asymmetric behaviour.26 The asymmetric behavior of hybrid-supercapacitors gives enhanced characteristics such as intrinsic shell area, atomic charge partition length, rapid and repeatable redox reactions, etc., which has the combined characteristics of both EDLC and pseudocapacitors respectively.25,27–29Fig. 3(b) shows the specific power–energy plot illustrating the storage capacity of asymmetrical capacitors occupying the specific region with an aqueous electrolyte, compared to other power devices such as EDLCs, conventional capacitors, asymmetric capacitors with organic electrolytes, and fuel cells. They have been proven to be one of the best energy storage devices in terms of specific power which is 100 times higher than that of batteries. In Fig. 3(c), out of all the conventional supercapacitors, hybrid-based supercapacitors exhibit an attainable performance focusing on characteristic features as shown in the plot. There has been less exploration of hybrid supercapacitors compared to the other two types, and researchers hypothesize that they may outperform the most promising class of supercapacitors available today in the near future and further developments have continued in order to tune the design and flexibility22 which is discussed further.
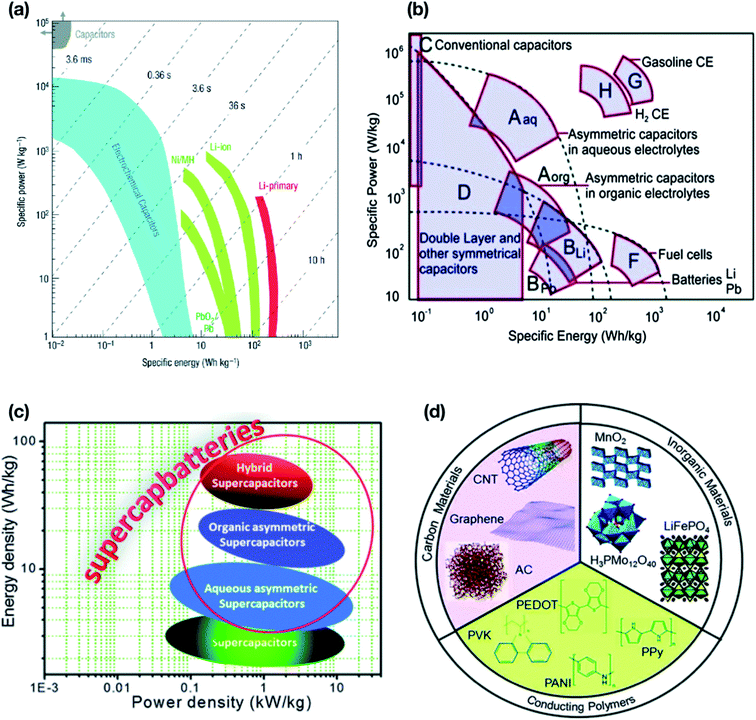 |
| | Fig. 3 (a) Typical Ragone plot showing power against energy density.473 (b) Specific energy/power performance for different energy storage devices.30 (c) Ragone plot showing the recent status of supercapacitors, asymmetric supercapacitors, and hybrid supercapacitors.30 (d) Illustration of various components used in the design of hybrid materials for energy storage.30 (a) has been adapted/reproduced from ref. 473 with permission from Nature Materials, Springer Nature.473 (c–e) have been adapted/reproduced from ref. 30 with permission from Chemical Society Reviews, Royal Society of Chemistry.30 | |
The merging of battery type faradaic or pseudocapacitive electrodes with a capacitive electrochemical double layer (EDL) electrode gives rise to hybrid supercapacitors that allow a reinforcing combination of properties of different components synergetically.30 This combination can reinforce the need for charge storage mechanisms in order to improve the overall performance. In consideration of the combinatorial strategy to develop hybrid supercapacitors, an optimal configuration is thus required for the mixing approach (composite electrodes). The mixing approach denotes the use of different electrodes in appropriate proportions concerning relative concentrations and particle sizes as are necessary for percolation paths keeping their chemical nature, crystal structure, and physiochemical properties stable. A growing number of hybrid materials for electrodes have been developed with improved power and energy densities. One such study by Dubal et al.30 proposed hybrid combinations of electroactive and conductive materials including a wide variety of inorganic species from oxides to polyoxometalate clusters, conducting organic materials and carbon-based electrodes, as depicted in Fig. 3(d). Also they provided a list of cathode and anode electrode materials used to optimize the performance, e.g., for the cathode, Li1−xNi1−y−zCoyMzO4, LixMn2O4, LiMnO2, MnO2, V2O5, LiV3O8, Li1−xVOPO4, and LixFePO4 specifically focusing on LixFePO4 and for the anode, a wide variety of materials as well as approaches have been investigated from lithium metal to graphite and carbons to alloys and recently metal nanocomposites have attracted great interest, of which core–shell LiMnPO4–LiFePO4 nanoparticles are an interesting example of hybrid supercapacitors. Revisiting all the materials aforementioned is beyond the scope of this review. However, in this review we will discuss some major advancements in two-dimensional nanotubular material based hybrid supercapacitors.
A general overview of the construction and mechanism of supercapacitors
Supercapacitors are devices that bridge the gap between capacitor elements and rechargeable batteries and are capable of storing charge/energy electrostatically by the help of two porous electrodes (usually made up of porous activated carbon)31 immersed in an electrolyte solution. They have been evolved to boost high energy storage capacity in terms of peak power demands or recapturing energy for which they are considered to be more effective than batteries. The three key elements of a supercapacitor are the separator, electrolyte, and electrodes, and their properties uniquely complement its overall performance. The dielectric strength, chemical inertness, porosity (ion-permeable) and shallow thickness are the most important requirements for a separator for effective working of the supercapacitor. The electrodes must also be highly porous, with the highest possible specific surface area, low electrical resistivity, high chemical stability, etc. The porous electrodes are separated by a small distance, liquid, or wet dielectric material. The electrodes and separator are impregnated within a liquid electrolyte (aqueous or organic)32–35 that allows the transfer of ions while providing insulation between the electrodes36,37 driven by the electrode surface area and separation between the electrodes which in-turn gives rise to double-layer (Helmholtz layer) capacitance. The positive and negative ionic charges within the electrolyte utilize the high surface area of electrodes and accumulate at the surface and compensate for the electric charge1,8,38–40 as shown in Fig. 4(a). As depicted by Chen et al.,41 a supercapacitor is composed of a multilayered structure including an inner compact layer, an outer compact layer and a diffuse layer which consists of solvated cations and anions. The electrode as shown in Fig. 4(b) is a porous carbon negative electrode that involves chemical bonding which in general is not purely physical nor chemical. The unsolvated anions and partially solvated cations in the compact layers are in the form of closely packed ions on the electrode surface and give rise to linear potential distributions with different gradients. For instance, in the diffuse layer, ion concentration and potential distribution deviate from linearity and reach a plateau towards the end of bulk electrolyte solution as shown in Fig. 4(b). The potential profile of the whole cell is given by eqn (1)where U is the voltage, and EP and EN represent positive and negative electrodes. As shown in Fig. 4(c), the potential in the electrolyte solution (Es) remains constant due to the mobility of ions. The ions from the electrolyte were adsorbed on the maximal surface area of the electrode in which electrodes make use of electrostatic attraction between ions and electrolyte that allows the formation of oppositely charged layers at the interface.42 Due to the high surface area of the electrodes, the charge storage mechanism and the performance on the basis of specific capacitance and energy density are better than those of conventional electrochemical capacitors.43 Supercapacitors can be classified into EDLCs and pseudocapacitors based on their energy storage mechanism, as shown in Fig. 4(d). The two main storage mechanisms in supercapacitors involve electric double-layer charge storage at the interface between the electrolyte and electrodes, and the pseudocapacitance, involving reversible fast faradaic redox reactions at the electrodes' surfaces. EDLCs are the most common commercially available supercapacitors where the charge is stored electrostatically or non-faradaically using a double layer (Helmholtz layer) in which the charge accumulates on the electrode surface by following the natural attraction of unlike charges diffused across the separator into the pores of the electrodes in the electrolyte medium Fig. 4(e).1,44 The mechanism is followed by the generation of two layers of polarized ions at the electrode interface due to the applied voltage. The two layers i.e., one layer within the solid electrode and the other with opposite polarity generated from dissolved and solvated ions distributed in the electrolyte move towards the polarized electrode which is separated by a monolayer of solvent molecules forming a molecular monolayer called the inner Helmholtz plane that adheres by physical adsorption on the electrode surface and separates the oppositely polarized ions from each other, forming a molecular dielectric. In pseudocapacitors, the charge is stored faradaically or electrochemically through the transfer of charge across the interface. This phenomenon is achieved through electrosorption, reduction–oxidation reactions, and an intercalation process as shown in Fig. 4(f).4,45 When voltage is applied at the capacitor terminals, the polarized ions in the electrolyte move to the opposite polarized electrode. The interface between the surfaces of the electrodes and the adjacent electrolyte forms an electric double-layer. The movement of one layer of ions at the electrode surface and the second layer of adjacent and solvated ions to the polarized electrode forms a static electric field which gives rise to double-layer capacitance. Accompanied by the electric double-layer, some de-solvated electrolyte ions also act as electron donors, permeate the separating solvent layer and are absorbed by the electrode's surface atoms that deliver their charge to the electrode resulting in a faradaic current. This faradaic charge transfer phenomenon gives rise to pseudocapacitance which is originated from a sequence of fast reversible redox reactions, electrosorption or an intercalation process between the electrode surface and electrolytes. The later devices are often misunderstood with a battery-type electrode storage mechanism: as per the definition, the pseudocapacitor's electrodes must be capacitive, that is the storage should be proportional to the applied voltage, while, in batteries, a flat discharge plateau is often observed. This behavior is characteristic of the electrode and cannot be applied in case of the asymmetric or hybrid device performance.46 In both, the storage mechanisms are synergetic between electrode and electrolyte materials.47 Generally, when the voltage is applied to a supercapacitor, ions in the electrolyte solution diffuse into the pores of the electrode of opposite charge and charge accumulates at the interface between the electrodes and the electrolyte, forming two charged layers (double layer) with an extremely small separation distance. The capacitance value C is proportional to the surface area A and is the reciprocal of the distance d between the two layers48,49 as depicted in eqn (2).where εr is the relative dielectric constant, and d is the thickness of the double layer with surface area A. The thickness of the double layer depends on the concentration of electrolytes and the size of ions. To achieve a higher capacitance, porous electrodes have been used to increase the surface area with an extremely large internal effective surface. Carbon technology is often used in these capacitors because it creates a very large surface area with a separation distance of just a few angstroms (0.1 nm), due to its porosity which is discussed further in this review.
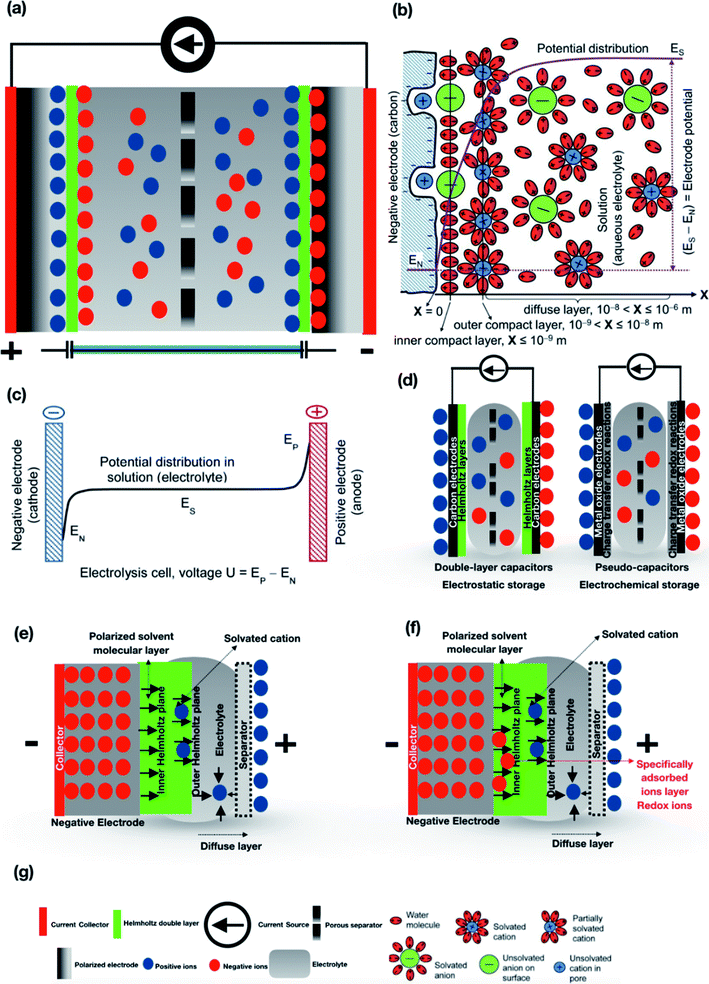 |
| | Fig. 4 (a) Schematic representation of basic construction of a supercapacitor. (b) Cross-sectional interface of the electrochemical double layer structure between a porous carbon negative electrode and an aqueous electrolyte. EN, EP, and Es are respectively the potentials of the negative and positive electrodes, and the electrolyte. (c) Potential distribution in the electrolyte solution between the negative and positive electrodes. (d) Energy storage mechanisms in supercapacitors e.g. electrostatic storage mechanism in double-layer capacitors & electrochemical storage mechanism in pseudo-capacitors. (e) The charge is stored electrostatically or non-faradaically using a double layer (Helmholtz layer) in which the charge accumulates on the electrode surface by following the natural attraction of unlike charges diffused across the separator into the pores of the electrodes in the electrolyte medium. The arrows represent the polarized solvent molecular layer in the inner Helmholtz plane and the arrows with positive ions represent solvated cations. (f) The charge is stored faradaically or electrochemically through the transfer of charge across the interface. This phenomenon is achieved through electrosorption, reduction–oxidation reactions, and an intercalation process. The arrows with negative ions represent redox ions and arrows with positive ions depict solvated cations. (g) Depiction of symbols in (a and b). (b, c and g) have been adapted/reproduced from ref. 41 with permission from Taylor & Francis.41 | |
Charge–discharge mechanism
Although supercapacitors have many advantages over batteries, they still lag due to some limiting factors, e.g., high cost per watt, low specific energy, high self-discharge, low cell voltage, etc. (Source: Maxwell Technologies, Inc.50). But in consideration of a sudden surge of energy, supercapacitors are slightly better than batteries due to their relatively fast charge–discharge cycle (Fig. 5(a)). They exhibit the capability to store more energy than conventional capacitors and are also capable of delivering it faster than rechargeable batteries along with a high tolerance towards charging and discharging cycles. Fig. 5(a) demonstrates a typical charge–discharge profile where supercapacitors supersede conventional batteries in terms of time taken to charge and discharge, respectively. According to the pie charts depicted in the figure, batteries take almost 80% of time to charge (light green color in recharge time) as well as to discharge (pink color in discharge time) in comparison to supercapacitors whereas the time to charge and discharge is comparatively shorter (less than 20%) in supercapacitors. In addition to having a limited number of charge–discharge cycles in batteries, the performance of the batteries' cycle life significantly deteriorated and shortened when a large pulsed discharging current with a peak greatly exceeding the average power consumption occurs. To reduce the peak current draw in battery-powered electronics with a highly fluctuating load, supercapacitors are widely exploited to mitigate such load current fluctuations in batteries.51Fig. 5(b) depicts a typical charge/discharge cycle of supercapacitors and batteries. Compared to hundreds of cycles for batteries, supercapacitors have the ability to withstand thousands of cycles before degrading and also have the ability to be deeply discharged when compared to batteries. The self-discharge rate of supercapacitors due to their composition is significantly higher than that of batteries. Fig. 5(c) illustrates the cyclic voltammograms of positive and negative electrodes in a three-electrode cell and galvanostatic charging and discharging plots for a two electrode cell for rechargeable batteries and supercapacitors respectively. Eqn (3) expresses the energy density of a supercapacitor as:| | 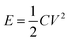 | (3) |
E is the energy provided by the supercapacitor, C is the capacitance of the supercapacitor, and V is the voltage of the supercapacitor. The potential for positive electrodes (Fig. 5(c)) tends towards positive in the graph and is always higher than that of negative electrodes due to electrical polarities. The electrical polarities generated are due to the fact that current always flows from positive to negative electrodes via an external circuit and the electrons flow from negative to positive electrodes. Generally, faradaic reactions that are generated from the interface of electrode|electrolyte are always present in rechargeable batteries but not in supercapacitors and because of this difference, the minimum cell voltage can decrease to zero in supercapacitors as shown in Fig. 5(c).
 |
| | Fig. 5 (a). Comparison of the charge and discharge cycles of batteries vs. supercapacitors. Pink color signifies the discharge time cycle and light green represents recharge timing (b). (c) The cyclic voltammograms of positive and negative electrodes in a three-electrode cell and galvanostatic charge and discharge plots for a two-electrode cell for rechargeable batteries and supercapacitors respectively. Umax and Umin are the maximum and minimum cell voltages; Udis is the average discharge voltage; τ and t: end times of the first charge and discharge cycle, τ ≥ (t − τ). 2τ and 2t: end times of the second charge and discharge cycle. (d) Charge–discharge voltage curves (referred from Battery University™ sponsored by Cadex Electronics Inc.474). (c and d) have been adapted/reproduced from ref. 41 and 474 with permission from Taylor & Francis41 and Battery University™ Copyright ©2003–2018 Cadex Electronics Inc. (Cadex)474 respectively. | |
The voltage and current characteristics upon charge and discharge of a supercapacitor are depicted in Fig. 5(d). During charge, the voltage linearly increases and the current drops when the capacitor is fully charged without any full-charge detection circuit and when the condition of a constant current supply is applied during discharge, the voltage drops linearly and the current increases. The charge/discharge process is unlimited in the case of supercapacitors but not in the case of rechargeable batteries which have a defined cycle life. A comparison can thus be established between batteries vs. supercapacitors in terms of charge time; supercapacitors take only 1–10 seconds to charge whereas batteries (lithium-ion) take 10–60 minutes. Likewise, the cycle life is around 1 million hours in comparison to 500 h in batteries. The optimum cell voltage is around 2.3 to 2.75 V in supercapacitors and 3.6 V in batteries (referred from Battery University™ sponsored by Cadex Electronics Inc.).
Efficient charge storage mechanisms: electrodes
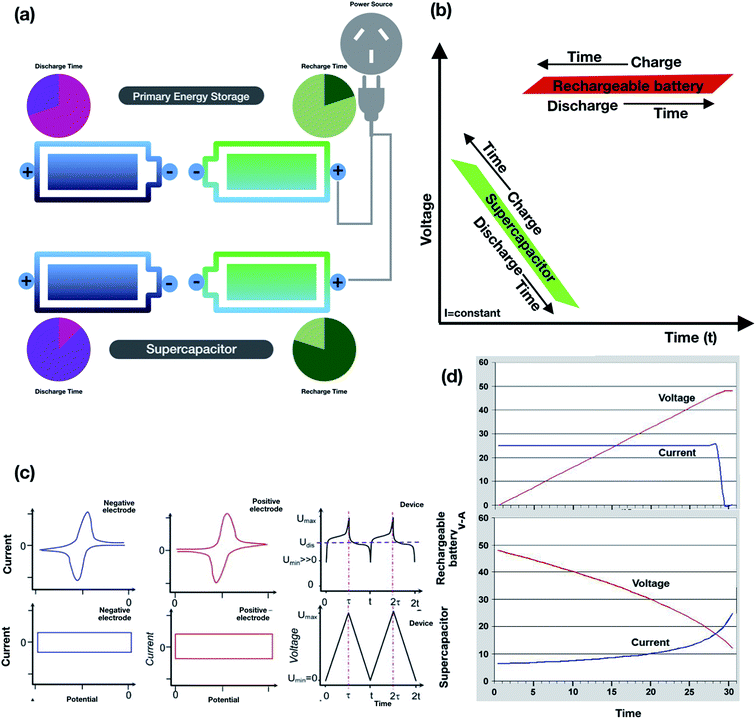
To improve the specific capacitance and charge storage, supercapacitors rely on electrode materials that are theoretically and experimentally supported. Mostly carbon-based materials are used in the fabrication of supercapacitors due to their high surface area, low cost, availability, and technologies supporting the production of carbon-based electrodes. Mostly EDLCs use carbon-based electrodes as the charge transfer mechanism strongly relies on the surface area accessible to electrolytic ions. The nature of the electrode material depends on various properties, e.g., carbon, is primarily focused on subclasses of EDLCs that is of great importance as they have a higher surface area, easy fabrication, and low-cost material. Various forms such as activated carbon, carbon nanotubes, and carbon aerogels can be used to store charges in EDLCs out of which we have mainly focused on nanotube structural conformations. There is an increasing demand in the usage of carbon nanotubes in EDLC electrode materials in the form of mesopores which are grown as entangled mat that allows continuous charge distribution on the available surface area. Physical mechanisms play a pivotal role in charge transfer where the surface area is utilized more efficiently to achieve higher capacitance.52,53 The diffusion of electrolytes is more facile in mesoporous networks resulting in low ESR (equivalent series resistance)54 and high power densities55 compared to other types of carbon-based electrodes.56–58 Influential parameters such as the pore sizes, pore shape, structure, surface functionality and electrical conductivity play a pivotal role in increasing the specific capacitance,59,60 for example, when using activated carbon, single and multiwalled carbon nanotubes, graphene, graphene oxide, etc.. One can further discuss the other influential parameters, e.g., pore size, in inducing an effect on the specific capacitance and performance of supercapacitors. Illustrative examples of carbon-based nanoporous materials are shown in Fig. 6(a–d).
