Tunable nonlinear optical responses and carrier dynamics of two-dimensional antimonene nanosheets†
Lei
Zhang
a,
Shah
Fahad
b,
Hao-Ran
Wu
a,
Tao-Tao
Dong
a,
Zi-Zhen
Chen
a,
Ze-Qi
Zhang
a,
Rui-Tong
Liu
a,
Xin-Ping
Zhai
a,
Xiang-Yang
Li
a,
Xian
Fei
a,
Qi-Wei
Song
a,
Zhe-Ji
Wang
a,
Li-Chuan
Chen
a,
Chun-Lin
Sun
a,
Yong
Peng
b,
Qiang
Wang
 *a and
Hao-Li
Zhang
*a and
Hao-Li
Zhang
 *ac
*ac
aState Key Laboratory of Applied Organic Chemistry (SKLAOC), Key Laboratory of Special Function Materials and Structure Design (MOE), College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: qiangwang@lzu.edu.cn; haoli.zhang@lzu.edu.cn
bKey Laboratory of Magnetism and Magnetic Materials of Ministry of Education, School of Physical Science and Technology, Lanzhou University, Lanzhou 730000, P. R. China
cTianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, Tianjin University, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin University, Tianjin 300072, P. R. China
First published on 8th August 2020
Abstract
Sb nanosheets, also known as antimonene, have received ever-growing consideration as a promising new type of two-dimensional (2D) material due to their many attractive properties. However, how their nonlinear optical (NLO) properties are affected by their nanosheet structure and measurement conditions remains unclear. Herein, we report a successful size-selective production method for Sb nanosheets, which is based on a combination of lithium ion intercalation, solvent exfoliation and size selection centrifugation. This high-yield and size-selective preparation method enables fundamental investigation on the relation of the intrinsic optical properties of Sb nanosheets. Nanosecond Z-scan measurements revealed a unique size-dependent broadband NLO response. When the average size is reduced from 3 micrometers to 50 nanometers, the Sb nanosheets exhibit a clear transition from saturable absorption to reversed saturable absorption. Ultrafast transient absorption spectroscopic investigation indicated that exciton cooling is significantly faster in a small nanosheet than in large ones, revealing that the different exciton relaxation dynamic plays key roles in the distinct size-tunable nonlinear optical response. This work paves new ways towards the mass production and practical application of antimonene.
New conceptsSb nanosheets have received ever-growing research interest recently as a new member of the 2D mono-elemental materials. However, their property investigation is still hindered by the lack of an efficient strategy for the large scale production of Sb nanosheets with decent control over their size distribution. Consequently, the fundamental understanding of the underlying mechanism associated with the nonlinear optical properties of Sb nanosheets is very limited. To tackle these challenges, we established a new gram scale preparation method of Sb nanosheets with high yield and good size-control. This new preparation method allows us to perform a systematic investigation on the NLO of Sb nanosheets with variable sizes under different laser conditions. For the first time, we demonstrate a distinct interconversion between SA and RSA behaviour depending on the size of the Sb nanosheets. We then performed the first transient absorption spectroscopic investigation on different Sb nanosheets, which unveiled that the diverse NLO response of the Sb nanosheets is correlated with their size dependent carrier dynamics. These results pave a new way to scale up the production of Sb nanosheets and their application in NLO devices. |
Introduction
Two-dimensional (2D) materials have drawn immense interest due to their attractive physical and chemical properties in the last decade.1–4 After the boom of graphene,5,6 other 2D materials are also being extensively explored, including transition-metal dichalcogenides (TMDs),7–9 layered double hydroxides (LDHs)10 and Mxene.11 Recently, the newly emerged VA group element-based 2D materials (P, As, Sb, and Bi) have attracted great attention.12,13 In spite of the poor stability of black phosphorus (BP),14–18 recent research has indicated that some other VA group pnictogens, for instance, Sb nanosheets, are significantly more stable under ambient conditions when they are shrunk into 2D materials.19–27 Moreover, recent works have also reported some engaging properties of Sb nanosheets, such as high Curie temperature in ferromagnetism,28 promising topological insulator phase,29,30 and impressive carrier mobility,31 making them highly desirable for future optical and electronic applications.Several methods have been applied to the fabrication of Sb nanosheets, including mechanical exfoliation,32 molecular beam epitaxy (MBE),33 and solvent-assisted exfoliation.24,34–37 However, most of the reported exfoliation methods are either not suitable for large quantity production or suffer from low yield or poor morphology control, which can be attributed to the strong interlayer interactions and ultrashort layer distance in bulk Sb. Meanwhile, ion intercalation has been widely applied in manufacturing various 2D materials.38–41 To date, only Na+ intercalation of antimony taking place under electrochemical conditions has been reported.42 Li+ intercalation has not been successfully applied to Sb, although Li+ has an ion diameter (0.180 nm) very close to the interlayer distance (0.216 nm) of Sb.
Many 2D materials have shown potential in manipulating laser pulses through third-order nonlinear optics (NLO).43 So far, studies on the NLO properties of Sb nanosheets are rare and lack in-depth analysis. Early work reported saturable absorption (SA) behavior on Sb nanosheets,44 which appears to be consistent with that predicted by theoretical calculation.45 In contrast, a recent study by Zhang et al. demonstrated that Sb nanosheets exhibited a transformation from SA to reverse saturable absorption (RSA) under high laser power irradiation.46 Both SA and RSA behaviors are of interest for many practical applications, such as phase modulators and laser protection, respectively. Therefore, it is crucial to establish a reliable method to control the SA and RSA behaviors of Sb nanosheets. More importantly, a fundamental understanding of the mechanism behind the conversion between SA and RSA behaviors has not yet been achieved. It is known that the optical nonlinearity of nanomaterials is strongly influenced by the size, excitation wavelength, pulse duration, etc.47 Therefore, it is critical to understand what factors dominate the NLO behaviors of the Sb nanosheets and the mechanism of NLO of Sb nanosheets with different sizes under variable laser power.
In this work, we developed an optimized intercalating exfoliation method to produce size-controllable Sb nanosheets, which is based on a combination of lithium ion intercalation, sonication-assisted solvent exfoliation, and gradient centrifugation. This method can be applied to gram scale production and achieve a yield up to 24.39%. The good control of the size-distribution of the produced Sb nanosheets allows systematical investigation of their size-dependent and intensity-dependent NLO for the first time. Transient absorption spectroscopies from femtosecond to nanosecond scale have been utilized to investigate the underlying mechanism. The strong and tunable nonlinear responses endow them with great potential in the fabrication of nonlinear photonic devices such as saturable absorbers and optical limiting filters.
