Exploring the structure evolution of MoS2 upon Li/Na/K ion insertion and the origin of the unusual stability in potassium ion batteries†
Xiaoqiong
Du‡
ab,
Xuyun
Guo‡
 a,
Jiaqiang
Huang‡
a,
Ziheng
Lu
a,
Jiaqiang
Huang‡
a,
Ziheng
Lu
 c,
Hong
Tan
ab,
Jian-Qiu
Huang
a,
Ye
Zhu
c,
Hong
Tan
ab,
Jian-Qiu
Huang
a,
Ye
Zhu
 *a and
Biao
Zhang
*a and
Biao
Zhang
 *ab
*ab
aDepartment of Applied Physics, Research Institute for Smart Energy, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China. E-mail: ye.ap.zhu@polyu.edu.hk; biao.ap.zhang@polyu.edu.hk
bThe Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen 518063, China
cShenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China
First published on 20th October 2020
Abstract
The recent revival of research on Na and K ion batteries has two benefits. It not only provides alternate energy storage technologies to Li ion batteries with potential cost advantages but also enhances our understanding of charge storage through systematic studies on alkali-metal ion batteries with increasing insertion ion sizes. Using MoS2 as a model material, the structure evolution upon the uptake of Li, Na, and K ions are compared through in situ TEM. Despite their larger size, insertion of K ions shows both the better electrochemical and structural stability. To understand this paradoxical and counter-intuitive phenomenon, in situ XRD is carried out to examine the phase transitions of MoS2 upon ion insertion, while ex situ TEM is further applied to closely examine the structures at the nanoscale. Complementary DFT calculations are performed to understand the kinetic/thermodynamic origins of the unusual stability. The result reveal that the less electrovalent K–S bond favors the intercalation process, resulting in preservation of the layered structure for stable cycling. This study provides a structural insight to design stable electrodes for the K-ion batteries.
New conceptsTwo-dimensional layered MoS2 is among the most promising anode materials for alkali-metal ion batteries due to its high theoretical capacity and large interlayer spacing. However, inferior cyclic performance caused by structure collapse limits its wide application. It is generally believed that severe capacity degradation associated with extensive volume expansion occurs in K ion batteries because of the large ion radius. Unusual stability is observed during the insertion of K ion, although it has a large size than that of Na and Li ions. The result differs from that observed in other anode materials such as Sb or Bi. With the help of in/ex situ TEM and in situ XRD, we reveal the different reaction mechanisms of MoS2 upon insertion of Li/Na/K ions. A conversion reaction (destroying the layered structure) is inclined to take place after Li-ion insertion, while the intercalation reaction (maintaining the layered structure) dominates the K ion uptake. The origin of this abnormal phenomenon is unveiled by the complementary DFT simulation, which shows suppression of the conversion reaction owing to the less electrovalent K–S bond. Our findings demonstrate that the charge radius does not directly determine the stability of a battery, and the reaction pathway also plays a critical role. |
Introduction
The advantages of Li ion batteries (LIBs) over other metal ion systems rely on both the low redox potential of Li+/Li (−3.04 V vs. SHE) and the small size of Li ion (0.76 Å).1 With concerns on the sustainability of lithium sources, research on sodium and potassium-based technologies has experienced a revival, considering the similar redox potentials of Na+/Na (−2.71 V vs. SHE) and K+/K (−2.93 V vs. SHE), which would enable the design of high-output-voltage batteries.2,3 Similarities between alkali-metal ions are expected to facilitate their development, as the knowledge on LIBs that has been accumulated in the past three decades can be largely applied to Na ion batteries (SIBs) and K ion batteries (PIBs).4,5 Indeed, analogous systems from the electrolytes to the electrode materials are adopted in alkali-metal ion batteries. For instance, carbonate-based solvents, including ethylene carbonate (EC), dimethyl carbonate (DMC) and diethyl carbonate (DEC), inherited from LIBs remain the most popular choices for the electrolytes in SIBs and PIBs.6–10 Many materials are capable of storing Li, Na and K ions with hard carbon anode and A3V2(PO4)2F3 (A represents Li, Na or K) cathode being the representative.11–16 However, a simple extrapolation from Li to Na and K systems is undoubtedly not enough. This is clearly reflected in the case of LiCoO2, the most successful cathode in LIBs, whose NaxCoO2 and KxCoO2 counterparts fail to deliver an attractive capacity and stability.17,18 While reasons for the variations are complex, the large ionic size of Na (1.02 Å) and K (1.38 Å) ion are considered to be partly responsible. The uptake of ions with a large radius can lead to enormous volume expansion and bring about potential structural damage to the electrode materials. An example for this are alloy anodes, which can accept several A ions to form AxM (A represents Li, Na or K, and M denotes alloy anodes such as Bi and Sb); much more significant volume expansion is induced in the formation of KxM than of LixM or NaxM.19,20 Consequently, stabilizing electrodes in PIBs is considered to be more challenging, necessitating efforts in both microstructure design and solid electrolyte interphase engineering.21–23Comparison studies between alkali-metal ion storage methods are expected to provide insights into the effects of ionic carriers on the structural evolution of electrodes, which are currently somewhat lacking, due likely to the absence of appropriate host materials. MoS2 has a large layer distance of 6.3 Å; therefore, it is an ideal material to accommodate alkali-metal ions with different sizes. It shows promising theoretical capacities of about 670 mA h g−1via a four-electron-transfer reaction when serving as the anode in LIBs, SIBs, and PIBs.24–30 Similar charge storage mechanisms have been reported, including the intercalation at a high voltage to form layered AMoS2 (eqn (1)) compounds and the following conversion reaction to precipitate molybdenum metal and A2S (eqn (2)).31–33 Herein, we fabricated MoS2/C nanofibers as a model material considering the advantages of the one-dimensional morphology for in situ experimental and statistics gathering. It was revealed that MoS2/C nanofibers in PIBs show the largest volume expansion of up to 140%, whereas the volume changes in LIBs and SIBs are only about 103% and 123%, respectively. Counter-intuitively, better structural stabilities and more stable cyclic performance during K ion insertion were observed. Complementary in situ/ex situ transmission electron microscopy (TEM) and theoretical calculations were conducted to study the origin of the difference in the structural transformations during Li, Na, and K ion insertion.