 |
| | Fig. 6 (a) Ordered structures with slit pores, nanotubes and asymmetric nanoporous carbons.42 (b) Symmetric supercapacitor based on metal oxide framework derived nanoporous carbon.475 (c) Stacked geometry model for a carbon-based electrode material consisting of GO electrodes with pure EMI + BF4−.476 (d) Porous electrodes made of carbide derived carbon immersed in a BMI-PF6 ionic liquid electrolyte.477 (a) has been adapted/reproduced from ref. 42 with permission from Springer Nature.42 (b) has been adapted/reproduced from ref. 475 with permission from the Royal Society of Chemistry.475 (c) has been adapted/reproduced from ref. 476 with permission from the Royal Society of Chemistry.476 (d) has been adapted/reproduced from ref. 477 with permission from American Chemical Society.476 | |
Effect of pore sizes on specific capacitance and performance
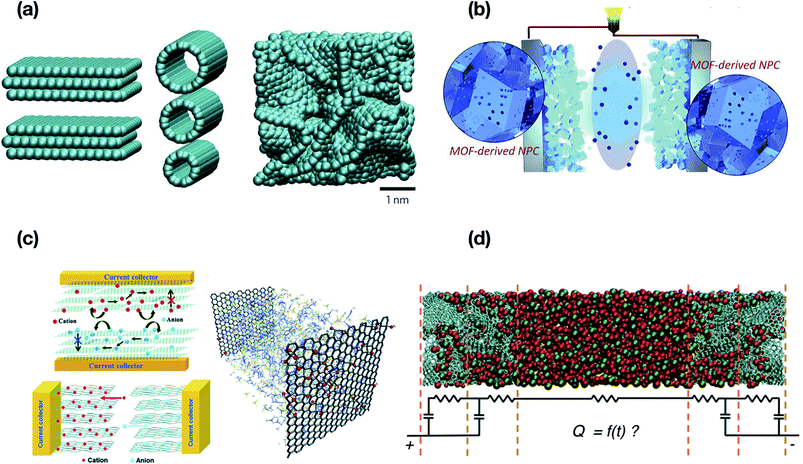
Gogotsi et al.61 made a major discovery on pore size effects on specific capacitance based on the contribution of unsolvated ions to capacity in purely double-layer electrodes using carbide-derived carbons with pore sizes smaller than 1 nm. They demonstrated the diffusion of ions through these pores upon desolvation, which contributes to increasing capacitance. Generally, the material used for electrodes is nowadays based on porous carbon that uses ion adsorption on the surface. The effect of pore size on capacitance may potentially improve the performance by maximizing the electrode surface area. As shown in Fig. 7(a), a detrimental effect on capacitance can be observed with the increase in pore size while the specific capacitance is normalized by the surface area of the electrodes.62–64Fig. 7(b) shows the optimization of electrodes to potentially improve the performance based on pore size. The specific capacitance is inversely proportional to the pore size, and one can easily note the difference in pore sizes leading to increased or decreased performance and specific capacitance. When the pore size is larger than the size of the ions, the contribution to capacitance is from the adjacent pore walls but having reduced specific capacitance,65 whereas the decrease in the size of the pores approximate to the crystallographic diameter of the ions led to increased capacitance as the solvation shell/pore is highly distorted and squeezes the ions allowing a closer approach of the ion center to the electrode surface.66–69
 |
| | Fig. 7 (a) Solvated ions residing in pores with varying adjacent pore walls. A decrease in the pore size leads to high or improved capacitance with the efficient approach of ion mobility towards the pore walls. The detrimental effect on capacitance can be observed with the increase in pore size as per Chmiola et al.62 (b) The plot depicts effect of synthesis temperature on the SSA, and the average pore size of CDC. Redline illustrates the SSA values and Blue line depicts the pore size. (b) has been adapted/reproduced from ref. 62 with permission from the American Association for the Advancement of Science.62 | |
By assuming that the carbon electrodes are cylindrical, the specific capacitance with respect to the specific surface area (SSA) can be depicted as given in eqn (4)
| | 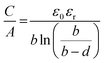 | (4) |
where
εr is the electrolyte dielectric constant,
ε0 is the dielectric constant in a vacuum,
b is the pore radius and
d is the distance between approaching electrolyte ions and the carbon surface.
70 One such theory has been experimentally demonstrated by Gamby
et al.,
71 who has shown that the specific capacitance of porous carbon electrodes showed a peculiar dependence on pore size. When the pore size is above 2 nm, the surface-specific capacitance decreases as the pore size decreases due to less accessibility for ions. But this pore dependence theory has been contradicted by Kiyohara
et al.,
72 stating that when the pore size is smaller than 2 nm the surface-specific capacitance increases as long as the pore size is larger than the ions which was illustrated by Monte Carlo simulations. The enhanced understanding of ionic transport in porous media can be demonstrated by the charge storage mechanism in ionic pores smaller than the size of solvated electrolyte ions. Furthermore, these findings also allow the design of specific capacitors for several applications such as hybrid electric vehicles. Further tuning the carbon porosity and designing carbon materials with a large volume of narrow but short pores may allow both energy and power characteristics to be improved. Kornyshev
et al.73 (
Fig. 8(a)) showed the accumulation of only one row of ions in a narrow nanopore in an unpolarised electrode using the two state ising model. Speaking about the pore radius, a tiny change in the pore radius might affect the voltage dependence on capacitance showing a smeared resonance as shown in
Fig. 8(b). The standard way of increasing the capacitance is to decrease the pore size of the electrodes as well as the thickness of pore walls. The model that they presented was based on several assumptions: (i) the propensity of cations and anions to occupy the pores in the electrode even in the unpolarized state of the electrode; (ii) during the polarisation of electrodes, co-ions tend to replace the counter ions; (iii) the ions are densely packed with no voids in between them; (iv) electrostatic interaction of ions in the pore is screened by polarization of pore walls.
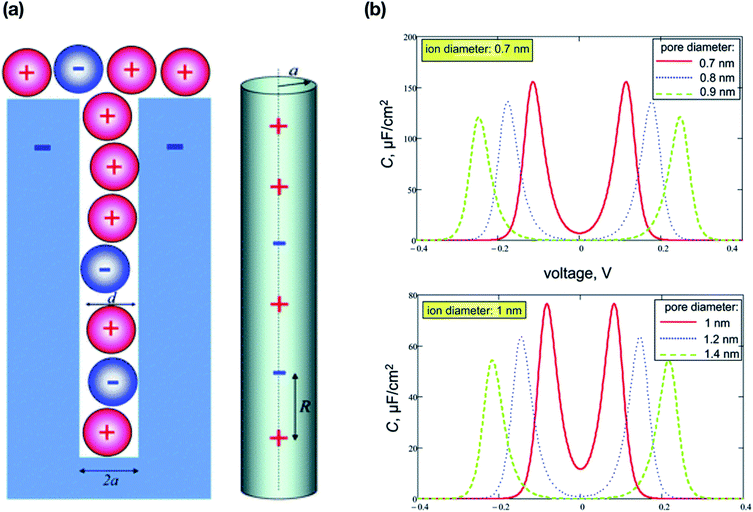 |
| | Fig. 8 (a) Schematic cross section of a negatively polarized electrode pore showing accommodation of one line of ions in the order of d < 2a < 2d. One dimensional lattice representation of distribution of ions. Reprinted with permission from Royal Society Publishing.73 (b) The specific voltage-dependent differential capacitance of a single pore per unit surface area. The effect of the diameter of the pore, shown for an ion diameter of 0.7 nm (upper panel) and 1 nm (lower panel); the effective dielectric constant of the pore interior, ε = 2. Due to saturation of charge density, the capacitance vanishes very quickly with voltage. (a and b) have been adapted/reproduced from ref. 73 with permission from the Royal Society of Chemistry.73 | |
To gain an insight into the intrinsic effects of nanopores on the electrochemical capacitive behavior, several experiments and hypotheses have been proposed. Itoi et al. proposed that heteroatom doping into porous carbon materials would potentially change the behavior of pore structures and sizes which in turn give noticeable pseudocapacitance in microporous carbon, e.g., ZTC (zeolite-template carbon).74 To increase the charge efficiency, the pore size distribution of electrode materials should be optimized for the removal of targeted ions by capacitive deionization through entrapment in EDLCs where the porous electrodes are polarized.75 Design and optimization of nanopores in graphene are critically important due to tunable porous channels which could potentially enhance the electrochemical storage capacity.76 Jung et al. showed that graphene aerogel supercapacitors exhibit a specific capacitance of 325 F g−1 at 1 A g−1 and an energy density of 45 W h kg−1 in a 0.5 M H2SO4 aqueous electrolyte with high electrochemical stability and electrode uniformity. Urita et al. proposed an ideal porous structure of carbon electrodes for EDLCs where the gravimetric capacitance was above ∼200 F g−1 even in an organic electrolyte by utilizing the carbon nanopore surface more effectively.77 For enhanced capacitive deionization (CDI), Gao et al. proposed that the usage of carbon electrodes with optimized chemical surface charge could extend the CDI working voltage window through discharge voltage which in turn increases the salt adsorption capacity.78 Several other studies proposed the characterization and effects of porous electrodes to meet the demands of three basic requirements: (i) increase the capacitance values, (ii) low resistance, and (iii) stability.77–90 Interesting work by Kalluri et al.91 reported atomistic simulations to study the effect of pore size and surface charge density on the capacitance of graphitic nanoporous carbon electrodes. They showed that through molecular dynamics (MD) simulations of pore-size dependent accumulation of aqueous electrolytes in slit-shaped graphitic carbon pores of different widths (0.65 nm to 1.6 nm), the capacitance of wider pores does not depend significantly on the applied potential window. Rather, the penetration of ions into pores becomes more difficult with decreasing pore width and increasing strength of the hydration shell which is in contrast to previously reported pore size effects. Another study by Song et al.92 reported the intrinsic ion selectivity effects of narrow hydrophobic pores using single-walled carbon nanotube membranes. They performed MD simulation studies with a pore radius of 3.4–6.1 Å, to show that Na+, K+, and Cl− face different free energy barriers when entering hydrophobic pores which gave consistent results with the work of Tai et al.93 MD simulations proposed earlier have been consistent with experimental results.94–120 Seminal studies by Kornyshev et al.73,121 proposed superionic states in EDLCs with nanoporous electrodes which are in agreement with the studies of Chmiola et al. & Largeot et al.,63,122 where they have explained the effect of an anomalous increase in capacitance with the decrease of pore size using image forces that exponentially screen out the electrostatic interaction of ions in the interior of pores. Also, basic assumptions for a nanopore model made by Kornyshev et al.73 led to future theoretical and experimental developments which may be useful for understanding what, in principle, nanoporous electrodes could deliver for energy storage. Apart from experimental and molecular atomistic observations, several first principles-based DFT (Density Functional Theory) theories have been proposed to exhibit the supercapacitor capacitance based on nanopore networks.123–136 The state of the art DFT methods have been proposed to calculate pore size distribution in various nanoporous materials.132,137–158 For instance, in the seminal studies of Seaton et al.,159 the pore size distribution from adsorption isotherms has been determined by DFT based methods in which they have suggested a method for modeling nitrogen adsorption on porous carbon by local mean-field approximations. Lastoskie et al.160 used non-local DFT within Tarazona smooth density approximation for modeling nitrogen adsorption on carbons. The pore size distribution range has been depicted below in the figure to understand the mechanism of how electrode porosity has a direct effect on particle to particle contact between the active material and the conductive diluent. By controlling porosity, higher intra-electrode conductivity can be achieved to ensure adequate electron exchange as well as sufficient void space for electrolyte access/transport.
Electrolyte materials and their applications
Apart from the most influential components such as electrodes, electrolytes have a greater impact on the performance of supercapacitors on the basis of ionic type, size, ion mobility, ionic conductivity, viscosity, thermal stability, and voltage window. We will cover some aspects of electrolyte materials to enable the readers to understand the importance of this in designing supercapacitors. The equivalent series resistance (ESR) is an important parameter for determining the power density of supercapacitors where the type of electrolyte used generally governs the charge/discharge rate. The electrolyte is usually the primary limiting operating voltage of the supercapacitor with its chemical stability issues.161 A schematic overview of the classification of electrolytes is illustrated in Fig. 9, comprising liquid electrolytes which include aqueous, non-aqueous, organic, ionic liquid, neutral, alkaline, mixed and acidic electrolytes; redox-active electrolytes that include gel polymers; solid-state/quasi-solid-state electrolytes which include inorganic solid-state electrolytes, solid polymer electrolytes, and gel polymer electrolytes.162 Considering the effects of electrolytes on increasing capacitance and power density, widening the potential window of the solution is one of the effective solutions. Higher priority must be given to the potential window in a range of working voltages for which the other parameters, e.g., power density, temperature range, salt solubility, material interaction, ion pore size, and electrochemical stability, under various conditions cannot be overlooked.163 Great efforts and multiple research activities are currently driven towards the development of advanced materials and configurations for enhanced energy densities, as well as cheaper preparation and processing methods. The aforementioned materials (electrodes and electrolytes) are widely fabricated, which in turn enables researchers to design novel material based supercapacitors. We will discuss the many efforts and approaches taken by researchers to design advanced electroactive materials and electrodes (specifically nanotubular materials) which is the main objective of this review.
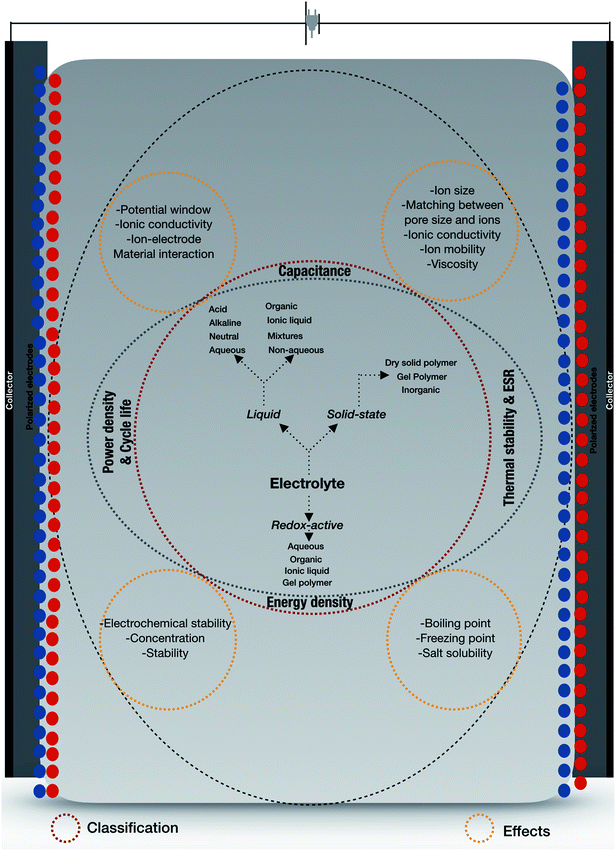 |
| | Fig. 9 Classification of electrolytes with electrochemical supercapacitor effects based on electrolytes. | |
Nanotubular networks
Since miniaturization of electronic components enables faster and more energy-efficient devices, micro- and nano-sized supercapacitors play a crucial role in the filtering of high-frequency noise in such systems. As capacitance is connected to physical size, any other technology would simply take too much space in microelectronic assemblies. Following these requirements, nanotube networks were found to be excellent candidates for supercapacitor materials (Fig. 10).
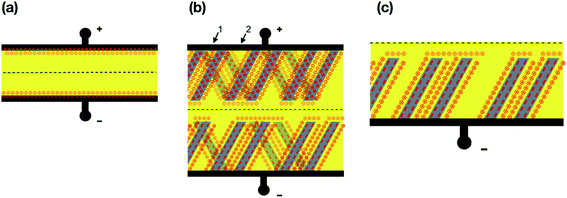 |
| | Fig. 10 (a) Schematic view of a charged capacitor (left) and (b) supercapacitor (right): solid black—electrodes; dashed—separator; yellow—electrolyte; nanotubes schematically shown in shades of gray; numbers indicate the hierarchical system of pores 1—small pores and 2 big ones; ” +”—positive charge; ” ⊖”—negative ion; ” −”—negative charge (electrons); ” ⊕”—positive ion. (c) Illustration of a part of a supercapacitor consisting of monolayers of nanotubes.167 | |
They can be made flexible (and even stretchable), are intrinsically porous and, being wrapped 2D sheets, possess an exceptional surface-to-volume ratio. Materials possessing tunable electronic properties can be used as electrodes within a wide range from insulating boron nitride nanotubes (BNNTs) to highly conductive carbon nanotubes (CNTs).164,165 One of the important discoveries made in 1991 by Sumio Iijima is of particular importance as the discovery of hollow, nanometer-sized tubes composed of graphitic carbon marked the beginning of an era in carbon nanotubes (Fig. 11).
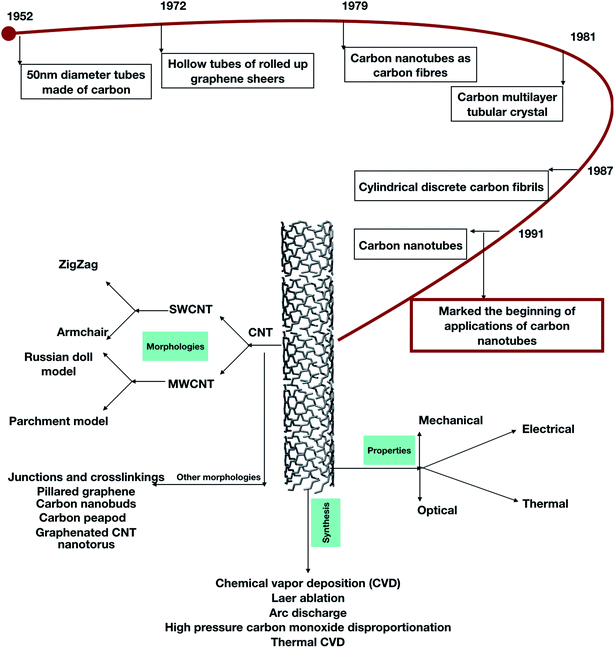 |
| | Fig. 11 A schematic diagram showing the discovery, morphologies, properties, and synthesis of carbon nanotubes. | |
Nanotubular materials are classified into three types: single-walled type (SW-type), multi-walled type (MW-type) and scrolled type (S-type) which are rectangular sheets rolled into single and concentric cylindrical forms with no edge atoms. S-type are generated from a single straight sheet keeping both ends free 166 (Fig. 12(a–d)). In SW-type nanotubes, due to rolling of sheets they undergo displacement of atoms which causes strain energy which is given by the following eqn (5) and (6):
where
N =
Ni +
Ne, and
Ni and
Ne are the numbers of internal and edge atoms respectively.
ε0 is the energy per atom of a periodic large sheet.
εe is the energy per one atom associated with edge atoms and
εs is the energy per atom due to strain. Also the strain energy is correlated with the diameter of the tube through
eqn (7)
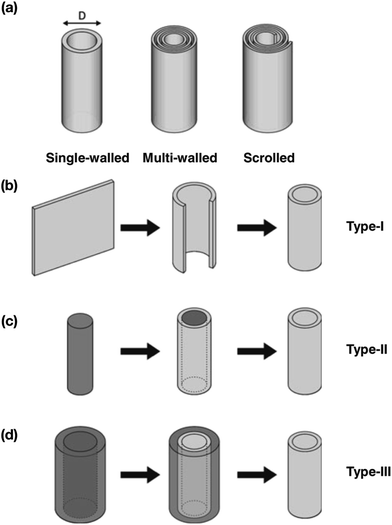 |
| | Fig. 12 (a). Type of nanotube. (b) Pathways of obtaining nanotubes, e.g., rolling of the sheet, (c) the surface of a core-type template, and (d) the internal surface of a sheath-type template. Reprinted with permission from Springer.478 (a–d) have been adapted/reproduced from ref. 478 with permission from Springer Nature.478 | |
Among the structural types of nanotubes, SW-type nanotubes are energetically more stable due to their topological configuration and excess energy due to strain unless the diameter of tubes is beyond a critical value e.g. Nεs < Nε0. Their unique topological and self-healing properties make these tubes very stable, which, however, require certain efforts for surface functionalization (Fig. 13), often a necessary requirement for tuning the electrode properties as well as matching the redox potential to that of the electrolyte. This is achieved either by aggressive surface treatments167 or wrapping CNTs with or around a polymer.168 Some polymers can form nanotubes on their own or around templates, with the notable example of the oldest known conducting polymer, polyaniline (PANI) nanotubes. It is worth mentioning that most of these materials are environment-friendly and/or are easy to dispose of. The discovery of fullerenes envisage a new horizon in synthesizing CNTs by rolling up graphene sheets into SWNTs and MWNTs that exhibit high stiffness and chirality169–171 which can be used in electronic devices as electrodes in supercapacitor applications172 due to their high electrical conductivity and flexibility. To facilitate the flow of electrons in CNTs, the fabrication and functionalization of the CNT networks is required. Generally, CNTs have an electron mean free path of ∼1 μm, an order of magnitude greater than conventional 2D materials. To allow the electrons to move freely and to obtain a better transport regime, the CNT networks present in the channel area (between the source and drain contacts) in conjunction with the electrodes require fabrication to observe the change in electrical signals which is the key factor in elucidating the mechanism of sensing. One such example has been reported by Gui et al.,173 where CNT van der Waals networks have been used to sense DNA nucleotides that showed sensing capabilities. The 3D networks with a high surface area and good electrical conductivity enable their use in various applications where the storage of gaseous species or ions is important. For instance, CNT networks in conjunction with metal oxides constitute efficient electrodes for electrochemical batteries and supercapacitors, in which the electrical conductivity and the large surface area of the CNT network are the key parameters for the battery performance.174,175 Considering lightweight supercapacitors, 3D nanotube networks with high electrical conductivity can be used as electrical conductors; CNT aerogels with densities of a few mg cm−3 could provide excellent substitutes for metal wires.176 A schematic view of CNT structures, effects and their application in supercapacitors is depicted in Fig. 13 with the conception of building CNTs from graphene177via van der Waals forces providing ultra-high-strength and light weight dimensions with conductive electrical and thermal properties.