Results and discussion
Scheme 1 illustrates the preparation of Sb nanosheets using Li+ intercalation. Sb bulk was ground well into a powder followed by the addition of n-butyl lithium (n-BuLi) and n-hexane to reflux, the mixture gradually turned to deep brown. After the reflux with n-BuLi for 48 h, the reaction was quenched by adding a large amount of ethanol. The precipitate was subsequently washed with ethanol and deionized water 9 times to remove lithium ions and organic compounds. After overnight vacuum drying, the black powder was collected and marked as Sb–Li washed. The intercalated product mixture without any washing step was dried and collected as well for further comparison, which is marked as Sb–Li unwashed. To obtain a highly concentrated Sb nanosheet dispersion, a mild sonication (100 W, 50 Hz) of Sb–Li washed powder in ethanol for 30 min is essential. Other organic solvents besides ethanol have also been tested following a similar protocol for the solubility of Sb nanosheets, and the best soluble results were found in ethanol. Gradient centrifugation of ethanol dispersion was carried out to separate size-ranged Sb nanosheets. Under 6 different relative centrifugal forces (RCF), Sb nanosheets in various sizes were obtained, and then their different NLO responses and photodynamic properties were investigated.Scanning electron microscopy (SEM) characterization on the starting material, Sb powder, revealed an obvious stacked layered structure (Fig. S1, ESI†), suggesting the possibility of exfoliating Sb powder into ultrathin structures. An atomic force microscope (AFM) was used to determine the thickness of exfoliated Sb nanosheets. As shown in Fig. 1a, a large flat platform with an average height around 3–6 nm was detected. Considering that the monolayer is 0.373 nm thick,20 the obtained nanosheets were around 8–16 layers. SEM also shows a clear nano-flake structure (Fig. 1b). Moreover, the transmission electron microscopy (TEM) results exhibit a typical twisted 2D structure revealing large size flexible nanosheets (Fig. 1c and Fig. S2, ESI†). The selected area electron diffraction (SAED) pattern exhibits uniform diffraction spots (Fig. 1d). Further analysis proves that the diffraction spots correspond to the (012) and (01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) crystal planes along the [
) crystal planes along the [![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 00] zone axis. These results confirmed that intercalated Sb nanosheets maintain the same crystal structure of bulk materials. The HR-TEM image in Fig. 1e reveals the lattice fringes of 0.310 nm, which can be assigned to the (012) plane of the Sb crystal, indicating the high crystal-feature quality of the prepared sample. The element-mapping analysis also confirmed strong Sb signals distributed on the surface of the nanosheets (Fig. 1f). All these characterizations prove the successful fabrication of Sb nanosheets.
00] zone axis. These results confirmed that intercalated Sb nanosheets maintain the same crystal structure of bulk materials. The HR-TEM image in Fig. 1e reveals the lattice fringes of 0.310 nm, which can be assigned to the (012) plane of the Sb crystal, indicating the high crystal-feature quality of the prepared sample. The element-mapping analysis also confirmed strong Sb signals distributed on the surface of the nanosheets (Fig. 1f). All these characterizations prove the successful fabrication of Sb nanosheets.
Fig. 2a shows that the obtained Sb nanosheets display a broad absorption covering the UV-vis to low-energy near-infrared (NIR) range. Several peaks in both unwashed samples (Sb–Li unwashed) and the washed sample (Sb–Li) can be assigned to the Sb crystal (PDF#35-0723) in XRD characterization (Fig. 2b). This result indicates that the crystalline structure of the Sb is maintained after Li+ intercalation and solvent exfoliation. The peak representing the (003) lattice face at 23.68° decreased after the intercalation, which implies that lithium ions can efficiently intercalate into the layers along the (003) direction. Fig. 2c compares the Raman spectra of Sb nanosheets with bulk Sb. Two peaks located at 114.8 cm−1 and 150.4 cm−1 can be assigned as the Eg and A1g modes of the Sb nanosheets, representing the in-plane vibrations and the out-of-plane vibrations, respectively. In comparison with bulk Sb, the two peaks of Sb nanosheets shifted slightly to higher wavenumbers for ∼5 cm−1, indicating that the bulk material was successfully exfoliated into the ultrathin 2D structure.41
X-ray photoelectron spectroscopy (XPS) measurements were carried out for surface chemical component analysis. The survey scan shows Sb 3d signals in both bulk and the nanosheets (Fig. S3, ESI†). Accurate chemical information was studied through the analysis of high-resolution XPS spectra and the results were exhibited in Fig. 2d. Six peaks can be deconvoluted in both Sb bulk and Sb nanosheets. The peaks located at 536.8 eV and 527.4 eV can be attributed to Sb 3d3/2 and Sb 3d5/2 of metal Sb, respectively. Meanwhile, a considerable oxide of antimony, mainly in the form of Sb2O3, was detected in both Sb bulk and Sb nanosheets. The peaks positioned at 529.7 eV and 539.1 eV were assigned to Sb 3d5/2 and Sb 3d3/2 of Sb2O3, respectively. The XPS peak located at 530.80 eV contains the overlapped component from both O 1s and Sb 3d5/2 signal. According to the literature, the peak located at 530.70 eV represents O 1s signals of metal oxides48,49 while the absorption oxygen signal is ascribed at 532.47 eV.50,51 Compared to the spectra of Sb bulk and Sb nanosheets, both metallic and oxidized components can be deconvoluted and the oxide of antimony cannot be avoided in the final product as the oxide signals are also detected in the starting bulk material. After the intercalation and washing steps, the Sb component in the XPS spectrum decreases from 30.0% to 9.2%. It is known that XPS is a surface sensitive technique in which only signals from the very surface components can be obtained.52 In order to estimate the thickness of surface oxides, we calculated the inelastic mean free path (IMFP) of Sb nanosheets,53 which is around 11.77 monolayers. We then estimated that the thickness of the surface oxides is about 3.03 nm (see detail in ESI†). Bulk Sb shows a dominant oxide signal due to the native oxide layer on the surface. Compared with the starting bulk Sb, the Sb nanosheets show increased oxide component, indicating that the repeated washing and sonication steps involving water could lead to an increase of surface oxides of antinomy, which is worth attention.54,55 The increase of surface oxide contents observed in Fig. 2d is consistent with the previous reports on Sb nanosheets produced by other top-down methods.25,34,37
We have also studied the solubility and stability of Sb nanosheets in various organic solvents, which is important for applications. Fig. 3 displays the solubility test of Sb nanosheets in ten representative organic solvents, including n-pentanol, ether, n-hexane, ethanol (EtOH), acetone (CH3COCH3), acetonitrile (CH3CN), dichloromethane (DCM), dimethyl formamide (DMF), N-methylpyrrolidone (NMP) and iso-propanol (IPA). Among the 10 solvents used in our 20 day tracking test of the Sb nanosheets, the ethanol suspension displayed the highest absorption value compared to other solvents, indicating that ethanol is the best solvent for Sb nanosheets, which results in the highest concentration of suspension in all the test solvents. However, even in the ethanol solution, the absorption decreased to 35% of the starting value in 9 days, and becomes stable afterward. The sediments gathered in the bottom of the tube were black, suggesting that some Sb nanosheets may aggregate and precipitate in the first few days.