| MoS2 + A → AMoS2 | (1) |
| AMoS2 + 3A → Mo + 2A2S | (2) |
Results and discussion
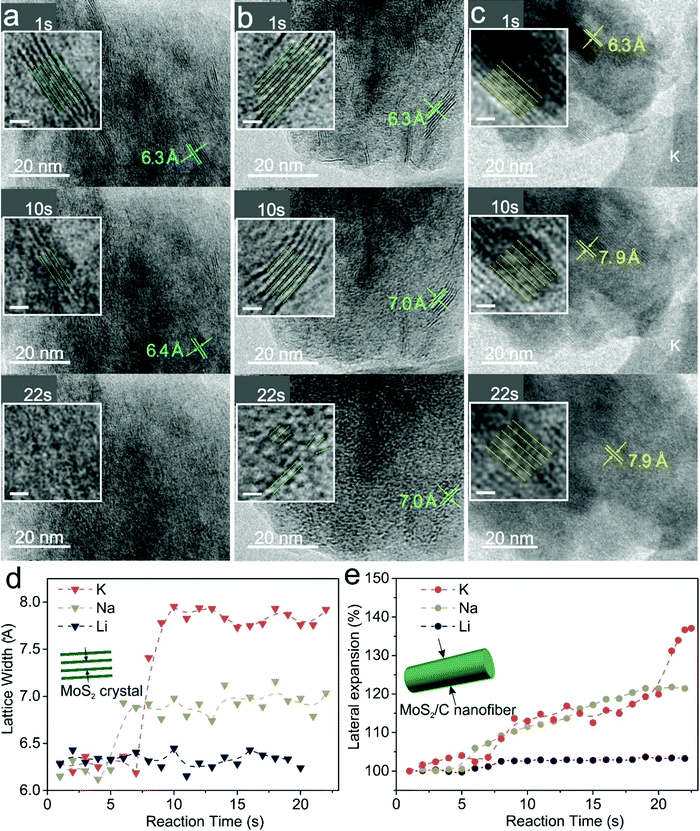
MoS2/C nanofibers were prepared by electrospinning (Fig. S1, ESI†). The crystalline phase of the nanofibers was identified by X-ray powder diffraction (XRD, Fig. S2a, ESI†) and is consistent with 2H-MoS2 (PDF#37-1492). A sharp peak at around 14.1° can be indexed to the (002) plane of MoS2, and one broad peak at about 21.2° belongs to carbon that originated from the carbonization of PAN. Thermogravimetry (TG, Fig. S2b, ESI†) was performed to analyze the carbon content in the sample, which was estimated to be ∼25%. The chemical state of Mo was examined by X-ray photoelectron spectroscopy (XPS, Fig. S3, ESI†); the Mo3d spectrum presented two pairs of peaks at 229.0 eV and 232.3 eV, corresponding to Mo(IV) and Mo(VI), respectively.34 The small amount of Mo(VI) species in the Mo3d spectrum may originate from the oxide layer of the MoS2/C nanofibers.35 The morphology of the MoS2/C nanofibers was investigated by scanning electron microscopy (SEM) and TEM. In Fig. S4a (ESI†), the MoS2/C nanofibers with an average diameter of about 100 nm present a uniform thread morphology after high-temperature treatment. The TEM images (Fig. S4b and c, ESI†) indicate that layered MoS2 crystals were homogeneously dispersed in the carbon nanofibers, and the selected area electron diffraction (SAED) pattern (Fig. S4b inset, ESI†) clearly shows the multi-crystalline feature of MoS2. In the high-resolution (HRTEM) images (Fig. S4d, ESI†), the MoS2 crystals consist of 3–5 layers with an interlayer distance of 6.3 Å.In situ TEM examinations were carried out to explore the charge storage processes of MoS2/C nanofibers after uptake of different sizes of alkali-metal ions with alkaline oxides on the surfaces as solid electrolytes and the alkaline metal as the reference/counter electrodes (Fig. S5, ESI†). As shown in Fig. 1a and b, after the first 10 seconds of lithiation and sodiation, the interlayer spacing of MoS2 increased from 6.3 Å to about 6.4 Å and 7.0 Å, respectively. Meanwhile, for the potassiation process, the interlayer spacing is enlarged to a striking 7.9 Å (Fig. 1c). Despite the expansion of the layer distance by the alkaline atom invasion, the lamellar structure could be retained well in the three types of batteries. These processes are mainly involved in the intercalation reaction, in which alkaline ions are inserted into MoS2 to form layered AMoS2 compounds, namely, LiMoS2, NaMoS2, and KMoS2.36–39 Regarding the deep reaction in the following 12 seconds, the layered crystals almost entirely disappeared in the case of lithiation because of the conversion of LiMoS2 to metallic Mo and Li2S. Some of the layered crystals were preserved during Na insertion; however, the MoS2 crystals were broken into small pieces. In comparison, the deep potassiation process of MoS2 displayed a different phenomenon from the Li and the Na counterparts, in which the layered crystals were unabridged, with an enlarged interlayer spacing of 7.9 Å. In situ TEM movies of these three processes are given in the ESI† (Movies S1–3). Electron energy loss spectroscopy (EELS) maps (Fig. S6–S8, ESI†) confirmed that Li, Na and K were successfully inserted into the MoS2/C nanofibers. The lattice width of the MoS2 crystals and the lateral length of the MoS2/C nanofibers were measured in real time, and the detailed statistics are given in Fig. 1d and e. The expansion of the interlayer distance became more severe with increasing intercalant size from Li to Na and K ions. Correspondingly, the lateral expansions of nanofibers after uptake of Li and Na ions were roughly calculated to be 103% and 123%, respectively, while the expansion reached nearly 140% for K ion uptake.