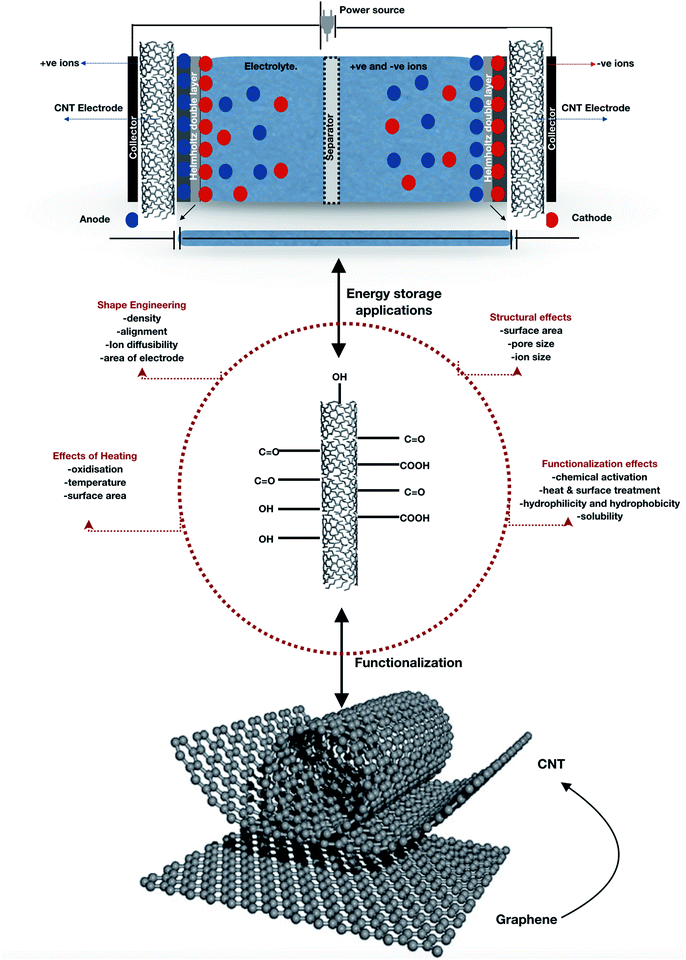 |
| | Fig. 13 CNT network for supercapacitor applications and its effects. | |
Various factors such as surface functionalization, heating, and structural effects with modified shape engineering influence the multiple applications of CNTs.178 For the first time, Niu et al.54 suggested that CNTs can be used as supercapacitors from the viewpoint of surface functionalization effects using nitric acid with functional groups on MWCNTs with a specific area of 430 m2 g−1, a gravimetric capacitance of 102 F g−1 and an energy density of 0.5 W h kg−1 obtained at 1 Hz on a single-cell device, using 38 wt% sulfuric acid as the electrolyte. Structural effects include the electrical charge accumulated due to electrostatic attraction on the surface of the electrode/electrolyte interface accessible by ions in the electrolyte. As CNTs possess a high surface area, the specific capacitance is always high in most cases; however, it also depends on the pore size distribution and conductivity. To explain the structural effects, Zhang et al.179 that CNTs with a diameter of about 25 nm and a specific area of 69.5 m2 g−1 had a specific capacitance of 14.1 F g−1 and showed excellent rate capability, better than those of entangled CNTs. To improve the graphitization of CNTs, heating is one of the major criteria to remove amorphous carbon and depends on the heating temperature and oxidization.180,181 With increasing temperature, the specific surface area increases, whereas the average pore diameter decreases and saturates at high temperatures. Chemical activation at surfaces of CNTs also enhances specific capacitance by surface functionalization.53,182,183 For instance, specific capacitance increased by almost seven times after chemical activation because of the microporosity of pure MWCNTs using chemical KOH activation.182 Another example of functionalization has been explained by Xiao et al.,184 in which the electrochemical performance was enhanced by the effect of annealing on different functional groups e.g., ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CO,
CO, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) COH and COOH at different temperatures to adjust the redox active functional groups on freestanding CNT films which was achieved at 200 °C that provides long term stability. Higher power density is another characteristic feature that enhances supercapacitor performance and can be achieved by shape engineering of CNTs. Highly densely packed SWCNTs showed higher capacitance and better performance for thick electrodes.185,186 Systematical investigations of the aforementioned factors can optimize CNTs for better performance and long-term stability. A new horizon for application of CNTs came into the limelight when Pankratov et al.187 reported on the first self-charging biosupercapacitor based on CtCDH/BOD (Corynascus thermophilus cellobiose dehydrogenase (CtCDH)/bilirubine oxidase (BOD) electrodes for direct electron transfer (DET) communication as shown in Fig. 14.
COH and COOH at different temperatures to adjust the redox active functional groups on freestanding CNT films which was achieved at 200 °C that provides long term stability. Higher power density is another characteristic feature that enhances supercapacitor performance and can be achieved by shape engineering of CNTs. Highly densely packed SWCNTs showed higher capacitance and better performance for thick electrodes.185,186 Systematical investigations of the aforementioned factors can optimize CNTs for better performance and long-term stability. A new horizon for application of CNTs came into the limelight when Pankratov et al.187 reported on the first self-charging biosupercapacitor based on CtCDH/BOD (Corynascus thermophilus cellobiose dehydrogenase (CtCDH)/bilirubine oxidase (BOD) electrodes for direct electron transfer (DET) communication as shown in Fig. 14.
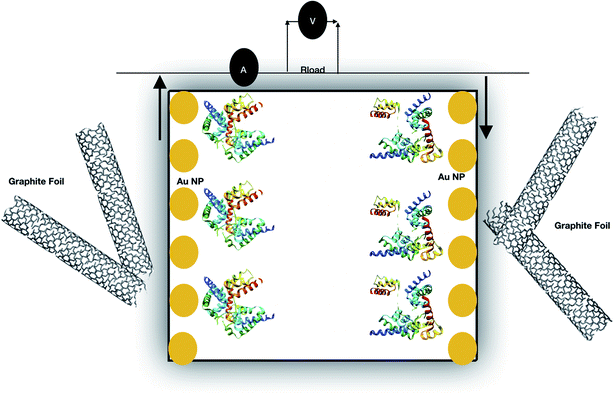 |
| | Fig. 14 Self-charging biological supercapacitor; capacitive side was built using graphite foil modified with a polyaniline/CNT composite and the charging side was prepared using an enzymatic fuel cell designed from 3D gold NP (yellow color) based nanobiostructures.190,479 | |
The capacitive part of the supercapacitor polyaniline/CNT composite was prepared by drop casting a suspension of nanotubes and electrodepositing a polyaniline film on the electrode surface.188 The charging part was comprised of CtCDH and Myrothecium verrucaria bilirubin oxidase (MvBOx), used as anodic and cathodic bio-elements, respectively, immobilized on gold electrodes modified with 20 nm AuNPs.189 The self-charging bio-supercapacitor provided a maximum power density of 1.2 mW cm−2 at 0.38 V improved by a factor of 170 in comparison to that of state-of-the-art EFCs albeit operating in a pulsed power mode.190 Carbon-based nanotube networks used for supercapacitors have been proven as promising candidates for energy storage applications. To get an overview of the specific capacitances of CNT based supercapacitors, we used a graphical illustration that is depicted in Fig. 15.
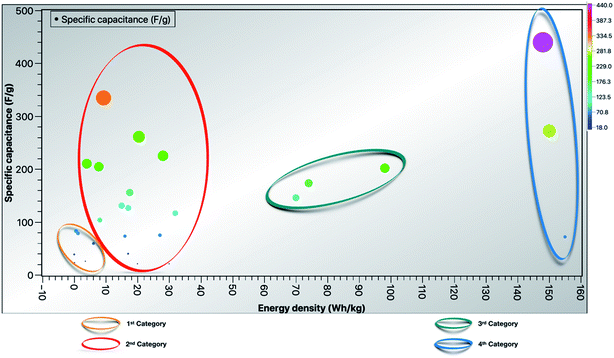 |
| | Fig. 15 Relational graph depicting specific capacitance vs. energy density in various nanotube network-based supercapacitors. | |
The plot was developed based on the relationship between specific capacitance (F g−1 on the y-axis) and energy density (W h kg−1 on the x-axis). The color bar scale represents the specific capacitance, and the category division was done based on energy density values obtained from the references as depicted in Fig. 15. The 1st category191–197 consists of supercapacitors with low energy density (0–10 W h kg−1) and specific capacitance below 100 F g−1, the 2nd category54,56,196,198–208 consists of supercapacitors with energy density in the range of 10–40 W h kg−1, the 3rd category209–211 consists of supercapacitors with an energy density range of 70–100 W h kg−1, and the 4th category212–214 consists of supercapacitors with energy density from 140–160 W h kg−1 and the description of the materials is tabulated in Table 3. Recent progress in CNT based high-performance supercapacitors has been immensely investigated using various techniques215–230 and more systematic investigations are needed to achieve high capacitance. However, using composite CNTs, e.g. with oxides, polymers or both, as electrodes for the stability of hybrid supercapacitor is still questionable.
Table 3 Relationship between specific capacitance (F g−1 on the y-axis) and energy density (W h kg−1 on the x-axis) in different materials as shown in Fig. 15
| Material |
Energy density (W h kg−1) |
Specific capacitance (F g−1) |
| CNTs |
0.02 |
39 |
| SWCNTs with transferred Cu |
0.13 |
23 |
| AMWCNTS |
0.55 |
83 |
| MWCNTs |
1.1 |
79 |
| CNTs |
3.56 |
25.6 |
| PAN based CNFs |
6 |
60 |
| MWCNTs |
7 |
18 |
| CNF-PAN + Nafion |
4 |
210 |
| CNF bacterial cellulose N, P-codoped |
7.76 |
205 |
| MWCNT catalyst removed by HNO3 |
8 |
104 |
| CNFs and PANI on PAN N2 doped |
9.2 |
335 |
| PAN/pitch base CNFs |
15 |
130.7 |
| VASWCNTs |
16 |
73 |
| CNF PAN + (PMHS) in DMF |
17 |
127 |
| Nanodiamond |
17 |
40 |
| Graphene sheet thermally reduced in water + IL solution which becomes gel in the end |
17.5 |
156 |
| MWCNTs transferred on Ni foil |
20 |
21 |
| CNF-bridged porous carbon by carbonization activation |
20.4 |
261 |
| VADWCNTs on CP |
27 |
75 |
| Carbon cloth/CNTs |
28 |
225 |
| Purified MWCNTs |
30 |
21 |
| (EG) exfoliated graphene |
31.9 |
117 |
| PAN-CNF + CNTS activation in KOH |
70 |
146 |
| Graphene sphere activated |
74 |
174 |
| CNFs@Ppy annealed at 900 °C |
98 |
202 |
| VAMWCNTs |
148 |
440 |
| Graphene water as a spacer |
150 |
273 |
| Graphene + CNTs on Ti |
155 |
72 |
Horizons of nanotube networks
Nanotube networks can be used as ultrathin separators made of BNNTs, where 0.5 m thickness is reported,231 but the most developed role of nanotube-based materials is in constituting supercapacitor electrodes. The reason why nanotubes perform better than, e.g., amorphous carbon (which is a cheaper solution) is believed to be nanotube organization in the hierarchical porous structure, with both micro- and meso-pores.178,232 This nanoscale order especially contributes to improved volumetric capacity, so that smaller devices can be created for microelectronic applications. Currently, CNT-based electrodes are the only ones where volumetric capacity (per cm3) can be twice the gravimetric capacity (per gram) and over 300 F cm−3, which means they can be relatively heavy in terms of weight but with small dimension. The theoretical limit for the capacitance of carbon-based materials in double-layer charge storage supercapacitors is just below 600 F g−1, as calculated from the specific capacitance of about 20 F cm−2 and specific surface area 3000 m2 g−1,43 and even smaller values were reported.164 Finite conductivity and geometrically limited full access to the whole surface area limit the specific capacitance of pure carbon-based double-layer charge storage supercapacitors to about 250 F g−1 and the practical energy storage limit is then below 10 W h kg−1. In the following, we will first discuss nanotube networks, and then review ways to overcome these limitations. One of the straightforward ways is to use pseudocapacitive or hybrid storage mechanisms, where template-made MgO2 nanotubes233 and similar arrangements hold a sort of a record.43 Another is to employ different (asymmetric) electrode materials to extend the operating voltage window beyond the electrolyte decomposition limit when different potential windows of the electrodes are used to maximize the operating voltage of the full device. In view of the requirements imposed on superconductor electrode materials, especially a hierarchical porous morphology, nanotube networks for such applications are grown in a controlled way. Different kinds of templates are used, like ZnO tetrapods, alumina porous templates, MgO4, or templates of catalyst particles deposited on a surface to facilitate the growth of a” forest” of nanotubes, often guided with an external field. Later followed strategies have often called on template-free growth methods.214,234 PANI nanotubes are synthesized using a sodium dodecyl sulfate template. Hierarchical carbon nanotube tube networks created by a direct wet chemical infiltration of dispersed CNTs into a porous ceramic network235 form self-entangled networks surrounding the ceramic network in the form of a nano-felt assembly, resulting in a double-hierarchical CNTT (CNT tube) architecture.165 Tubes form an open pore structure with pores in the range of several micrometers, which is beneficial for supercapacitor applications due to the ease of surface accessibility. Also, CNTT–polymer composites were built over these networks. In 2017, a lightweight material aerographite was introduced (Fig. 16). Aerographite236 is a 3D interconnected carbon foam with a hollow tetrapodal morphology. The electrical conductivity of aerographite is strongly dependent on the wall thickness, the degree of graphitization and the ambient temperature. The wall thickness of aerographite, in turn, can be controlled by a stepwise reduction of solid arms of the sacrificial ZnO template with respect to synthesis time, in which wall thicknesses between 3 and 22 nm can be easily achieved (Fig. 16(a–f)).
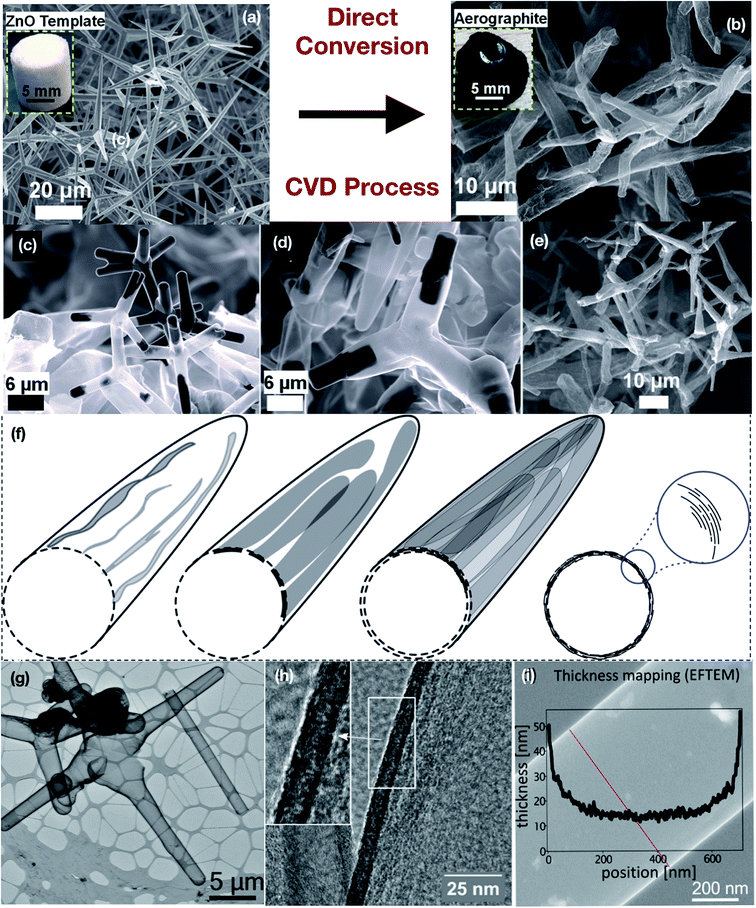 |
| | Fig. 16 (a and b) Highly porous ZnO tetrapodal network template transformed into a nano-microtubular carbon based aerographite tetrapod network through the CVD process. Hydrogen gas molecules selectively etch ZnO and toluene gas simultaneously deposits carbon at high temperatures inside the CVD chamber. (a) The SEM image demonstrates the tetrapodal geometry of ZnO structures. (b) Converted carbon based network tetrapodal morphology of the aerographite network. The conversion process takes place via selective removal of ZnO.385 SEM images of aerographite conversion from ZnO: (c) partially converted, (d) almost converted, and (e) completely converted (crumbled aerographite network).385,480,481 (f) Schematic of sacrificial growth of aerographite tubular carbon arms over ZnO arms through a belt-like growth process. Aerographite structures retain the initial ZnO template architecture which is mainly tetrapodal. The aerographite tetrapods are hollow from inside with a nano-microtubular arm morphology480,481 in contrast to the initial ZnO template which is solid. (g) TEM image of typical aerographite particles in the ‘normal’ hollow, closed graphitic shell modification on a lacey carbon grid. (h) HR-TEM image with the parallel view of an aerographite closed shell with a low curvature (diameter of the tubular ligament ≈ 2000 nm). (i) EFTEM was used to investigate the thickness of the intact hollow closed shell aerographite ligament (red line indicates the locations where the thickness measurement was performed). (a–i) have been adapted/reproduced from ref. 385 with permission from Elsevier.385 | |
The decrease of the wall thickness leads to a reduced electrical conductivity of untreated aerographite (Fig. 16(b)). In contrast, the conductivity of annealed aerographite increased with reducing the wall thicknesses.236 Aerographite shows a metallic conductive behavior that can be changed to semiconducting by further high-temperature treatment236 due to the surface defects, charge carrier density and graphitization that increases the electrical conductivity in thin-walled structures and shows low bandgap energies as the bandgap energy is dependent on the wall thickness of aerographite. The unique combination of a high surface area, light weight, large porosity, tunable conductivity, etc., of aerographite makes it a suitable candidate with high potential in the direction of supercapacitor and other energy-related applications.