Based on the theory of Hansen solubility parameters (HSP, δ),56 a good solvent exhibits a similar HSP with the solute, which can be evaluated through HSP distance Ra:
| δ2 = δD2 + δP2 + δH2 | (1) |
| Ra2 = 4(δD,solv − δD,solu)2 + (δP,solv − δP,solu)2 + (δH,solv − δH,solu)2 | (2) |
As shown in Scheme 1, different size-ranged Sb nanosheets were prepared through a gradient centrifugation procedure.57 Employing ethanol as a solvent, six rotating speeds were used corresponding to the gravitational acceleration approximately 70g, 600g, 1700g, 3400g, 5600g and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, respectively (Fig. S4, ESI†). The yield under each rotation speed was calculated by weighing the corresponding sediment collected from each centrifugation step (Table S1, ESI†). The overall yield of purified Sb nanosheets is estimated to be above 24% with the 122 mg amount of starting materials, much higher than that of the bath sonication methods reported in the literature (Table S2, ESI†). We also compared the liquid phase sonication and Li+ intercalation in producing Sb nanosheets (Table S1 and Fig. S5, ESI†). Under the same preparation time and starting mass (122 mg) of Sb powder, the yield of intercalation (24.39%) is over four times higher than that of liquid phase sonication (5.89%), indicating that intercalation is an efficient method to synthesize Sb nanosheets. Since the dispersion experiment proved that ethanol is the best solvent for Sb nanosheets, we also prepared the Sb nanosheet dispersion in ethanol through 48h sonication. The yield utilizing 122 mg of Sb powder as a starting material is 6.78%, higher than that through isopropanol and water mixture (5.89%). Furthermore, we compared the yields of different starting masses (Table S2 and Fig. S6, ESI†). The yields of 122 mg, 244 mg and 1220 mg starting masses are 24.39%, 19.64% and 15.20%, respectively. Even through the yield decreases with the increase of Sb bulk powder in gram scale preparation, as the stirring and sonication become less efficient, the total yield of 15.20% is still desirable.
000g, respectively (Fig. S4, ESI†). The yield under each rotation speed was calculated by weighing the corresponding sediment collected from each centrifugation step (Table S1, ESI†). The overall yield of purified Sb nanosheets is estimated to be above 24% with the 122 mg amount of starting materials, much higher than that of the bath sonication methods reported in the literature (Table S2, ESI†). We also compared the liquid phase sonication and Li+ intercalation in producing Sb nanosheets (Table S1 and Fig. S5, ESI†). Under the same preparation time and starting mass (122 mg) of Sb powder, the yield of intercalation (24.39%) is over four times higher than that of liquid phase sonication (5.89%), indicating that intercalation is an efficient method to synthesize Sb nanosheets. Since the dispersion experiment proved that ethanol is the best solvent for Sb nanosheets, we also prepared the Sb nanosheet dispersion in ethanol through 48h sonication. The yield utilizing 122 mg of Sb powder as a starting material is 6.78%, higher than that through isopropanol and water mixture (5.89%). Furthermore, we compared the yields of different starting masses (Table S2 and Fig. S6, ESI†). The yields of 122 mg, 244 mg and 1220 mg starting masses are 24.39%, 19.64% and 15.20%, respectively. Even through the yield decreases with the increase of Sb bulk powder in gram scale preparation, as the stirring and sonication become less efficient, the total yield of 15.20% is still desirable.
The obtained Sb nanosheets were firstly characterized by Raman, infrared and UV-vis-NIR spectra (Fig. S7, ESI†). The Raman peaks exhibit a redshift with the increase of centrifugation speed. Several peaks appear in the region of 500–600 cm−1 in high rotation speed samples in infrared spectra, which can be assigned to Sb–O signal. The absorption of the NIR region decreases with the increase of rotation speed in the UV-vis-NIR spectra. Representative AFM images and the corresponding height profile are exhibited in Fig. S8 (ESI†). TEM and dynamic light scattering (DLS) were then used for better size analysis and statistics information on size distribution (Table 1 and Fig. S9, ESI†), which revealed a decrease in the particle sizes from micrometer to nanometer through the gradient centrifugation, indicating the successful size-separation of the Sb nanosheets.
| Centrifugal speed (rpm) | 1000 | 3000 | 5000 | 7000 | 9000 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Relative centrifugal force (g) | 70 | 600 | 1700 | 3400 | 5600 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
||
| Size from TEM (nm) | 6500 | 978.0 | 175.0 | 150.0 | 56.3 | 44.0 | ||
| Size from DLS (nm) | >μm | >μm | 212.3 | 175.4 | 77.8 | 63.5 | ||
| NLO | β (×10−10 m W−1) | 4.68 μJ | −2.4 | −2.1 | −2.1 | −4.5 | −2.1 | −0.43 |
| 13.80 μJ | −1.5 | −1.6 | −0.16 | −2.6 | −0.96 | 1.05 | ||
| 40.10 μJ | −1.0 | −0.19 | −0.24 | −0.78 | −2.1 | 0.44 | ||
| I S (×1011 W m−2) | 4.68 μJ | 8.0 | 13.7 | 15.0 | 6.41 | 8.21 | 26.2 | |
| 13.80 μJ | 13.2 | 17.6 | 3.50 | 0.28 | 0.24 | — | ||
| 40.10 μJ | 15.9 | 2.2 | 1.25 | 0.28 | 0.26 | — | ||
| TAS | Nanosecond scale | τ 1 (ns) | 193.16 (61.43%) | 89.63 (57.79%) | 59.26 (56.41%) | 201.64 (28.76%) | 341.25 (24.22%) | 132.83 (54.11%) |
| τ 2 (ns) | 35.96 (38.57%) | 31.13 (42.21%) | 26.90 (43.59%) | 53.80 (71.24%) | 74.43 (75.78%) | 53.80 (45.89%) | ||
| Femtosecond scale | τ 1 (ps) | 3.32 ± 0.48 (62.5%) | 3.28 ± 0.27 (62.5%) | 3.13 ± 0.26 (72.1%) | 3.12 ± 0.20 (71.7%) | 2.78 ± 0.20 (75.9%) | 2.77 ± 0.23 (70.2%) | |
| τ 2 (ps) | 293.3 ± 83.5 (62.5%) | 213.1 ± 35.2 (23.2%) | 191.4 ± 34.3 (23.2%) | 145.4 ± 18.8 (26.7%) | 106.1 ± 16.0 (23.3%) | 75.16 ± 9.7 (29.11%) | ||
| τ 3 > ns | 12.2% | 7.57% | 4.97% | 1.64% | 0.78% | 0.69% | ||
It is worth noting that the size of the nanosheets also affects their solubility. During the solubility test, ethanol shows the best performance of all of the gradient centrifuged samples (Fig. S10, ESI†). The previous study by Qu et al. has suggested that 2-butanol is a good solvent for Sb nanosheets.36 Fig. S10 (ESI†) shows that 2-butanol displayed a good solubility to small nanosheets that were prepared through 3k (600g) rotation speed, as its HSP is close to that of 2-pentanol (δ2-pentanol = 22.2, Table S3, ESI†). Knowing the Hansen solubility parameter of Sb nanosheets is crucial for the future development of new solvent blends for efficient exfoliation. Apart from ethanol, NMP, an extensively used solvent for the exfoliation and dispersion of 2D materials, also displayed an adequate soluble performance to Sb nanosheets. Meanwhile, a clear oxidation process of Sb in water was observed. The sediment from the Sb nanosheet water suspension displays a white color and some new peaks (141, 189, 217, 254, 292, and 498 cm−1) in the Raman spectra (Fig. S11, ESI†). These new peaks are in agreement with the signals of Sb2O3,58 confirming that Sb nanosheets were gradually oxidized in water. Further high-resolution XPS spectra of the white precipitate proved that Sb is mainly in the form of oxides (Fig. S3, ESI†).
