Open Access Article
Open Access ArticlePhoto/thermo-responsive and size-switchable nanoparticles for chemo-photothermal therapy against orthotopic breast cancer†
Ying
Bi‡
ab,
Miao
Wang‡
ab,
Lirong
Peng‡
a,
Lifo
Ruan
bc,
Mengxue
Zhou
bc,
Yi
Hu
 *bc,
Jun
Chen
*bc,
Jun
Chen
 *bc and
Jimin
Gao
*a
*bc and
Jimin
Gao
*a
aZhejiang Provincial Key Laboratory for Technology & Application of Model Organisms, School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou, Zhejiang 325035, China. E-mail: jimingao64@163.com
bCAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, Multi-disciplinary Research Division, Institute of High Energy Physics, Chinese Academy of Sciences (CAS), Beijing 100049, China. E-mail: chenjun@ihep.ac.cn; huyi@ihep.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 17th December 2019
Abstract
Tumor penetration of nanocarriers is still an unresolved challenge for effective drug delivery. Herein, we described a size-switchable nanoplatform in response to an external near-infrared (NIR) laser for transcellular drug delivery. The nanoplatform was constructed with a poly(N-isopropylacrylamide) (PNIPAM)-based nanogel encapsulating chitosan-coated single-walled carbon nanotubes, followed by loading a chemotherapeutic drug, doxorubicin (DOX). In mice bearing orthotopic breast tumors, the photothermal effect from single-walled carbon nanotubes upon NIR irradiation potently inhibited tumor growth. The antitumor effect of the nanomedicine with NIR irradiation might be attributed to its capability of transcellular transport and tumor penetration in mice. In addition, the nanomedicine with NIR irradiation could elicit an antitumor response by increasing cytotoxic T cells and decreasing myeloid-derived suppressor cells. These results validated the application of photo/thermo-responsive nanomedicine in the orthotopic model of breast cancer.
Despite the advances in developing nanocarriers for tumor-targeted drug delivery in the past decade, the translation of research results into clinics remains challenging.1 One of the major hindrances is the insufficient penetration of nanocarriers in solid tumors.2 Typically, nanocarriers can penetrate only a few cell layers post extravasation from the blood vessels.2,3 Moreover, the uneven intratumoral distribution of delivered drugs may eventually result in compromised therapeutic efficacy regardless of treatment modalities.4 To address the penetration issue, utilization of stimuli-responsive nanocarriers to overcome the complex physiological and pathological barriers is an intriguing strategy.5,6 It is broadly acknowledged that the physicochemical characteristics, including size, surface charge and morphology, of nanoparticles substantially influence their tumor penetration and intracellular delivery.7–10 Therefore, the nanocarriers with a size-switchable property upon tumor-confined stimuli will potentially enhance tumor penetration.
Photothermal therapy (PTT) by means of light-triggered heating through light-absorbing photothermal agents provides a spatiotemporally controllable tool for cancer treatment.11 In addition to the direct ablation of tumor cells by light-induced hyperthermia, elevation of temperature can ameliorate the permeability of blood vessels inside tumors and boost the release of entrapped therapeutic payloads. For example, Kirui and co-workers have characterized how mild PTT could enhance the penetration of macromolecules in tumors.12 Therefore, photothermal effects may act synergistically with size-switchable nanoparticles to enhance their tumor penetration ability.