Nanostructured materials for advanced supercapacitors
Nanotechnology has undoubtedly opened a new avenue enabling new materials and technology for energy storage applications. Out of all the new materials proposed to date for energy storage applications, graphene has proven to be the mother of all graphitic forms because of its capacity to be wrapped into 0D buckyballs, a roll of 1D nanotubes and a stacked 3D graphite form.237 These materials continuously spur the development of high capacitive, cost-effective, and highly efficient energy storage. To mitigate the deficiencies imposed by lithium-ion batteries due to the lack of lithium resources,238–241 nanostructured electrodes play a pivotal role as they possess structure-dependent advantages accelerating the reaction kinetics in the diffusion process as well as achieving good cycling stability. The diversified advantages of various nanostructures for high performance energy storage applications have attracted many researchers to delve into designing electrode materials in many forms such as e.g. low dimensional architectures (0D, 1D, 2D, and 3D), and hierarchical & hollow nanostructures which include single-shelled hollow spheres, tubular nanostructures, hollow polyhedrons, yolk-shelled nanostructures, multi-shelled nanostructures and frame like nanostructures.242 The characteristics of all the diversified nanostructures can be summarized as follows: 0D nanostructures can be amorphous or crystalline and exhibit various shapes and forms being single or polycrystalline, composed of single or multiple chemical elements respectively, generally nanomaterials. 1D nanostructures comprise nanotubes, nanorods, and nanowires being a standalone material or embedded within another medium. 2D nanostructures exhibit plate-like shapes including nanofilms, nanolayers and nanocoatings being single or multi-layered structures deposited on a substrate. 3D nanostructures are not confined within the range of nanoscale in any dimension arbitrarily characterized above 100 nm, composed of multiple arrangements of nanosized crystals in different orientations which contain the dispersion of nanoparticles, bundles of nanowires and multiple nanolayers. Nanostructured materials (NSMs) have been characterized on the basis of dimensionality.243 0D NSMs comprise uniform particle arrays, e.g. quantum dots,244 heterogeneous particle arrays, core–shell quantum dots, onions, hollow spheres, and nanolenses. 1D NSMs are ideal systems having a large number of novel phenomena at the nanoscale level. The size and dimensionality of 1D NSMs depends on the structural and functional properties such as nanowires, nanorods,245 nanotubes,246 nanobelts,247 nanoribbons,248 and hierarchical nanostructures.249 2D nanostructures have two dimensions outside of the nanometric size range and comprise junctions, branched structures,250 nanoprisms, nanoplates,251 nanosheets,252 nanowalls253 and nanodisks.254 3D NSMs have a large specific surface area and other superior properties over their bulk counterparts arising from the quantum size effect, e.g. nanoballs,255 nanocoils,256 nanocones, nanopillars257 and nanoflowers258 (Fig. 17). Particular importance has been given to design heterogeneous nanostructures both theoretically and experimentally for energy storage applications. A schema devised by Liu et al.259 (Fig. 18) shows the nanostructured materials used as electrodes acquire high energy densities at high rate capabilities which can be dramatically enhanced if optimal configured nanostructured materials can be chosen. They have classified them into 4 major divisions such as 0-D consisting of core–shell nanoparticles, nanospheres and composite nanoparticles, 1-D consisting of CNTs and nanowire arrays, 2-D consisting of graphene based composites and carbon coated nanobelts and finally 3-D nanostructures consisting of mesoporous–microporous composite electrodes. Some of the previously reported nanostructures comprising the aforementioned structures have significantly improved the capacitance and storage capabilities for energy storage applications. 0D or quasi-zero-dimensional materials are atomic clusters and nanoparticles which are spatially confined molecular systems possessing an aspect ratio from one to infinity. They can be used as electrode materials with a nanoscale crystalline particle size and show better discharge life due to their high specific surface area eventually improving the power density. Some of the typical examples considering 0D nanoparticles for supercapacitor application include hydrous IrO2 (ref. 260) (specific capacitance 550 F g−1), SnO2,261 and amorphous RuO2 (ref. 262) (specific capacitance 720 F g−1). He et al.263 found that particle size has an intrinsic effect on reaction kinetics to achieve better cycling stability in 0D nanocrystals. Combining 0D active materials with carbonaceous materials is an effective strategy to improve the efficiency of energy storage.264 Similarly 1D NSMs are also able to solve the problem of low specific surface area and provide improvisation for electrode active-materials. Zhao et al.265 investigated the nanosizing effects of ultrasmall FeSe2 with a high surface area which exhibits enhanced electrochemical performance with a high capacity of 500 mA h g−1, good rate capability of 250 mA h g−1 at 10 A g−1, and superior cycling stability over 400 cycles. 1D nanostructures including metal oxide frameworks,266–270 carbon-based materials271–278 and metal chalcogenides279,280 have distinctive accelerated charge transfer properties. Ou et al.281 synthesized rGO-coated Sb2Se3 nanorods that showed a current density of 0.1 A g−1, a good rate capability of 386 mA h g−1 at a current density of 2 A g−1, and excellent stability up to 500 cycles. In recent years, 2D nanostructured materials have been a focus in materials research due to their low dimensional characteristics and bulk properties. Their unique shape-dependent characteristics have evolved to their usage in sensors, photocatalysts, energy storage applications, and nanoreactors.282–284 Hierarchical nanostructures or 3D nanostructures exhibit great cycling stability259 and reaction kinetics due to their 3D configurations,285–290 whereas hollow nanostructures owing to their well-defined shell and interior void have versatile features providing extra space for strain and buffering large volume change during reaction improving the cycling stability.291–306 Owing to the characteristics of nanostructured materials, the performance can be notably differentiated. Hybrid supercapacitors comprising of 3D nanostructures have greater efficiency due to the stoichiometry and arrangement of electrode materials. Li et al.303 fabricated ultrathin MoS2 nanosheet arrays vertically anchored on bagasse-derived three-dimensional (3D) porous carbon frameworks for an efficient increase in conductivity, high specific capacity and sodium-ion diffusion rates. Gu et al.304 investigated four-layered bismuth oxyhalogens for EDLC's capacitive and pseudo-capacitive behavior. They concluded that due to the faradaic reaction mechanism, BiOI exhibits the highest capacitance among the four bismuth oxyhalogens; due to the high electronegativity of F, BiOF has a higher capacitance than BiOCl and BiOBr and due to the larger interlayer distance, BiOBr has a higher capacitance than BiOCl. Similar work has been proposed for the fine layered structure and properties of Bi2SiO5 and Bi2O2CO3.305
 |
| | Fig. 17 Typical SEM and TEM images of NSMs, e.g. 0D: (a) quantum dots, (b) nanoparticle arrays, (c) core–shell nanoparticles, (d) hollow cubes, and (e) nanospheres. 1D: (a) nanowires, (b) nanorods, (c) nanotubes, (d) nanobelts, (e) nanoribbons, and (f) hierarchical nanostructures. 2D: (a) junctions (continuous islands), (b) branched structures, (c) nanoplates, (d) nanosheets, (e) nanowalls and (f) nanodisks. 3D: (a) nanoballs (dendritic structures), (b) nanocoils, (c) nanocones, (d) nanopillars and (e) nanoflowers. This figure has been adapted/reproduced from ref. 243 with permission from Elsevier.243 | |
 |
| | Fig. 18 Schematic illustration of different heterogeneous materials based on structural complexity. This figure has been adapted/reproduced from ref. 259 with permission from the Royal Society of Chemistry.259 | |
The imminent fortuity of graphene-based supercapacitors
In this current review, we will now focus on lightweight supercapacitors that have an imminent opportunity to be used widely in the near future, and one such example is graphene where we can expect the scenario of using viable graphene-based supercapacitors using advanced energy storage and recovery solutions.306,307 Graphene is a thin layer of pure carbon endowed with an abundance of specific features, such as being the best known conductor, environmentally friendly and sustainable, a tightly packed hexagonal honeycomb lattice procuring high strength as it is the thinnest compound with high strength and light absorption characteristics, and it can replace activated carbon in supercapacitors due to its high surface area, light weight and elasticity.308 Due to its wide range of applicability for instance usage in ultra-fast computers, transforming transportation (cars and aircraft), weaving washable wearables, flexible and transparent solar cells, cleaning up oil spills, desalinating seawater, preventing microbial infections etc., graphene has been considered as potential electrode material for electrochemical energy storage devices.309 The theoretical upper limit of storing charge previously was estimated to be 550 F g−1 based on graphene-based supercapacitors which are compared to current supercapacitors that have a limit of 150 F g−1. One advantage of using graphene as an electrode material is that it doesn't depend on the distribution of pores like other carbon-based materials and has a high surface area that is readily accessible to the electrolytes. Utilizing the intrinsic surface area and surface capacitance, the stacking of graphene layers has been avoided to increase the efficiency of the heterogeneous electron transfer kinetics. For that purpose curved graphene layers were introduced for electrode material preparation where an energy density of 85.6 W h kg−1 at room temperature and 136 W h kg−1 at 80 °C were measured at a current density of 1 A g−1.6 Although many approaches have been used to synthesize graphene for supercapacitor applications to prevent agglomerations that tend to restack back to graphite,200,201,310,311 a novel low temperature based exfoliation of graphene due to unique surface chemistry has been obtained which has a high capacitance rate and good energy storage performance.312 The restacking and aggregation nature of graphene motivated by strong π-π interaction compromises the capacitance values and leads to reduced conductivity. To overcome the problem of restacking, 3D graphene has attracted a lot of attention .285,313–319 Conventional supercapacitors use activated carbon as an electrode material whose stability has been compromised for the automobile industry due to instability and high voltage and temperature operations. The typical voltage of single cells using activated carbon is limited to 2.8 V, and the stacking of a large number of cells is thus necessary to achieve 300 to 500 V for automobiles that require high stability and high operating voltage. Single-walled carbon nanotubes (SWCNTs) are superior to ACs in terms of voltage stability and can achieve up to 4.0 V at room temperature. Recently, Nomura et al.320 reported a new electrode material, a seamless mesoporous carbon sheet consisting of continuous graphene walls with a very small number of carbon edge sites (Fig. 19(a–e), which are the origin of corrosion reactions. Using this new material, it is possible to assemble symmetric supercapacitors with excellent stability under 3.5 V@60 °C and 4.4 V@25 °C conditions (Fig. 19(f–j)), even using a conventional organic electrolyte. Moreover, high-voltage operation at 4.4 V makes it possible to achieve 2.7 times higher energy density compared to conventional ACs.
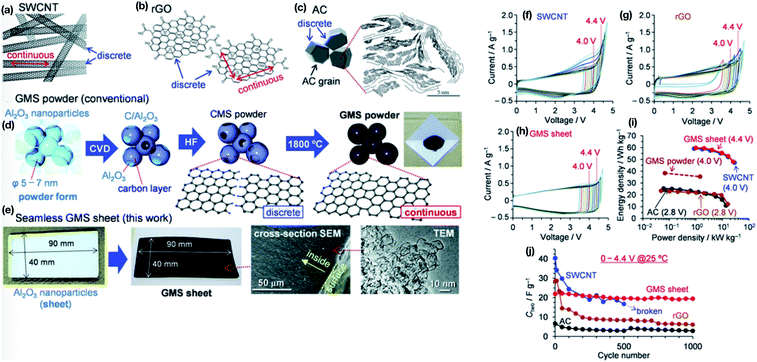 |
| | Fig. 19 (a) Carbon materials used in the work of Nomura et al. where they have demonstrated a superstable mesoporous carbon sheet made of edge-free graphene walls. The figure depicts (a) SWCNTs (single-walled carbon nanotubes), (b) rGO (reduced graphene oxide), (c) AC (activated carbon), (d) GMS (graphene mesosponge) powder preparation, and (e) sheet-moulded Al2O3 nanoparticles and a GMS sheet with cross-sectional SEM and TEM images. (f) Cyclic voltammetric scans of (a) SWCNTs, (g) rGO (reduced graphene oxide) and (h) GMS (graphene mesosponge) performed at 10 mV s−1 in 1.5 M TEMA-BF4/PC. (i) Ragone plot measured at upper limit voltage. (j) Capacitance vs. cycle numbers. (a–j) have been adapted/reproduced from ref. 320 with permission from the Royal Society Of Chemistry.320 | |
As far as the applications of graphene-based supercapacitors are concerned, several properties modulate the preparation of graphene-based materials to be widely used in different applications that have an intrinsic effect on physical and chemical properties. Properties321 and characteristics are depicted in Fig. 20(c). An important physical property of supercapacitor electrode materials is electrical conductivity which is imposed by graphene due to fast charge transfer between the electrodes.26 Another crucial aspect of graphene is surface functionalities where heteroatom doping and introducing functional groups are possible, for instance, oxygen, nitrogen, and sulfur and phenol, keto, ether, and quinone groups respectively.15,322–324 Other properties such as the edges, surface area, pore size and interlayer distance between the graphene sheets play a major role in increasing capacitance and power density by the mechanism of synthesis for which physicochemical properties have been affected in order to produce better performances.321 Apart from physicochemical properties, some of the mechanical properties such as stiffness, strength, and toughness affect the multi-functionality of such materials which in turn is a challenge to the industry and scientists to explore further.325 The development of supercapacitors is driven by their applications (Fig. 20(a)). They include primarily integrated energy sources, especially as a buffer in front of a rechargeable battery. Such use prolongs the battery life and enables fast energy recycling, e.g., from breaking of electric vehicles. Supercapacitors can be made flexible with applications in self-powered (especially piezo and small temperature difference powered devices) and wearable electronics (Fig. 20(b)). The other main aspects of graphene-based biological supercapacitors may enable improved pacemakers and implantable medical devices using ions derived from body fluids which could lead to long-lasting cardiac pacemakers. They work on the principle of graphene layered with a modified human protein as an electrode which is typically 1 μm thick, flexible and twisted inside the body without any mechanical damage while improving the charge storing capacity326 as depicted in Fig. 20(d). The application is not limited to biomedical applications but has attracted many areas of research such as bioengineering, drug delivery, tissue engineering, biotechnology and bioinformatics327 (Fig. 20(e)). Another specific example is the antibacterial activity of graphene oxides that have potential effects on therapeutics and disease diagnosis328 (Fig. 19(f)). Since graphene induces some electrostatic effects the lipid bilayer acts as an electrolyte and graphene as an electrode to induce charge transfer, thereby accelerating the antibacterial activity through reactive oxygen species (ROS) and oxidative stress329 (Fig. 20(g)). From environmental aspects, graphene-based materials have additional advantages in addressing global environmental challenges.
 |
| | Fig. 20 (a) Application of graphene-based supercapacitors in various sectors. (b) Graphene-based supercapacitors or miniaturized bioelectronics. (c) Properties affecting the synthesis of graphene-based supercapacitors varying from 2D graphene to 3D curved graphene. (d) Improved pacemakers and implantable medical devices. (e) Graphene-based supercapacitors for tissue engineering. (f) Graphene-based supercapacitors for drug-delivery. (g) Antibacterial activity of graphene with altered biological properties. | |
Graphene-based materials can be used as a sorbent or photocatalytic material for environmental decontamination for future water treatment and desalination membranes. The exceptional properties of graphene-based materials have also shown progress in the adsorption of several contaminants, metal ions, organic compounds, and gaseous pollutants. Also, graphene-based membranes such as nanoporous graphene membrane sheets and graphene-oxide barriers have an additional advantage in water treatment, water desalination, capacitive deionization, and antimicrobial activity whereas reduced graphene oxide (rGO) is widely used in gas sensing technology (chemical and biological sensing).330–354 The next important factor influencing EDLC performance is quantum capacitance from electronic structure theory that arises from the limited Density of States (DOS) contribution near the Fermi level due to band filling/emptying during the charging of EDLCs.355 The effects of quantum capacitance have been firstly observed by Tao et al., on single-layer graphene in an electrochemical cell that showed good agreement with DFT predictions.356 Later, Ruoff et al.357 studied the layer effect on the capacitance for few-layer graphene electrodes, and Downard et al.358 measured the quantum capacitance of aryldiazonium modified few-layer graphene electrodes. Quantum capacitance can be obtained from the electrode DOS directly357,359–364 and is relevant for the studies of gated graphene and nanotubes for capacitance–voltage profiling. Ji et al.357 measured the capacitance of one to five-layer graphene in EDLCs where they found that capacitance has been suppressed near neutrality and is abnormally enhanced for thickness below a few layers. Recently, Raha et al.365 investigated a forest-like 3D carbon structure formed from reduced graphene oxide used as an electrode material for high-performance supercapacitors where they validated the effect of quantum capacitance on a graphite sheet based supercapacitor by means of DFT and experimental studies. Zhan et al.366 investigate a novel C60-modified graphene supercapacitor material, which highlights a complex interplay among the surface morphology, electronic structure, and interfacial capacitance, suggesting general improvement strategies for optimizing carbon-based supercapacitor materials. An effective strategy to construct novel electrode materials with high energy storage density has been illustrated by Li et al.,367 using N-doped graphene quantum dots for high-performance flexible supercapacitors. Song et al.368 and many other researchers369–378 investigated graphene oxide as a supercapacitor electrode material to study the quantum capacitance by means of DFT studies. These observations may shed light on developing new theoretical models based on DFT studies for the improvement of the energy density of carbon-based supercapacitors.
Two-dimensional nanotubular materials for hybrid supercapacitors
Few capacity records are held by nanotube network-based supercapacitors. The reason lies in a generally higher specific surface area of activated carbon materials. However, these structures often lack the required hierarchical morphology. Therefore exceptional capacitances are obtained only at slow voltage scan rates. At about 5 to 10 mV s−1 scan rates capacities reach the order of 500 F g−1, which will probably be improved by the time this review is published,379,380 and the same is true for rationally stacked graphene.381 Although carbon nanotube network-based supercapacitors so far generally demonstrated slightly lower capacitances, there are some notable exceptions. CVD grown multi-walled CNTs on nickel catalyst particles attached to microfibrous carbon paper and functionalized with carbon aerogel coating yielded 524 F g−1 capacitance.176,381 Here the hierarchical morphology was created from the aerogel to the CNT network and over to microfibers. This result can be 2 to 6 times improved (up to 3000 F g−1) by activating the CNTs, however, at the expense of lower stability.382 Lower stability with capacitance decreasing with cycling can be understood from self-healing of activated nanotubes, and opening the interior of the tubes with activation provides rate-dependent results. Already in 2011 a polyaniline–MnO2 nanotube nanocomposite supercapacitor was demonstrated to have 626 F g−1 specific capacitance and a corresponding energy density of 17.8 W h kg−1.383 The way to improve these results is to employ pseudocapacitive charge storage. However, for this purpose the currently used oxide and polymer nanotube networks have an insufficient surface area and conductivity compared to CNTs, graphene and activated carbon.384 Therefore, it is desirable to develop functionalized carbon nanotube networks suitable for the hybrid storage mechanism. For the first time, the utilization of an ultralight and extremely porous nano-microtubular aerographite tetrapodal network as an electrode interface for electrochemical capacitive energy storage was reported by Parlak et al., in ref. 385. A simple and robust electrode fabrication strategy based on surface functionalized aerographite with optimum selected porosity leads to high specific capacitance (640 F g−1) and high energy (14.2 W h kg−1) and power densities (9.67 × 103 W kg−1). The study also indicates that aerographite shows hybrid capacitive behavior, bearing a double layer and pseudocapacitance simultaneously. Recently Zhou et al.386 showed the fabrication of flexible electrodes based on nanocarbon electronic conductors combined with pseudocapacitive materials for wearable supercapacitors. Novel flexible densified horizontally aligned carbon nanotube arrays (HACNTs) with a controlled nanomorphology for improved ion transport are introduced and combined with a conformally coated poly(3-methylthiophene) (P3MT) conducting polymer to impart pseudocapacitance. The resulting P3MT/HACNT nanocomposite electrodes exhibit a high areal capacitance of 3.1 F cm−2 at 5 mA cm−2, with areal capacitance remaining at 1.8 F cm−2 even at a current density of 200 mA cm−2. The asymmetric supercapacitor cell also delivers more than 1–2 orders of magnitude improvement in both areal energy and power density over the state-of-the-art cells. Yi et al.387 demonstrated a hybrid supercapacitor constructed from a Si-based anode (with boron doping) and a porous carbon cathode that delivers a capacity of 685 mA Hg−1 at a high current density of 6.4 A g−1. The hybrid supercapacitor exhibits a high energy density of 128 W h kg−1 at 1229 W kg−1 with a long cycling life (capacity retention of 70% after 6000 cycles and low self-discharge rate (voltage retention of 82% after 50 hours)). Some of the recent advancements and progress in nanotubular materials for hybrid-based supercapacitors are listed in Table 4.
Table 4 Progress in nanotubular materials for hybrid supercapacitors
| Material |
Energy density |
Specific/volumetric capacitance |
Cycles |
Specific power (W kg−1) |
Reference |
| Mo oxide nanotube arrays |
— |
2000 F cm−3 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
450
|
| MoS2 nanosheets |
26 W h kg−1 |
26.7 F g−1 |
6000 |
750 W kg−1 |
451
|
| Poly(3-oligo(ethylene oxide))thiophene (PD2ET) with SWCNTs |
22.5 W h kg−1 |
399 F g−1 |
8000 |
— |
452
|
| MnO2−X/MWCNT |
— |
∼259.6 F g−1 |
>10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
453
|
| (GO)/carbon nanotube (CNT) foams |
— |
56.69 F g |
2000 |
— |
454
|
| POM (polyoxometalate) based inorganic–organic hybrids |
|
986–1611 F g−1 |
1000 |
|
455
|
| CoS2/NSC-CNT-p |
14.1 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W h kg−1 W h kg−1 |
544.2 ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F g−1 F g−1 |
2000 |
|
456
|
| Bi-metal (Ni wire) organic framework nanosheets Ni@CoNi-MOF/CNT |
31.3 mW h cm−3 |
100 F cm−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
457
|
| Ag micro/nanoparticles (MNPs) in MWCNTs |
— |
1083 F g−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
458
|
| Polypyrrole-based hybrid nanostructures |
|
64 F g−1 |
500 |
|
459
|
| TiO2-VACNT (vertically aligned carbon nanotube) hybrid |
|
16.24 mF cm−2 |
5000 |
|
460
|
| Vertically aligned CNT-TiO2 |
0.041 μW h cm−2 |
6.436 mF cm−2 |
2000 |
|
461
|
| Porous carbon nanotube-graphene hybrid fibers (CNT-GFs) |
4.83 mW h cm−3 |
60.75 F cm−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
223
|
| Zn–Co–S@HTCSs//Fe2O3@PPNTs |
85.12 W h kg−1 |
∼149 mA Hg−1 |
6000 |
460 W kg−1 |
462
|
| Carbon nanosheet (CN)/CNT |
— |
110 mF cm−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
463
|
| Copper hexacyanoferrate (CuHCF)/MWCNT |
60.4 W h kg−1 |
989 F g−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.5 kW kg−1 |
464
|
| N-Doped carbon nanonet flakes (NCNFs) |
— |
613 F g−1 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
465
|
| Copper (Cu) hydroxide nanotube array grafted nickel aluminum layered double hydroxide nanosheets (CH NTAs@NiAl LDH NSs) |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991.5 μW cm−2 991.5 μW cm−2 |
250 μAh cm−2 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
466
|
| CNT/MnO2 |
8.14 mW h cm−3 |
492 F cm−3 |
7000 |
— |
467
|
| Ni6MnO8@CNTs |
58.2 W h kg−1 |
711 F g−1 |
5000 |
831.4 W kg−1 |
468
|
| Carbon fiber-reinforced cellulose nanofiber/multiwalled carbon nanotubes (CF-CNF/MWCNT-HAs) |
8.93 mW h cm−3 |
19.4 F cm−3 |
3000 |
|
469
|
| TiO2 nanotube arrays (TNTAs) TNTAs/C/MnO2 |
465 mW h m−2 |
492 mF cm−2 |
3000 |
— |
470
|
| Ultrafine tin dioxide (SnO2)/few-walled carbon nanotubes (FWNTs) |
30.63 kW kg−1 |
220.5 F g−1 |
1000 |
512.79 W kg−1 |
471
|
| Zn2GeO4/CNT-O |
— |
120 F g−1 |
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
472
|
Metal oxide (MOx) based nanotube networks for hybrid supercapacitors
The current focus is synthesizing multifunctional materials with ultralow density, a high surface area, and mechanical robustness which are emphasized in energy storage devices.388 These nanostructured materials have been devised to functionalize in energy storage devices being fabricated on the basis of a high surface area in which electrolyte ions can easily permeate, diffuse with high porosity through these functionalized electrode materials.389–391 Many efforts have been devoted to incorporating metal oxide frameworks into graphene sheets resulting in 3D composite electrodes392 as shown in Fig. 21.
