Nano-makisu: highly anisotropic two-dimensional carbon allotropes made by weaving together nanotubes†
Lei
Zhao
 ab,
Wei
Liu
ab,
Wei
Liu
 *cd,
WenCai
Yi
*cd,
WenCai
Yi
 e,
Tao
Hu
e,
Tao
Hu
 bd,
Dalar
Khodagholian
b,
FengLong
Gu
bd,
Dalar
Khodagholian
b,
FengLong
Gu
 f,
Haiqing
Lin
d,
Eva
Zurek
f,
Haiqing
Lin
d,
Eva
Zurek
 g,
Yonghao
Zheng
g,
Yonghao
Zheng
 *ah and
Maosheng
Miao
*ah and
Maosheng
Miao
 *bd
*bd
aSchool of Optoelectronic Science and Engineering, University of Electronic Science and Technology of China (UESTC), Chengdu, 610054, P. R. China. E-mail: zhengyonghao@uestc.edu.cn
bDepartment of Chemistry & Biochemistry, California State University Northridge, Northridge, CA 91330, USA. E-mail: mmiao@csun.edu
cDepartment of Optical Engineering, Zhejiang A&F University, Hangzhou, 311300, P. R. China. E-mail: weiliu@zafu.edu.cn
dBeijing Computational Science Research Center, Beijing, 100193, P. R. China
eSchool of Physics and Physical Engineering, Qufu Normal University, Qufu, 273165, P. R. China
fKey Laboratory of Theoretical Chemistry of Environment, Ministry of Education, School of Chemistry and Environment, South China Normal University, Guangzhou, 510006, P. R. China
gDepartment of Chemistry, State University of New York at Buffalo, Buffalo, NY 14260-3000, USA
hCentre for Applied Chemistry, University of Electronic Science and Technology of China (UESTC), Chengdu, 611731, P. R. China
First published on 27th November 2019
Abstract
Graphene and carbon nanotubes (CNT) are the representatives of two-dimensional (2D) and one-dimensional (1D) forms of carbon, both exhibiting unique geometric structures and peculiar physical and chemical properties. Herein, we propose a family or series of 2D carbon-based highly anisotropic Dirac materials by weaving together an array of CNTs by direct C–C bonds or by graphene ribbons. By employing first-principles calculations, we demonstrate that these nano-makisus are thermally and dynamically stable and possess unique electronic properties. These 2D carbon allotropes are all metals and some nano-makisus show largely anisotropic Dirac cones, causing very different transport properties for the Dirac fermions along different directions. The Fermi velocities in the kx direction could be ∼170 times higher than those in the ky direction, which is the strongest anisotropy among 2D carbon allotropes to the best of our knowledge. This intriguing feature of the electronic structure has only been observed in heavy element materials with strong spin–orbit coupling. These results indicate that carbon based materials may have much broader applications in future nanoelectronics.
Introduction
Carbon is the most versatile element, forming a plethora of compounds and allotropes, because it can form bonds in various hybridizations of atomic orbitals. Hence, it is one of the most common components in matter, and it comprises a wide range of systems from living tissues, minerals, to functional materials.1–3 The discovery of fullerenes in 1985 set off a tide of new research devoted towards the synthesis of novel carbon allotropes, including the synthesis of carbon nanotubes and the exfoliation of graphene.4–7 As the most stable two-dimensional (2D) carbon material, graphene exhibits many unusual physical properties such as ballistic carrier transport, room-temperature quantum Hall effect, ambipolar field effect and extremely high conductivity, etc.7–10 These peculiar properties render graphene as a promising candidate for applications in many future technologies.11,12 The success of graphene has inspired the search for other novel 2D carbon materials with very different structures and lattice topologies. Although most of the 2D carbon allotropes are energetically metastable, many of them, such as graphdiyne, have been realized experimentally.13,14 Furthermore, the new 2D carbon allotropes often exhibit unique properties that might outperform graphene in various aspects, including potential superconductivity, a negative Poisson's ratio, and possess distorted or anisotropic Dirac cones, etc.,14–17 which are associated with their unique lattice topologies. These results illustrate the importance of the structure–property relationship in carbon allotropes.For many years, it was believed that many geometric features of graphene are necessary preconditions for the existence of a Dirac cone. However, after the discovery of several new 2D Dirac carbon allotropes, such as α-, β-, and 6,6,12-graphyne,14 phagraphene,17 and S-, D-, E-, and r-graphene,18,19 it turns out that a strictly planar structure, chemically equivalent and sp2-hybridized carbon atoms, a honeycomb lattice, and even a hexagonal symmetry are not prerequisites for the existence of Dirac cones. Many of these 2D carbon allotropes show largely distorted Dirac cones leading to electronic properties that are complementary to that of graphene.14,20,21 In some Dirac materials, the Dirac cones are remarkably anisotropic, causing very different transport properties of the Dirac fermions in different directions.20,22–27 Such unique properties can provide more tunability for novel properties and applications.28,29 However, such largely anisotropic Dirac cones are rare in carbon based materials.20
Carbon nanotubes (CNTs), as a one-dimensional (1D) form of carbon, are cylindrical carbon allotropes composed of sp2-hybridized carbon atoms that form hexagonal networks.30 They exhibit many novel properties such as extraordinary strength, as well as unique electronic, thermal and mechanical properties, which give them great potential in a wide range of applications.31,32 CNTs have been widely used in composite and coating materials, drug delivery, heterogeneous catalysis, energy storage and biotechnology.33–36 Furthermore, the hollow tubular structure enables CNTs to be used as effective gas and metal containers, as well as host nanoreactors to promote some chemical reactions through nanoscale confinement.37,38 Hydrostatic/non-hydrostatic pressure was applied on CNTs to build new carbon allotropes both experimentally and theoretically.39–45 Due to the unique tubular structure, CNTs usually have a very high length-to-diameter ratio, which makes many properties of CNTs extremely anisotropic.