Based on the scalable production of size-ranged Sb nanosheets, we have investigated the NLO properties of various sizes of Sb nanosheets systematically through the open-aperture Z-scan technique (Scheme S1, ESI†). Size-ranged Sb nanosheets were re-dispersed in NMP for all of the NLO measurements. NMP is a high boiling point solvent that showed neither SA nor RSA signals (Fig. S12, ESI†), so any NLO behaviour of the suspension should originate from the suspended Sb nanosheets.47 Meanwhile, XPS results indicate a large content of Sb2O3 on the very surface of the Sb nanosheets. To clarify the influence of the oxides, Z-scan response of Sb2O3 was also collected for comparison. The obtained Z-scan curve is the normalized transmittance (Tnorm) as a function of the sample position. Herein, we used Tnorm, which equals the ratio of nonlinear and linear transmittance. The Z-scan data were fitted to the nonlinear transmission equation using a sum of two nonlinear absorptions with opposite signs:
 | (3) |
Fig. 4a–f illustrates the nanosecond Z-scan data and fitting results, where the transmission of different samples increases when the input laser intensity increases (input intensity is maximum at z = 0), signifying strong saturable absorption (SA) behavior at low laser energy (4.68 μJ). However, with the increase of the laser power to 13.8 μJ, several samples (1700g, 3400g, 5600g) exhibit slight RSA around the focus (z = 0 mm). With further increasing laser power to 40.1 μJ, more obvious RSA behaviors around z = 0 mm were detected accompanied by the SA signal. Similar intensity-dependent transformation from SA to RSA has been previously observed in Sb nanosheets and other materials.46,59,60 Intensity dependent optical limiting response from carbon nanotubes61 is ascribed to the nonlinear scattering of the nanomaterial suspension. Meanwhile, switching from saturable absorption to optical limiting with laser intensity has been observed in MoTe2,62 black phosphorus63 and MoS2.64 The transition from SA to RSA is usually explained as the excited-state absorption (ESA)62 or two-photon absorption (TPA).63 Therefore, the intensity-dependent transition from SA to RSA of Sb nanosheets can be explained as the result of competition between SA and RSA under the influence of laser intensity.65 Under low intensity, bleaching of the ground state absorption is dominant, thus giving the SA signal. When the intensity rises further, ESA of the nanosheets became dominant and the sample started to exhibit increasingly strong RSA behaviors.66
Meanwhile, not only an intensity-dependent NLO but also a strong size-dependent NLO behavior can be clearly observed from Fig. 4. The SA signal can be detected in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample only at the laser power of 4.68 μJ. Moreover, the nonlinear absorption coefficient (β) of the 10
000g sample only at the laser power of 4.68 μJ. Moreover, the nonlinear absorption coefficient (β) of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample is also smaller than other samples (Table 1), indicating a weak SA property compared to the others. However, the normalized transmittance of the 10
000g sample is also smaller than other samples (Table 1), indicating a weak SA property compared to the others. However, the normalized transmittance of the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample maintains around 1.0 at far field from the focus point and decreases quickly at Z = 0 mm with the further rise of laser energy (up to 40.1 μJ), reflecting a typical RSA behavior. In contrast, the 600–5600g samples displayed SA or SA accompanying RSA signals under the same laser power. With the sample moving from the far field to the focus point (Z = 0 mm), the normalized transmittance of these samples firstly increased and then decreased, displaying a downward valley around the focus point overlapping with a broad upward band. Moreover, compared to the dramatic nonlinear optical response of Sb nanosheets, the Z-scan results of Sb2O3 displayed a weak response, indicating that the RSA signal mainly originates from the Sb nanosheets and not from the surface oxide (Fig. S13, ESI†). The size-dependent RSA behavior of Sb nanosheets is much easier to observe than the energy-dependent behavior. This phenomenon indicates that the NLO behavior of Sb nanosheets is more efficiently tuned through variable sizes. The RSA property can be observed at low laser power in small size Sb nanosheets while it can only be detected at high laser power in moderate rotation speed prepared samples, such as 600g, 1700g, and 3400g and the accompanying SA signal cannot be ignored. The above analysis reveals that size separation is critical to control the NLO of the Sb nanosheets. The small mismatch between the original data and fitting curves may come from the scattering, which is a common phenomenon that appeared in the Z-scan test of the nanomaterials.66–68 The size-dependent nonlinear optics have also been reported in MoS2,47 WS2,69 and BP.70 Our previous work demonstrated a transition from SA to RSA response in MoS2 with a decrease in the size of the nanosheets.47 The RSA behavior in these small-sized nanosheets is usually attributed to the edge and quantum confinement effects.47,69 However, a systematic investigation on the size-dependent NLO response of Sb nanosheets has not been reported.
000g sample maintains around 1.0 at far field from the focus point and decreases quickly at Z = 0 mm with the further rise of laser energy (up to 40.1 μJ), reflecting a typical RSA behavior. In contrast, the 600–5600g samples displayed SA or SA accompanying RSA signals under the same laser power. With the sample moving from the far field to the focus point (Z = 0 mm), the normalized transmittance of these samples firstly increased and then decreased, displaying a downward valley around the focus point overlapping with a broad upward band. Moreover, compared to the dramatic nonlinear optical response of Sb nanosheets, the Z-scan results of Sb2O3 displayed a weak response, indicating that the RSA signal mainly originates from the Sb nanosheets and not from the surface oxide (Fig. S13, ESI†). The size-dependent RSA behavior of Sb nanosheets is much easier to observe than the energy-dependent behavior. This phenomenon indicates that the NLO behavior of Sb nanosheets is more efficiently tuned through variable sizes. The RSA property can be observed at low laser power in small size Sb nanosheets while it can only be detected at high laser power in moderate rotation speed prepared samples, such as 600g, 1700g, and 3400g and the accompanying SA signal cannot be ignored. The above analysis reveals that size separation is critical to control the NLO of the Sb nanosheets. The small mismatch between the original data and fitting curves may come from the scattering, which is a common phenomenon that appeared in the Z-scan test of the nanomaterials.66–68 The size-dependent nonlinear optics have also been reported in MoS2,47 WS2,69 and BP.70 Our previous work demonstrated a transition from SA to RSA response in MoS2 with a decrease in the size of the nanosheets.47 The RSA behavior in these small-sized nanosheets is usually attributed to the edge and quantum confinement effects.47,69 However, a systematic investigation on the size-dependent NLO response of Sb nanosheets has not been reported.
The size-dependent NLO behavior may be associated with several factors, such as quantum confinement, defects,71 temperature,59 chemical composition36,72 and bandgaps.73,74 The carrier relaxing process in Sb nanosheets is strongly dependent on the band structure of the materials. According to recent theoretical studies, the band gap of Sb transforms from a metal to a semiconductor with the thickness decreasing from trilayer to monolayer.26 However, the experimental results indicate that Sb nanosheets from exfoliation exhibited narrow band gaps and were observed to be 0.8–1.44 eV,36 due to the oxide on the surface. Zhang et al. simulated the band structure of Sb with surface oxides, and indicated that the band gap is tunable covering a wide range from 1.51 eV at low oxidation degree to 0 eV at high oxidation degree.72 All the available quantitative analysis indicated that the band gaps of Sb nanosheets are smaller than the photon energy we used (2.33 eV) in Z-scan measurement. Therefore, it is believed that the electrons could be pumped to the bottom of the conductive band of Sb nanosheets under our experimental conditions.