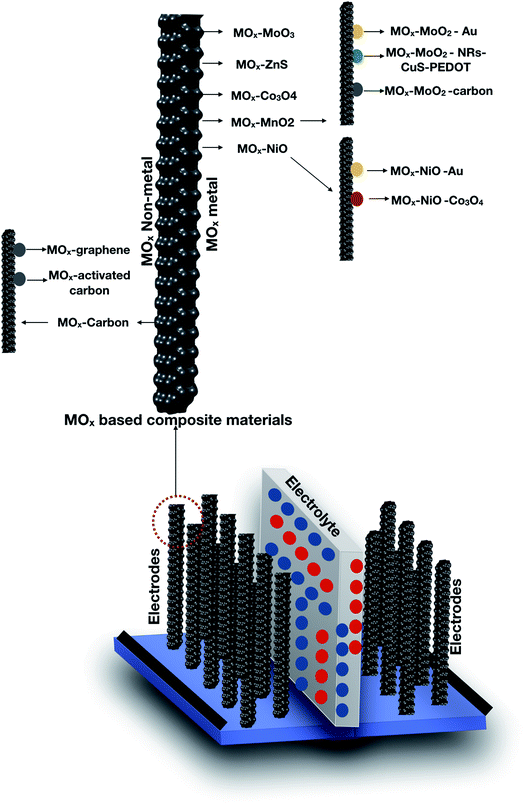 |
| | Fig. 21 Metal oxide (MOx) based composite materials for advanced supercapacitors, including composites doped with metal and nonmetallic materials. | |
We will give some instances where nanostructured metal oxides have been used as a framework for advanced supercapacitors. As reported by Sharma et al.,393 hollow nanostructures of copper oxides with various morphologies have counteracted electrochemical stabilization in their solid counterparts where higher specific capacitance values through cyclic voltammetry and charge–discharge studies have been reported compared to copper oxide-based composites with CNTs, graphene and rGO. The specific capacitance as reported was estimated to be 144 F g−1 at a current density of 1.0 A g−1 as compared to conventional Cu2O/rGO,394 microstructures,395 and nanoparticle-MWCNTs396 with capacitance values of 31 F g−1, 173 F g−1, and 132 F g−1 at current density values of 0.1 A g−1, 0.1 A g−1, and 2.5 A g−1 respectively. The first metal oxides (RuO2), which brought limelight to pseudo-capacitors with very high specific capacitance values was never used as a framework for energy storage devices,397 due to high toxicity and cost. Rather alternative approaches e.g. hybrid composites were considered. For example, RuO2/CNT (anode) based composite materials delivered high power with the aim of developing hybrid capacitors in combination with Co(OH)2/CNTs (cathode) without loss of energy where the maximum energy density and specific power density of the cell reach the value of 23.7 W h kg−1 and 8.1 kW kg−1, respectively.398 For a high-performance supercapacitor, MnO2 as a pseudocapacitive material has been extensively investigated for fabricating fiber shaped supercapacitors in combination with CNTs. Gong et al. investigated399 a fiber shaped supercapacitor with coaxial Ni as the current collector based on a CNT/MnO2 hybrid nanostructure shell with a capacitance of 231 mF cm−1. It works on the principle of redox interactions and ion intercalation/deintercalation and has vast applications in wearable electronic devices. Recently, vanadium oxide (V2O5), a cheap transition metal oxide in combination with CNTs, has become a promising candidate in supercapacitor application.400 It possesses high porosity and high pore volume that ensures electrolyte deposition on high surface area electrodes within the range of nanometer-scale showing a high specific capacity of 452 mA h g−1 and retaining up to 65% of the capacity while discharging. Mandal et al.401 investigated the same with a thin film coating of ∼6 nm V2O5 that has a high capacity of 680 mA h g−1 with a 67% retention rate during discharge cycles. Low-cost zinc oxide (ZnO) based composite materials have a greater impact on advanced supercapacitors due to their viable properties such as good electrochemical reversibility, eco-friendliness, good cycling stability and simple preparation. Kalpana et al. first reported a ZnO/carbon aerogel402 composite where ZnO electrode porosity improves charge/discharge cycles with the capacitance of the mixed oxide electrode/carbon aerogel being 375 F g−1 at 75 mA cm−2. Wu et al.403 successfully synthesized ZnO/rGO compounds with uniform incorporation of RGO sheets into the ZnO matrix and achieved a specific capacitance of up to 308 F g−1 at 1 A g−1, and only 6.5% of the available capacity was attenuated after more than 1500 cycles. Du et al. reported that a rGO and honeycomb-like ZnO particle mixed material exhibited an improved capacitance of 231 F g−1 at 0.1 A g−1 due to the presence of spacers and also at the same time flower-like ZnO decorated rGO was reported with a specific capacitance of 149 F g−1 at 0.1 A g−1.404,405 Bi et al.406 demonstrated the molecular dynamics simulation of the structure and performance of conductive metal–organic framework (MOF) electrodes for supercapacitors with room temperature ionic liquids. They applied jump-wise voltages between two identical electrodes and monitored the charge dynamics of applied voltage. They achieved a gravimetric energy density of ∼57 W h kg−1 at a cell voltage of 4 V. The MOF with the smallest pore diameter (0.81 nm) gives 8.8 μF cm−2 at −1.1 and +1.5 V and a pore diameter of 1.57 nm delivers a capacitance of ∼10 μF cm−2 from −0.5 to +0.5 V. Also, to obtain the best gravimetric performance, they have chosen MOFs with the largest pore size ∼2.39 nm that delivered a higher energy of 57 W h kg−1 and power density of 135–390 kW kg−1 at a cell voltage of 4 V.
Summary & outlook
In this review, we have summarized and systematically outlined the conventional strategies associated with the development of nanotube network-based supercapacitor materials, their properties, and their implications for supercapacitors' performance. CNTs used for supercapacitor electrodes generally provide lower specific capacitance than in the case of activated carbon. Nevertheless, the mesoporous structure of CNTs results in higher electrode conductivity and consequently higher specific power. Functionalization with metallic oxides or conductive polymers in the CNT material results in higher specific capacitance if such a composite is used for supercapacitor electrodes. Composites such as MoS2 nanosheets anchored on N-doped carbon microspheres with pseudocapacitive properties achieve excellent rate capacity and cycling stability407 which shows good application potential, along with a maximum energy density of 120 W h kg−1 and capacity retention of 85.5% over 4000 cycles. But, with the ever-increasing demand for energy storage applications, lithium-ion batteries cannot meet such requirements due to lack of lithium resources and uneven global distribution. Researchers are now focused on potassium ion storage technology as a promising substitute for energy storage applications. Chen et al. proposed disordered, large interlayer spacing and oxygen-rich carbon nanosheets for potassium ion hybrid capacitors which promisingly showed a high energy density of 149 W h kg−1, an ultrahigh power output of 21 kW kg−1, and a long cycling life (80% capacity retention after 5000 cycles).408 As another such example 3D hierarchical NiCo2O4 nanosheets/carbon nanotubes/carbon cloth as a flexible electrode material for electrochemical capacitors showed a maximum specific capacitance of 1518 F g−1 at a scan rate of 5 mV s−1 in 2 mol L−1 KOH aqueous solution and excellent stability under the conditions of long term cycling, mechanical bending and twisting conditions.409 A recent paper on a metal oxide-based supercapacitor using copper oxide nanoparticles with a multi-walled nanotube nanocomposite yielded a specific capacitance of 452.8 F g−1 at a 10 mV s−1 scan rate. Also, the nanocomposite exhibited good cycling stability with 90% capacity retention over 500 charge–discharge cycles.410 3D polyaniline nanothorns on buckypaper as an electrode exhibit a high specific capacitance of 742 F g−1 at 1 A g−1 in a 1 M H2SO4 electrolyte and a capacitance retention of 76% after 2000 cycles.411 As a supercapacitor electrode material, NiCo2S4 nanotubes with a unique hollow and spinous structure exhibit a good specific capacitance (630 F g−1 at 1 A g−1), low internal resistance Rs (0.68 Ω) and high capacitance retention (91% after 3000 cycles) at 10 A g−1.412 Some of the recent developments in high-performance supercapacitors using nanotubular networks have opened a new avenue for exploring techniques, specifically electrode characterization and modulations, to achieve high power density, capacity retention, and good cyclability.215,216,223–227,264,386,413–433 Although the advancements and implementations of nanotubular networks in supercapacitors have significantly achieved better storage capabilities and performance, tailoring and optimization of materials are still in a developmental phase where cost-effectiveness and good performance are some of the key concerns in practical applicability. In an attempt to develop a combination of high power and energy densities, hybrid technology is one of the challenging aspects. For that we need better understanding of surface chemistry between electrode materials and electrolytes to improve the interfacial interactions favorable for enhanced charge transfer. Nanoarchitectures play a vital role in establishing a structure–property synergy between different components such as porous electrode materials. Thus, surface-functionalized engineered materials need to be intensively explored to obtain an optimized supercapacitor which can deal with the energy crisis we are facing today.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors acknowledge the financial support from the Swedish Research Council.
References
- R. Kötz and M. Carlen, Electrochim. Acta, 2000, 45, 2483–2498 CrossRef.
- J. Ho, T. R. Jow and S. Boggs, IEEE Electr. Insul. Mag., 2010, 26, 20–25 Search PubMed.
- T. Garite, B. Snell, D. Walker and V. Darrow, Obstet. Gynecol., 1995, 86, 411–416 CrossRef CAS.
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539 CrossRef CAS.
-
K. Naoi, P. Simon, J. Electrochem. Soc., 2008, 17, 34–37 Search PubMed.
- C. Liu, Z. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef.
-
A. Yu, A. Davies and Z. Chen, in Electrochemical Technologies for Energy Storage and Conversion, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2012, vol. 1, pp. 317–382 Search PubMed.
-
P. Simon and Y. Gogotsi, in Materials for Sustainable Energy, Co-Published with Macmillan Publishers Ltd, UK, 2010, pp. 138–147 Search PubMed.
- R. Berenguer, Bol. Grupo Español Carbón, 2015, 9–13 Search PubMed.
- A. Lewandowski, A. Olejniczak, M. Galinski and I. Stepniak, J. Power Sources, 2010, 195, 5814–5819 CrossRef CAS.
- Y. Chen, X. Zhang, D. Zhang, P. Yu and Y. Ma, Carbon, 2011, 49, 573–580 CrossRef CAS.
- F. Wang, S. Xiao, Y. Hou, C. Hu, L. Liu and Y. Wu, RSC Adv., 2013, 3, 13059 RSC.
- L. Chang and Y. Hang Hu, Compr. Energy Syst., 2018, 2–5, 663–695 CrossRef.
- E. Frackowiak, Q. Abbas and F. Béguin, J. Energy Chem., 2013, 22, 226–240 CrossRef CAS.
-
F. Béguin and E. Frackowiak, Supercapacitors, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 Search PubMed.
- Z. Tan, G. Chen and Y. Zhu, Science, 2015, 1, 211–225 CAS.
- L. Demarconnay, E. G. Calvo, L. Timperman, M. Anouti, D. Lemordant, E. Raymundo-Piñero, A. Arenillas, J. A. Menéndez and F. Béguin, Electrochim. Acta, 2013, 108, 361–368 CrossRef CAS.
- G. P. Pandey, Y. Kumar and S. A. Hashmi, Indian J. Chem., Sect. A: Inorg., Phys., Theor. Anal., 2010, 49, 743–751 Search PubMed.
- G. Feng, S. Li, V. Presser and P. T. Cummings, J. Phys. Chem. Lett., 2013, 4, 3367–3376 CrossRef CAS.
- C. Arbizzani, S. Beninati, M. Lazzari, F. Soavi and M. Mastragostino, J. Power Sources, 2007, 174, 648–652 CrossRef CAS.
- P. T. Ha, H. Moon, B. H. Kim, H. Y. Ng and I. S. Chang, Biosens. Bioelectron., 2010, 25, 1629–1634 CrossRef CAS PubMed.
-
A. Javaid, in Activated Carbon Fiber and Textiles, Elsevier, 2017, pp. 281–303 Search PubMed.
- A. Muzaffar, M. B. Ahamed, K. Deshmukh and J. Thirumalai, Renewable Sustainable Energy Rev., 2019 Search PubMed.
- W. Zuo, R. Li, C. Zhou, Y. Li, J. Xia and J. Liu, Adv. Sci., 2017 Search PubMed.
- A. G. Pandolfo and A. F. Hollenkamp, J. Power Sources, 2006, 157, 11–27 CrossRef CAS.
- P. Gómez-Romero, O. Ayyad, J. Suárez-Guevara and D. Muñoz-Rojas, J. Solid State Electrochem., 2010, 14, 1939–1945 CrossRef.
- D. Tie, S. Huang, J. Wang, J. Ma, J. Zhang and Y. Zhao, Energy Storage Mater., 2019, 21, 22–40 CrossRef.
- E. S. Kim, E. H. Ahn, T. Dvir and D. H. Kim, Int. J. Nanomedicine, 2014, 9, 1–5 CrossRef CAS.
- D. P. Dubal, O. Ayyad, V. Ruiz and P. Gómez-Romero, Chem. Soc. Rev., 2015, 44, 1777–1790 RSC.
- Y. Wang and Y. Xia, Adv. Mater., 2013, 25, 5336–5342 CrossRef CAS.
- Y. Huang, Y. Li, Q. Gong, G. Zhao, P. Zheng, J. Bai, J. Gan, M. Zhao, Y. Shao, D. Wang, L. Liu, G. Zou, D. Zhuang, J. Liang, H. Zhu and C. Nan, ACS Appl. Mater. Interfaces, 2018, 10, 16572–16580 CrossRef CAS.
- H.-C. Wu, Y.-P. Lin, E. Lee, W.-T. Lin, J.-K. Hu, H.-C. Chen and N.-L. Wu, Mater. Chem. Phys., 2009, 117, 294–300 CrossRef CAS.
- K. Ku, B. Kim, H. Chung and W. Kim, Synth. Met., 2010, 160, 2613–2617 CrossRef CAS.
- Z. Ren, Y. Li and J. Yu, iScience, 2018, 9, 138–148 CrossRef CAS.
- C. U. Jeong, S.-Y. Lee, J. Kim, K. Y. Cho and S. Yoon, J. Power Sources, 2018, 398, 193–200 CrossRef CAS.
- L. Tong, K. H. Skorenko, A. C. Faucett, S. M. Boyer, J. Liu, J. M. Mativetsky, W. E. Bernier and W. E. Jones, J. Power Sources, 2015, 297, 195–201 CrossRef CAS.
- A. Burke, J. Power Sources, 2000, 91, 37–50 CrossRef CAS.
- A. S. Aricò, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef PubMed.
- A. Chu and P. Braatz, J. Power Sources, 2002, 112, 236–246 CrossRef CAS.
- G. Z. Chen, Int. Mater. Rev., 2017, 62, 173–202 CrossRef CAS.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P.-L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS.
- Y. Shao, M. F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn and R. B. Kaner, Chem. Rev., 2018, 118, 9233–9280 CrossRef CAS PubMed.
- J. R. Miller and A. F. Burke, Electrochem. Soc. Interface, 2008, 17, 53–57 CAS.
- B. E. Conway, V. Birss and J. Wojtowicz, J. Power Sources, 1997, 66, 1–14 CrossRef CAS.
- T. Brousse, D. Bélanger and J. W. Long, J. Electrochem. Soc., 2015, 162, A5185–A5189 CrossRef CAS.
- Y. Zhang, X. Cui, L. Zu, X. Cai, Y. Liu, X. Wang and H. Lian, Materials, 2016, 9, 734 CrossRef PubMed.
- J. R. Miller and A. F. Burke, Electrochem. Soc. Interface, 2008, 17, 53–57 CAS.
-
D. Shin, Y. Kim, J. Seo, N. Chang, Y. Wang and M. Pedram, in Proceedings Design, Automation and Test in Europe, Date, 2011 Search PubMed.
-
Cadex Electronics Inc., How Does a Supercapacitor Work, 2019 Search PubMed.
- R. A. Dougal, S. Liu and R. E. White, IEEE Trans. Compon. Packag. Technol., 2002, 25, 120–131 CrossRef.
- E. Frackowiak and F. Béguin, Carbon, 2001, 39, 937–950 CrossRef CAS.
- E. Frackowiak, K. Metenier, V. Bertagna and F. Beguin, Appl. Phys. Lett., 2000, 77, 2421–2423 CrossRef CAS.
- C. Niu, E. K. Sichel, R. Hoch, D. Moy and H. Tennent, Appl. Phys. Lett., 1997, 70, 1480–1482 CrossRef CAS.
- K. H. An, W. S. Kim, Y. S. Park, H. J. Jeong, Y. C. Choi, J. M. Moon, D. J. Bae, S. C. Lim and Y. H. Lee, AIP Conf. Proc., 2001, 590, 241–244 CrossRef CAS.
- C. Du, J. Yeh and N. Pan, Nanotechnology, 2005, 16, 350–353 CrossRef CAS.
- B. J. Yoon, S. H. Jeong, K. H. Lee, H. Seok Kim, C. Gyung Park and J. Hun Han, Chem. Phys. Lett., 2004, 388, 170–174 CrossRef CAS.
- A. Muhulet, F. Miculescu, S. I. Voicu, F. Schütt, V. K. Thakur and Y. K. Mishra, Mater. Today Energy, 2018, 9, 154–186 CrossRef.
- H. Yang, S. Kannappan, A. S. Pandian, J.-H. Jang, Y. S. Lee and W. Lu, Nanotechnology, 2017, 28, 445401 CrossRef PubMed.
- K. Yu, Z. Wen, H. Pu, G. Lu, Z. Bo, H. Kim, Y. Qian, E. Andrew, S. Mao and J. Chen, J. Mater. Chem. A, 2013, 1, 188–193 RSC.
- Y. Gogotsi, A. Nikitin, H. Ye, W. Zhou, J. E. Fischer, B. Yi, H. C. Foley and M. W. Barsoum, Nat. Mater., 2003, 2, 591–594 CrossRef CAS PubMed.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 CrossRef CAS PubMed.
- C. Kjølseth, H. Fjeld, Ø. Prytz, P. I. Dahl, C. Estournès, R. Haugsrud and T. Norby, Solid State Ionics, 2010, 181, 268–275 CrossRef.
- A. J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 CrossRef PubMed.
- R. de Levie and A. Vogt, J. Electroanal. Chem., 1992, 341, 353–360 CrossRef CAS.
- J. Dzubiella and J.-P. Hansen, J. Chem. Phys., 2005, 122, 234706 CrossRef CAS PubMed.
- J. Marañón Di Leo and J. Marañón, J. Mol. Struct.: THEOCHEM, 2005, 729, 53–57 CrossRef.
- M. Carrillo-Tripp, H. Saint-Martin and I. Ortega-Blake, Phys. Rev. Lett., 2004, 93, 168104 CrossRef PubMed.
- T. Ohkubo, T. Konishi, Y. Hattori, H. Kanoh, T. Fujikawa and K. Kaneko, J. Am. Chem. Soc., 2002, 124, 11860–11861 CrossRef CAS PubMed.
- Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882–1889 CrossRef CAS PubMed.
- J. Gamby, P. L. Taberna, P. Simon, J. F. Fauvarque and M. Chesneau, J. Power Sources, 2001, 101, 109–116 CrossRef CAS.
- K. Kiyohara, T. Sugino and K. Asaka, J. Chem. Phys., 2010, 132, 144705 CrossRef PubMed.
- A. A. Kornyshev, Faraday Discuss., 2013, 164, 117 RSC.
- H. Itoi, H. Nishihara and T. Kyotani, Langmuir, 2016, 32, 11997–12004 CrossRef CAS PubMed.
- L. Han, K. G. Karthikeyan, M. A. Anderson and K. B. Gregory, J. Colloid Interface Sci., 2014, 430, 93–99 CrossRef CAS PubMed.
- S. M. Jung, D. L. Mafra, C. Te Lin, H. Y. Jung and J. Kong, Nanoscale, 2015, 7, 4386–4393 RSC.
- K. Urita, C. Urita, K. Fujita, K. Horio, M. Yoshida and I. Moriguchi, Nanoscale, 2017, 9, 15643–15649 RSC.
- X. Gao, S. Porada, A. Omosebi, K. L. Liu, P. M. Biesheuvel and J. Landon, Water Res., 2016, 92, 275–282 CrossRef CAS PubMed.
- H. L. K. S. Mosch, O. Akintola, W. Plass, S. Höppener, U. S. Schubert and A. Ignaszak, Langmuir, 2016, 32, 4440–4449 CrossRef CAS PubMed.
- M. Sevilla and A. B. Fuertes, ChemSusChem, 2016, 9, 1880–1888 CrossRef CAS PubMed.
- J.-G. Li, Y.-F. Ho, M. M. M. Ahmed, H.-C. Liang and S.-W. Kuo, Chem.–Eur. J., 2019, 18, 6208–6216 Search PubMed.
- H. Itoi, H. Nishihara and T. Kyotani, Langmuir, 2016, 32, 11997–12004 CrossRef CAS PubMed.
- C. Lian, X. Kong, H. Liu and J. Wu, J. Phys.: Condens. Matter, 2016, 28, 464008 CrossRef PubMed.
- A. Aldalbahi, M. Rahaman, M. Almoigli, A. Meriey and K. Alharbi, Nanomaterials, 2018, 8, 19 CrossRef PubMed.
- S. M. Jung, D. L. Mafra, C. Te Lin, H. Y. Jung and J. Kong, Nanoscale, 2015, 7, 4386–4393 RSC.
- Y. Lee, S. Noh, M. S. Kim, H. J. Kong, K. Im, O. S. Kwon, S. Kim and H. Yoon, Nanoscale, 2016, 8, 11940–11948 RSC.
- M. Seredych, E. Rodríguez-Castellõn and T. J. Bandosz, ChemSusChem, 2015, 8, 1955–1965 CrossRef CAS PubMed.
- G. Wu, Y. Hu, Y. Liu, J. Zhao, X. Chen, V. Whoehling, C. Plesse, G. T. M. Nguyen, F. Vidal and W. Chen, Nat. Commun., 2015, 6, 7258 CrossRef CAS PubMed.
- A. Pramanik, S. Maiti and S. Mahanty, Dalton Trans., 2015, 44, 14604–14612 RSC.
- K. Lee, H. Song, K. H. Lee, S. H. Choi, J. H. Jang, K. Char and J. G. Son, ACS Appl. Mater. Interfaces, 2016, 8, 22516–22525 CrossRef CAS PubMed.
- R. K. Kalluri, M. M. Biener, M. E. Suss, M. D. Merrill, M. Stadermann, J. G. Santiago, T. F. Baumann, J. Biener and A. Striolo, Phys. Chem. Chem. Phys., 2013, 15, 2309–2320 RSC.
- C. Song and B. Corry, J. Phys. Chem. B, 2009, 113, 7642–7649 CrossRef CAS PubMed.
- K. Tai, S. Haider, A. Grottesi and M. S. P. Sansom, Eur. Biophys. J., 2009, 38, 347–354 CrossRef CAS PubMed.
- L. Yang, B. H. Fishbine, A. Migliori and L. R. Pratt, J. Am. Chem. Soc., 2009, 131, 12373–12376 CrossRef CAS PubMed.
- Y. Shim and H. J. Kim, ACS Nano, 2009, 3, 1693–1702 CrossRef CAS PubMed.