In this work, we propose a family of carbon allotropes, by interlinking an array of zigzag single-walled carbon nanotubes (SWNTs), whose structures are analogous to makisu, a mat woven from bamboo and cotton string and used in Japanese cuisine. These nano-makisus might combine the advantages of both graphene and CNTs. Using density functional theory calculations, their stability and electronic properties are studied thoroughly. The results show that these carbon allotropes are dynamically stable, and they are all metallic. Moreover, highly anisotropic Dirac cones are found in their band structures. The unusual electronic transport properties predicted by the calculations suggest that nano-makisus could be a new family of candidate materials for future nanoelectronics.
Methodology
The calculations were performed using first-principles density functional theory (DFT) as implemented in the Vienna ab initio simulation package (VASP).46–48 The exchange and correlation were obtained within the generalized gradient approximation (GGA), using the functional of Perdew, Burke, and Ernzerhof (PBE).49 The projector augmented wave (PAW) method was used to treat the core states.50,51 Periodic boundary conditions (PBC) were applied, and the vacuum between sheets in neighboring cells along the (001) direction was set to be larger than 15 Å to avoid spurious interactions between periodic images. For the electronic structure calculations, plane waves with a kinetic energy cutoff of 500 eV were adopted to expand the valence electron (2s22p2) wavefunctions. Brillouin zone (BZ) integrations were carried out using Γ-centered sampling grids with a resolution of 2π × 0.2 Å−1. Structural relaxation was performed using the conjugate gradient (CG) scheme until the force components were converged to within 0.01 eV Å−1, and the self-consistent field calculations were stopped when the energy difference between two steps was smaller than 1 × 10−8 eV per atom. First-principles molecular dynamic simulations under constant temperature and volume (NVT) were performed to examine thermal stability. The phonon spectra were recorded to examine dynamical stability using the PHONOPY package.52Results and discussion
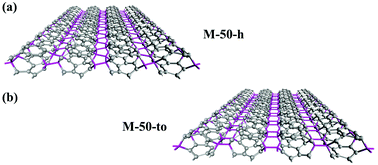
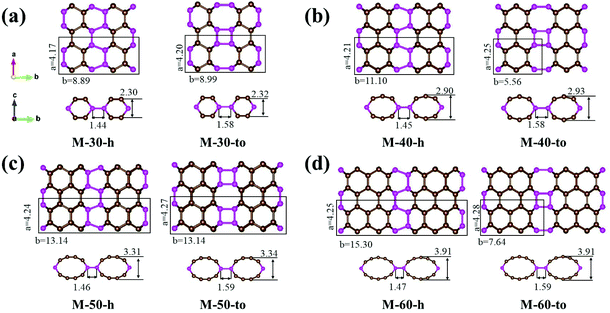
Nano-makisus are constructed by connecting zigzag (n,0), n = 3–6, SWNTs along the radial directions of the nanotubes. The neighboring nanotubes are connected by sp3-carbons. The structures of two representative nano-makisus connected by direct C–C bonds are shown in Fig. 1a and b. Two different ways of connecting the neighboring zigzag SWNTs are studied in this work. In one style, the SWNTs are linked together via hexagonal rings alone (shown in Fig. 1a), while in the other style, the nanotubes are linked via a combination of tetragons and octagons (shown in Fig. 1b). Similar connectivities can be found in previously reported structures (bct C4, Cco-C8, etc.).53–55 The constructed structures are denoted by the chiral indices (n,m) of the SWNTs, together with the way in which the neighboring nanotubes are connected. Accordingly, we obtained M-30-h/to, M-40-h/to, M-50-h/to and M-60-h/to nano-makisus, where “M” represents nano-makisu, the numbers correspond to the chiral indices of the nanotubes, “h” represents the hexagon connection and “to” represents the tetragon-octagon connection.The top and side views of the optimized atomic configurations of eight nano-makisus are shown in Fig. 2a–d. The pristine SWNTs only consist of sp2-hybridized carbon atoms located in a honeycomb lattice, corresponding to a coordination number of 3. When two neighbouring nanotubes are connected, the connecting carbon atoms will have 4 neighbouring atoms, and therefore are in a sp3 hybridized state. As shown in Fig. 2, in each nano-makisu, the pink-coloured sp3-hybridized carbon atoms are on the same plane and sandwiched between the sp2-hybridized carbon atoms. After optimization, the cross sections of carbon nanotubes will change from circular to elliptical to reduce the stress caused by the sp3 hybridization of the connecting carbon atoms. The nanotubes with larger radii also show larger deformation. In most cases, the nano-makisu unit cell consists of two SWNTs, namely, the number of carbon atoms per nano-makisu unit cell is twice as many as that in the unit cell of the nanotube as shown in Table 1. However, nano-makisu M-40-to and M-60-to are exceptions, due to the fact that zigzag (4,0), (6,0) SWNTs and the connecting tetragons and octagons have a mirror symmetry. Therefore, the unit cells of nano-makisu M-40-to and M-60-to consist of only one SWNT, and they have the same number of carbon atoms per unit cell as that in zigzag (4,0) and (6,0) SWNTs, respectively. The distance between two neighbouring nanotubes in nano-makisu mainly depends on the connection style and is slightly affected by the size of the nanotubes. In “to”-type nano-makisu, the distance between two nanotubes is 1.58–1.59 Å, which is slightly further than that of typical sp3–sp3 C–C bonds, while the distances between nanotubes in “h”-type nano-makisu are much shorter, ranging from 1.44–1.47 Å, rendering strong sp2 components in these C atoms.