The third-order NLO is tightly related to the electron excited-state dynamic process, hence, excited carrier dynamics on size-ranged Sb nanosheets were studied. Nanosecond transient absorption spectra (ns-TAS) of the gradient centrifuged samples were collected first (Scheme S2, ESI†). A long transient bleaching phenomenon in nanosecond transient absorption was detected in all samples (Table 1 and Fig. S14, ESI†). SA is often induced by the long exciton bleaching, which probably originates from the trap states in 2D materials.75,76 These traps are often accompanied by exfoliation procedures due to mechanical defect site oxidation. Indeed, the long recovery time on the nanosecond and microsecond scale may contribute to the observed SA behavior at low laser irradiation power. Under low excitation irradiance, the number of absorbed photons is in direct proportion to the laser power, resulting in the linear absorption. With the increase of pulse energy, most of the carriers are excited from the ground state. When the incident intensity is strong enough to reach the saturation limit, nearly all electron states in the bottom of the conduction band are filled with excited electrons.59,60 The remaining photons will not be absorbed and the SA process takes place.
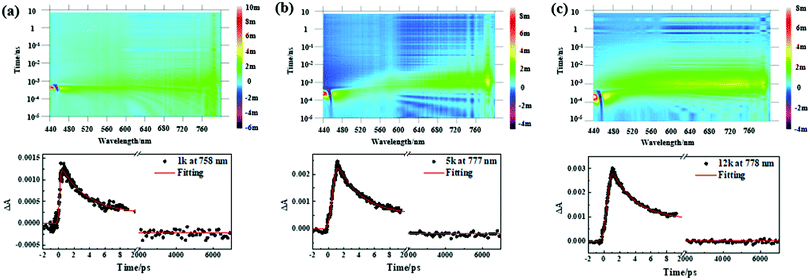
Furthermore, femtosecond transient absorption spectroscopy (fs-TAS) was carried out to reveal the ultrafast exciton dynamics (Scheme S3, ESI†). Fig. 5 and Fig. S15 (ESI†) display the bird's eye plot (wavelength vs. time vs. intensity) of the transient absorption spectra for the Sb nanosheets prepared through different centrifuging speeds. The fs-TAS spectra of Sb2O3 and NMP were collected as well for comparison (Fig. S16 and S17, ESI†). No noticeable response from the two samples was detected under either low or high pump energy. Therefore, it is concluded that the observed nonlinear optical response mainly originates from the Sb component and not from the surface oxide. The signal rise in Fig. 5 is not instantaneous, which implies that the autocorrelation of the pump and probe pulses should be taken into account.77 Through our fitting software (Surface Xplorer), the influence of instrument response function (IRF) including the autocorrelation of the pump and probe pulses has been considered, which could be reflected in the fitting curves around zero not being instantaneously raised, agreeing well with the experimental data. Moreover, autocorrelation also influences the assignment of lifetimes in ultrafast carrier dynamics. The coherence interference effect should also be considered when the extracted lifetime is shorter than the autocorrelation regime. The shortest lifetime is several picoseconds in our test, out of the autocorrelation region (±141 fs), demonstrating that the coherence spike does not influence our fitting results. The fitting results are demonstrated below their corresponding bird's eye plots and listed in Table 1. Three lifetime components can be extracted in all of the samples. Consistent with that observed in the nanosecond scale transient absorption spectra, the fitting results here also exhibit a long ns-component exceeded the 7 ns time window for the femtosecond transient absorption spectra in all of the samples. This long lifetime component indicates the existence of a long exciton bleaching time up to μs timescale, which is in good agreement with the ns transient absorption results. With the decrease of the size distribution of the Sb nanosheets, the ns component is decreased from 12.2% in the 70g sample to 0.69% in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample. Table 1 summarizes the detailed parameters, including the sizes collected through TEM and DLS, NLO parameters including the negative nonlinear absorption coefficient (β), saturable intensity (Is), and excited-state dynamics obtained from transient absorption spectra on both the nanosecond (Fig. S14, ESI†) and femtosecond scale for different Sb nanosheets (Fig. 5 and Fig. S15, ESI†).
000g sample. Table 1 summarizes the detailed parameters, including the sizes collected through TEM and DLS, NLO parameters including the negative nonlinear absorption coefficient (β), saturable intensity (Is), and excited-state dynamics obtained from transient absorption spectra on both the nanosecond (Fig. S14, ESI†) and femtosecond scale for different Sb nanosheets (Fig. 5 and Fig. S15, ESI†).
Based on the nanosecond and femtosecond transient absorption data, it is possible to put together a clear picture of the carrier dynamic process and the origin of the NLO. For instance, in the 70g, 600g and 1700g samples with the probe wavelength at their maximum absorption value in the corresponding transient absorption spectra, the decay curves can be extracted into three lifetime values included ∼3 ps, ∼200–300 ps (19.80–3.3%) and >7 ns (∼10%), corresponding to the intraband thermalization, interband and trap state-mediated carrier relaxation, respectively.76 Upon photoexcitation, photogenerated excitons formed on the fs scale, which can be reflected by the ΔA value, reached a positive maximum value and then underwent a fast intraband thermalization on the 3 ps timescale. Then a slower electron–hole recombination process occurred (interband recombination of several hundred ps and other longer recombination paths reaching the ns time window maximum). However, when the size further decreases, such as in the 3400g, 5600g and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g samples, the interband carrier recombination component became faster. Meanwhile, the ultra-long lifetime component (>7 ns) extracted in the 3400g, 5600g and 10
000g samples, the interband carrier recombination component became faster. Meanwhile, the ultra-long lifetime component (>7 ns) extracted in the 3400g, 5600g and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g samples decreased apparently, and eventually became negligible in the 10
000g samples decreased apparently, and eventually became negligible in the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample.
000g sample.
The fast interband carrier recombination in small size Sb nanosheets may be caused by the spatial confinement effect,47,59 the diffusion length of the carriers upon exciton in small Sb nanosheets is restricted thus decreasing the possibility for the carriers to encounter defects.78 As a result, the ns scale component became insignificant. Since the trap state induced ultralong lifetime component may contribute to the SA behavior, the decreased ns component in small nanosheets makes the SA negligible. Moreover, the electronic feature in small Sb nanosheets may be different from that in large nanosheets, which has been observed in other 2D materials such as TMDs47 and BP.70 The difference in the electronic structure can efficiently induce distinct excited state dynamic behavior.