To exclude potential artifacts from the in situ TEM setup, such as the use of A2O solid electrolytes, we further performed in situ XRD and ex situ TEM on real LIBs, SIBs and PIBs to investigate the unexpected structural stability of MoS2 at various insertion/extraction stages of Li/Na/K ions. The in situ XRD patterns of MoS2/C nanofibers for K ion storage in the first and second cycles are presented in Fig. 2a. During the first discharging process, the intensity of the MoS2 peak located at 14.1° gradually decreases and a new peak at about 10.6° becomes more prominent, which can be assigned to K0.4MoS2 compound (PDF#27-0421). Then, the K0.4MoS2 peak shifts to 11.1° after continuous K ion interpolation, where the formed compound is defined as KxMoS2 (x > 0.4). Further insertion of K ions leads to the fully potassiated phase KMoS2 pertaining to a broad peak at 11.3°, which agrees well with the 7.9 Å interlayer spacing observed in TEM. Turning to the charging processes, the wide peak of 11.3° shifts back to 11.1° and no obvious MoS2 peaks emerge, indicating that KMoS2 can only return to KxMoS2 (0.4 < x < 1.0) and not to pristine MoS2. For the following discharging and charging processes, only KMoS2 and KxMoS2 appear on the stage, and the corresponding XRD peaks show periodic right and left shifts. The K ions shuttle between KxMoS2 and KMoS2, which ensures that the phase transition is completely reversible in the cycles. It should be noted that the interlayer spacing of KxMoS2 shrinks during K ion insertion, while it extends during K ion extraction. This inverse relationship between the interlayer spacing and K ion insertion/extraction can be explained by the 2H-1T phase transformation.40–42 In the case of LIBs (Fig. S9a, ESI†), the peak of the (002) facet gradually shifts to 13.7° after Li ion insertion, which can be attributed to the intercalated product LiMoS2.42 When discharging to 0 V, no obvious peaks can be recognized, indicating that the MoS2 is transformed to small/amorphous Mo and Li2S.43 When charging back to 3 V, no MoS2 peaks are found, which indicates that the layered structures of MoS2 largely vanish and cannot be recovered after Li ion insertion/extraction. In the first discharging process of SIBs (Fig. S9b, ESI†), two new peaks at 11.7° and 12.5° are observed, corresponding to Na0.5MoS2 and NaMoS2, respectively.33 After full discharge to 0 V, most of the layered NaMoS2 is decomposed, as suggested by the low intensity of the peak at 12.5°. This phenomenon is similar to the Li case, in which no peaks can be found when fully charged at 3 V, implying that amorphous or negligible crystallized MoS2 dominats after Na ion extraction.
The above in situ observations are fully consistent with the ex situ TEM characterizations of the first cycle of real PIBs. The pristine MoS2 crystals in the nanofibers are shown in Fig. S10a and b (ESI†). When discharging to 1.0 V, a large interlayer spacing of 8.3 Å associated with K0.4MoS2 was observed (Fig. S10c and d, ESI†), corresponding to the 10.6° peak in the in situ XRD. However, this was not probed by in situ TEM, probably due to the low-resolution limitation of real-time observation and the fast reaction process of K0.4MoS2.44 After discharging to 0.5 V (Fig. S10e and f, ESI†), a slightly shrunken layer distance of 8.0 Å was found, arising from the 2H-1T phase transformation; this is related to the 11.1° peak from KxMoS2 according to the in situ XRD results. Some unreacted MoS2 can be seen with the original interlayer distance of 6.3 Å. For the fully discharged state (Fig. S10g and h, ESI†), most of the MoS2 was converted to KMoS2 with a contractive distance of 7.9 Å, as observed by both in situ TEM and XRD. Back to 3 V (Fig. S10i and j, ESI†), the interlayer distance is extended again to 8.0 Å, indicating that KMoS2 reverts to the KxMoS2 species. Due to the statistic advantage of the nanofiber composite, the MoS2 crystals are scattered in the nanofibers independently; thus, the layer numbers and lateral length can be readily measured. As shown in Fig. 2b, the layer numbers of MoS2 crystal in nanofibers in different states were counted. The original MoS2 presented 3–5 layers, which was almost unchanged upon charge/discharge, confirming the stability of the layered crystals and the reversible reaction between KMoS2 and KxMoS2.