| System | Carbons | ΔE/atom (eV) | sp3 (%) | C–C (sp2–sp2) | C–C (sp2–sp3) | C–C (sp3–sp3) |
|---|---|---|---|---|---|---|
| Graphene | 2 | 0 | 0 | 1.42 | — | — |
| SWNT (3,0) | 12 | 1.29 | 0 | 1.49 | — | — |
| SWNT (4,0) | 16 | 0.77 | 0 | 1.46 | — | — |
| SWNT (5,0) | 20 | 0.49 | 0 | 1.44 | — | — |
| SWNT (6,0) | 24 | 0.35 | 0 | 1.43 | — | — |
| M-30-h | 24 | 0.80 | 0.17 | 1.49 | 1.57 | 1.54 |
| M-30-to | 24 | 0.89 | 0.17 | 1.49 | 1.56 | 1.58 |
| M-40-h | 32 | 0.55 | 0.13 | 1.47 | 1.56 | 1.55 |
| M-40-to | 16 | 0.60 | 0.13 | 1.45 | 1.54 | 1.58 |
| M-50-h | 40 | 0.39 | 0.10 | 1.45 | 1.55 | 1.56 |
| M-50-to | 40 | 0.43 | 0.10 | 1.45 | 1.53 | 1.57 |
| M-60-h | 48 | 0.29 | 0.08 | 1.44 | 1.54 | 1.57 |
| M-60-to | 24 | 0.32 | 0.08 | 1.44 | 1.53 | 1.59 |
To further study the bonding between two neighbouring nanotubes, the electron localization function (ELF) was calculated and the detailed results are shown in Fig. S1.† The electrons between two nanotubes are highly localized, indicating the formation of covalent bonds. The nano-makisu structures are composed of both sp2-hybridized tube and sp3-hybridized connecting carbon atoms, shown in brown and pink in Fig. 2, respectively. Therefore, there are three types of carbon–carbon bonds in nano-makisus, namely, sp2–sp2, sp2–sp3 and sp3–sp3 bonds. As the diameter of the nanotubes increases, the curvature of the nanotube surface becomes smaller. When the nanotubes are connected to form nano-makisus, the deformation of nanotubes will also reduce the surface curvature. This will make the three C–C bonds formed by sp2-hybridized carbon atoms closer to the ideal trigonal planar geometry. Therefore, as the nanotubes become larger, the average bond lengths of sp2–sp2 and sp2–sp3 C–C bonds in nano-makisus will gradually approach the typical bond length without any tension (1.40 and 1.51 Å). The sp3–sp3 bond length in “h”-type nano-makisu is much closer to the typical sp3–sp3 bond length (1.54 Å) than that in “to”-type nano-makisu, which can be attributed to the formation of large tension tetragons in “to”-type nano-makisu. The detailed data are shown in Table 1. From M-30-h/to to M-60-h/to, the average sp2–sp2 and sp2–sp3 carbon bond lengths decrease, while the average bond length of sp3–sp3 increases, which clearly demonstrates the stretching process of carbon nanotubes.
The thermodynamic stability of nano-makisus was investigated by calculating their total energies using first-principles calculations. The obtained results are shown in Table 1. Compared with graphene, the most stable 2D carbon allotrope, nano-makisus are 0.29–0.89 eV per carbon higher in energy. However, nano-makisus possess lower energies than many previously reported carbon allotropes, such as α-graphyne and penta-graphene.14,56 Meanwhile, the energies of nano-makisus are comparable with two carbon allotropes previously proposed by our group, bubble-wrap carbons (0.36–0.81 eV per atom less stable than graphene) and egg-tray graphenes (0.22–0.33 eV per atom less stable than graphene), as listed in Table S1.† With increasing diameter of the nanotubes, nano-makisus become more planar and the energy differences relative to graphene tend to become smaller due to the smaller tension in larger nanotubes. Moreover, the energies of “to”-type nano-makisus are somewhat higher than that of “h”-type, which can be attributed to the fact that hexagons are more stable than tetragons and octagons. It is noteworthy that the energies of nano-makisus are lower than that of the corresponding pristine SWNTs, which means pristine SWNTs, especially those with small radii, tend to connect with each other spontaneously and form nano-makisus. For example, the reactions from separated SWNTs to M-30-h/to and M-40-h/to show no barrier (Fig. S2†). This is partially because the connection between two neighbouring nanotubes can relieve the strain inside carbon nanotubes. In particular, the energy reduction from SWNT (3,0) to M-30-h/to is the largest (Fig. S3†). Fig. 3 shows how the energy difference per atom relative to graphene changes as a function of the percentage of sp3-hybridized carbon atoms in each unit cell for each nano-makisu. We found that the energies of nano-makisus possess a proportional relationship with the ratio of sp3 carbon atoms, that is, a nano-makisu with a higher sp3 carbon ratio will have a higher energy.