Besides the suboptimal tumor penetration of nanocarriers, the deficiency of research in orthotopic tumor models might also impede the clinical translation of nanomedicines. The majority of preclinical studies of nanomedicines have exploited murine subcutaneous tumor models. However, these subcutaneous tumor models may not resemble the corresponding orthotopic tumor models in terms of vascular and stromal microenvironments.13–15 Compared with subcutaneous tumor models, orthotopic tumor models generally better simulate the physiological situation and therefore can be a more reliable tool for evaluating the therapeutic effects of nanomedicines against cancers.16 In particular, examination of tumor penetration of nanocarriers in orthotopic tumor models is necessary, as the tumor vasculature influences the tumor penetration of nanoparticles.17,18
We have developed a size-switchable nanocarrier in response to a near-infrared (NIR) laser for targeted drug delivery.18–20 We used thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) nanogels, which can shrink upon an increase in temperature,21,22 to encapsulate chitosan-wrapped carbon nanotubes as a NIR-responsive PTT agent.23 When loaded with a chemotherapeutic agent doxorubicin (DOX), the nanoparticles have been shown to have potent antitumor activity in both subcutaneous and orthotopic mouse models of bladder cancer.18,19 Herein, we aimed to further validate the antitumor effect of DOX-loaded nanoparticles (NP-DOX) in a mouse model of orthotopic breast cancer and examine the tumor penetration of nanoparticles upon NIR irradiation (Scheme 1). In addition, our data suggested that NP-DOX with NIR irradiation could elicit an antitumor immune response.
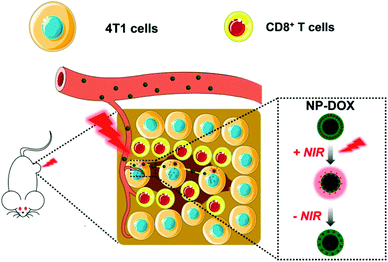 | ||
| Scheme 1 Schematic illustration of NIR-responsive and size-switchable nanomedicines for chemo–photothermal therapy. This scheme uses elements from Servier Medical Art (https://www.smart.servier.com). | ||
Results and discussion
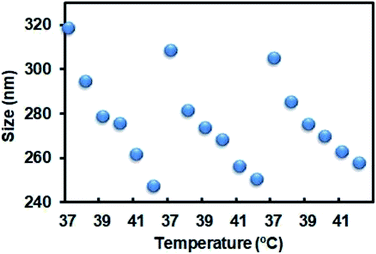
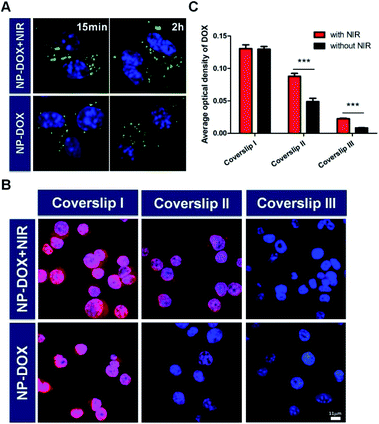
The nanoparticles in this study were prepared according to a reported protocol.19 One major component of the NPs, PNIPAM, features a thermoresponsive phase transition that might change the size of the NPs in response to the surrounding temperature. To demonstrate the thermoresponsive size change of NP-DOX, we measured the size of NP-DOX at different temperatures by dynamic light scattering (DLS). As shown in Fig. 1, when the temperature was increased from 37 °C to 42 °C, the hydrodynamic size of NP-DOX changed from ∼320 nm to ∼240 nm. Afterward, we decreased the temperature to 37 °C, and the size of NP-DOX was back to ∼300 nm (Fig. 1). The size shrinkage of nanoparticles underwent little decay after three rounds of temperature changes, thereby suggesting that the nanoparticles were relatively stable.At the cellular level, lysosomes of 4T1 cells incubated with the NPs for a predetermined time were stained with green-colored fluorescent LysoTracker and observed by confocal microscopy. Without NIR, the lysosomes were generally observed as green fluorescent dots (Fig. 2A). By contrast, after NIR irradiation, the green dot-like lysosomes in the cells were fused with the neighboring dots, indicating the destabilization of lysosomes (Fig. 2A). The destabilization of lysosomes, which could be ascribed to the NIR-induced photothermal effect, may facilitate lysosomal escape of the NPs.24 Next, we asked whether DOX was able to pass through the neighboring cells. As seen in Fig. 2B, the red fluorescence of DOX indicated the uptake of NP-DOX in the cells. At the beginning, the cellular uptake of NP-DOX was similar with or without NIR (Fig. 2B and C). However, DOX fluorescence from NP-DOX + NIR irradiation was much stronger than that from NP-DOX in coverslip II (1.8 times) and III (2.7 times) (Fig. 2B and C). These results suggested that NIR irradiation enhanced the transcellular transport of DOX.