The mechanism behind the optical limiting behavior of nanomaterials is usually believed to be associated with nonlinear absorption and scattering. The nonlinear scattering induced optical limiting behavior has been reported in suspensions of carbon nanotubes,79 graphene,80,81 MoS2 nanotubes82 or nanosheets,83 which is commonly attributed to the laser-induced bubbles of the solvents. It has been reported that the lifetimes of the bubbles are on the nanosecond scale.84 To verify the optical limiting mechanism in our system, the Z-scan performance of polymethyl methacrylate (PMMA) thin film containing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g sample was measured, as shown in Fig. S18 (ESI†). The film exhibited strong optical limiting behavior, which indicates that the RSA behavior of Sb nanosheets largely originates from nonlinear absorption instead of scattering, as the film excluded the bubble effects.85,86
000g sample was measured, as shown in Fig. S18 (ESI†). The film exhibited strong optical limiting behavior, which indicates that the RSA behavior of Sb nanosheets largely originates from nonlinear absorption instead of scattering, as the film excluded the bubble effects.85,86
Moreover, the mechanism behind RSA is usually considered as the ESA or TPA mechanism. It is known that the lifetime of TPA is normally very short. For instance, the lifetime of TPA in graphene oxide was reported to be less than 60 ps.87 The excited-state lifetime of Sb nanosheets in our TAS results was over nanoseconds, much longer than the reported lifetime of the TPA process in other 2D materials. Such a long lifetime is consistent with an ESA process. Therefore, we attribute the RSA behavior of Sb nanosheets to the ESA mechanism. Actually, with the increase of pump energy from 400 nJ to 2.4 μJ, the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g samples present nearly no negative signals, revealing that ESA is obvious (Fig. S19–S21, ESI†). The ESA is considered to be the reason inducing RSA behavior. Based on the above investigation on the carrier dynamics of the Sb nanosheets, we may explain the RSA behavior of small Sb nanosheets as follows. Due to the strong confinement effect and the possible existence of edge states in smaller Sb nanosheets, the ESA process is dominant,47,59 which induces RSA behavior.65,66 For the manipulation of the light, the third-order nonlinear optics based on Sb nanosheets exhibit quite a difference in size range, indicating the application potential for model locking and laser protecting. Our ultrafast transient absorption research on the size effect provides new understanding of this new 2D material.
000g samples present nearly no negative signals, revealing that ESA is obvious (Fig. S19–S21, ESI†). The ESA is considered to be the reason inducing RSA behavior. Based on the above investigation on the carrier dynamics of the Sb nanosheets, we may explain the RSA behavior of small Sb nanosheets as follows. Due to the strong confinement effect and the possible existence of edge states in smaller Sb nanosheets, the ESA process is dominant,47,59 which induces RSA behavior.65,66 For the manipulation of the light, the third-order nonlinear optics based on Sb nanosheets exhibit quite a difference in size range, indicating the application potential for model locking and laser protecting. Our ultrafast transient absorption research on the size effect provides new understanding of this new 2D material.
Conclusions
In conclusion, we have developed an efficient and facile strategy for the preparation of high-quality and size-tunable Sb nanosheets. This method is based on a combination of Li+ intercalation, sonication-assisted solvent exfoliation, and gradient centrifugation. We have obtained the Hansen solubility parameter of Sb nanosheets through solubility tests in different organic solvents, which is important for the development of more efficient and nontoxic exfoliation methods. Z-scan tests performed on a series of size-ranged Sb nanosheets revealed the strong size and intensity-dependent NLO behavior. Large nanosheets displayed stable SA behavior, while a transformation tendency from SA to RSA was observed with either a decrease in the size of the nanosheet or an increase of laser energy. The Sb nanosheets with an average lateral size of below 50 nm displayed a strong RSA behavior even under laser power as low as 13.8 μJ, suggesting potential application in an optical limiter. Our work demonstrates that size control is critical to regulate the NLO of Sb nanosheets. Transient absorption analysis revealed that size-induced fast exciton dynamics and strong ESA behavior contribute to the distinct NLO performance in small Sb nanosheets. The results in this work provide clear guideline to tune the NLO of Sb nanosheets and bring a further understanding of the unique optical properties of Sb nanomaterials, which are important to explore their potential applications.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Ministry of Science and Technology of China (2017YFA0204903) and the National Natural Science Foundation of China (NSFC 51733004, 51525303, 21673106, 21702085, 21602093 and 21572086). The authors thank beam line BL14B1 (Shanghai Synchrotron Radiation Facility) for providing the beam time. We thank Mr Bo Ma and Xin-Hai Yan for their help in synthesis. We wish to thank the Electron Microscopy Center of Lanzhou University for the microscopy and microanalysis of our specimens.References
- H. Zhang, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2015, 48, 56–64 CrossRef CAS PubMed.
- Y. Sun, S. Gao, F. Lei, C. Xiao and Y. Xie, Acc. Chem. Res., 2015, 48, 3–12 CrossRef CAS PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192–200 CrossRef CAS PubMed.
- M. Chhowalla, Z. Liu and H. Zhang, Chem. Soc. Rev., 2015, 44, 2584–2586 RSC.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- F. Cui, Q. Feng, J. Hong, R. Wang, Y. Bai, X. Li, D. Liu, Y. Zhou, X. Liang, X. He, Z. Zhang, S. Liu, Z. Lei, Z. Liu, T. Zhai and H. Xu, Adv. Mater., 2017, 29, 1705015 CrossRef PubMed.
- A. I. Khan and D. O'Hare, J. Mater. Chem., 2002, 12, 3191–3198 RSC.
- B. Anasori, M. R. Lukatskaya and Y. Gogotsi, Nat. Rev. Mater., 2017, 2, 16098 CrossRef CAS.
- C. Xing, L. Liu, D. Fan, Z. Peng and H. Zhang, FlatChem, 2019, 13, 8–24 CrossRef CAS.
- K. H. Kim, H. Y. Park, J. Shim, G. Shin, M. Andreev, J. Koo, G. Yoo, K. Jung, K. Heo, Y. Lee, H. Y. Yu, K. R. Kim, J. H. Cho, S. Lee and J. H. Park, Nanoscale Horiz., 2020, 5, 654–662 RSC.
- J. Kang, J. D. Wood, S. A. Wells, J. H. Lee, X. Liu, K. S. Chen and M. C. Hersam, ACS Nano, 2015, 9, 3596–3604 CrossRef CAS PubMed.
- R. Gusmao, Z. Sofer and M. Pumera, Angew. Chem., Int. Ed., 2017, 56, 8052–8072 CrossRef CAS PubMed.
- A. Carvalho, M. Wang, X. Zhu, A. S. Rodin, H. B. Su and A. H. C. Neto, Nat. Rev. Mater., 2016, 1, 16061 CrossRef CAS.
- T. Ahmed, S. Balendhran, M. N. Karim, E. L. H. Mayes, M. R. Field, R. Ramanathan, M. Singh, V. Bansal, S. Sriram, M. Bhaskaran and S. Walia, npj 2D Mater. Appl., 2017, 1, 18 CrossRef.
- W. Lei, G. Liu, J. Zhang and M. Liu, Chem. Soc. Rev., 2017, 46, 3492–3509 RSC.
- G. Wang, R. Pandey and S. P. Karna, ACS Appl. Mater. Interfaces, 2015, 7, 11490–11496 CrossRef CAS PubMed.
- P. Ares, F. Aguilar-Galindo, D. Rodriguez-San-Miguel, D. A. Aldave, S. Diaz-Tendero, M. Alcami, F. Martin, J. Gomez-Herrero and F. Zamora, Adv. Mater., 2016, 28, 6332–6336 CrossRef CAS PubMed.
- M. Pumera and Z. Sofer, Adv. Mater., 2017, 29, 1605299 CrossRef PubMed.
- X. Wu, Y. Shao, H. Liu, Z. Feng, Y. L. Wang, J. T. Sun, C. Liu, J. O. Wang, Z. L. Liu, S. Y. Zhu, Y. Q. Wang, S. X. Du, Y. G. Shi, K. Ibrahim and H. J. Gao, Adv. Mater., 2017, 29, 1605407 CrossRef PubMed.