- H. Sui, J. Yao and L. Zhang, Computation, 2015, 3, 687–700 CrossRef CAS.
- M. Holmboe and I. C. Bourg, J. Phys. Chem. C, 2014, 118, 1001–1013 CrossRef CAS.
- J. Geske and M. Vogel, Mol. Simul., 2017, 43, 13–18 CrossRef CAS.
- D. Liu, C. Zheng, Q. Yang and C. Zhong, J. Phys. Chem. C, 2009, 113, 5004–5009 CrossRef CAS.
- S. Wang, Q. Feng, F. Javadpour, T. Xia and Z. Li, Int. J. Coal Geol., 2015, 147–148, 9–24 CrossRef CAS.
- A. Boţan, B. Rotenberg, V. Marry, P. Turq and B. Noetinger, J. Phys. Chem. C, 2011, 115, 16109–16115 CrossRef.
- A. Özgür Yazaydin and R. W. Thompson, Microporous Mesoporous Mater., 2009, 123, 169–176 CrossRef.
- L. Connelly, H. Jang, F. Teran Arce, S. Ramachandran, B. L. Kagan, R. Nussinov and R. Lal, Biochemistry, 2012, 51, 3031–3038 CrossRef CAS PubMed.
- M. L. Fernández, M. Risk, R. Reigada and P. T. Vernier, Biochem. Biophys. Res. Commun., 2012, 423, 325–330 CrossRef PubMed.
- A. Sharma, S. Namsani and J. K. Singh, Mol. Simul., 2015, 41, 414–422 CrossRef CAS.
- J. Vatamanu, D. Bedrov and O. Borodin, Mol. Simul., 2017, 43, 838–849 CrossRef CAS.
- R. Capone, H. Jang, S. A. Kotler, B. L. Kagan, R. Nussinov and R. Lal, Biochemistry, 2012, 51, 776–785 CrossRef CAS PubMed.
-
A. V. Korchuganov, V. M. Chernov, K. P. Zolnikov, D. S. Kryzhevich and S. G. Psakhie, Inorg. Mater. Appl. Res., 2016, 7, 648–657 Search PubMed.
- Y. Hu, D. Devegowda, A. Striolo, A. Phan, T. A. Ho, F. Civan and R. F. Sigal, SPE J., 2014, 20, 112–124 CrossRef.
- Y. Ichikawa, K. Kawamura, N. Theramast and K. Kitayama, Mech. Mater., 2004, 36, 487–513 CrossRef.
- J. Azamat, A. Khataee and F. Sadikoglu, RSC Adv., 2016, 6, 94911–94920 RSC.
- Y. Tao, Q. Xue, Z. Liu, M. Shan, C. Ling, T. Wu and X. Li, ACS Appl. Mater. Interfaces, 2014, 6, 8048–8058 CrossRef CAS PubMed.
- V. Karanikola, A. F. Corral, H. Jiang, A. Eduardo Sáez, W. P. Ela and R. G. Arnold, J. Membr. Sci., 2015, 483, 15–24 CrossRef CAS.
- D. Dubbeldam and R. Q. Snurr, Mol. Simul., 2007, 33, 305–325 CrossRef CAS.
- I. C. Bourg and C. I. Steefel, J. Phys. Chem. C, 2012, 116, 11556–11564 CrossRef CAS.
- F. Y. Jiang, Y. Bouret and J. T. Kindt, Biophys. J., 2004, 87, 182–192 CrossRef CAS PubMed.
- C. L. Rountree, R. K. Kalia, E. Lidorikis, A. Nakano, L. Van Brutzel and P. Vashishta, Annu. Rev. Mater. Res., 2002, 32, 377–400 CrossRef CAS.
- A. O. Imdakm and T. Matsuura, J. Membr. Sci., 2005, 262, 117–128 CrossRef CAS.
- M. M. A. Shirazi, A. Kargari, A. F. Ismail and T. Matsuura, Desalination, 2016, 377, 73–90 CrossRef CAS.
- A. O. Imdakm and T. Matsuura, J. Membr. Sci., 2004, 237, 51–59 CrossRef CAS.
- A. A. Lee, S. Kondrat and A. A. Kornyshev, Phys. Rev. Lett., 2014, 113, 048701 CrossRef PubMed.
- C. Largeot, C. Portet, J. Chmiola, P.-L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730–2731 CrossRef CAS PubMed.
- G. Feng and P. T. Cummings, J. Phys. Chem. Lett., 2011, 2, 2859–2864 CrossRef CAS.
- S. Wang, H. Li, H. Sawada, C. S. Allen, A. I. Kirkland, J. C. Grossman and J. H. Warner, Nanoscale, 2017, 9, 6417–6426 RSC.
- H. Zhou, X. Chen, L. Wang, X. Zhong, G. Zhuang, X. Li, D. Mei and J. Wang, Phys. Chem. Chem. Phys., 2015, 17, 24420–24426 RSC.
- Z. Jin, Fluid Phase Equilib., 2018, 458, 177–185 CrossRef CAS.
- L. Zhu, Y. Jin, Q. Xue, X. Li, H. Zheng, T. Wu and C. Ling, J. Mater. Chem. A, 2016, 4, 15015–15021 RSC.
- B. Jin, X. Zhang, F. Li, N. Zhang, Z. Zong, S. Cao, Z. Li and X. Chen, Phys. Chem. Chem. Phys., 2019, 21, 6126–6132 RSC.
- L. Zhang, Y. Xiong, Y. Li, M. Wei, W. Jiang, R. Lei and Z. Wu, Fuel, 2017, 204, 1–11 CrossRef CAS.
- C. R. Clarkson, J. Wood, S. Burgis, S. Aquino and M. Freeman, SPE Reservoir Eval. Eng., 2013, 15, 648–661 CrossRef.
-
A. V. Neimark and P. I. Ravikovitch, 2000, pp. 51–60.
- Z. Li, Z. Jin and A. Firoozabadi, SPE J., 2014, 19, 1096–1109 CrossRef.
-
M. Thommes, R. Guillet-Nicolas and K. A. Cychosz, in Mesoporous Zeolites: Preparation, Characterization and Applications, 2015, pp. 349–384 Search PubMed.
- R. Zaleski, A. Kierys, M. Dziadosz, J. Goworek and I. Halasz, RSC Adv., 2012, 2, 3729–3734 RSC.
- F. Béguin, K. Kierzek, M. Friebe, A. Jankowska, J. Machnikowski, K. Jurewicz and E. Frackowiak, Electrochim. Acta, 2006, 51, 2161–2167 CrossRef.
- D. Henderson, J. Colloid Interface Sci., 2012, 374, 345–347 CrossRef CAS PubMed.
- J. P. Olivier, J. Porous Mater., 1995, 2, 9–17 CrossRef CAS.
- S. Yorgun and D. Yildiz, J. Taiwan Inst. Chem. Eng., 2015, 53, 122–131 CrossRef CAS.
- C. M. Lastoskie and K. E. Gubbins, Adv. Chem. Eng., 2000, 128, 41–50 CAS.
- J. Landers, G. Y. Gor and A. V. Neimark, Colloids Surf., A, 2013, 437, 3–32 CrossRef CAS.
- J. Fu, Y. Liu, Y. Tian and J. Wu, J. Phys. Chem. C, 2015, 119, 5374–5385 CrossRef CAS.
- V. R. Cooper, L. Kong and D. C. Langreth, Phys. Procedia, 2010, 3, 1417–1430 CrossRef CAS.
- T. Yamamoto, A. Endo, Y. Inagi, T. Ohmori and M. Nakaiwa, J. Colloid Interface Sci., 2005, 284, 614–620 CrossRef CAS PubMed.
- M. Thommes and K. A. Cychosz, Adsorption, 2014, 20, 233–250 CrossRef CAS.
- C. Cazorla and S. A. Shevlin, Dalton Trans., 2013, 42, 4670–4676 RSC.
-
M. Thommes, K. A. Cychosz and A. V. Neimark, in Novel Carbon Adsorbents, 2012, pp. 107–145 Search PubMed.
- J. Landers, G. Y. Gor and A. V. Neimark, Colloids Surf., A, 2013, 437, 3–32 CrossRef CAS.
- P. I. Ravikovitch, G. L. Haller and A. V. Neimark, Adv. Colloid Interface Sci., 1998, 76–77, 203–226 CrossRef CAS.
- S. Zhou and A. Bongiorno, Acc. Chem. Res., 2014, 47, 3331–3339 CrossRef CAS PubMed.
- D. W. Oxtoby, Annu. Rev. Mater. Res., 2002, 32, 39–52 CrossRef CAS.
- P. I. Ravikovitch, A. Vishnyakov, R. Russo and A. V. Neimark, Langmuir, 2000, 16, 2311–2320 CrossRef CAS.
-
S. Lowell, J. E. Shields, M. A. Thomas and M. Thommes, Characterization of porous solids and powders: surface area, pore size and density, Springer Science & Business Media, 2012, vol. 16 Search PubMed.
- D. Saha, S. Deng and Z. Yang, J. Porous Mater., 2009, 16, 141–149 CrossRef CAS.
- J. N. Caguiat, D. W. Kirk and C. Q. Jia, Carbon, 2014, 72, 47–56 CrossRef CAS.
- M. Matena, J. Björk, M. Wahl, T.-L. Lee, J. Zegenhagen, L. H. Gade, T. A. Jung, M. Persson and M. Stöhr, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 125408 CrossRef.
- M. Thommes, Chem.-Ing.-Tech., 2010, 82, 1059–1073 CrossRef CAS.
- S. Figueroa-Gerstenmaier, J. B. Avalos, L. D. Gelb, K. E. Gubbins and L. F. Vega, Langmuir, 2003, 19, 8592–8604 CrossRef CAS.
- C. G. Van De Walle and A. Janotti, Phys. Status Solidi B, 2011, 248, 19–27 CrossRef CAS.
- N. A. Seaton, J. P. R. B. Walton and N. Quirke, Carbon, 1989, 27, 853–861 CrossRef CAS.
- C. Lastoskie, K. E. Gubbins and N. Quirke, J. Phys. Chem., 1993, 97, 4786–4796 CrossRef CAS.
- X. Chen, R. Paul and L. Dai, Natl. Sci. Rev., 2017, 4, 453–489 CrossRef CAS.
- J. Yi, Z. Huo, A. M. Asiri, K. A. Alamry and J. Li, Prog. Chem., 2018, 30, 1624–1633 Search PubMed.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7484–7539 RSC.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- F. Schütt, S. Signetti, H. Krüger, S. Röder, D. Smazna, S. Kaps, S. N. Gorb, Y. K. Mishra, N. M. Pugno and R. Adelung, Nat. Commun., 2017, 8, 1215 CrossRef PubMed.
-
T. Sekino, Inorganic and Metallic Nanotubular Materials, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, vol. 117 Search PubMed.
- V. V. N. Obreja, Phys. E, 2008, 40, 2596–2605 CrossRef CAS.
- M. F. Mousavi, M. Hashemi, M. S. Rahmanifar and A. Noori, Electrochim. Acta, 2017, 228, 290–298 CrossRef CAS.
- R. Saito, G. Dresselhaus and M. S. Dresselhaus, Chem. Phys. Lett., 1992, 195, 537–542 CrossRef CAS.
-
R. Saito, G. Dresselhaus and M. S. Dresselhaus, Physical Properties of Carbon Nanotubes, Imperial College Press and Distributed by World Scientific Publishing Co., 1998 Search PubMed.
- R. H. Baughman, Science, 2002, 297, 787–792 CrossRef CAS PubMed.
-
A. L. Elías, N. Perea-López, L. P. Rajukumar, A. McCreary, F. López-Urías, H. Terrones and M. Terrones, in Nanotube Superfiber Materials, Elsevier, 2014, pp. 457–493 Search PubMed.
- E. L. Gui, L.-J. Li, K. Zhang, Y. Xu, X. Dong, X. Ho, P. S. Lee, J. Kasim, Z. X. Shen, J. A. Rogers and Mhaisalkar, J. Am. Chem. Soc., 2007, 129, 14427–14432 CrossRef CAS PubMed.
- C. Y. Lee, H. M. Tsai, H. J. Chuang, S. Y. Li, P. Lin and T. Y. Tseng, J. Electrochem. Soc., 2005, 152, A716 CrossRef CAS.
- J. Y. Lee, K. Liang, K. H. An and Y. H. Lee, Synth. Met., 2005, 150, 153–157 CrossRef CAS.
- T. Bordjiba, M. Mohamedi and L. H. Dao, Adv. Mater., 2008, 20, 815–819 CrossRef CAS.
- M. Seifert, Angew. Chem., Int. Ed., 2017, 56, 7351 CrossRef CAS.
- H. Pan, J. Li and Y. P. Feng, Nanoscale Res. Lett., 2010, 5, 654–668 CrossRef CAS PubMed.
- H. Zhang, G. P. Cao and Y. S. Yang, Nanotechnology, 2007, 18, 195607 CrossRef.
- Y. Soneda, J. Yamashita, M. Kodama, H. Hatori, M. Toyoda and M. Inagaki, Appl. Phys. A, 2006, 82, 575–578 CrossRef CAS.
- Y. Soneda, M. Toyoda, Y. Tani, J. Yamashita, M. Kodama, H. Hatori and M. Inagaki, J. Phys. Chem. Solids, 2004, 65, 219–222 CrossRef CAS.
- G. Agar, Johnson Matthey Technol. Rev., 2014, 58, 221–223 CrossRef.
- C. G. Liu, M. Liu, F. Li and H. M. Cheng, Appl. Phys. Lett., 2008, 92, 143108 CrossRef.
- X. Xiao, T. Li, Z. Peng, H. Jin, Q. Zhong, Q. Hu, B. Yao, Q. Luo, C. Zhang, L. Gong, J. Chen, Y. Gogotsi and J. Zhou, Nano Energy, 2014, 6, 1–9 CrossRef CAS.
- D. N. Futaba, K. Hata, T. Yamada, T. Hiraoka, Y. Hayamizu, Y. Kakudate, O. Tanaike, H. Hatori, M. Yumura and S. Iijima, Nat. Mater., 2006, 5, 987–994 CrossRef CAS PubMed.
- H. Pan, C. K. Poh, Y. P. Feng and J. Lin, Chem. Mater., 2007, 19, 6120–6125 CrossRef CAS.
- D. Pankratov, Z. Blum, D. B. Suyatin, V. O. Popov and S. Shleev, ChemElectroChem, 2014, 1, 343–346 CrossRef.
- M. N. Hyder, S. W. Lee, F. Ç. Cebeci, D. J. Schmidt, Y. Shao-Horn and P. T. Hammond, ACS Nano, 2011, 5, 8552–8561 CrossRef CAS PubMed.
- X. Wang, M. Falk, R. Ortiz, H. Matsumura, J. Bobacka, R. Ludwig, M. Bergelin, L. Gorton and S. Shleev, Biosens. Bioelectron., 2012, 31, 219–225 CrossRef CAS PubMed.
- P. Bollella, R. Ludwig and L. Gorton, Appl. Mater. Today, 2017 Search PubMed.
- M. Kaempgen, J. Ma, G. Gruner, G. Wee and S. G. Mhaisalkar, Appl. Phys. Lett., 2007, 90, 264104 CrossRef.
- C. L. Pint, N. W. Nicholas, S. Xu, Z. Sun, J. M. Tour, H. K. Schmidt, R. G. Gordon and R. H. Hauge, Carbon, 2011, 49, 4890–4897 CrossRef CAS.
- L. Gao, A. Peng, Z. Y. Wang, H. Zhang, Z. Shi, Z. Gu, G. Cao and B. Ding, Solid State Commun., 2008, 146, 380–383 CrossRef CAS.
- R. Reit, J. Nguyen and W. J. Ready, Electrochim. Acta, 2013, 91, 96–100 CrossRef CAS.
- S. Dörfler, I. Felhösi, T. Marek, S. Thieme, H. Althues, L. Nyikos and S. Kaskel, J. Power Sources, 2013, 227, 218–228 CrossRef.
- B.-H. Kim, K. S. Yang, Y. A. Kim, Y. J. Kim, B. An and K. Oshida, J. Power Sources, 2011, 196, 10496–10501 CrossRef CAS.
- S. Talapatra, S. Kar, S. K. Pal, R. Vajtai, L. Ci, P. Victor, M. M. Shaijumon, S. Kaur, O. Nalamasu and P. M. Ajayan, Nat. Nanotechnol., 2006, 1, 112–116 CrossRef CAS PubMed.
- B. Kim, H. Chung and W. Kim, Nanotechnology, 2012, 23, 155401 CrossRef PubMed.
- Y.-K. Hsu, Y.-C. Chen, Y.-G. Lin, L.-C. Chen and K.-H. Chen, J. Mater. Chem., 2012, 22, 3383 RSC.
- K. Kakaei, M. D. Esrafili and A. Ehsani, Interface Sci. Technol., 2019, 339–386 Search PubMed.
- M. A. Pope, S. Korkut, C. Punckt and I. A. Aksay, J. Electrochem. Soc., 2013, 160, A1653–A1660 CrossRef CAS.
- C. Tran and V. Kalra, J. Power Sources, 2013, 235, 289–296 CrossRef CAS.
- L.-F. Chen, Z.-H. Huang, H.-W. Liang, H.-L. Gao and S.-H. Yu, Adv. Funct. Mater., 2014, 24, 5104–5111 CrossRef CAS.
- F. Miao, C. Shao, X. Li, K. Wang and Y. Liu, J. Mater. Chem. A, 2016, 4, 4180–4187 RSC.
- T. Hiraoka, A. Izadi-Najafabadi, T. Yamada, D. N. Futaba, S. Yasuda, O. Tanaike, H. Hatori, M. Yumura, S. Iijima and K. Hata, Adv. Funct. Mater., 2010, 20, 422–428 CrossRef CAS.
- B.-H. Kim, K. S. Yang, H.-G. Woo and K. Oshida, Synth. Met., 2011, 161, 1211–1216 CrossRef CAS.
- C. Du and N. Pan, Nanotechnology, 2006, 17, 5314–5318 CrossRef CAS.
- Y. Jiang, J. Yan, X. Wu, D. Shan, Q. Zhou, L. Jiang, D. Yang and Z. Fan, J. Power Sources, 2016, 307, 190–198 CrossRef CAS.
- Y. Qiu, G. Li, Y. Hou, Z. Pan, H. Li, W. Li, M. Liu, F. Ye, X. Yang and Y. Zhang, Chem. Mater., 2015, 27, 1194–1200 CrossRef CAS.
- T. Kim, G. Jung, S. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed.
- L.-F. Chen, X.-D. Zhang, H.-W. Liang, M. Kong, Q.-F. Guan, P. Chen, Z.-Y. Wu and S.-H. Yu, ACS Nano, 2012, 6, 7092–7102 CrossRef CAS PubMed.
- X. Yang, J. Zhu, L. Qiu and D. Li, Adv. Mater., 2011, 23, 2833–2838 CrossRef CAS PubMed.
- Q. Cheng, J. Tang, J. Ma, H. Zhang, N. Shinya and L.-C. Qin, Phys. Chem. Chem. Phys., 2011, 13, 17615 RSC.
- W. Lu, L. Qu, K. Henry and L. Dai, J. Power Sources, 2009, 189, 1270–1277 CrossRef CAS.
- P. Lu, X. Wang, L. Wen, X. Jiang, W. Guo, L. Wang, X. Yan, F. Hou, J. Liang, H.-M. Cheng and S. X. Dou, Small, 2019, 15, e1805064 CrossRef PubMed.
- S. N. Ansari, M. Saraf, A. K. Gupta and S. M. Mobin, Chem.–Asian J., 2019, 14, 3566–3571 CrossRef CAS PubMed.
- C. R. Chen, H. Qin, H. P. Cong and S. H. Yu, Adv. Mater., 2019, 31, e1900573 CrossRef PubMed.
- M. Tahir, L. He, W. A. Haider, W. Yang, X. Hong, Y. Guo, X. Pan, H. Tang, Y. Li and L. Mai, Nanoscale, 2019, 11, 7761–7770 RSC.
- Y. Zhou, Y. Zhu, B. Xu and X. Zhang, Chem. Commun., 2019, 55, 4083–4086 RSC.
- C. Pereira, R. S. Costa, L. Lopes, B. Bachiller-Baeza, I. Rodriguez-Ramos, A. Guerrero-Ruiz, P. B. Tavares, C. Freire and A. M. Pereira, Nanoscale, 2019, 11, 3397 RSC.
- X. Guan, D. Kong, Q. Huang, L. Cao, P. Zhang, H. Lin, Z. Lin and H. Yuan, Polymers, 2019, 11, 178 CrossRef PubMed.
- Y. Chen, B. Xu, J. Gong, J. Wen, T. Hua, C. W. Kan and J. Deng, ACS Appl. Mater. Interfaces, 2019, 11, 2120–2129 CrossRef CAS PubMed.
- H. Park, R. B. Ambade, S. H. Noh, W. Eom, K. H. Koh, S. B. Ambade, W. J. Lee, S. H. Kim and T. H. Han, ACS Appl. Mater. Interfaces, 2019, 11, 9011–9022 CrossRef CAS PubMed.
- W. Zhou, Y. Du, J. Zeng, F. Liu and Y. Zhu, Nanoscale, 2019, 11, 7624–7633 RSC.
- F. Daneshvar, A. Aziz, A. M. Abdelkader, T. Zhang, H.-J. Sue and M. E. Welland, Nanotechnology, 2019, 30, 015401 CrossRef PubMed.
- H. Niu, Y. Zhang, Y. Liu, N. Xin and W. Shi, J. Colloid Interface Sci., 2019, 539, 545–552 CrossRef CAS PubMed.
- X. Fu, A. Chen, Y. Yu, S. Hou and L. Liu, Chem.–Asian J., 2019, 14, 634–639 CrossRef CAS PubMed.
- X. Guan, L. Cao, Q. Huang, D. Kong, P. Zhang, H. Lin, W. Li, Z. Lin and H. Yuan, Polymers, 2019, 11, 973 CrossRef CAS PubMed.