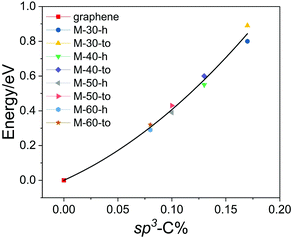 | ||
| Fig. 3 The energy difference per atom relative to graphene and how it changes as a function of the percentage of sp3-hybridized carbon atoms in each unit cell for each nano-makisu. | ||
To examine the dynamic stability of nano-makisus, we calculated the phonon band structure along high-symmetry directions, and the results are shown in Fig. S4.† The phonon spectra of all nano-makisus with the exception of one, M-50-h, show no imaginary frequencies, indicating they are dynamically stable.
The imaginary frequencies in the phonon spectrum of M-50-h are extremely small and are likely artefacts present from the convergence of the phonon calculation, since the calculations of the force constants for a two-dimensional carbon allotrope usually require better convergence and larger supercells for frozen phonons, and the unit cell of M-50-h is already quite large. However, positive phonon frequencies cannot ensure that the material is stable at finite temperatures. In order to further confirm the thermal stability of these predicted structures, ab initio molecular dynamics simulations (AIMD) were carried out. The simulations were run for 6 ps containing 4000 MD steps at temperatures up to 1000 K. The nano-makisu structures did not experience serious disruption or dissociation, indicating that these 2D carbon materials possess good stability. The final configurations for each molecular dynamic simulation are shown in Fig. S5.†
Although the total energy calculations and the MD simulations indicate that nano-makisus could stay stable, their synthesis remains a challenging task. However, recently there has been extensive experimental progress in the fabrication of low dimensional carbon allotropes via several routes, including bottom-up chemical synthesis57 and the compression of bundles of zigzag SWNTs. The latter route is more promising. As a matter of fact, the studies of SWNTs under high pressures and high temperatures have shown the conversion of the sp2 carbons to sp3 carbons.44,58–60 Besides, the simulated X-ray diffraction patterns of the theoretically predicted bct C4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 and Cco-C8
53 and Cco-C8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 structures are in good agreement with the experimental data, revealing the 4-, 6- and 8-member carbon rings in bct C4 and Cco-C8 and the presence of the sp3 interlinking carbons that are very similar to those found in nano-makisus. These experimental studies strongly suggest that the proposed nano-makisus could be fabricated, and as shown by our calculations they can remain stable once they are made. Indeed, many allotropes with energies higher than nano-makisus such as graphdiyne,13 a C20 cage61 and the smallest carbon nanotube62 (0.77, 1.14 and 1.15 eV per atom less stable than graphene, respectively) have already been synthesized.
54 structures are in good agreement with the experimental data, revealing the 4-, 6- and 8-member carbon rings in bct C4 and Cco-C8 and the presence of the sp3 interlinking carbons that are very similar to those found in nano-makisus. These experimental studies strongly suggest that the proposed nano-makisus could be fabricated, and as shown by our calculations they can remain stable once they are made. Indeed, many allotropes with energies higher than nano-makisus such as graphdiyne,13 a C20 cage61 and the smallest carbon nanotube62 (0.77, 1.14 and 1.15 eV per atom less stable than graphene, respectively) have already been synthesized.
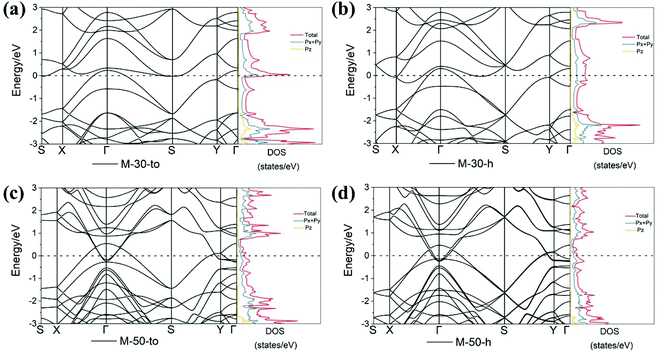
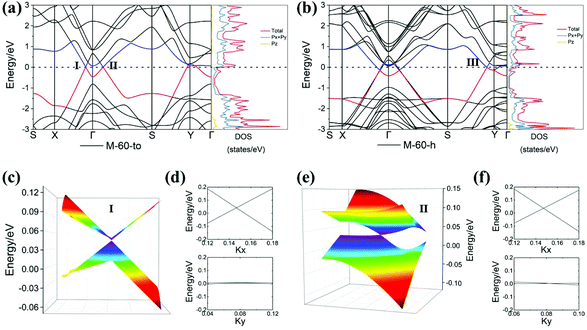
Next, we turn our attention to study the electronic properties of nano-makisus. The obtained band structures and density of states (DOS) calculated at the PBE level of theory are shown in Fig. 4–6, and the results reveal that all the nano-makisus are metallic. By comparing the band structures of different nano-makisus, we find that the connecting style of two neighbouring nanotubes (“h” and “to”) in nano-makisus has little impact on the electronic properties of M-30-to/h and M-50-to/h. For example, the band structures of M-30-to and M-30-h are very similar (Fig. 4a and b). The same is found for M-50-to and M-50-h (Fig. 4c and d).