- S. M. Beladi-Mousavi, A. M. Pourrahimi, Z. Sofer and M. Pumera, Adv. Funct. Mater., 2019, 29, 1807004 Search PubMed.
- C. Gibaja, D. Rodriguez-San-Miguel, P. Ares, J. Gomez-Herrero, M. Varela, R. Gillen, J. Maultzsch, F. Hauke, A. Hirsch, G. Abellan and F. Zamora, Angew. Chem., Int. Ed., 2016, 55, 14345–14349 CrossRef CAS PubMed.
- R. Gusmao, Z. Sofer, D. Bousa and M. Pumera, Angew. Chem., Int. Ed., 2017, 56, 14417–14422 CrossRef CAS PubMed.
- S. Zhang, Z. Yan, Y. Li, Z. Chen and H. Zeng, Angew. Chem., Int. Ed., 2015, 54, 3112–3115 CrossRef CAS PubMed.
- S. Guo, W. Zhou, B. Cai, K. Zhang, S. Zhang and H. Zeng, Nanoscale Horiz., 2019, 4, 1145–1152 RSC.
- X. Tang, L. Hu, T. W. Fan, L. Zhang, L. P. Zhu, H. Li, H. L. Liu, J. Y. Liang, K. D. Wang, Z. J. Li, S. C. Ruan, Y. P. Zhang, D. Y. Fan, W. C. Chen, Y. J. Zeng and H. Zhang, Adv. Funct. Mater., 2019, 29, 1808746 CrossRef.
- Z. Y. Hu, J. F. Gao, S. L. Zhang, J. J. Zhao, W. H. Zhou and H. B. Zeng, Phys. Rev. Mater., 2019, 3, 074005 CrossRef CAS.
- X. Wang, G. Bian, C. Xu, P. Wang, H. Hu, W. Zhou, S. A. Brown and T. C. Chiang, Nanotechnology, 2017, 28, 395706 CrossRef PubMed.
- S. Zhang, M. Xie, F. Li, Z. Yan, Y. Li, E. Kan, W. Liu, Z. Chen and H. Zeng, Angew. Chem., Int. Ed., 2016, 55, 1666–1669 CrossRef CAS PubMed.
- P. Ares, F. Zamora and J. Gomez-Herrero, ACS Photonics, 2017, 4, 600–605 CrossRef CAS.
- T. Lei, C. Liu, J.-L. Zhao, J.-M. Li, Y.-P. Li, J.-O. Wang, R. Wu, H.-J. Qian, H.-Q. Wang and K. Ibrahim, J. Appl. Phys., 2016, 119, 015302 CrossRef.
- C. Gibaja, M. Assebban, I. Torres, M. Fickert, R. Sanchis-Gual, I. Brotons, W. S. Paz, J. J. Palacios, E. G. Michel, G. Abellan and F. Zamora, J. Mater. Chem. A, 2019, 7, 22475–22486 RSC.
- J. N. Gu, Z. G. Du, C. Zhang, J. G. Ma, B. Li and S. B. Yang, Adv. Energy Mater., 2017, 7, 1700447 CrossRef.
- X. Wang, J. He, B. Zhou, Y. Zhang, J. Wu, R. Hu, L. Liu, J. Song and J. Qu, Angew. Chem., Int. Ed., 2018, 57, 8668–8673 CrossRef CAS PubMed.
- W. Z. Lin, Y. P. Lian, G. Zeng, Y. Y. Chen, Z. H. Wen and H. H. Yang, Nano Res., 2018, 11, 5968–5977 CrossRef CAS.
- A. J. Cooper, N. R. Wilson, I. A. Kinloch and R. A. W. Dryfe, Carbon, 2014, 66, 340–350 CrossRef CAS.
- W. Sirisaksoontorn, A. A. Adenuga, V. T. Remcho and M. M. Lerner, J. Am. Chem. Soc., 2011, 133, 12436–12438 CrossRef CAS PubMed.
- G. Bepete, E. Anglaret, L. Ortolani, V. Morandi, K. Huang, A. Penicaud and C. Drummond, Nat. Chem., 2017, 9, 347–352 CrossRef CAS PubMed.
- L. Zhang, L. F. Gao, L. X. Li, C. X. Hu, Q. Q. Yang, Z. Y. Zhu, R. Peng, Q. Wang, Y. Peng, J. Jin and H. L. Zhang, Mater. Chem. Front., 2018, 2, 1700–1706 RSC.
- F. Li, M. Xue, J. Li, X. Ma, L. Chen, X. Zhang, D. R. MacFarlane and J. Zhang, Angew. Chem., Int. Ed., 2017, 56, 14718–14722 CrossRef CAS PubMed.
- X. Tian, R. Wei, Q. Guo, Y. J. Zhao and J. Qiu, Adv. Mater., 2018, 30, e1801638 CrossRef PubMed.
- Y. F. Song, Z. M. Liang, X. T. Jiang, Y. X. Chen, Z. J. Li, L. Lu, Y. Q. Ge, K. Wang, J. L. Zheng, S. B. Lu, J. H. Ji and H. Zhang, 2D Mater., 2017, 4, 045010 CrossRef.
- Y. F. Xu, B. Peng, H. Zhang, H. Z. Shao, R. J. Zhang and H. Y. Zhu, Ann. Phys., 2017, 529, 1600152 CrossRef.
- F. Zhang, M. Wang, Z. Wang, K. Han, X. Liu and X. Xu, J. Mater. Chem. C, 2018, 6, 2848–2853 RSC.
- K. G. Zhou, M. Zhao, M. J. Chang, Q. Wang, X. Z. Wu, Y. Song and H. L. Zhang, Small, 2015, 11, 694–701 CrossRef CAS PubMed.
- L. Tan, A. Tang, Y. Zou, M. Long, Y. Zhang, J. Ouyang and J. Chen, Sci. Rep., 2017, 7, 3281 CrossRef PubMed.
- H. Wang, Y.-n. Wang, Y. Sun, X. Pan, D. Zhang and Y. F. Tsang, Process Saf. Environ. Prot., 2018, 113, 40–47 CrossRef CAS.
- A. Spitzer and H. Lüth, Surf. Sci., 1985, 160, 353–361 CrossRef CAS.
- L. Bodenes, A. Darwiche, L. Monconduit and H. Martinez, J. Power Sources, 2015, 273, 14–24 CrossRef CAS.
- B. D. Ratner and D. G. Castner, Surface Analysis – The Principal Techniques, 2009 Search PubMed.
- M. P. Seah and W. A. Dench, Surf. Interface Anal., 1979, 1, 2–11 CrossRef CAS.
- M. Bat-Erdene, G. R. Xu, M. Batmunkh, A. S. R. Bati, J. J. White, M. J. Nine, D. Losic, Y. Chen, Y. Wang, T. Y. Ma and J. G. Shapter, J. Mater. Chem. A, 2020, 8, 4735–4739 RSC.
- M. Assebban, C. Gibaja, M. Fickert, I. Torres, E. Weinreich, S. Wolff, R. Gillen, J. Maultzsch, M. Varela, S. T. J. Rong, K. P. Loh, E. G. Michel, F. Zamora and G. Abellaan, 2D Mater., 2020, 7, 025039 CrossRef.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, 2007 Search PubMed.