Ex situ TEM characterizations were also carried out on LIBs and SIBs in the fully discharged state for comparison with PIBs. As shown in Fig. 2d and the inset, only dense Mo particles with a lattice-plane spacing of 2.2 Å (PDF#42-1120) were discovered in the discharged LIBs, with no trace of layered phases; this implies that deep conversion dominates. The small metallic Mo particles may be undetectable by in situ XRD. A small number of layered crystals were presented in the discharged SIBs (Fig. 2e), which are smaller and contain fewer layers with an expanded interlayer spacing of 7.0 Å, in agreement with the in situ TEM and in situ XRD observation in Fig. 1b and Fig. S9b (ESI†). In contrast, many broad layered crystals with a large interlayer spacing of 7.9 Å were preserved in the discharged PIBs (Fig. 2f). The lateral lengths of the layered crystals in both SIBs and PIBs were counted in the fully discharged state (Fig. 2c). The average lateral length of the pristine MoS2 crystals in the nanofibers was about 5.2 nm. This value in SIBs dramatically decreased to ca 3.4 nm after sodiation. The layered crystals that vanished from the LIBs and SIBs were presumably converted to Mo and Li2S/Na2S species through conversion reactions. On the other hand, the lateral length (4.9 nm) in PIBs did not show a significant change after discharging to 0 V, further proving the better stability of the layered crystals during K ion storage.
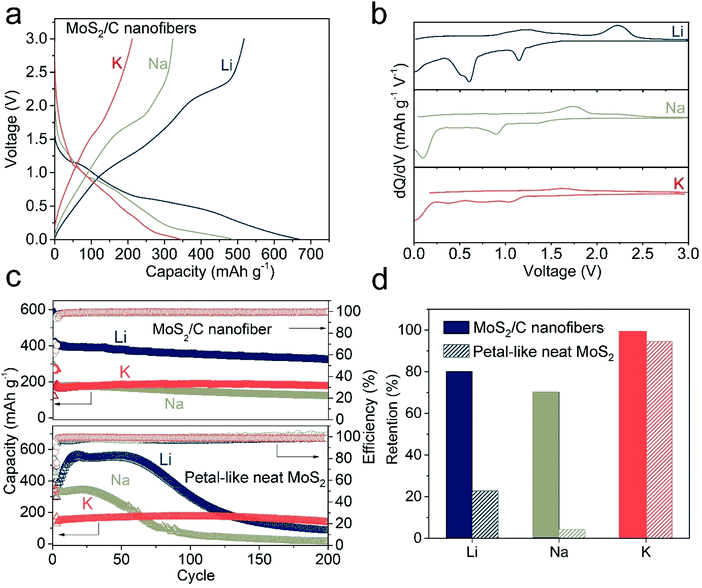
The electrochemical performance of the MoS2/C nanofibers was tested in LIBs, SIBs, and PIBs to explore the consequences of the structural transformation on the cyclic stability. The first discharge and charge profiles of the MoS2/C nanofibers in LIBs, SIBs, and PIBs are compared in Fig. 3a. Much longer plateaus were observed in the LIBs and SIBs, which is indicative of their distinct reaction paths. This can be seen more clearly in the dQ/dV plots, where sharp peaks are presented during Li and Na insertion (Fig. 3b). Consistent with the observed structural stability, the MoS2/C nanofibers demonstrated the most stable behavior in PIBs, with a capacity retention of about 99.4% after 200 cycles (Fig. 3c and d). The MoS2/C nanofibers present good rate performance for PIBs (Fig. S11, ESI†), which is not affected by the larger radius of K ion, showing reversible capacities of 212, 196.2, 186.4, and 175.2 mA h g−1 under increasing current densities of 50, 100, 200 and 400 mA g−1, respectively. Note that the pure carbon nanofibers have a capacity of only 100 mA h g−1 (Fig. S12, ESI†). Considering the 75 wt% mass loading of MoS2 in the composite, MoS2 alone in the composite delivered a capacity of about 200 mA h g−1 at a high current density of 500 mA g−1. In contrast, relatively rapid capacity decreases were observed in the LIBs and SIBs, which retained only 80.1% and 70.1% of the initial capacity, respectively. As is well known, the carbon host in the nanofibers is conducive to better cycling performance for active materials.45–47 To exclude the interference of carbon, petal-like neat MoS2 was synthesized by the hydrothermal method. As shown in Fig. S13a (ESI†), the prepared neat MoS2 presents a pure 2H-MoS2 phase (PDF#37-1492) without any carbon signal. In the TEM images (Fig. S13b, c and b inset, ESI†), neat MoS2 presents a petal-like morphology with a diameter of ca. 200 nm and is polycrystalline, as revealed by SAED. The HRTEM image (Fig. S13d, ESI†) clearly displays a layered structure with an interlayer spacing of 6.3 Å, which is the same as the MoS2/C nanofibers. The cyclic performance of neat MoS2 in the LIBs, SIBs and PIBs is given in Fig. 3c. Apparent capacity degradation in the LIBs and SIBs was observed. In contrast, the neat MoS2 in PIBs realizes the most stable capacity of 170 mA h g−1 after 200 cycles, with retention of 94.5% (Fig. 3d). The integrity of the petal-like particles was maintained (Fig. S14, ESI†) and the layered structures were retained to a large extent, with an expanded d-spacing of 7.7 Å after prolonged cycling. Without protection from the carbon nanofibers, the electrochemical stabilities of MoS2 show a more striking contrast between the three types of batteries. It is reasonable to conclude that MoS2 intrinsically shows a more stable cyclic performance in PIBs than in LIBs and SIBs.