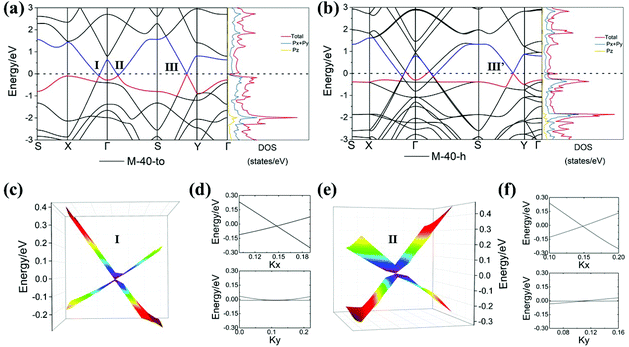
The band structures and DOS of M-40-to/h are shown in Fig. 5a and b. Interestingly, crossing points with linear bands were found in both band structures, indicating possible Dirac points in the first BZ. In M-40-to, two crossing points, denoted as I and II, are found at (0.1033, 0.0, 0.0) along the line from X to Γ and at (0.1077, 0.1097, 0.0) along the line from Γ to S, respectively. The 3D band structures around these two crossing points (Fig. 5c and e) clearly reveal that they are indeed Dirac points. The two Dirac cones lie slightly below the Fermi level, which indicates that M-40-to is a self-doped Dirac material and the electrons act as charge carriers at both Dirac point I and II. Remarkably, these two Dirac cones show a highly anisotropic character. In order to further study its effects on transport properties, we obtained the Fermi velocities by calculating the slope of the linear bands along two perpendicular directions (x and y) using the expression vf = E(q)/ħ|q|. For Dirac cone I, the slopes of the two crossing bands in the kx direction in reciprocal space are equal to −33.52 and +13.30 eV Å, which correspond to Fermi velocities of 8.11 × 105 and 3.22 × 105 m s−1. These two velocities are close to that of 8.22 × 105 m s−1 in graphene, while in the ky direction, the slopes are −0.74 and +0.70 eV Å, which correspond to the Fermi velocities of 1.80 × 104 and 1.68 × 104 m s−1. The Fermi velocities in the kx direction are 19 to 45 times higher than that in the ky direction, indicating the highly anisotropic transport properties. The anisotropy is also confirmed by the band dispersions near point I parallel or perpendicular to the high symmetry X–Γ line as shown in Fig. 5d and f. For Dirac cone II, the slopes in the kx direction are −32.24 (vfx = 7.80 × 105 m s−1) and +16.57 eV Å (vfx = 4.01 × 105 m s−1), and in the ky direction they are −0.19 (vfy = 4.56 × 103 m s−1) and +4.46 eV Å (vfy = 1.08 × 105 m s−1). The Fermi velocities in the kx direction are 4 to 171 times higher than that in the ky direction, which shows a stronger anisotropy when compared with other 2D Dirac carbon allotropes reported previously14,17,19–21,63–65 (Table S2†). M-40-h possesses four Dirac cones at positions similar to those of M-40-to (Fig. 5b). This is caused by the fact that M-40-h is lower in symmetry, and this doubles the size of its unit cell and correspondingly the bands are “folded”. Partial charge density distributions of the two Dirac bands in the proximity of the Fermi level demonstrate that point I and II have similar charge distribution and the electron densities in the highest occupied band (VBM) are delocalized, while the electron densities in the lowest unoccupied band (CBM) are highly localized at sp2 carbons in the nanotubes (Fig. S7b†).
Besides, there is another crossing point of linear bands in both M-40-to and M-40-h. They are located at (0.1270, 0.5, 0.0) (Dirac point III) as shown in Fig. 5a and (0.1247, 0.5, 0.0) (Dirac point III′) as shown in Fig. 5b along the line from S to Y in the first BZ, respectively. The 3D band structures of these two points (Fig. S6a and S6c†) also reveal that they are Dirac points. For Dirac cone III in M-40-to, the slopes of the band energies and the corresponding Fermi velocities of the two crossing bands in the kx direction are −34.87 (vfx = 8.43 × 105 m s−1) and +29.42 eV Å (vfx = 7.11 × 105 m s−1), and in the ky direction the values are −0.88 (vfy = 2.14 × 104 m s−1) and +0.90 eV Å (vfy = 2.18 × 104 m s−1). The Fermi velocities in the kx direction are 33 to 39 times higher than that in the ky direction. The corresponding values in M-40-h are −34.91 (vfx = 8.44 × 105 m s−1) and +23.71 eV Å (vfx = 5.73 × 105 m s−1) in the kx direction, and −32.61 (vfy = 7.89 × 105 m s−1) and +19.62 eV Å (vfy = 4.74 × 105 m s−1) in the ky direction. These values are comparable with that of graphene and other carbon Dirac allotropes predicted in previous studies.14,17,19 Partial charge density distributions of the highest occupied band show that the holes are delocalized at Dirac point III in M-40-to, and localized at the sp2 carbons at Dirac point III′ in M-40-h. In contrast, the electron density in the lowest unoccupied bands shows that the electrons are localized at sp2 carbons in M-40-to and partially localized in sp2 nanotube carbons in M-40-h (Fig. S7c and d†).