- M. K. Jha, K. Hata and C. Subramaniam, ACS Appl. Mater. Interfaces, 2019, 11, 18285–18294 CrossRef CAS PubMed.
- Z. Chang, X. Sang, Y. Song, X. Sun and X. X. Liu, Dalton Trans., 2019, 48, 6812–6816 RSC.
- E. P. Gilshteyn, D. Amanbayev, A. S. Anisimov, T. Kallio and A. G. Nasibulin, Sci. Rep., 2017, 7, 17449 CrossRef PubMed.
- Q. Wang, J. Yan and Z. Fan, Energy Environ. Sci., 2016, 9, 729–762 RSC.
- M. Huang, Y. Zhang, F. Li, L. Zhang, R. S. Ruoff, Z. Wen and Q. Liu, Sci. Rep., 2015, 4, 3878 CrossRef PubMed.
- T. Chen and L. Dai, Mater. Today, 2013, 16, 272–280 CrossRef CAS.
- Y. K. Mishra, S. Kaps, A. Schuchardt, I. Paulowicz, X. Jin, D. Gedamu, S. Freitag, M. Claus, S. Wille, A. Kovalev, S. N. Gorb and R. Adelung, Part. Part. Syst. Charact., 2013, 30, 775–783 CrossRef CAS.
- J. Marx, A. Brouschkin, S. Roth, D. Smazna, Y. K. Mishra, H. Wittich, K. Schulte, R. Adelung and B. Fiedler, Synth. Met., 2018, 235, 145–152 CrossRef CAS.
- K. Shehzad, Y. Xu, C. Gao and X. Duan, Chem. Soc. Rev., 2016, 45, 5541–5588 RSC.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS.
- S. W. Kim, D. H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- A. K. Shukla and T. Prem Kumar, Wiley Interdiscip. Rev.: Energy Environ., 2013, 2, 14–30 CAS.
- X. Hu, W. Zhang, X. Liu, Y. Mei and Y. Huang, Chem. Soc. Rev., 2015, 44, 2376–2404 RSC.
- Y. Fang, X.-Y. Yu and X. W. (David) Lou, Matter, 2019, 1, 90–114 CrossRef.
- J. N. Tiwari, R. N. Tiwari and K. S. Kim, Prog. Mater. Sci., 2012, 57, 724–803 CrossRef CAS.
- Y.-T. Kim, J. H. Han, B. H. Hong and Y.-U. Kwon, Adv. Mater., 2010, 22, 515–518 CrossRef CAS PubMed.
- T. Okada, K. Kawashima, Y. Nakata and X. Ning, Jpn. J. Appl. Phys., 2005, 44, 688–691 CrossRef CAS.
- H. Xia, J. Feng, H. Wang, M. O. Lai and L. Lu, J. Power Sources, 2010, 195, 4410–4413 CrossRef CAS.
- G.-R. Li, Z.-P. Feng, J.-H. Zhong, Z.-L. Wang and Y.-X. Tong, Macromolecules, 2010, 43, 2178–2183 CrossRef CAS.
- J.-M. Park, K. S. Nalwa, W. Leung, K. Constant, S. Chaudhary and K.-M. Ho, Nanotechnology, 2010, 21, 215301 CrossRef PubMed.
- L. M. Cao, H. Tian, Z. Zhang, X. Y. Zhang, C. X. Gao and W. K. Wang, Nanotechnology, 2004, 15, 139–142 CrossRef CAS.
- B. B. Nayak, D. Behera and B. K. Mishra, J. Am. Ceram. Soc., 2010, 93, 3080–3083 CrossRef CAS.
- A. K. P. Mann and S. E. Skrabalak, Chem. Mater., 2011, 23, 1017–1022 CrossRef CAS.
- P. F. Siril, A. Lehoux, L. Ramos, P. Beaunier and H. Remita, New J. Chem., 2012, 36, 2135 RSC.
- S. Vizireanu, S. D. Stoica, C. Luculescu, L. C. Nistor, B. Mitu and G. Dinescu, Plasma Sources Sci. Technol., 2010, 19, 034016 CrossRef.
- S.-H. Jung, E. Oh, K.-H. Lee, Y. Yang, C. G. Park, W. Park and S.-H. Jeong, Cryst. Growth Des., 2008, 8, 265–269 CrossRef CAS.
- L. Wang and Y. Yamauchi, Chem. Mater., 2009, 21, 3562–3569 CrossRef CAS.
- J. N. Wang, L. F. Su and Z. P. Wu, Cryst. Growth Des., 2008, 8, 1741–1747 CrossRef CAS.
- J. Liu, J. Essner and J. Li, Chem. Mater., 2010, 22, 5022–5030 CrossRef CAS.
- W. Lei, D. Liu, P. Zhu, X. Chen, J. Hao, Q. Wang, Q. Cui and G. Zou, CrystEngComm, 2010, 12, 511–516 RSC.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384–1404 RSC.
- H. Elzanowska, E. Miasek and V. I. Birss, Electrochim. Acta, 2008, 53, 2706–2715 CrossRef CAS.
- J. Mu, B. Chen, Z. Guo, M. Zhang, Z. Zhang, C. Shao and Y. Liu, J. Colloid Interface Sci., 2011, 356, 706–712 CrossRef CAS.
- T. P. Gujar, V. R. Shinde, C. D. Lokhande, W.-Y. Kim, K.-D. Jung and O.-S. Joo, Electrochem. Commun., 2007, 9, 504–510 CrossRef CAS.
- M. He, K. Kravchyk, M. Walter and M. V. Kovalenko, Nano Lett., 2014, 14, 1255–1262 CrossRef CAS.
- Q. Xu, W. Li, L. Ding, W. Yang, H. Xiao and W.-J. Ong, Nanoscale, 2019, 11, 1475–1504 RSC.
- F. Zhao, S. Shen, L. Cheng, L. Ma, J. Zhou, H. Ye, N. Han, T. Wu, Y. Li and J. Lu, Nano Lett., 2017, 17, 4137–4142 CrossRef CAS.
- R. S. Devan, R. A. Patil, J. H. Lin and Y. R. Ma, Adv. Funct. Mater., 2012, 22, 3326–3370 CrossRef CAS.
- M. Ge, C. Cao, J. Huang, S. Li, Z. Chen, K. Q. Zhang, S. S. Al-Deyab and Y. Lai, J. Mater. Chem. A, 2016, 4, 6772–6801 RSC.
- J. Tian, Z. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920–6937 RSC.
- G. Zhang, X. Xiao, B. Li, P. Gu, H. Xue and H. Pang, J. Mater. Chem. A, 2017, 5, 8155–8186 RSC.
- T. Zhu, H. Bin Wu, Y. Wang, R. Xu and X. W. Lou, Adv. Energy Mater., 2012, 2, 1497–1502 CrossRef CAS.
- S. Peng, G. Jin, L. Li, K. Li, M. Srinivasan, S. Ramakrishna and J. Chen, Chem. Soc. Rev., 2016, 45, 1225–1241 RSC.
- S. Zhai, L. Wei, H. E. Karahan, X. Chen, C. Wang, X. Zhang, J. Chen, X. Wang and Y. Chen, Energy Storage Mater., 2019, 19, 102–123 CrossRef.
- Y. Liang, D. Wu and R. Fu, Sci. Rep., 2013, 3, 1119 CrossRef.
- C. Cheng and H. J. Fan, Nano Today, 2012, 7, 327–343 CrossRef CAS.
- Q. Wei, F. Xiong, S. Tan, L. Huang, E. H. Lan, B. Dunn and L. Mai, Adv. Mater., 2017, 29, 1602300 CrossRef.
- J. Le Xie, C. X. Guo and C. M. Li, Energy Environ. Sci., 2014, 7, 2559–2579 RSC.
- Z. Yin and Q. Zheng, Adv. Energy Mater., 2012, 2, 179–218 CrossRef CAS.
- B. I. Kharisov, O. V. Kharissova, B. O. García, Y. P. Méndez and I. G. De La Fuente, RSC Adv., 2015, 5, 105507–105523 RSC.
- W. Zhao, C. Zhang, F. Geng, S. Zhuo and B. Zhang, ACS Nano, 2014, 8, 10909–10919 CrossRef CAS PubMed.
- E. Lhuillier, S. Pedetti, S. Ithurria, B. Nadal, H. Heuclin and B. Dubertret, Acc. Chem. Res., 2015, 48, 22–30 CrossRef CAS.
- X. Ou, C. Yang, X. Xiong, F. Zheng, Q. Pan, C. Jin, M. Liu and K. Huang, Adv. Funct. Mater., 2017, 27, 1606242 CrossRef.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- C. Marichy, M. Bechelany and N. Pinna, Adv. Mater., 2012, 24, 1017–1032 CrossRef CAS.
- L. Yu, H. Hu, H. Bin Wu and X. W. D. Lou, Adv. Mater., 2017, 29 Search PubMed.
- H. Jiang, P. S. Lee and C. Li, Energy Environ. Sci., 2013, 6, 41–53 RSC.
- P. Yu, X. Zhang, D. Wang, L. Wang and Y. Ma, Cryst. Growth Des., 2009, 9, 528–533 CrossRef CAS.
- X.-Y. Yu, L. Yu and X. W. D. Lou, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- J. Wang, Y. Cui and D. Wang, Adv. Mater., 2019, 31, 1801993 CrossRef PubMed.
- P. Tartaj and J. M. Amarilla, Chem. Commun., 2014, 50, 2077–2088 RSC.
- H. Hu, B. Y. Guan and X. W. Lou, Chem, 2016, 1, 102–113 CAS.
- J. Hong, J. Y. Han, H. Yoon, P. Joo, T. Lee, E. Seo, K. Char and B. S. Kim, Nanoscale, 2011, 3, 4515–4531 RSC.
- G. Zhang, L. Yu, H. E. Hoster and X. W. Lou, Nanoscale, 2013, 5, 877–881 RSC.
- P. Liu and L. Zhang, Crit. Rev. Solid State Mater. Sci., 2009, 34, 75–87 CrossRef CAS.
- C. K. Ranaweera, Z. Wang, E. Alqurashi, P. K. Kahol, P. R. Dvornic, B. K. Gupta, K. Ramasamy, A. D. Mohite, G. Gupta and R. K. Gupta, J. Mater. Chem. A, 2016, 4, 9014–9018 RSC.
- Y. E. Miao, W. Fan, D. Chen and T. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 4423–4428 CrossRef CAS.
- B. Y. Guan, X. Y. Yu, H. Bin Wu and X. W. D. Lou, Adv. Mater., 2017, 29 Search PubMed.
- Z. A. Qiao, B. Guo, A. J. Binder, J. Chen, G. M. Veith and S. Dai, Nano Lett., 2013, 13, 207–212 CrossRef CAS PubMed.
- B. T. Zhu, Z. Wang, S. Ding, J. S. Chen and X. W. Lou, RSC Adv., 2011, 1, 397–400 RSC.
- C. Y. Cao, W. Guo, Z. M. Cui, W. G. Song and W. Cai, J. Mater. Chem., 2011, 21, 3204–3209 RSC.
- F. Xu, Z. Tang, S. Huang, L. Chen, Y. Liang, W. Mai, H. Zhong, R. Fu and D. Wu, Nat. Commun., 2015, 6, 7221 CrossRef.
- C. Liu, J. Wang, J. Li, R. Luo, J. Shen, X. Sun, W. Han and L. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 18609–18617 CrossRef CAS.
- L. Yu, L. Zhang, H. Bin Wu and X. W. D. Lou, Angew. Chem., Int. Ed., 2014, 53, 3711–3714 CrossRef CAS.
- Y. Li, H. Wang, B. Huang, L. Wang, R. Wang, B. He, Y. Gong and X. Hu, J. Mater. Chem. A, 2018, 6, 14742–14751 RSC.
- W. Gu, L. Yang, F. Teng, Z. U. Abiden and F. Zhao, Energy Technol., 2019, 7, 1900460 CrossRef.
- W. Gu, F. Teng, Z. Liu, Z. Liu, W. Hao, A. Zhang and Y. Teng, J. Electroanal. Chem., 2017, 804, 185–191 CrossRef CAS.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, L. B. Alemany, W. Lu and J. M. Tour, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS.
-
R. Mertens, Graphene Supercapacitors Introd, News, https://www.graphene-info.com/graphene-supercapacitors Search PubMed.
-
T. Pallone, https://www.globalspec.com, 2018.
- A. S. Aricò, P. Bruce, B. Scrosati, J. M. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef.
- Z. S. Iro, C. Subramani and S. S. Dash, Int. J. Electrochem. Sci., 2016, 11, 10628–10643 CrossRef CAS.
- W. Lv, D. M. Tang, Y. B. He, C. H. You, Z. Q. Shi, X. C. Chen, C. M. Chen, P. X. Hou, C. Liu and Q. H. Yang, ACS Nano, 2009, 3, 3730–3736 CrossRef CAS.
- S. He and W. Chen, Nanoscale, 2015, 7, 6957–6990 RSC.
- L. Manjakkal, C. G. Núñez, W. Dang and R. Dahiya, Nano Energy, 2018, 51, 604–612 CrossRef CAS.
- X. H. Xia, D. L. Chao, Y. Q. Zhang, Z. X. Shen and H. J. Fan, Nano Today, 2014, 9, 785–807 CrossRef CAS.
- B. G. Choi, M. Yang, W. H. Hong, J. W. Choi and Y. S. Huh, ACS Nano, 2012, 6, 4020–4028 CrossRef CAS PubMed.
- X. Dong, J. Wang, J. Wang, M. B. Chan-Park, X. Li, L. Wang, W. Huang and P. Chen, Mater. Chem. Phys., 2012, 134, 576–580 CrossRef CAS.
- H. Sun, L. Mei, J. Liang, Z. Zhao, C. Lee, H. Fei, M. Ding, J. Lau, M. Li, C. Wang, X. Xu, G. Hao, B. Papandrea, I. Shakir, B. Dunn, Y. Huang and X. Duan, Science, 2017, 356, 599–604 CrossRef CAS PubMed.
- X. Dong, X. Wang, L. Wang, H. Song, H. Zhang, W. Huang and P. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 3129–3133 CrossRef CAS PubMed.
- K. Nomura, H. Nishihara, N. Kobayashi, T. Asada and T. Kyotani, Energy Environ. Sci., 2019, 12, 1542–1549 RSC.
- W. Yang, M. Ni, X. Ren, Y. Tian, N. Li, Y. Su and X. Zhang, Curr. Opin. Colloid Interface Sci., 2015, 20, 416–428 CrossRef CAS.
- F. Béguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 26, 2219–2251 CrossRef PubMed.
- D. W. Wang and D. Su, Energy Environ. Sci., 2014, 7, 576–591 RSC.
- D. Hulicova-Jurcakova, M. Seredych, G. Q. Lu and T. J. Bandosz, Adv. Funct. Mater., 2009, 19, 438–447 CrossRef CAS.
- D. G. Papageorgiou, I. A. Kinloch and R. J. Young, Prog. Mater. Sci., 2017, 90, 75–127 CrossRef CAS.
- I. M. Mosa, A. Pattammattel, K. Kadimisetty, P. Pande, M. F. El-Kady, G. W. Bishop, M. Novak, R. B. Kaner, A. K. Basu, C. V. Kumar and J. F. Rusling, Adv. Energy Mater., 2017, 7, 1700358 CrossRef.
- T. P. Dasari Shareena, D. McShan, A. K. Dasmahapatra and P. B. Tchounwou, Nano-Micro Lett., 2018, 10 CAS.
- S. K. Verma, E. Jha, P. K. Panda, J. K. Das, A. Thirumurugan, M. Suar and S. K. S. Parashar, Nanomedicine, 2018, 13, 43–68 CrossRef CAS.
- X. Zou, L. Zhang, Z. Wang and Y. Luo, J. Am. Chem. Soc., 2016, 138, 2064–2077 CrossRef CAS PubMed.
- F. Perreault, A. Fonseca De Faria and M. Elimelech, Chem. Soc. Rev., 2015, 44, 5861–5896 RSC.
- Y. Wang, Z. Li, J. Wang, J. Li and Y. Lin, Trends Biotechnol., 2011, 29, 205–212 CrossRef CAS.
- Z. Wang and B. Mi, Environ. Sci. Technol., 2017, 51, 8229–8244 CrossRef CAS.
- G. Lalwani, M. D'Agati, A. M. Khan and B. Sitharaman, Adv. Drug Delivery Rev., 2016, 105, 109–144 CrossRef CAS.
- K. Lü, G. X. Zhao and X. K. Wang, Chin. Sci. Bull., 2012, 57, 1223–1234 CrossRef.
- S. Wang, H. Sun, H. M. Ang and M. O. Tadé, Chem. Eng. J., 2013, 226, 336–347 CrossRef CAS.
- K. Hu, D. D. Kulkarni, I. Choi and V. V. Tsukruk, Prog. Polym. Sci., 2014, 39, 1934–1972 CrossRef CAS.
- B. Luo, S. Liu and L. Zhi, Small, 2012, 8, 630–646 CrossRef CAS.
- A. B. Seabra, A. J. Paula, R. De Lima, O. L. Alves and N. Durán, Chem. Res. Toxicol., 2014, 27, 159–168 Search PubMed.
- A. Kamyshny and S. Magdassi, Small, 2014, 10, 3515–3535 CrossRef CAS.
-
C. Ameta, R. Ameta and S. Kothari, in Microwave-Assisted Organic Synthesis, A Green Chemical Approach, 2014, pp. 253–286 Search PubMed.
- V. C. Sanchez, A. Jachak, R. H. Hurt and A. B. Kane, Chem. Res. Toxicol., 2012, 25, 15–34 Search PubMed.
- M. S. Mauter and M. Elimelech, Environ. Sci. Technol., 2008, 42, 5843–5859 CrossRef CAS PubMed.
-
S. C. Ray, Applications of Graphene and Graphene-Oxide Based Nanomaterials, 2015 Search PubMed.
- R. Leary and A. Westwood, Carbon, 2011, 49, 741–772 CrossRef CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- A. Chavez-Valdez, M. S. P. Shaffer and A. R. Boccaccini, J. Phys. Chem. B, 2013, 117, 1502–1515 CrossRef CAS.
- M. Holzinger, A. Le Goff and S. Cosnier, Front. Chem., 2014, 2, 63 Search PubMed.
- M. Pumera, Chem. Soc. Rev., 2010, 39, 4146–4157 RSC.
- W. Hu, C. Peng, W. Luo, M. Lv, X. Li, D. Li, Q. Huang and C. Fan, ACS Nano, 2010, 4, 4317–4323 CrossRef CAS.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- S. Goenka, V. Sant and S. Sant, J. Controlled Release, 2014, 173, 75–88 CrossRef CAS.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668–674 RSC.
- J. Bai and B. Zhou, Chem. Rev., 2014, 114, 10131–10176 CrossRef CAS.
- M. Hu, Z. Yao and X. Wang, Ind. Eng. Chem. Res., 2017, 56, 3477–3502 CrossRef CAS.
- C. Zhan, C. Lian, Y. Zhang, M. W. Thompson, Y. Xie, J. Wu, P. R. C. Kent, P. T. Cummings, D. E. Jiang and D. J. Wesolowski, Adv. Sci., 2017, 4 CAS.
- J. Xia, F. Chen, J. Li and N. Tao, Nat. Nanotechnol., 2009, 4, 505–509 CrossRef CAS.
- H. Ji, X. Zhao, Z. Qiao, J. Jung, Y. Zhu, Y. Lu, L. L. Zhang, A. H. MacDonald and R. S. Ruoff, Nat. Commun., 2014, 5, 3317 CrossRef.
- P. A. Brooksby, A. K. Farquhar, H. M. Dykstra, M. R. Waterland and A. J. Downard, J. Phys. Chem. C, 2015, 119, 25778–25785 CrossRef CAS.
- S. Luryi, Appl. Phys. Lett., 1988, 52, 501–503 CrossRef.
- S. Dröscher, P. Roulleau, F. Molitor, P. Studerus, C. Stampfer, K. Ensslin and T. Ihn, Phys. Scr., 2012, T146, 014009 CrossRef.
- E. Paek, A. J. Pak and G. S. Hwang, J. Electrochem. Soc., 2013, 160, A1–A10 CrossRef CAS.
- M. D. Stoller, C. W. Magnuson, Y. Zhu, S. Murali, J. W. Suk, R. Piner and R. S. Ruoff, Energy Environ. Sci., 2011, 4, 4685–4689 RSC.
- L. Li, C. Richter, S. Paetel, T. Kopp, J. Mannhart and R. C. Ashoori, Science, 2011, 332, 825–828 CrossRef CAS PubMed.
- A. A. Shylau, J. W. Kłos and I. V. Zozoulenko, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 205402 CrossRef.
- H. Raha, B. Manna, D. Pradhan and P. K. Guha, Nanotechnology, 2019, 30, 435404 CrossRef PubMed.
- C. Zhan, T. A. Pham, M. R. Cerón, P. G. Campbell, V. Vedharathinam, M. Otani, D. E. Jiang, J. Biener, B. C. Wood and M. Biener, ACS Appl. Mater. Interfaces, 2018, 10, 36860–36865 CrossRef CAS PubMed.
- Z. Li, X. Liu, L. Wang, F. Bu, J. Wei, D. Pan and M. Wu, Small, 2018, 14, 1801498 CrossRef PubMed.
- C. Song, J. Wang, Z. Meng, F. Hu and X. Jian, ChemPhysChem, 2018, 19, 1579–1583 CrossRef CAS PubMed.
- M. Mousavi-Khoshdel, E. Targholi and M. J. Momeni, J. Phys. Chem. C, 2015, 119, 26290–26295 CrossRef CAS.
- S. Ratha, S. R. Marri, N. A. Lanzillo, S. Moshkalev, S. K. Nayak, J. N. Behera and C. S. Rout, J. Mater. Chem. A, 2015, 3, 18874–18881 RSC.