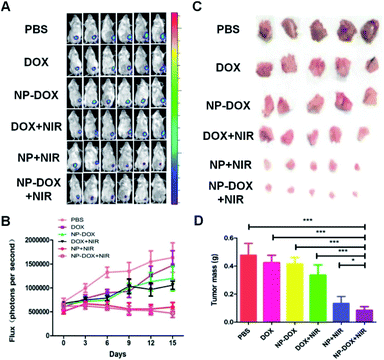
Encouraged by the excellent tumor-targeting and tumor penetration ability of NP-DOX with NIR irradiation, we proceeded to evaluate its antitumor activity in mice bearing orthotopic breast tumors. When the average sizes of tumors reached about 60 mm3, the mice were administered with PBS, free DOX, NP-DOX, DOX plus NIR irradiation, NP plus NIR irradiation and NP-DOX plus NIR irradiation. Tumor volumes of the mice were recorded every three days. From the results of the tumor volumes, we found that NP and NP-DOX with NIR irradiation could potently inhibit tumor growth in mice (Fig. 3A and B). By contrast, the tumors continued to grow in mice upon the other four treatments (Fig. 3A and B). These results suggested that NIR-induced hyperthermia was able to suppress tumor growth. According to our previous report,19 carbon nanotubes embedded in the nanocarriers could convert NIR efficiently into heat and consequently increase the tumor temperature to be higher than 50 °C. Such a high temperature prevented tumor growth. After the mice were sacrificed, the tumors from the NP-DOX + NIR group were significantly smaller than those from the NP + NIR group (Fig. 3C and D). These results suggested that there might be synergistic chemo–photothermal effects against tumors.
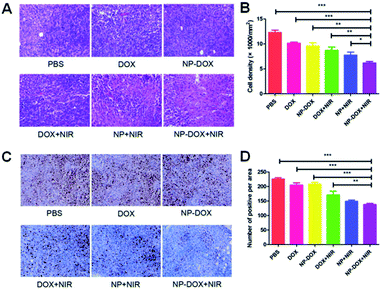
Hematoxylin and eosin (H&E) staining was employed to evaluate the antitumor efficacy of the nanoparticles. From H&E images (Fig. 4A), more apoptotic or necrotic cells were found in the NP-DOX plus NIR group in comparison to PBS and free DOX groups (Fig. 4B). Fig. 4C displays the results of the Ki-67 assay. With the aid of NIR irradiation, brown dots were reduced by NP-DOX (Fig. 4D), which indicated a potent inhibitory effect on tumor cell proliferation.
Based on the superior therapeutic outcome of the photothermal and chemotherapy, we next asked whether the dying tumor cells might release a large amount of antigens and elicit local immune response.25 In particular, tumor cells undergoing apoptosis could provide antigens for dendritic cells.26 As professional antigen-presenting cells, dendritic cells play key roles in the initiation and regulation of anticancer immunity.27 Therefore, we further studied the effects of NPs on dendritic cells. CD11c+ dendritic cells are representative antigen-presenting cells for the induction of antitumor immunity.28 We found that NP-DOX with NIR irradiation significantly increased the percentage of CD11c+ dendritic cells, as compared with DOX and NP-DOX without NIR irradiation (Fig. S1A†). Similarly, NP-DOX with NIR irradiation significantly increased the percentage of CD80+CD86+ cells, as compared with DOX and NP-DOX without NIR irradiation (Fig. S1B†). These cells are mature dendritic cells which may induce a cytotoxic T cell response.29 Based on these findings, we next tested the effects of DOX and NP-DOX on CD8+ cells that are cytotoxic T cells combating cancer cells.30 We found that NP-DOX with NIR irradiation also increased the percentage of CD8+ cells (Fig. S2A†), while that of T helper cells CD4+ remained almost unchanged (Fig. S2B†). NP-DOX/NIR-induced increase in CD8+ cells was in agreement with the increase in CD80+CD86+ cells. Besides the anticancer immunity, we have also examined the negative regulator such as CD4+FoxP3+ cells, which are regulatory T cells (Tregs) and could be immunologic barriers for CD8+ cells-mediated antitumor immune response.30 Results indicated that the levels of CD4+FoxP3+ cells were similar between DOX, NP-DOX and NP-DOX with NIR irradiation (Fig. S3†). These results suggested that NP-DOX with NIR irradiation might not suppress CD8+ cell-mediated antitumor immune response. Furthermore, we found that CD11b+Gr-1+ myeloid-derived suppressor cells were significantly reduced by NP-DOX with NIR irradiation (Fig. S4†). As these cells have been suggested to be responsible for lung metastasis of breast cancer,31 the NP-DOX/NIR-induced decrease in CD11b+Gr-1+ cells might prevent the tumor metastasis. These results provided preliminary data of NP-DOX/NIR-associated immune response. Further mechanistic studies would be required to elucidate how the immunologic system is activated by nanomedicines against cancer.