- C. Backes, B. M. Szydlowska, A. Harvey, S. Yuan, V. Vega-Mayoral, B. R. Davies, P. L. Zhao, D. Hanlon, E. J. Santos, M. I. Katsnelson, W. J. Blau, C. Gadermaier and J. N. Coleman, ACS Nano, 2016, 10, 1589–1601 CrossRef CAS PubMed.
- B. S. Naidu, M. Pandey, V. Sudarsan, R. K. Vatsa and R. Tewari, Chem. Phys. Lett., 2009, 474, 180–184 CrossRef CAS.
- R. Chen, X. Zheng and T. Jiang, Opt. Express, 2017, 25, 7507–7519 CrossRef CAS PubMed.
- F. Zhang, Z. X. Wu, Z. P. Wang, D. L. Wang, S. L. Wang and X. G. Xu, RSC Adv., 2016, 6, 20027–20033 RSC.
- J. Wang and W. J. Blau, Appl. Phys. B: Lasers Opt., 2008, 91, 521–524 CrossRef CAS.
- C. Quan, M. He, C. He, Y. Huang, L. Zhu, Z. Yao, X. Xu, C. Lu and X. Xu, Appl. Surf. Sci., 2018, 457, 115–120 CrossRef CAS.
- X. Zheng, R. Chen, G. Shi, J. Zhang, Z. Xu, X. Cheng and T. Jiang, Opt. Lett., 2015, 40, 3480–3483 CrossRef CAS PubMed.
- Q. Ouyang, H. Yu, K. Zhang and Y. Chen, J. Mater. Chem. C, 2014, 2, 6319–6325 RSC.
- K. X. Wang, N. N. Dong, Z. W. Liu, M. K. Shi, B. Zhang, J. Wang and Y. Chen, Polym. Chem., 2019, 10, 6003–6009 RSC.
- F. Zhang, K. Q. Chen, X. T. Jiang, Y. Z. Wang, Y. Q. Ge, L. M. Wu, S. X. Xu, Q. L. Bao and H. Zhang, J. Mater. Chem. C, 2018, 6, 8977–8983 RSC.
- B. P. Biswal, S. Valligatla, M. Wang, T. Banerjee, N. A. Saad, B. M. K. Mariserla, N. Chandrasekhar, D. Becker, M. Addicoat, I. Senkovska, R. Berger, D. N. Rao, S. Kaskel and X. Feng, Angew. Chem., Int. Ed., 2019, 58, 6896–6900 CrossRef CAS PubMed.
- Z. Li, N. Dong, Y. Zhang, J. Wang, H. Yu and F. Chen, APL Photonics, 2018, 3, 080802 CrossRef.
- H. Long, L. Tao, C. Y. Tang, B. Zhou, Y. Zhao, L. Zeng, S. F. Yu, S. P. Lau, Y. Chai and Y. H. Tsang, Nanoscale, 2015, 7, 17771–17777 RSC.
- Y. H. Xu, X. F. Jiang, Y. Q. Ge, Z. N. Guo, Z. K. Zeng, Q. H. Xu, H. Zhang, X. F. Yu and D. Y. Fan, J. Mater. Chem. C, 2017, 5, 3007–3013 RSC.
- X. Zhang, S. Zhang, Y. Xie, J. Huang, L. Wang, Y. Cui and J. Wang, Nanoscale, 2018, 10, 17924–17932 RSC.
- S. Zhang, W. Zhou, Y. Ma, J. Ji, B. Cai, S. A. Yang, Z. Zhu, Z. Chen and H. Zeng, Nano Lett., 2017, 17, 3434–3440 CrossRef CAS PubMed.
- S. F. Zhang, X. Y. Zhang, H. Wang, B. H. Chen, K. Wu, K. P. Wang, D. Hanlon, J. N. Coleman, J. P. Chen, L. Zhang and J. Wang, Opt. Mater. Express, 2016, 6, 3159–3168 CrossRef CAS.
- Y. X. Zhang, D. Z. Lu, H. H. Yu and H. J. Zhang, Adv. Opt. Mater., 2019, 7, 1800886 CrossRef.
- L. F. Gao, J. Y. Xu, Z. Y. Zhu, C. X. Hu, L. Zhang, Q. Wang and H. L. Zhang, Nanoscale, 2016, 8, 15132–15136 RSC.
- Q. Q. Yang, R. T. Liu, C. Huang, Y. F. Huang, L. F. Gao, B. Sun, Z. P. Huang, L. Zhang, C. X. Hu, Z. Q. Zhang, C. L. Sun, Q. Wang, Y. L. Tang and H. L. Zhang, Nanoscale, 2018, 10, 21106–21115 RSC.
- K. Wang, B. M. Szydlowska, G. Wang, X. Zhang, J. J. Wang, J. J. Magan, L. Zhang, J. N. Coleman, J. Wang and W. J. Blau, ACS Nano, 2016, 10, 6923–6932 CrossRef CAS PubMed.
- W. L. Wilson, P. F. Szajowski and L. E. Brus, Science, 1993, 262, 1242–1244 CrossRef CAS PubMed.
- B. Anand, R. Podila, P. Ayala, L. Oliveira, R. Philip, S. S. Sai, A. A. Zakhidov and A. M. Rao, Nanoscale, 2013, 5, 7271–7276 RSC.
- G. K. Lim, Z. L. Chen, J. Clark, R. G. S. Goh, W. H. Ng, H. W. Tan, R. H. Friend, P. K. H. Ho and L. L. Chua, Nat. Photonics, 2011, 5, 554–560 CrossRef CAS.
- J. Wang, Y. Hernandez, M. Lotya, J. N. Coleman and W. J. Blau, Adv. Mater., 2009, 21, 2430–2435 CrossRef CAS.
- K. P. Loh, H. Zhang, W. Z. Chen and W. Ji, J. Phys. Chem. B, 2006, 110, 1235–1239 CrossRef CAS PubMed.
- K. Wang, J. Wang, J. Fan, M. Lotya, A. O'Neill, D. Fox, Y. Feng, X. Zhang, B. Jiang, Q. Zhao, H. Zhang, J. N. Coleman, L. Zhang and W. J. Blau, ACS Nano, 2013, 7, 9260–9267 CrossRef CAS PubMed.
- V. Kotaidis, C. Dahmen, G. von Plessen, F. Springer and A. Plech, J. Chem. Phys., 2006, 124, 184702 CrossRef CAS PubMed.
- M. Zhao, K. Liu, Y.-D. Zhang, Q. Wang, Z.-G. Li, Y.-L. Song and H.-L. Zhang, Mater. Horiz., 2015, 2, 619–624 RSC.
- B. M. Szydłowska, A. Graf, A. Kelly, W. J. Blau, M. C. Gather, J. Zaumseil and C. Backes, J. Mater. Chem. C, 2020, 8(31), 10805–10815 RSC.
- Z.-B. Liu, X. Zhao, X.-L. Zhang, X.-Q. Yan, Y.-P. Wu, Y.-S. Chen and J.-G. Tian, J. Phys. Chem. Lett., 2011, 2, 1972–1977 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, synthetic procedures, detailed characterization of SEM, XPS, yield calculation, TEM, TAS and related discussion. See DOI: 10.1039/d0nh00262c |
| This journal is © The Royal Society of Chemistry 2020 |