- B. SanthiBhushan, M. S. Khan, V. K. Bohat and A. Srivastava, IEEE Trans. Nanotechnol., 2018, 17, 205–211 CAS.
- Y. Liu, J. Li, W. Li, Y. Li, F. Zhan, H. Tang and Q. Chen, Int. J. Hydrogen Energy, 2016, 41, 10354–10365 CrossRef CAS.
- P. Hirunsit, M. Liangruksa and P. Khanchaitit, Carbon, 2016, 108, 7–20 CrossRef CAS.
- Y. Zhao, S. Huang, M. Xia, S. Rehman, S. Mu, Z. Kou, Z. Zhang, Z. Chen, F. Gao and Y. Hou, Nano Energy, 2016, 28, 346–355 CrossRef CAS.
- S. Gupta, B. Aberg, S. Carrizosa and N. Dimakis, Materials, 2016, 9, 615 CrossRef.
- Y. Xu, Y. Feng, X. Li, G. Hu, Y. Luo, L. Sun, T. Tang, J. Wen, H. Wang and M. Li, Int. J. Electrochem. Sci., 2017, 12, 8820–8831 CrossRef CAS.
- C. Qu, L. Zhang, W. Meng, Z. Liang, B. Zhu, D. Dang, S. Dai, B. Zhao, H. Tabassum, S. Gao, H. Zhang, W. Guo, R. Zhao, X. Huang, M. Liu and R. Zou, J. Mater. Chem. A, 2018, 6, 4003–4012 RSC.
- A. Pathak, A. S. Gangan, S. Ratha, B. Chakraborty and C. S. Rout, J. Phys. Chem. C, 2017, 121, 18992–19001 CrossRef CAS.
- J. Kim, J. H. Jeon, H. J. Kim, H. Lim and I. K. Oh, ACS Nano, 2014, 8, 2986–2997 CrossRef CAS PubMed.
- Z. Li, Z. Xu, H. Wang, J. Ding, B. Zahiri, C. M. B. Holt, X. Tan and D. Mitlin, Energy Environ. Sci., 2014, 7, 1708–1718 RSC.
- Z.-S. Wu, K. Parvez, A. Winter, H. Vieker, X. Liu, S. Han, A. Turchanin, X. Feng and K. Müllen, Adv. Mater., 2014, 26, 4552–4558 CrossRef CAS.
- G. Wang, R. Liang, L. Liu and B. Zhong, Electrochim. Acta, 2014, 115, 183–188 CrossRef CAS.
- Jaidev, R. I. Jafri, A. K. Mishra and S. Ramaprabhu, J. Mater. Chem., 2011, 21, 17601 RSC.
- S. Saha, P. Samanta, N. C. Murmu and T. Kuila, J. Energy Storage, 2018, 17, 181–202 CrossRef.
- O. Parlak, Y. Kumar Mishra, A. Grigoriev, M. Mecklenburg, W. Luo, S. Keene, A. Salleo, K. Schulte, R. Ahuja, R. Adelung, A. P. F. Turner and A. Tiwari, Nano Energy, 2017, 34, 570–577 CrossRef CAS.
- Y. Zhou, X. Wang, L. Acauan, E. Kalfon-Cohen, X. Ni, Y. Stein, K. K. Gleason and B. L. Wardle, Adv. Mater., 2019, e1901916 CrossRef PubMed.
- R. Yi, S. Chen, J. Song, M. L. Gordin, A. Manivannan and D. Wang, Adv. Funct. Mater., 2014, 24, 7433–7439 CrossRef CAS.
- K. L. Stano, S. Faraji, R. Hodges, O. Yildiz, B. Wells, H. I. Akyildiz, J. Zhao, J. Jur and P. D. Bradford, Small, 2016, 12, 2432–2438 CrossRef CAS PubMed.
- C. D. Lokhande, D. P. Dubal and O.-S. Joo, Curr. Appl. Phys., 2011, 11, 255–270 CrossRef.
- F. Shi, L. Li, X. Wang, C. Gu and J. Tu, RSC Adv., 2014, 4, 41910–41921 RSC.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanoscale, 2013, 5, 72–88 RSC.
- X. Huang, X. Qi, F. Boey and H. Zhang, Chem. Soc. Rev., 2012, 41, 666–686 RSC.
- V. Sharma, I. Singh and A. Chandra, Sci. Rep., 2018, 8, 1307 CrossRef PubMed.
- B. Li, H. Cao, G. Yin, Y. Lu and J. Yin, J. Mater. Chem., 2011, 21, 10645–10648 RSC.
- L. Chen, Y. Zhang, P. Zhu, F. Zhou, W. Zeng, D. D. Lu, R. Sun and C. Wong, Sci. Rep., 2015, 5, 9672 CrossRef PubMed.
- M. A. S. M. Haniff, S. M. Hafiz, K. A. Wahid, Z. Endut, M. I. Syono, N. M. Huang, S. A. Rahman and I. A. Azid, J. Mater. Sci., 2017, 52, 6280–6290 CrossRef CAS.
- V. Subramanian, S. C. Hall, P. H. Smith and B. Rambabu, Solid State Ionics, 2004, 175, 511–515 CrossRef CAS.
- X. F. Wang, Z. You and D. B. Ruan, Chin. J. Chem., 2006, 24, 1126–1132 CrossRef CAS.
- W. Gong, B. Fugetsu, Z. Wang, I. Sakata, L. Su, X. Zhang, H. Ogata, M. Li, C. Wang, J. Li, J. Ortiz-Medina, M. Terrones and M. Endo, Commun. Chem., 2018, 1, 16 CrossRef.
- J. S. Sakamoto and B. Dunn, J. Electrochem. Soc., 2002, 149, A26 CrossRef CAS.
- S. Mandal, J. M. Amarilla, J. Ibáñez and J. M. Rojo, J. Electrochem. Soc., 2002, 148, A24 CrossRef.
- D. Kalpana, K. S. Omkumar, S. S. Kumar and N. G. Renganathan, Electrochim. Acta, 2006, 52, 1309–1315 CrossRef CAS.
- H. Wu, S. Lin, C. Chen, W. Liang, X. Liu and H. Yang, Mater. Res. Bull., 2016, 83, 434–441 CrossRef CAS.
- G. Du, Y. Li, L. Zhang, X. Wang, P. Liu, Y. Feng and X. Sun, Mater. Lett., 2014, 128, 242–244 CrossRef CAS.
- A. Meng, J. Shao, X. Fan, J. Wang and Z. Li, RSC Adv., 2014, 4, 60300–60305 RSC.
-
S. Bi, M. Chen, R. Wang, J. Feng, M. Dinca, A. A. Kornyshev and G. Feng, 2019, 1–19, arXiv:1903.00279 [cond-mat.mtrl-sci].
- J. Jiang, Y. Zhang, Y. An, L. Wu, Q. Zhu, H. Dou and X. Zhang, Small Methods, 2019, 1900081 CrossRef.
- J. Chen, B. Yang, H. Hou, H. Li, L. Liu, L. Zhang and X. Yan, Adv. Energy Mater., 2019, 9, 1803894 CrossRef.
- J. N. Zhang, P. Liu, L. N. Jin, C. Jin and S. W. Bian, ChemistrySelect, 2017, 2, 8618–8624 CrossRef CAS.
- R. Paulose and M. Raja, J. Nanosci. Nanotechnol., 2019, 19, 8151–8156 CrossRef.
- X. Wang, H. Wei, X. Liu, W. Du, X. Zhao and X. Wang, Nanotechnology, 2019, 30, 325401 CrossRef CAS.
- W. Huang, A. Zhang, H. Liang, R. Liu, J. Cai, L. Cui and J. Liu, J. Colloid Interface Sci., 2019, 549, 140–149 CrossRef CAS.
- B. Liang, Z. Zheng, M. Retana, K. Lu, T. Wood, Y. Ai, X. Zu and W. Zhou, Nanotechnology, 2019, 30, 295401 CrossRef CAS PubMed.
- L.-P. Lv, C. Zhi, Y. Gao, X. Yin, Y. Hu, D. Crespy and Y. Wang, Nanoscale, 2019, 11, 13996–14009 RSC.
- S. Cho, I. Jung, L. Zhang, S. Yoo, J. H. Won, S. B. Jung, L. Liu and S. Park, Nanotechnology, 2019, 30, 425401 CrossRef.
- Y. Yang, S.-W. Ng, D. Chen, J. Chang, D. Wang, J. Shang, Q. Huang, Y. Deng and Z. Zheng, Small, 2019, e1902071 CrossRef.
- X. Wang, Y. Zhang, J. Zheng, X. Liu and C. Meng, J. Colloid Interface Sci., 2019, 554, 191–201 CrossRef CAS.
- B. Zhang, J. Li, F. Liu, T. Wang, Y. Wang, R. Xuan, G. Zhang, R. Sun and C. Wong, Chem.–Eur. J., 2019, 25, 11715–11724 CrossRef CAS PubMed.
- M. Hu, C. Cui, C. Shi, Z.-S. Wu, J. Yang, R. Cheng, T. Guang, H. Wang, H. Lu and X. Wang, ACS Nano, 2019, 13, 6899–6905 CrossRef CAS PubMed.
- Z. Yang and Z. Chen, Nanotechnology, 2019, 30, 245402 CrossRef CAS PubMed.
- C. Zhang, H. Li, A. Huang, Q. Zhang, K. Rui, H. Lin, G. Sun, J. Zhu, H. Peng and W. Huang, Small, 2019, 15, e1805493 CrossRef.
- L. Wu, K. Zhang, X. Zhu, S. Cao, D. Niu and X. Feng, Langmuir, 2019, 35, 5125–5129 CrossRef CAS.
- J. Liu and P. Liu, J. Colloid Interface Sci., 2019, 542, 1–7 CrossRef CAS.
- S. Ramesh, K. Karuppasamy, H. M. Yadav, J.-J. Lee, H.-S. Kim, H.-S. Kim and J.-H. Kim, Sci. Rep., 2019, 9, 6034 CrossRef.
- U. Kamran, Y. J. Heo, J. W. Lee and S. J. Park, Micromachines, 2019, 234 CrossRef.
- W. Ma, M. Li, X. Zhou, J. Li, Y. Dong and M. Zhu, ACS Appl. Mater. Interfaces, 2019, 11, 9283–9290 CrossRef CAS.
- B. Cheng, R. Cheng, F. Tan, X. Liu, J. Huo and G. Yue, Nanoscale Res. Lett., 2019, 14, 66 CrossRef.
- K. Trzcinski, M. Szkoda, A. P. Nowak, M. Lapinski and A. Lisowska-Oleksiak, Beilstein J. Nanotechnol., 2019, 10, 483–493 CrossRef CAS.
- S. C. Dhanabalan, B. Dhanabalan, X. Chen, J. S. Ponraj and H. Zhang, Nanoscale, 2019, 11, 3046–3101 RSC.
- F. Hekmat, S. Shahrokhian and S. Rahimi, Nanoscale, 2019, 11, 2901–2915 RSC.
- M. Manoj, C. Muhamed Ashraf, M. Jasna, K. M. Anilkumar, B. Jinisha, V. S. Pradeep and S. Jayalekshmi, J. Colloid Interface Sci., 2019, 535, 287–299 CrossRef CAS.
- Z. Zhang, Z. Yao, Y. Meng, D. Li, Q. Xia and Z. Jiang, Inorg. Chem., 2019, 58, 1591–1598 CrossRef CAS.
- H. Li, Z. Li, Z. Wu, M. Sun, S. Han, C. Cai, W. Shen, X. Liu and Y. Fu, J. Colloid Interface Sci., 2019, 549, 105–113 CrossRef CAS PubMed.
- Z. Tan, G. Chen and Y. Zhu, Nanocarbons Adv. Energy Storage, 2015, 1, 211–225 CAS.
- Y. Kou, Y. Xu, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753–8757 CrossRef CAS.
- A. Izadi-Najafabadi, T. Yamada, D. N. Futaba, M. Yudasaka, H. Takagi, H. Hatori, S. Iijima and K. Hata, ACS Nano, 2011, 5, 811–819 CrossRef CAS.
- Z. Tang, C. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS.
- K. Naoi, W. Naoi, S. Aoyagi, J. Miyamoto and T. Kamino, Acc. Chem. Res., 2013, 46, 1075–1083 CrossRef CAS.
- H. C. Chien, W. Y. Cheng, Y. H. Wang and S. Y. Lu, Adv. Funct. Mater., 2012, 22, 5038–5043 CrossRef CAS.
- X. Yang, C. Cheng, Y. Wang, L. Qiu and D. Li, Science, 2013, 341, 534–537 CrossRef CAS.
- L. Mai, H. Li, Y. Zhao, L. Xu, X. Xu, Y. Luo, Z. Zhang, W. Ke, C. Niu and Q. Zhang, Sci. Rep., 2013, 3, 1718 CrossRef.
- L. Zhang, F. Zhang, X. Yang, G. Long, Y. Wu, T. Zhang, K. Leng, Y. Huang, Y. Ma, A. Yu and Y. Chen, Sci. Rep., 2013, 3, 1408 CrossRef.
- Z. S. Wu, X. Feng and H. M. Cheng, Natl. Sci. Rev., 2014, 1, 277–292 CrossRef CAS.
- R. Signorelli, D. C. Ku, J. G. Kassakian and J. E. Schindall, Proc. IEEE, 2009, 97, 1837–1847 CAS.
- C. Wang, M. Osada, Y. Ebina, B. W. Li, K. Akatsuka, K. Fukuda, W. Sugimoto, R. Ma and T. Sasaki, ACS Nano, 2014, 8, 2658–2666 CrossRef CAS.
- M. F. El-Kady, M. Ihns, M. Li, J. Y. Hwang, M. F. Mousavi, L. Chaney, A. T. Lech and R. B. Kaner, Proc. Natl. Acad. Sci., 2015, 112, 4233–4238 CrossRef CAS PubMed.
- Z. Peng, J. Lin, R. Ye, E. L. G. Samuel and J. M. Tour, ACS Appl. Mater. Interfaces, 2015, 7, 3414–3419 CrossRef CAS.
- N. Choudhary, C. Li, H.-S. Chung, J. Moore, J. Thomas and Y. Jung, ACS Nano, 2016, 10, 10726–10735 CrossRef CAS.
- C. Arbizzani, M. Mastragostino and F. Soavi, J. Power Sources, 2001, 100, 164–170 CrossRef CAS.
- J. Bowen, H. Seyedsina, C. Hongqi, M. Shiva, N. T. Nguyen, Y. Min, A. Marco and S. Patrik, Appl. Mater. Today, 2019, 17, 227–235 CrossRef.
- S. García-Dalí, J. I. Paredes, J. M. Munuera, S. Villar-Rodil, A. Adawy, A. Martínez-Alonso and J. M. D. Tascón, ACS Appl. Mater. Interfaces, 2019, 0, acsami.9b13484 Search PubMed.
- M. Zhou, Y. Li, Q. Gong, Z. Xia, Y. Yang, X. Liu, J. Wang and Q. Gao, ChemElectroChem, 2019, 6, 4595–4607 CrossRef CAS.
- D. Shin, H. Hwang, T. Yeo, S. Park, T. Kim, J. Lee and W. Choi, Carbon, 2019, 152, 746–754 CrossRef CAS.
- Y.-R. Son and S.-J. Park, Chem. Eng. J., 2019, 373, 1020–1029 CrossRef CAS.
- N. Du, L. Gong, L. Fan, K. Yu, H. Luo, S. Pang, J. Gao, Z. Zheng, J. Lv and B. Zhou, ACS Appl. Nano Mater., 2019, 2, 3039–3049 CrossRef.
- K. Lei, J. Ling, J. Zhou, H. Zou, W. Yang and S. Chen, Mater. Res. Bull., 2019, 116, 59–66 CrossRef CAS.
- M. Hong, C. Zhou, S. Xu, X. Ye, Z. Yang, L. Zhang, Z. Zhou, N. Hu and Y. Zhang, J. Power Sources, 2019, 423, 80–89 CrossRef CAS.
- T. Yeo, J. Lee, D. Shin, S. Park, H. Hwang and W. Choi, J. Mater. Chem. A, 2019, 7, 9004–9018 RSC.
- L. Wang, C. Zhang, X. Jiao and Z. Yuan, Nano Res., 2019, 12, 1129–1137 CrossRef CAS.
- P. Avasthi, A. Kumar and V. Balakrishnan, ACS Appl. Nano Mater., 2019, 2, 1484–1495 CrossRef CAS.
- P. Avasthi and V. Balakrishnan, Adv. Mater. Interfaces, 2019, 6, 1801842 CrossRef.
- F. Hekmat, H. Hosseini, S. Shahrokhian and H. E. Unalan, Energy Storage Mater. DOI:10.1016/j.ensm.2019.09.022.
- P. He, Z. Ding, X. Zhao, J. Liu, Q. Huang, J. Peng and L.-Z. Fan, Carbon, 2019, 155, 453–461 CrossRef CAS.
- F. Song, D. Huo, J. Hu, H. Huang, J. Yuan, J. Shen and A.-J. Wang, Nanotechnology, 2019, 30, 505401 CrossRef.
- D. Xia, J. Quan, G. Wu, X. Liu, Z. Zhang, H. Ji, D. Chen, L. Zhang, Y. Wang, S. Yi, Y. Zhou, Y. Gao and R. Jin, Nanomaterials, 2019, 9, 1225 CrossRef CAS PubMed.
- S. Chandra Sekhar, G. Nagaraju, B. Ramulu, S. K. Hussain, D. Narsimulu and J. S. Yu, Nano Res., 2019, 12, 2597–2608 CrossRef CAS.
- W. Gong, B. Fugetsu, Z. Wang, T. Ueki, I. Sakata, H. Ogata, F. Han, M. Li, L. Su, X. Zhang, M. Terrones and M. Endo, Carbon, 2019, 154, 169–177 CrossRef CAS.
- J. Liu, T. Xiong, T. Liu, C. Yang, H. Jiang and X. Li, Electrochim. Acta, 2019, 320, 134627 CrossRef CAS.
- L. Xia, X. Li, Y. Wu, S. Hu, Y. Liao, L. Huang, Y. Qing and X. Lu, Chem. Eng. J., 2020, 379, 122325 CrossRef CAS.
- Z. Zhang, Z. Xu, Z. Yao, Y. Meng, Q. Xia, D. Li and Z. Jiang, J. Alloys Compd., 2019, 805, 396–403 CrossRef CAS.
- J. Chen, Int. J. Electrochem. Sci., 2019, 7293–7302 CrossRef CAS.
- P. Liu, Q. Ru, P. Zheng, Z. Shi, Y. Liu, C. Su, X. Hou, S. Su and F. Chi-Chung Ling, Chem. Eng. J., 2019, 374, 29–38 CrossRef CAS.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS.
- Battery UniversityTM sponsored by Cadex Electronics Inc., Copyr. ©2003–2018 Cadex Electron. Inc.
- R. R. Salunkhe, Y. Kamachi, N. L. Torad, S. M. Hwang, Z. Sun, S. X. Dou, J. H. Kim and Y. Yamauchi, J. Mater. Chem. A, 2014, 2, 19848–19854 RSC.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- C. Péan, C. Merlet, B. Rotenberg, P. A. Madden, P. L. Taberna, B. Daffos, M. Salanne and P. Simon, ACS Nano, 2014, 8, 1576–1583 CrossRef PubMed.
-
T. Sekino, Inorganic and Metallic Nanotubular Materials, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, vol. 117 Search PubMed.
- M. Carbone, L. Gorton and R. Antiochia, Electroanalysis, 2015 Search PubMed.
- A. Schuchardt, T. Braniste, Y. K. Mishra, M. Deng, M. Mecklenburg, M. A. Stevens-Kalceff, S. Raevschi, K. Schulte, L. Kienle, R. Adelung and I. Tiginyanu, Sci. Rep., 2015, 5, 8839 CrossRef CAS PubMed.
- S. Garlof, M. Mecklenburg, D. Smazna, Y. K. Mishra, R. Adelung, K. Schulte and B. Fiedler, Carbon, 2017, 111, 103–112 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Anton
Grigoriev
a,
Anton
Grigoriev
 a,
Yogendra Kumar
Mishra
a,
Yogendra Kumar
Mishra
 b and
Rajeev
Ahuja
b and
Rajeev
Ahuja
 *c
*c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000


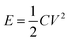



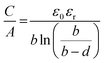





![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) CO,
CO, ![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) COH and COOH at different temperatures to adjust the redox active functional groups on freestanding CNT films which was achieved at 200 °C that provides long term stability. Higher power density is another characteristic feature that enhances supercapacitor performance and can be achieved by shape engineering of CNTs. Highly densely packed SWCNTs showed higher capacitance and better performance for thick electrodes.185,186 Systematical investigations of the aforementioned factors can optimize CNTs for better performance and long-term stability. A new horizon for application of CNTs came into the limelight when Pankratov et al.187 reported on the first self-charging biosupercapacitor based on CtCDH/BOD (Corynascus thermophilus cellobiose dehydrogenase (CtCDH)/bilirubine oxidase (BOD) electrodes for direct electron transfer (DET) communication as shown in Fig. 14.
COH and COOH at different temperatures to adjust the redox active functional groups on freestanding CNT films which was achieved at 200 °C that provides long term stability. Higher power density is another characteristic feature that enhances supercapacitor performance and can be achieved by shape engineering of CNTs. Highly densely packed SWCNTs showed higher capacitance and better performance for thick electrodes.185,186 Systematical investigations of the aforementioned factors can optimize CNTs for better performance and long-term stability. A new horizon for application of CNTs came into the limelight when Pankratov et al.187 reported on the first self-charging biosupercapacitor based on CtCDH/BOD (Corynascus thermophilus cellobiose dehydrogenase (CtCDH)/bilirubine oxidase (BOD) electrodes for direct electron transfer (DET) communication as shown in Fig. 14.





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W h kg−1
W h kg−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F g−1
F g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991.5 μW cm−2
991.5 μW cm−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000