We further performed ex situ TEM to examine the morphologies of the MoS2/C nanofibers cycled 200 times. As shown in Fig. 4a and the inset, all the lamellar structural MoS2 disappeared and changed into dense nanoparticles after cycling in LIBs. These particles can be indexed to Mo phase with a lattice plane spacing of 2.2 Å (PDF#42-1120), which is one of the conversion products. The layered crystals could no longer be found in the SIBs after 200 cycles, and the materials changed to loose particles with low contrast (Fig. 4c). The SAED pattern (Fig. 4c inset) suggests an amorphous phase without prominent diffraction spots or rings, which can be inferred from the Li case to be amorphous Mo species. Interestingly, many layered crystals with a layer distance of about 7.6 Å were observed in the PIBs (Fig. 4e and inset). There are fewer layered crystals than in the first fully discharged one (Fig. 2f), possibly because part of the layered MoS2 may have been consumed through a deep conversion reaction. The reaction schematics are described in Fig. 4b, d and f: the layered crystals in LIBs/SIBs were easily converted to dense/loose particle structures after long cycling, whereas they were most stable in the PIBs, explaining the best cyclic stability of the latter in the electrochemical tests.
 | ||
| Fig. 4 Ex situ TEM images and schematics of MoS2/C nanofibers in (a and b) LIBs, (c and d) SIBs and (e and f) PIBs after 200 cycles. | ||
The exceptional stability upon large K ion insertion suggests that different thermodynamic/kinetic processes govern the intercalation and conversion. Density functional theory (DFT) computations were carried out to study the mechanisms. We first calculated the reaction enthalpies of conversion from the intercalated compounds AMoS2 (A represents Li, Na, or K) to A2S and Mo. These values can serve as a descriptor of the thermodynamic driving forces for the conversion reactions. We specifically choose the intermediate intercalated compounds as the reference for our calculation because they are the last intercalated compounds before the conversion reaction actually occurs in these three systems. The results are shown in Fig. 5a, Table S1, and Fig. S15a (ESI†). The decomposition enthalpies of the AMoS2-intercalated compounds into Mo and A2S are −4.46 eV f.u.−1, −3.16 eV f.u.−1, and −3.30 eV f.u.−1 for A = Li, Na, and K, respectively. This indicates a smaller thermodynamic driving force in the case of K for the conversion to take place, leading to the preservation of a large quantity of the lamellar structures. It is also interesting to note that within the accuracy of the DFT framework (and when neglecting the temperature effect), all the computed reaction enthalpies are negative, indicating that the conversion reactions are energetically favorable to take place even at a relatively low degree of alkalization. Therefore, we speculate that the kinetic factor plays a critical role in the conversion reaction as well because these intercalated compounds are observed in experiments. Due to the lack of reliable methods to directly compute the energy barrier for the complicated phase transition, we analyzed the bond characteristics of AMoS2, which may reflect the ease of the conversion reactions. The charge distributions of LiMoS2, NaMoS2, and KMoS2 were investigated by the charge density difference (Fig. 5b and Fig. S15b, ESI†), which was defined by subtracting the electron densities of Li/Na/K and MoS2 from the electron density of LiMoS2/NaMoS2/KMoS2. Taking KMoS2 as an example, it is clear in Fig. 5b that some charges accumulate between K and S atoms, suggesting their interaction or bonding. To provide a better-quantified view, Fig. 5c and Fig. S16 (ESI†) demonstrate the two-dimensional charge density differences along the planes through the Li/Na/K and Mo–S atoms. Interestingly, the charge depletion of Li atom (down to −0.07 e Bohr−3) is more severe than that of K atom (down to −0.03 e Bohr−3). The more polar feature of the charge distribution between Li and S suggests that the Li–S bond is more electrovalent than the K–S bond.48 The polarity is further supported by the Bader analysis (Fig. 5d and Tables S2–S4, ESI†); the Bader charges of alkaline atoms in LiMoS2, NaMoS2, and KMoS2 are +0.87, +0.83 and +0.74 e, respectively. The different polarities between the Li–S and K–S bonds may arise from the more dispersed electron cloud of K due to the larger number of electrons. Regarding the conversion reaction, it was conjectured that the more polar bonds between Li and S atoms kinetically facilitate the formation of Li2S by reducing the charge transfer barrier. Briefly, the conversion reaction in the K–MoS2 system is suppressed thermodynamically (by the lower formation energy) and kinetically (by the less electrovalent K–S bond).