Conclusions
In summary, we have demonstrated that photo/thermo-responsive nanomedicines could provide synergistic chemo–photothermal therapy in mice bearing orthotopic breast tumors. The death of tumor cells upon NP-DOX and NIR might elicit CD8+ cell-mediated antitumor immune response to further enhance the antitumor effects of NP-DOX. The findings in this study validated the application of photo/thermo-responsive nanomedicines in the orthotopic tumor model.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21574136, 81573110, 11875269), the CAS Youth Innovation Promotion Association Program (2015008), Science Foundation of National Health Commission (WKJ-ZJ-1928), Science and Technology Major Projects of Wenzhou (ZJ2017014, 2018ZY001) and the Hundred Talents Program of CAS.References
- J. J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- R. Tong, H. D. Hemmati, R. Langer and D. S. Kohane, J. Am. Chem. Soc., 2012, 134, 8848–8855 CrossRef CAS PubMed.
- J. J. Chen, J. X. Ding, Y. C. Wang, J. J. Cheng, S. X. Ji, X. L. Zhuang and X. S. Chen, Adv. Mater., 2017, 29, 1701170 CrossRef PubMed.
- H. Kobayashi, R. Watanabe and P. L. Choyke, Theranostics, 2014, 4, 81–89 CrossRef CAS PubMed.
- M. X. Zhou, K. K. Wen, Y. Bi, H. R. Lu, J. Chen, Y. Hu and Z. F. Chai, Curr. Top. Med. Chem., 2017, 17, 2319–2334 CAS.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- M. X. Zhou, H. Huang, D. Q. Wang, H. R. Lu, J. Chen, Z. F. Chai, S. Q. Yao and Y. Hu, Nano Lett., 2019, 19, 3671–3675 CrossRef CAS PubMed.
- P. Zhang, J. Wang, H. Chen, L. Zhao, B. Chen, C. Chu, H. Liu, Z. Qin, J. Liu, Y. Tan, X. Chen and G. Liu, J. Am. Chem. Soc., 2018, 140, 14980–14989 CrossRef PubMed.
- K. K. Wen, M. X. Zhou, H. R. Lu, Y. Bi, L. F. Ruan, J. Chen and Y. Hu, ACS Biomater. Sci. Eng., 2018, 4, 4244–4254 CrossRef CAS.
- M. X. Zhou, X. C. Zhang, J. Xie, R. X. Qi, H. R. Lu, S. Leporatti, J. Chen and Y. Hu, Nanomaterials, 2018, 8, 952 CrossRef PubMed.
- Y. J. Liu, P. Bhattarai, Z. F. Dai and X. Y. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- D. K. Kirui, E. J. Koay, X. J. Guo, V. Cristini, H. F. Shen and M. Ferrari, Nanomedicine, 2014, 10, 1487–1496 CrossRef CAS PubMed.
