Self-assembled Zn phthalocyanine as a robust p-type selective contact in perovskite solar cells†
Ece
Aktas
 ab,
Jesús
Jiménez-López
ab,
Jesús
Jiménez-López
 ac,
Kobra
Azizi
d,
Tomas
Torres
ac,
Kobra
Azizi
d,
Tomas
Torres
 *def and
Emilio
Palomares
*def and
Emilio
Palomares
 *ag
*ag
aInstitute of Chemical Research of Catalonia (ICIQ-BIST), Avda. PaÏsos Catalans, 16, Tarragona, E-43007, Spain. E-mail: epalomares@iciq.es
bDepartament de Química-Física I Inorgànica, URV, E-43007, Spain
cDepartmant d’Enginyeria Electrònica, Eèctrica I Automàtica, URV, Tarragona, E-43007, Spain
dDepartamento de Química Orgánica, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain
eIMDEA-Nanociencia, Campus de Cantoblanco, 28049 Madrid, Spain
fInstitute for Advanced Research in Chemical Sciences (IAdChem) Universidad Autónoma de Madrid, 28049 Madrid, Spain
gICREA, Passeig Lluís Companys 23, E-08010, Spain
First published on 11th August 2020
Abstract
The use of self-assembled monolayers (SAMs) as selective charge extracting layers in perovskite solar cells is a great approach to replace the commonly used charge selective contacts, as they can easily modify the interface to enhance the final solar cell performance. Here, we report a novel synthetic approach of the commonly known zinc phtalocyanine (ZnPc) molecule TT1, widely employed in dye-sensitized solar cells and previously used in perovskite solar cells. TT1 is used as a p-type selective contact, and it demonstrates its ability to form SAM on top of the indium tin oxide (ITO) transparent electrode, obtaining higher efficiencies compared to Pedot:PSS based perovskite solar cells. The differences observed, with an enhanced open-circuit voltage and overall efficiency in TT1 devices are correlated with differences in energetics rather than recombination kinetics.
New conceptsWe report in this communication for the first time the use of a zinc phathalocyanine self-assembled monolayer (SAM) as an efficient and robust selective contact for highly efficient perovskite solar cells (ca. 15% under standard sun simulated light). In contrast with previous work, that described the use of phthalocyanines thin films as a hole transport layer, here we use a self-organized layer that suffices for achieving higher efficiencies. In addition, the novelty of the synthesis that approaches 94% product yield, as well as the easy-to-prepare SAM selective contact are two remarkable facts. The methodology could be used in flexible substrates (plastic) as it does not require high temperature steps. We believe that our work paves the way to exploring these types of molecules and their related counterparts (porphyrins) as SAMs in thin film solar cells. |
Introduction
Hybrid lead halide perovskites represent the latest example of efficient thin films for the conversion of sunlight into electrical current.1 Solar cells made using such materials have reached efficiencies over 25% under standard measurement conditions.2 Importantly, the photoactive material must be sandwiched between a p-type and an n-type selective contact to efficiently extract charges obtaining high power conversion efficiencies. When an n-type semiconductor is deposited onto the transparent conducting oxide (TCO) the solar cell configuration is denoted as n–i–p or regular perovskite solar cells. In contrast, when a p-type organic semiconductor thin film is deposited onto the TCO the solar cell configuration is denoted as p–i–n or inverted.3 Among the most commonly used p-type selective contacts, it is possible to find polymers, such as PTAA (poly[bis(4-phenyl)(2,4,6-trimethyl-phenyl)amine]),4 or low molecular weight molecules such as spiro-OMeTAD (N2,N2,N2′,N2′,N7,N7,N7′,N7′-octakis(4-methoxyphenyl)-9,9′-spirobi[9H-fluorene]-2,2′,7,7′-tetramine).5–7 In regular architectures, metallic oxides such as SnO2 or TiO28,9 are commonly used as n-type selective contacts while for inverted architectures it is fullerenes and their derivatives the most employed selective contact.10 The highest reported efficiencies have been obtained using metallic oxides in a regular architecture, but it requires high processing temperatures. However, inverted architectures offer a great alternative, they employ easier fabrication procedures with low processing temperatures that allow this architecture to be implemented into flexible substrates, and the efficiencies obtained match the values obtained from regular architectures.11In inverted perovskite solar cells, alternative approaches to the use of thin organic films, as p-type selective contacts, have included the use of self-assembled monolayers (SAMs).12,13 SAMs attach to the surface on which they are deposited by chemical bonding, and they have been demonstrated as an effective approach to modify interface properties.14 While the most common use of SAMs has been as an interlayer between the perovskite and the selective contact,15 there are already some examples to be used as selective contact itself. Our group and others have shown that this approach is appealing for the achievement of efficient solar cells.16,17 The advantages of SAMs are that they do not require the use of chemical dopants to oxidize the organic thin film, also SAMs provide a homogeneous perovskite film formation on top of the ITO that ensures increased Voc and FF.
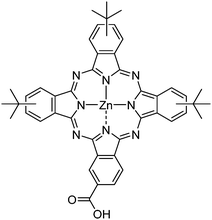
In this publication, we employ zinc carboxyphthalocyanine TT1 as p-type selective contact deposited as a SAM in inverted perovskite solar cells.18 TT1 is a well-known phthalocyanine, widely used in dye sensitized solar cells (DSSC) and that has demonstrated its facility to attach to metallic oxides,19–21 such as indium tin oxide (ITO). Additionally, it has also been employed in perovskite solar cells as a thin film on top of the perovskite layer.22 Here, using a novel synthetic route, we use for the first time TT1 as a p-type contact using the SAM approach. TT1 already provides tri-tert-butyl groups at the periphery of the moieties, which prevents the formation of molecular aggregates.19 We obtain efficient perovskite solar cells and we investigate the origin of such differences, which accounts for differences in energetics rather than recombination kinetics.
Results and discussion
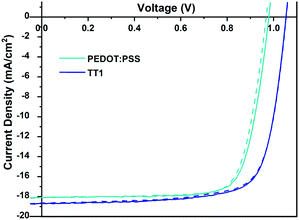
Herein, we developed the first direct transformation of hydroxyl methyl phthalocyanine into its corresponding carboxyl derivative (TT1) catalyzed by ZnO in high yield (Chart 1). The dehydrogenation of hydroxy methyl phthalocyanine was performed in zinc oxide and potassium hydroxide solution in mesitylene to yield TT1 in 96% yield and hydrogen gas as the only by-product (for more details see Scheme S1, ESI†). The final compound (TT1) was fully characterized by 1H NMR, LC/Mass, and Maldi-TOF.Fig. 1 shows the current density vs. voltage (J–V) curves for perovskite solar cells made using PEDOT:PSS, an ionic polymer, used as our reference, and TT1 SAMs.
As can be seen, both devices show negligible hysteresis (differences in the forward/reverse J–V measured curves) and TT1 based solar cells show larger open-circuit voltage (Voc). In fact, the measured voltage is substantially larger than the Voc measured for perovskite solar cells using a thin film of TT1 as the HTM.22 Device fabrication specifications can be found in the ESI,† as well as the characterization of TT1 SAMs and MAPI (methyl ammonium lead iodide) perovskite layer.
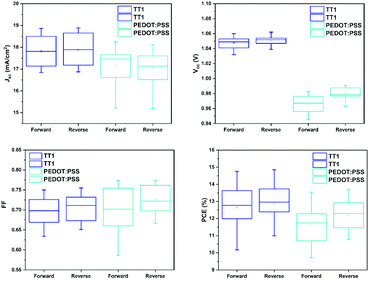
Of utmost importance is the fact that, on average, TT1 based perovskite solar cells always show better device performance (Fig. 2). The statistical distribution of the cell parameters was achieved from more than 20 devices (Table 1). In light of the better efficiencies obtained with TT1, we decided to study in depth the perovskite morphology and the device properties.
| HTMs | Scan | Voc (V) | Jsc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PEDOT:PSS | Fwd | 0.967 ± 0.05 | 17.17 ± 1.5 | 69.9 ± 1.0 | 11.62 ± 2.09 (13.71) |
| Rev | 0.984 ± 0.04 | 17.04 ± 1.7 | 72.6 ± 0.8 | 12.18 ± 1.5 (13.68) | |
| TT1 | Fwd | 1.045 ± 0.01 | 17.85 ± 1.0 | 68.7 ± 0.6 | 12.89 ± 1.96 (14.85) |
| Rev | 1.049 ± 0.01 | 17.92 ± 1.0 | 69.7 ± 0.5 | 13.11 ± 1.0 (14.11) |
As can be seen in Fig. 3, a closer look to the hybrid lead halide perovskite thin film grown onto the TT1 SAM or the PEDOT:PSS polymer layer did not show any relevant difference, obtaining high quality perovskite films, with a thickness of approximately 350 nm (Fig. S8, ESI†). Yet, the deposition of the TT1 changes the ITO surface wettability attending to the change in the contact angle measurements (see Fig. S6, ESI†). Next, we carried out transient optoelectronic techniques under operando conditions in order to study the origin of the differences observed in the Voc between both p-type contacts when used in complete devices.
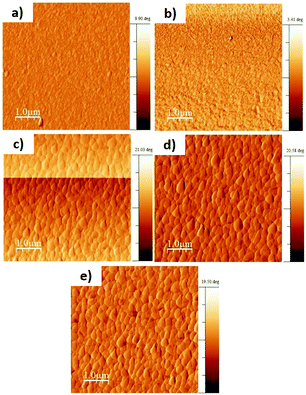 | ||
| Fig. 3 Topographical atomic force microscopy (AFM) pictures of (a) TT1/ITO, (b) PEDOT:PSS/ITO, (c) MAPI/ITO, (d) MAPI/PEDOT:PSS/ITO and (e) MAPI/TT1/ITO electrodes. The scale bar is 1 micrometer. | ||
The use of transient optoelectronic techniques, such as transient photovoltage (TPV), transient photocurrent (TPC), or differential capacitance (DiffCap) has been demonstrated as a useful approach to study charge recombination and charge storage on operating devices. In this case, we will use these techniques to study what is the origin of the differences observed on the Voc, if they are related to changes in the energetics, or, if this is associated with different carrier kinetics.23–26 The description of the techniques and data treatment can be found in the ESI.†
Fig. 4 illustrates the differences in energy levels of the different materials used as p-type selective contacts. All energy values have been previously reported in the scientific literature.22
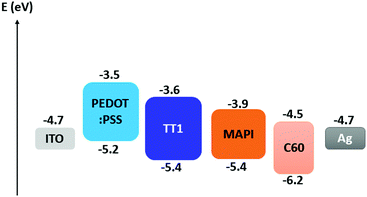 | ||
| Fig. 4 Energy level diagram of the different materials used in the fabrication of the MAPI based solar cells. | ||
At first glance, the deeper HOMO (highest occupied molecular orbital) energy value for TT1 would be responsible for the gain in Voc registered in the solar cells. Thus, the differences in Voc could be explained by the differences in energetics between the PEDOT:PSS and the TT1 molecule.
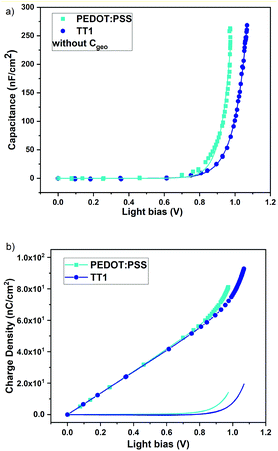
The DiffCap measurements (Fig. 5) also agree with the differences in energy between the PEDOT:PSS film and the TT1 SAM with a shift of the exponential curves registered for different voltages close to the maxVoc corresponding to 1 sun irradiation. Two different regimes are observed in Fig. 5. First, a constant part, related to the geometric capacitance in the device, related to the charges stored in the contacts and electrodes.25,27 The second regime, the exponential part, is related to the chemical capacitance. Once the contacts are depleted with charges, they start accumulating in the bulk of the perovskite.25 The difference between both exponential curves is ∼100 mV, in good agreement with the experimental values recorded for the devices at 1 sun (TT1Voc = 1.05 V and PEDOT:PSSVoc = 0.98 V). In this case, the differences in the exponential tail are what we expected. The Voc will increment with the quasi Fermi level splitting (QFLS) with the light bias until the contacts are depleted with charges, therefore we expect that the QFLS will be also correlated with the HOMO values of the p-type selective contacts. A greater QFLS is expected for TT1, which is confirmed by the differences observed in Fig. 5.
Once the differences in the DiffCap measurements were registered we turned on the analysis of the carrier recombination dynamics in these devices. The TPV decays were registered under the same illumination conditions used for the DiffCap. Fig. 6 illustrates the differences in carrier recombination kinetics for both types of solar cells studied in this work. The measured kinetics were fitted to eqn (1).
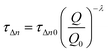 | (1) |
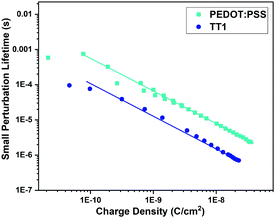 | ||
| Fig. 6 Carrier lifetime at different charge densities measured from the exponential part of the measurements shown in Fig. 5. The solid lines correspond to the fittings to eqn (1). | ||
From the fitting to eqn (1), we obtained a carrier recombination order δ of 1.90 and 1.94 for PEDOT:PSS and for the TT1 based devices, respectively. Although we found that PEDOT:PSS presents slower recombination dynamics compared to TT1, the δ values confirm our hypothesis; the differences in Voc observed between inverted MAPI solar cells, using fullerene as an n-type selective contact and PEDOT:PSS or TT1 SAMs as a p-type selective contact, are due to the difference in energetics and not due to different carrier recombination kinetics.
Conclusions
In conclusion, we have developed a highly efficient synthesis of benchmark phthalocyanine TT1, and we have demonstrated that TT1 SAMs can be used as efficient p-type selective contacts in MAPI perovskite solar cells with efficiencies close to 15% @1 sun under sun-simulated light (1.5 AMG spectra). The devices show voltages over 1 V due to the correct alignment of the HOMO energy level with the MAPI perovskite VB. In contrast, PEDOT:PSS devices, used as a control, show lower Voc due to higher HOMO energy values. The measured device capacitance, as well as the evaluation of the carrier recombination order under operando conditions, supported the observation that the Voc differences are due to the differences in HOMO energy value and not due to faster or slower carrier recombination dynamics at the solar cells. These results shown herein open new avenues for the use of robust molecules such as phthalocyanines18,29–31 and porphyrins as efficient p-type SAM contacts in thin film solar cells.Author contributions
TT, and KA synthesized TT1. EA and JJ fabricated the devices and carried out the photo-physical characterization under the supervision of EP. All authors participated in the discussion of the results and the manuscript writing and, finally, approved the submission.Conflicts of interest
There are no conflicts to declare.Acknowledgements
EA, JJ and EP thank MINECO (projects CTQ2013-47183, CTQ 2017-89814-P and CTQ2017-85393-P) and SGR-AGAUR 2017SGR00978. EP is also thankful to ICIQ and ICREA for economical support. Prof. Torres thanks the ERA-NET/European Commission/MINECO, (UNIQUE, SOLAR-ERA.NET Cofound 2 No. 008/PCI2019-111889-2). IMDEA Nanociencia acknowledges support from the “Severo Ochoa” Programme for Centres of Excellence in R&D (MINECO, Grant SEV2016-0686).Notes and references
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- Best Research-Cell Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html.
- M. Saliba, J.-P. Correa-Baena, C. M. Wolff, M. Stolterfoht, N. Phung, S. Albrecht, D. Neher and A. Abate, Chem. Mater., 2018, 30, 4193–4201 CrossRef CAS.
- M. Stolterfoht, C. M. Wolff, Y. Amir, A. Paulke, L. Perdigón-Toro, P. Caprioglio and D. Neher, Energy Environ. Sci., 2017, 10, 1530–1539 RSC.
- C. Rodríguez-Seco, M. Méndez, C. Roldán-Carmona, R. Pudi, M. K. Nazeeruddin and E. J. Palomares, Angew. Chem., Int. Ed., 2020, 59, 5303–5307 CrossRef PubMed.
- Q. Wang, E. Mosconi, C. Wolff, J. Li, D. Neher, F. De Angelis, G. P. Suranna, R. Grisorio and A. Abate, Adv. Energy Mater., 2019, 9, 1900990 CrossRef.
- S. Paek, P. Qin, Y. Lee, K. T. Cho, P. Gao, G. Grancini, E. Oveisi, P. Gratia, K. Rakstys, S. A. Al-Muhtaseb, C. Ludwig, J. Ko and M. K. Nazeeruddin, Adv. Mater., 2017, 29, 1–7 CrossRef PubMed.
- J. Wang, K. Datta, C. H. L. Weijtens, M. M. Wienk and R. A. J. Janssen, Adv. Funct. Mater., 2019, 29, 1905883 CrossRef CAS.
- M. M. Tavakoli, P. Yadav, R. Tavakoli and J. Kong, Adv. Energy Mater., 2018, 8, 1–9 Search PubMed.
- K. Lee, H. Yu, J. W. Lee, J. Oh, S. Bae, S. K. Kim and J. Jang, J. Mater. Chem. C, 2018, 6, 6250–6256 RSC.
- D. Yang, T. Sano, Y. Yaguchi, H. Sun, H. Sasabe and J. Kido, Adv. Funct. Mater., 2019, 29, 1–6 Search PubMed.
- E. Yalcin, M. Can, E. Aktas, R. Pudi, W. Cambarau, S. Demic and E. Palomares, Energy Environ. Sci., 2019, 12, 230–237 RSC.
- A. Al-Ashouri, A. Magomedov, M. Roß, M. Jošt, M. Talaikis, G. Chistiakova, T. Bertram, J. A. Márquez, E. Köhnen, E. Kasparavičius, S. Levcenco, L. Gil-Escrig, C. J. Hages, R. Schlatmann, B. Rech, T. Malinauskas, T. Unold, C. A. Kaufmann, L. Korte, G. Niaura, V. Getautis and S. Albrecht, Energy Environ. Sci., 2019, 12, 3356–3369 RSC.
- R. Qiao and L. Zuo, J. Mater. Res., 2018, 33, 387–400 CrossRef CAS.
- G. Tumen-Ulzii, T. Matsushima, D. Klotz, M. R. Leyden, P. Wang, C. Qin, J.-W. Lee, S.-J. Lee, Y. Yang and C. Adachi, Commun. Mater., 2020, 1, 31 CrossRef.
- C. M. Wolff, L. Canil, C. Rehermann, N. Ngoc Linh, F. Zu, M. Ralaiarisoa, P. Caprioglio, L. Fiedler, M. Stolterfoht, S. Kogikoski, I. Bald, N. Koch, E. L. Unger, T. Dittrich, A. Abate and D. Neher, ACS Nano, 2020, 14, 1445–1456 CrossRef CAS PubMed.
- K. Choi, H. Choi, J. Min, T. Kim, D. Kim, S. Y. Son, G.-W. Kim, J. Choi and T. Park, Sol. RRL, 2020, 4, 1900251 CrossRef.
- M. Urbani, G. de la Torre, M. K. Nazeeruddin and T. Torres, Chem. Soc. Rev., 2019, 48, 2738–2766 RSC.
- J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martinez-Ferrero, E. Palomares, J. Ko, M. Graetzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358–8362 CrossRef CAS PubMed.
- M. Garcia-Iglesias, J.-J. Cid, J.-H. Yum, A. Forneli, P. Vazquez, M. K. Nazeeruddin, E. Palomares, M. Gratzel and T. Torres, Energy Environ. Sci., 2011, 4, 189–194 RSC.
- L. Cabau, C. Vijay Kumar, A. Moncho, J. N. Clifford, N. López and E. Palomares, Energy Environ. Sci., 2015, 8, 1368–1375 RSC.
- Y. Zhang, S. Paek, M. Urbani, M. Medel, I. Zimmermann, K. T. Cho, M. Ince, M. K. Nazeeruddin and T. Torres, ACS Appl. Energy Mater., 2018, 1, 2399–2404 CrossRef CAS.
- E. Palomares, N. F. Montcada, M. Méndez, J. Jiménez-López, W. Yang and G. Boschloo, in Characterization Techniques for Perovskite Solar Cell Materials, Elsevier, 2020, pp. 161–180 Search PubMed.
- J. Jiménez-López and E. Palomares, Nanoscale, 2019, 11, 20024–20029 RSC.
- I. Gelmetti, N. F. Montcada, A. Pérez-Rodríguez, E. Barrena, C. Ocal, I. García-Benito, A. Molina-Ontoria, N. Martín, A. Vidal-Ferran and E. Palomares, Energy Environ. Sci., 2019, 12, 1309–1316 RSC.
- C. M. Wolff, P. Caprioglio, M. Stolterfoht and D. Neher, Adv. Mater., 2019, 31(52), 1902762 CrossRef CAS PubMed.
- D. Kiermasch, L. Gil-Escrig, A. Baumann, H. J. Bolink, V. Dyakonov and K. Tvingstedt, J. Mater. Chem. A, 2019, 7, 14712–14722 RSC.
- C. G. Shuttle, B. O’Regan, A. M. Ballantyne, J. Nelson, D. D. C. Bradley, J. de Mello and J. R. Durrant, Appl. Phys. Lett., 2008, 92, 093311 CrossRef.
- T. Duong, J. Peng, D. Walter, J. Xiang, H. Shen, D. Chugh, M. Lockrey, D. Zhong, J. Li, K. Weber, T. P. White and K. R. Catchpole, ACS Energy Lett., 2018, 3, 2441–2448 CrossRef CAS.
- Y. Feng, Q. Hu, E. Rezaee, M. Li, Z. Xu, A. Lorenzoni, F. Mercuri and M. Muccini, Adv. Energy Mater., 2019, 1901019 CrossRef.
- M. Haider, C. Zhen, T. Wu, G. Liu and H. M. Cheng, J. Mater. Sci. Technol., 2018, 34, 1474–1480 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00443j |
| This journal is © The Royal Society of Chemistry 2020 |