Conclusion
The alkali-metal ion storage mechanisms of Li, Na and K were studied by complementary in situ experimental and calculation approaches. A massive volume expansion of about 140% occurs upon K ion insertion; however, unexpected stability is observed. In situ TEM was used to examine the structural evolution of layered MoS2 upon insertion of Li, Na and K ions, and the results indicated that most of the layered crystals are preserved in PIBs. The detailed reaction paths were elucidated by in situ XRD and ex situ TEM. It is revealed that a large ratio of intercalation reactions occurs during K ion uptake, giving rise to better structural and electrochemical stability than that obtained upon Li or Na ion insertion. Layered MoS2 tends to be transformed into tiny particles by deep conversion reactions in the cases of LIBs and SIBs. Assisted by DFT calculations, we unveiled the thermodynamic and kinetic origins of the anomalous stability in the insertion of K ions; it relies on the lower electrovalence of the K–S bond, which arises from the larger dispersed electron clouds of K atom than of Li and Na atoms.Experimental section
Preparation of MoS2/C nanofibers, carbon nanofibers and petal-like neat MoS2
MoS2/C nanofibers were prepared by electrospinning. Typically, polyacrylonitrile (PAN, 0.5 g) was dissolved in dimethylformamide (DMF, 10 mL). Ammonium tetra-thiomolybdate ((NH4)2MoS4, 0.8 g) was added under continuous stirring to obtain a uniform solution. Electrospinning was conducted under a high voltage of 18 kV with a feed rate of 30 μL min−1 to obtain a film on Al foil. The film precursor was first stabilized at 250 °C for 4 hours in a muffle furnace before annealing at 900 °C for 2 hours under argon atmosphere. After the annealing, (NH4)2MoS4 decomposed to MoS2, and PAN turned into carbon. The pure carbon nanofibers were prepared by the same method without adding (NH4)2MoS4. In comparison, petal-like neat MoS2 was synthesized by a hydrothermal method.49 Ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O, 0.5 mmol) and thiourea (NH2CSNH2, 7 mmol) were dissolved in deionized (DI, 35 mL) water. The solution was maintained in a 50 mL Teflon-lined stainless steel autoclave at 220 °C for 18 hours. The final product was washed with DI water and ethanol before drying at 80 °C overnight.Electrochemical performance measurements
All cells were assembled via a two-electrode CR2032 coin half-cell in an argon-filled glovebox. MoS2/C nanofibers and carbon nanofibers were used as freestanding electrodes in the battery tests. Neat MoS2 electrodes were mixed with vapor-grown carbon fibers (VGCF), carbon black (Super P) and carboxymethyl cellulose (CMC) at a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form a tape on the Cu current collector, which was dried at 80 °C overnight under vacuum. 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) with a volume ratio of 1
1 to form a tape on the Cu current collector, which was dried at 80 °C overnight under vacuum. 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 M NaPF6 in propylene carbonate (PC) with 3 vol% FEC and 1 M potassium bis(fluorosulfony)imide (KFSI) in EC/PC (volume ratio of 1
1, 1 M NaPF6 in propylene carbonate (PC) with 3 vol% FEC and 1 M potassium bis(fluorosulfony)imide (KFSI) in EC/PC (volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were adopted as electrolytes for the LIB, SIB, and PIB, respectively. Glass fiber membranes (Whatman, GF/D) were used as separators. All batteries were tested on a LAND battery test system.
1) were adopted as electrolytes for the LIB, SIB, and PIB, respectively. Glass fiber membranes (Whatman, GF/D) were used as separators. All batteries were tested on a LAND battery test system.
Characterizations
The X-ray powder diffraction (XRD) patterns were collected by a Rigaku Smartlab instrument with a Cu-Kα radiation source at 45 kV and 200 mA. For the in situ XRD measurements, an XRD cell with a beryllium window was employed. The morphologies of the MoS2/C nanofibers were examined on a scanning electron microscope (SEM, JEOL JSM-6335F). Thermogravimetric analysis (TGA) was conducted using a TGA/DSC3+ (Mettler Toledo) from 50 °C to 650 °C with a heating rate of 15 °C min−1 in air. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific Nexsa. TEM and scanning TEM (STEM) were performed using a JEOL JEM-2100F TEM/STEM operated at 200 kV and equipped with a Gatan Enfina electron spectrometer. For the in situ TEM study, the MoS2/C nanofibers were loaded onto an electrochemically etched micro-size tungsten tip and fixed with conductive silver epoxy. Another tungsten tip with submicron size at the top was used to scratch a fresh alkaline metal (Li, Na, or K) surface in an argon-filled glove box and was attached to a piezo-driven biasing probe built into the Nanofactory TEM scanning tunneling microscopy (TEM-STM) holder. Once the sealed holder was taken out of the glovebox, it was inserted into the TEM column as quickly as possible for immediate in situ study by TEM. The native A2O (A represents Li, Na or K) on the alkaline metal surface served as a solid electrolyte. A voltage bias of −5 V between the alkaline metal tip and MoS2 tip was applied, which was higher than those applied in the coin cells. It was necessary to use a slightly higher potential in the in situ TEM measurements to drive alkaline ions (Li+, Na+ and K+) through the solid electrolytes due to the poor ionic conductivity of solid electrolytes and the high resistance between these two electrodes.44 In this case, the driving force in in situ TEM may have been larger than that in the coin cells; however, effective changes were not observed in comparison with the ex situ TEM results.50,51 Therefore, the in situ TEM results were reliable. The three in situ videos were taken at 200, 250 and 100k× magnifications for Li, Na and K with a 0.5 s exposure time, respectively. The corresponding dose rates for the Li, Na, and K systems (units of the number of electrons per square angstrom per second, e− Å−2 s−1) were recorded and calibrated, and they equaled 443, 764 and 122 e− Å−2 s−1, respectively. The ex situ TEM samples were prepared by disassembling the coin cell in the glovebox and washing with dimethyl carbonate (DMC) to remove the residual electrolyte.DFT simulations
DFT calculations were conducted under the generalized gradient approximation (GGA) parameterized by Perdew–Burke–Ernzerhof (PBE) performed in the Vienna Ab initio Simulation Package (VASP).52–54 To account for the van der Waals interactions, the optimized PBE functional was applied.55 The energy cutoff for the plane-wave basis set was 500 eV, while the k-point was sampled with a spacing of less than 0.05 Å−1. The convergence criteria for electron self-consistency and force were 10−6 eV and 0.02 eV Å−1, respectively.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work is supported by the Hong Kong Research Grants Council through the General Research Fund (Project No. 15305219), the Key Project for Basic Research of Shenzhen (No. JCYJ20170818104125570), and the Hong Kong Polytechnic University (ZVRP, and 1-ZE30).Notes and references
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS.