- W. Zhang, W. Fan, S. Rachagani, Z. Zhou, S. M. Lele, S. K. Batra and J. C. Garrison, Sci. Rep., 2019, 9, 11117 CrossRef PubMed.
- A. S. Fung, C. Lee, M. Yu and I. F. Tannock, BMC Cancer, 2015, 15, 112 CrossRef PubMed.
- E. Chan, A. Patel, W. Heston and W. Larchian, BJU Int., 2009, 104, 1286–1291 CrossRef PubMed.
- J. J. Killion, R. Radinsky and I. J. Fidler, Cancer Metastasis Rev., 1998, 17, 279–284 CrossRef PubMed.
- W. Shi, Y. X. Yin, Y. Wang, B. Zhang, P. Tan, T. Jiang, H. Mei, J. Deng, H. F. Wang, T. Guo, Z. Q. Pang and Y. Hu, Oncotarget, 2017, 8, 32212–32227 Search PubMed.
- H. Gao, Y. Bi, X. Wang, M. Wang, M. X. Zhou, H. R. Lu, J. M. Gao, J. Chen and Y. Hu, ACS Biomater. Sci. Eng., 2017, 3, 3628–3634 CrossRef CAS.
- H. Gao, Y. Bi, J. Chen, L. R. Peng, K. K. Wen, P. Ji, W. F. Ren, X. Q. Li, N. Zhang, J. M. Gao, Z. F. Chai and Y. Hu, ACS Appl. Mater. Interfaces, 2016, 8, 15103–15112 CrossRef CAS PubMed.
- Y. P. Qin, J. Chen, Y. Bi, X. H. Xu, H. Zhou, J. M. Gao, Y. Hu, Y. L. Zhao and Z. F. Chai, Acta Biomater., 2015, 17, 201–209 CrossRef CAS PubMed.
- D. Q. Wang, H. Huang, M. X. Zhou, H. R. Lu, J. Chen, Y. T. Chang, J. M. Gao, Z. F. Chai and Y. Hu, Chem. Commun., 2019, 55, 4051–4054 RSC.
- D. Wang, M. Zhou, H. Huang, L. Ruan, H. Lu, J. Zhang, J. Chen, J. Gao, Z. Chai and Y. Hu, ACS Appl. Bio Mater., 2019, 2, 3178–3182 CrossRef CAS.
- Y. Liu, H. P. Li, J. Xie, M. X. Zhou, H. Huang, H. R. Lu, Z. F. Chai, J. Chen and Y. Hu, Biomater. Sci., 2017, 5, 1022–1031 RSC.
- X. Yang, B. Fan, W. Gao, L. Li, T. Li, J. Sun, X. Peng, X. Li, Z. Wang, B. Wang, R. Zhang and J. Xie, Int. J. Nanomed., 2018, 13, 4333–4344 CrossRef CAS PubMed.
- A. Balasubramani, Science, 2019, 364, 1247 Search PubMed.
- M. L. Albert, Nat. Rev. Immunol., 2004, 4, 223–231 CrossRef CAS PubMed.
- P. Giovanelli, T. A. Sandoval and J. R. Cubillos-Ruiz, Trends Immunol., 2019, 40, 699–718 CrossRef CAS PubMed.
- H. Lee, H. J. Lee, I. H. Song, W. S. Bang, S. H. Heo, G. Gong and I. A. Park, In Vivo, 2018, 32, 1561–1569 CrossRef CAS PubMed.
- J. Lee, S. N. Sait and M. Wetzler, Int. Immunol., 2004, 16, 1377–1389 CrossRef CAS PubMed.
- B. Farhood, M. Najafi and K. Mortezaee, J. Cell. Physiol., 2019, 234, 8509–8521 CrossRef CAS PubMed.
- Y. L. Hsu, M. C. Yen, W. A. Chang, P. H. Tsai, Y. C. Pan, S. H. Liao and P. L. Kuo, Breast Cancer Res., 2019, 21, 23 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00652d |
| ‡ These authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2020 |