The electronic structures of M-60-to/h are shown in Fig. 6. Similar to M-40-to, two crossing points of linear bands are found along the high symmetry line X–Γ at (0.0909, 0.0, 0.0) (denoted as point I) and along the high symmetry line Γ–S at (0.0889, 0.0884, 0.0) (denoted as point II) in the first BZ.
Different to M-40-to, these two points lie slightly above the Fermi level, causing self-doped holes to be charge carriers. As shown in Fig. 6c and e, the calculated 3D band structures around points I and II clearly reveal that they are anisotropic Dirac points. For Dirac cone I, the slopes of the band energies of the two bands in reciprocal space and the corresponding Fermi velocities are equal to −34.73 (vfx = 8.40 × 105 m s−1) and +27.97 eV Å (vfx = 6.76 × 105 m s−1) in the kx direction, and −0.69 (vfy = 1.67 × 104 m s−1) and +0.62 eV Å (vfy = 1.49 × 104 m s−1) in the ky direction. For Dirac cone II, the slopes and the Fermi velocities are −34.18 (vfx = 8.26 × 105 m s−1) and +26.57 eV Å (vfx = 6.42 × 105 m s−1) in the kx direction, and −3.81 (vfy = 9.20 × 104 m s−1) and +0.38 eV Å (vfy = 9.20 × 103 m s−1) in the ky direction. The Fermi velocities in the kx direction are 9 to 70 times higher than that in the ky direction. The highly anisotropic properties can also be inferred from Fig. 6d and f. The partial charge densities show that the electrons at point I and II are both highly localized at sp2 carbons as shown in Fig. S9a.†
Similar to M-40-h, M-60-h possesses one Dirac point (denoted as point III) located at (0.0773, 0.5, 0.0) along the line from S to Y. The calculated 3D bands in the vicinity of this point reveal that it is a highly anisotropic Dirac cone as displayed in Fig. S8.† The slopes of the crossing bands and the Fermi velocities are −33.21 (vfx = 8.03 × 105 m s−1) and −12.77 eV Å (vfx = 3.09 × 105 m s−1) in the kx direction, and −2.20 (vfy = 5.32 × 104 m s−1) and −1.79 eV Å (vfy = 4.34 × 104 m s−1) in the ky direction, which also illustrates the anisotropy of M-60-h. The partial charge density distributions of the two Dirac bands in the proximity of the Fermi level at Dirac point III in M-60-h show that the electrons at both the lower and upper cones are partially localized at sp2 carbons in nanotubes, as seen in Fig. S9b.† Interlinked carbon nanotubes and fullerenes have been observed or predicted before.66,67 In comparison with those allotropes, nano-makisus are two dimensional materials featuring anisotropic Dirac cones. It is interesting that the electronic structure of bct-C12,68 a three-dimensional interlinked nanotube allotrope, is also very anisotropic. It shows linear dispersion (for Dirac particles) in one direction (kz) and conventional metallic bands in the other two directions.
Conclusions
In summary, we proposed a series of 2D carbon materials with orderly arranged hollow tubular structures named nano-makisus. Their stabilities, structural features and electronic properties are studied by means of first-principles calculations. Depending on the size of the tubes, many nano-makisus are energetically very stable compared to other 2D carbon allotropes. Molecular dynamics calculations indicated that nano-makisus can withstand temperatures up to 1000 K. The electronic structure calculations demonstrated that these 2D carbon allotropes are all metallic. Some nano-makisus possess highly anisotropic Dirac cones with Fermi velocities varying from 4.56 × 103 to 8.44 × 105 m s−1 along different k directions. The Fermi velocities in the kx direction could be ∼170 times higher than that in the ky direction, which is the largest anisotropy among 2D carbon allotropes. The strong propensity of the nanotubes toward interlinking under high pressure might provide a feasible route to fabricate this new family of 2D carbon allotropes. The high and direction-dependent Fermi velocities give nano-makisus great potential for future use in direction-dependent electronic devices. Furthermore, the hollow tubular structures may also enable nano-makisus to effectively constrain gas and metal species, and promote some chemical reactions through nanoscale confinement.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. M. acknowledges the support of the NSF CAREER award 1848141 and ACF PRF fund 50249-UN16. This work was also supported by the National Natural Science Foundation of China (61604101), the Scientific Research Foundation of UESTC for Young Teacher (ZYGX2016KYQD134). W. L. acknowledges the Scientific Research and Developed Fund of Zhejiang A&F University (No. 2019FR005 and 2019FR006), the National Natural Science Foundation of China (11975206) for financial support. E. Z. acknowledges the NSF (DMR-1827815) for financial support. Part of the calculations are performed on NSF-funded XSEDE resources (TGDMR130005) especially on the Stampede cluster run by Texas Advanced Computing Centre.References
- A. Hirsch, Nat. Mater., 2010, 9, 868 CrossRef CAS PubMed.
- K. Kinoshita, Carbon – Electrochemical and Physicochemical properties, John Wiley and Sons, Inc., 1st edn, 1988 Search PubMed.
- E. Frackowiak, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- H. W. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- S. Iijima and T. Ichihashi, Nature, 1993, 363, 603 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- Y. Zhang, Y.-W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- P. Avouris and F. Xia, MRS Bull., 2012, 37, 1225–1234 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- G. Li, Y. Li, H. Liu, Y. Guo, Y. Li and D. Zhu, Chem. Commun., 2010, 46, 3256–3258 RSC.
- D. Malko, C. Neiss, F. Viñes and A. Görling, Phys. Rev. Lett., 2012, 108, 086804 CrossRef PubMed.
- H. Terrones, M. Terrones, E. Hernández, N. Grobert, J.-C. Charlier and P. Ajayan, Phys. Rev. Lett., 2000, 84, 1716 CrossRef CAS PubMed.
- W. Liu, J.-Y. Liu, J. Xia, H.-Q. Lin and M.-S. Miao, Nanoscale, 2018, 10, 11328–11334 RSC.
- Z. Wang, X.-F. Zhou, X. Zhang, Q. Zhu, H. Dong, M. Zhao and A. R. Oganov, Nano Lett., 2015, 15, 6182–6186 CrossRef CAS PubMed.
- W. Liu, M. Miao and J.-Y. Liu, J. Mater. Chem. C, 2015, 3, 9921–9927 RSC.
- L.-C. Xu, R.-Z. Wang, M.-S. Miao, X.-L. Wei, Y.-P. Chen, H. Yan, W.-M. Lau, L.-M. Liu and Y.-M. Ma, Nanoscale, 2014, 6, 1113–1118 RSC.
- C. Su, H. Jiang and J. Feng, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 075453 CrossRef.
- L. Zhang, Z. Wang, Z. M. Wang, S. Du, H.-J. Gao and F. Liu, J. Phys. Chem. Lett., 2015, 6, 2959–2962 CrossRef CAS PubMed.
- V. Pardo and W. E. Pickett, Phys. Rev. Lett., 2009, 102, 166803 CrossRef PubMed.
- V. Pardo and W. E. Pickett, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 035111 CrossRef.
- G. Lee, M. A. Farhan, J. S. Kim and J. H. Shim, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 245104 CrossRef.
- J. Park, G. Lee, F. Wolff-Fabris, Y. Koh, M. Eom, Y. Kim, M. Farhan, Y. Jo, C. Kim and J. Shim, Phys. Rev. Lett., 2011, 107, 126402 CrossRef PubMed.
- Z. Liu, B. Zhou, Y. Zhang, Z. Wang, H. Weng, D. Prabhakaran, S.-K. Mo, Z. Shen, Z. Fang and X. Dai, Science, 2014, 343, 864–867 CrossRef CAS PubMed.
- B. Feng, J. Zhang, S. Ito, M. Arita, C. Cheng, L. Chen, K. Wu, F. Komori, O. Sugino and K. Miyamoto, Adv. Mater., 2018, 30, 1704025 CrossRef PubMed.
- Y. Feng, Z. Wang, C. Chen, Y. Shi, Z. Xie, H. Yi, A. Liang, S. He, J. He and Y. Peng, Sci. Rep., 2014, 4, 5385 CrossRef CAS PubMed.
- T. Wehling, A. M. Black-Schaffer and A. V. Balatsky, Adv. Phys., 2014, 63, 1–76 CrossRef CAS.
- T. W. Odom, J.-L. Huang, P. Kim and C. M. Lieber, J. Phys. Chem. B, 2000, 104, 2794–2809 CrossRef CAS.
- V. N. Popov, Mater. Sci. Eng., R, 2004, 43, 61–102 CrossRef.
- M. F. De Volder, S. H. Tawfick, R. H. Baughman and A. J. Hart, Science, 2013, 339, 535–539 CrossRef CAS PubMed.
- E. Bekyarova, Y. Ni, E. B. Malarkey, V. Montana, J. L. McWilliams, R. C. Haddon and V. Parpura, J. Biomed. Nanotechnol., 2005, 1, 3–17 CrossRef CAS PubMed.
- A. Bianco, K. Kostarelos and M. Prato, Curr. Opin. Chem. Biol., 2005, 9, 674–679 CrossRef CAS PubMed.
- D. N. Futaba, K. Hata, T. Yamada, T. Hiraoka, Y. Hayamizu, Y. Kakudate, O. Tanaike, H. Hatori, M. Yumura and S. Iijima, Nat. Mater., 2006, 5, 987 CrossRef CAS PubMed.
- J. Planeix, N. Coustel, B. Coq, V. Brotons, P. Kumbhar, R. Dutartre, P. Geneste, P. Bernier and P. Ajayan, J. Am. Chem. Soc., 1994, 116, 7935–7936 CrossRef CAS.
- J. Hu, Y. Bando, J. Zhan, C. Zhi and D. Golberg, Nano Lett., 2006, 6, 1136–1140 CrossRef CAS PubMed.
- J. Zhang, D. Zhao, D. Xiao, C. Ma, H. Du, X. Li, L. Zhang, J. Huang, H. Huang and C. L. Jia, Angew. Chem., Int. Ed., 2017, 56, 1850–1854 CrossRef CAS PubMed.
- M. Hu, Z. Zhao, F. Tian, A. R. Oganov, Q. Wang, M. Xiong, C. Fan, B. Wen, J. He and D. Yu, Sci. Rep., 2013, 3, 1331 CrossRef PubMed.
- T. Yildirim, O. Gülseren, Ç. Kılıç and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 12648 CrossRef CAS.
- Z. Zhao, X.-F. Zhou, M. Hu, D. Yu, J. He, H.-T. Wang, Y. Tian and B. Xu, J. Superhard Mater., 2012, 34, 371–385 CrossRef.
- S. Saxena and T. A. Tyson, ACS Nano, 2010, 4, 3515–3521 CrossRef CAS PubMed.
- Q. Li, Y. Ma, A. R. Oganov, H. Wang, H. Wang, Y. Xu, T. Cui, H.-K. Mao and G. Zou, Phys. Rev. Lett., 2009, 102, 175506 CrossRef PubMed.
- A. Merlen, N. Bendiab, P. Toulemonde, A. Aouizerat, A. San Miguel, J.-L. Sauvajol, G. Montagnac, H. Cardon and P. Petit, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 035409 CrossRef.
- J. Proctor, M. Halsall, A. Ghandour and D. Dunstan, Phys. Status Solidi B, 2007, 244, 147–150 CrossRef CAS.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133 CrossRef.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758 CrossRef CAS.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 134106 CrossRef.
- K. Umemoto, R. M. Wentzcovitch, S. Saito and T. Miyake, Phys. Rev. Lett., 2010, 104, 125504 CrossRef PubMed.
- Z. Zhao, B. Xu, X.-F. Zhou, L.-M. Wang, B. Wen, J. He, Z. Liu, H.-T. Wang and Y. Tian, Phys. Rev. Lett., 2011, 107, 215502 CrossRef PubMed.
- H. Cheng, G. P. Pez and A. C. Cooper, Nano Lett., 2003, 3, 585–587 CrossRef CAS.
- Z. Shunhong, Z. Jian, W. Qian, C. Xiaoshuang, K. Yoshiyuki and J. Puru, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 2372–2377 CrossRef PubMed.
- S. Hitosugi, T. Yamasaki and H. Isobe, J. Am. Chem. Soc., 2012, 134, 12442–12445 CrossRef CAS PubMed.
- V. N. Khabashesku, Z. Gu, B. Brinson, J. L. Zimmerman, J. L. Margrave, V. A. Davydov, L. S. Kashevarova and A. V. Rakhmanina, J. Phys. Chem. B, 2002, 106, 11155–11162 CrossRef CAS.
- P. V. Teredesai, A. Sood, D. Muthu, R. Sen, A. Govindaraj and C. Rao, Chem. Phys. Lett., 2000, 319, 296–302 CrossRef CAS.
- M. Yao, Z. Wang, B. Liu, Y. Zou, S. Yu, W. Lin, Y. Hou, S. Pan, M. Jin and B. Zou, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 205411 CrossRef.
- H. Prinzbach, A. Weiler, P. Landenberger, F. Wahl, J. Wörth, L. T. Scott, M. Gelmont, D. Olevano and B. v. Issendorff, Nature, 2000, 407, 60 CrossRef CAS.
- X. Zhao, Y. Liu, S. Inoue, T. Suzuki, R. Jones and Y. Ando, Phys. Rev. Lett., 2004, 92, 125502 CrossRef CAS PubMed.
- M. Zhao, W. Dong and A. Wang, Sci. Rep., 2013, 3, 3532 CrossRef PubMed.
- W.-J. Yin, Y.-E. Xie, L.-M. Liu, R.-Z. Wang, X.-L. Wei, L. Lau, J.-X. Zhong and Y.-P. Chen, J. Mater. Chem. A, 2013, 1, 5341–5346 RSC.
- L. Li, X. Kong and F. M. Peeters, Carbon, 2019, 141, 712–718 CrossRef CAS.
- Z. Zhao, B. Xu, L.-M. Wang, X.-F. Zhou, J. He, Z. Liu, H.-T. Wang and Y. Tian, ACS Nano, 2011, 5, 7226–7234 CrossRef CAS PubMed.
- L. Wang, B. Liu, H. Li, W. Yang, Y. Ding, S. V. Sinogeikin, Y. Meng, Z. Liu, X. C. Zeng and W. L. Mao, Science, 2012, 337, 825–828 CrossRef CAS PubMed.
- X. Dong, M. Hu, J. He, Y. Tian and H.-T. Wang, Sci. Rep., 2015, 5, 10713 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Electron localization function (ELF), phonon spectra, ab initio molecular dynamics simulations (AIMD), band structure of Dirac point III in M-40-to, Dirac point III’ in M-40-h, Dirac point III in M-60-h and partial charge densities of M-40-to/h and M-60-to/h. See DOI: 10.1039/c9nr08069d |
| This journal is © The Royal Society of Chemistry 2020 |