- K. Chayambuka, G. Mulder, D. L. Danilov and P. H. L. Notten, Adv. Energy Mater., 2018, 8, 1800079 CrossRef.
- J. Y. Hwang, S. T. Myung and Y. K. Sun, Adv. Funct. Mater., 2018, 28, 1802938 CrossRef.
- H. Tan, X. Lin, J. Huang, J. Huang, M. Shi, X. Du and B. Zhang, Nanoscale, 2019, 11, 11445–11450 RSC.
- J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng, Y. Li, H. Liu and S. Dou, Adv. Sci., 2017, 4, 1700146 CrossRef.
- Z. X. Wei, D. X. Wang, M. L. Li, Y. Gao, C. Z. Wang, G. Chen and F. Du, Adv. Energy Mater., 2018, 8, 1801102 CrossRef.
- D. Ni, W. Sun, Z. Wang, Y. Bai, H. Lei, X. Lai and K. Sun, Adv. Energy Mater., 2019, 9, 1900036 CrossRef.
- J. Zheng, Y. Yang, X. Fan, G. Ji, X. Ji, H. Wang, S. Hou, M. R. Zachariah and C. Wang, Energy Environ. Sci., 2019, 12, 615–623 RSC.
- L. Ji, M. Gu, Y. Shao, X. Li, M. H. Engelhard, B. W. Arey, W. Wang, Z. Nie, J. Xiao, C. Wang, J. G. Zhang and J. Liu, Adv. Mater., 2014, 26, 2901–2908 CrossRef CAS.
- W. Zhang, Z. Wu, J. Zhang, G. Liu, N.-H. Yang, R.-S. Liu, W. K. Pang, W. Li and Z. Guo, Nano Energy, 2018, 53, 967–974 CrossRef CAS.
- X. Lin, X. Du, P. S. Tsui, J.-Q. Huang, H. Tan and B. Zhang, Electrochim. Acta, 2019, 316, 60–68 CrossRef CAS.
- Z. L. Jian, Z. Y. Xing, C. Bommier, Z. F. Li and X. L. Ji, Adv. Energy Mater., 2016, 6, 1501874 CrossRef.
- X. Lin, J. Huang and B. Zhang, Carbon, 2019, 143, 138–146 CrossRef CAS.
- Z. Liu, Y.-Y. Hu, M. T. Dunstan, H. Huo, X. Hao, H. Zou, G. Zhong, Y. Yang and C. P. Grey, Chem. Mater., 2014, 26, 2513–2521 CrossRef CAS.
- X. Lin, J. Huang, H. Tan, J.-Q. Huang and B. Zhang, Energy Storage Mater., 2019, 16, 97–101 CrossRef.
- B. Zhang, R. Dugas, G. Rousse, P. Rozier, A. M. Abakumov and J. M. Tarascon, Nat. Commun., 2016, 7, 10308 CrossRef CAS.
- K. Mizushima, P. Jones, P. Wiseman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783–789 CrossRef CAS.
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Chem. Rev., 2014, 114, 11636–11682 CrossRef CAS.
- A. Darwiche, C. Marino, M. T. Sougrati, B. Fraisse, L. Stievano and L. Monconduit, J. Am. Chem. Soc., 2012, 134, 20805–20811 CrossRef CAS.
- H. Kim, J. C. Kim, M. Bianchini, D. H. Seo, J. Rodriguez-Garcia and G. Ceder, Adv. Energy Mater., 2018, 8, 1702384 CrossRef.
- J. Huang, X. Lin, H. Tan and B. Zhang, Adv. Energy Mater., 2018, 8, 1703496 CrossRef.
- F. Xie, L. Zhang, B. Chen, D. Chao, Q. Gu, B. Johannessen, M. Jaroniec and S.-Z. Qiao, Matter, 2019, 1, 1681–1693 CrossRef.
- Q. Zhang, J. Mao, W. K. Pang, T. Zheng, V. Sencadas, Y. Chen, Y. Liu and Z. Guo, Adv. Energy Mater., 2018, 8, 1703288 CrossRef.
- T. Wang, S. Chen, H. Pang, H. Xue and Y. Yu, Adv. Sci., 2017, 4, 1600289 CrossRef.
- J. Wang, J. Liu, D. Chao, J. Yan, J. Lin and Z. X. Shen, Adv. Mater., 2014, 26, 7162–7169 CrossRef CAS.
- L. David, R. Bhandavat and G. Singh, ACS Nano, 2014, 8, 1759–1770 CrossRef CAS.
- Y. Wang, L. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2016, 55, 7423–7426 CrossRef CAS.
- Y. Fang, D. Luan, Y. Chen, S. Gao and X. W. D. Lou, Angew. Chem., Int. Ed., 2020, 59, 7178–7183 CrossRef CAS.
- M. Han, Z. Lin and J. Yu, J. Mater. Chem. A, 2019, 7, 4804–4812 RSC.
- Z. Li, K. Jiang, F. Khan, A. Goswami, J. Liu, A. Passian and T. Thundat, Sci. Adv., 2019, 5, eaav2820 CrossRef.
- L. Zhang, D. Sun, J. Kang, J. Feng, H. A. Bechtel, L. W. Wang, E. J. Cairns and J. Guo, Nano Lett., 2018, 18, 1466–1475 CrossRef CAS.
- X. Du, J. Huang, X. Guo, X. Lin, J.-Q. Huang, H. Tan, Y. Zhu and B. Zhang, Chem. Mater., 2019, 31, 8801–8809 CrossRef CAS.
- X. Wang, X. Shen, Z. Wang, R. Yu and L. Chen, ACS Nano, 2014, 8, 11394–11400 CrossRef CAS.
- G. K. Veerasubramani, M.-S. Park, G. Nagaraju and D.-W. Kim, J. Mater. Chem. A, 2019, 7, 24557–24568 RSC.
- Y. Fang, X. Y. Yu and X. W. Lou, Angew. Chem., Int. Ed., 2019, 131, 7826–7830 CrossRef.
- L. Wang, Z. Xu, W. Wang and X. Bai, J. Am. Chem. Soc., 2014, 136, 6693–6697 CrossRef CAS.
- F. X. Li, J. L. Zou, L. J. Cao, Z. Q. Li, S. Gu, Y. Liu, J. Q. Zhang, H. T. Liu and Z. G. Lu, J. Phys. Chem. C, 2019, 123, 5067–5072 CrossRef CAS.
- A. Andersen, S. M. Kathmann, M. A. Lilga, K. O. Abrecht, R. T. Hallen and D. H. Mei, J. Phys. Chem. C, 2012, 116, 1826–1832 CrossRef CAS.
- P. Gao, L. Wang, Y. Zhang, Y. Huang and K. Liu, ACS Nano, 2015, 9, 11296–11301 CrossRef CAS.
- B. Chen, H. Lu, J. Zhou, C. Ye, C. Shi, N. Zhao and S.-Z. Qiao, Adv. Energy Mater., 2018, 8, 1702909 CrossRef.
- S. Hao, X. Shen, M. Tian, R. C. Yu, Z. X. Wang and L. Q. Chen, Nano Energy, 2017, 41, 217–224 CrossRef CAS.
- K. Leng, Z. Chen, X. Zhao, W. Tang, B. Tian, C. T. Nai, W. Zhou and K. P. Loh, ACS Nano, 2016, 9208–9215 CrossRef CAS.
- Z. Zhu, Y. Tang, W. R. Leow, H. Xia, Z. Lv, J. Wei, X. Ge, S. Cao, Y. Zhang, W. Zhang, H. Zhang, S. Xi, Y. Du and X. Chen, Angew. Chem., Int. Ed., 2019, 58, 3521–3526 CrossRef CAS.
- Z. Fan, L. Zhang, D. Baumann, L. Mei, Y. Yao, X. Duan, Y. Shi, J. Huang, Y. Huang and X. Duan, Adv. Mater., 2019, 31, 1900608 CrossRef.
- W. Wang, B. Jiang, C. Qian, F. Lv, J. Feng, J. Zhou, K. Wang, C. Yang, Y. Yang and S. Guo, Adv. Mater., 2018, 30, 1801812 CrossRef.
- H. Huang, J. Cui, G. Liu, R. Bi and L. Zhang, ACS Nano, 2019, 13, 3448–3456 CrossRef CAS.
- P. Xiong, J. Wu, M. Zhou and Y. Xu, ACS Nano, 2020, 14, 1018–1026 CrossRef CAS.
- H. L. Li, K. Yu, H. Fu, B. J. Guo, X. Lei and Z. Q. Zhu, J. Phys. Chem. C, 2015, 119, 7959–7968 CrossRef CAS.
- S. Zhang, B. V. Chowdari, Z. Wen, J. Jin and J. Yang, ACS Nano, 2015, 9, 12464–12472 CrossRef CAS.
- M. T. McDowell, S. W. Lee, J. T. Harris, B. A. Korgel, C. Wang, W. D. Nix and Y. Cui, Nano Lett., 2013, 13, 758–764 CrossRef CAS.
- Y. Wu, S. Hu, R. Xu, J. Wang, Z. Peng, Q. Zhang and Y. Yu, Nano Lett., 2019, 19, 1351–1358 CrossRef.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS.
- J. Klimes, D. R. Bowler and A. Michaelides, J. Phys.: Condens. Matter, 2010, 22, 022201 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0nh00517g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |