Ni-Catalyzed electrophile driven regioselective arylative cyclization of ortho-functional diaryl acetylenes for the synthesis of pyridine and indene derivatives†
Maneesh Kumar Reddy
Singam
ab,
Attunuri
Nagireddy
ab,
Manda
Rajesh
ab,
Veeramalla
Ganesh
ab and
Maddi Sridhar
Reddy
 *ab
*ab
aDepartment of OSPC, CSIR-Indian Institute of Chemical Technology, Habsiguda, Hyderabad 500007, India. E-mail: msreddy@iict.res.in
bAcademy of Scientific and Innovative Research, New Delhi 110001, India
First published on 18th November 2019
Abstract
A regioselective carbonickelation followed by cyclization, an arylative cyclization, of ortho functional diaryl acetylenes is achieved apparently through an electrophile driven alkyne polarization. A series of selectively substituted di aryl isoquinoline, pyridine and indene derivatives are thus accessed from diarylacetylenes with azide, carbonyl and cyanide tethers.
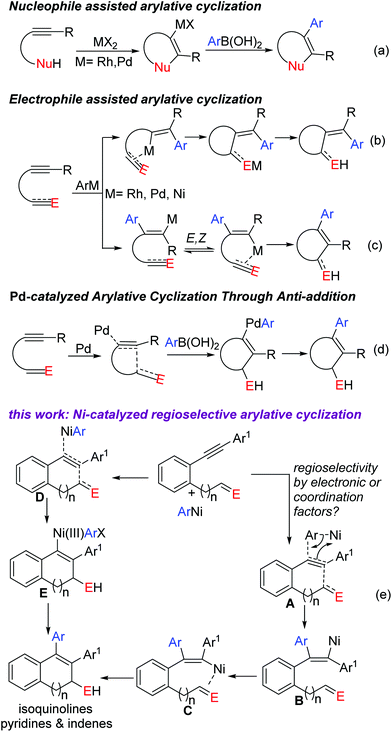
Aryl metallation of alkynes is an outstanding pathway for synthesizing multisubstituted aryl olefins.1–6 The resultant alkenyl-metal center is highly carbophilic/nucleophilic and is thus captured in tandem by various electrophiles for complex and desired outcomes. Intramolecular captures (overall an arylative cyclization) occur obviously with high ease to afford cyclic scaffolds. These arylative cyclizations are complimentary to the electrophilic cyclizations (Fig. 1a) of nucleophile tethered alkynes where arylation (oxidative coupling) follows cyclization in contrast to this approach which comprises cyclization after arylation (aryl metallation). Use of ArB(OH)2 as the aryl source made this approach more practical and convenient. An extensive study has been performed to unveil the compatibility of various metal complexes (based on Rh, Pd, Cu, Ru, Co and Ir) with ArB(OH)2 in this reaction (Fig. 1b–d). Discovery of the compatibility of economical Ni
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 complexes with ArB(OH)2 made this approach further reliable. Seminal studies by Liu,4a Lam4b–d and us4e–g opened this new avenue for further exploration. Most of the reactions were proved to undergo syn aryl nickelation followed by cis– trans isomerization. This isomerisation can be ceased at one end if one of these two forms is arrested by some means, usually by an internal electrophile capture4a–f or by co-ordination with an internal ligand (Fig. 1c).4g
4 complexes with ArB(OH)2 made this approach further reliable. Seminal studies by Liu,4a Lam4b–d and us4e–g opened this new avenue for further exploration. Most of the reactions were proved to undergo syn aryl nickelation followed by cis– trans isomerization. This isomerisation can be ceased at one end if one of these two forms is arrested by some means, usually by an internal electrophile capture4a–f or by co-ordination with an internal ligand (Fig. 1c).4g
On the other hand, regioselectivity in these aryl metallations is usually governed by steric or electronic bias.5 Terminal alkynes4f–g,5a–b and monoaryl (or monoalkenyl) internal alkynes5c–e have a clear steric or electronic bias and pose no regioselectivity issues. But with unsymmetrical dialkyl or diaryl acetylenes, regioselectivity is obviously a major issue to be addressed. In the case of alkyl alkynes with carbonyl, nitrile and allylether tethers (although unsymmetrical with no steric bias), a regioselective aryl metallation was achieved6 with chelation assistance where it was always syn-selective towards an exo olefin formation upon cyclization. But there is a huge dearth of similar studies on diaryl acetylenes.7
In continuation of our interest in novel carbometallation and nucleometallation of alkynes,4e–g,8 we herein report a highly regioselective arylative cyclization of ortho functionalized diaryl acetylenes to construct a wide range of biologically and pharmaceutically interesting pyridine and indene derivatives (Fig. 1e).9 We reason that this unusual regioselectivity is promoted by the internal electrophile since there are no apparent electronic or steric differences between two aryls. The electrophile must have induced the polarization in the alkyne (A) before the arylnickelation (B) by the nickel complex (path I). The other possibility (path II) is, in analogy to Pd(0) addition on the alkyne as reported by Tsukamoto2f–g,7b (Fig. 1d), the simultaneous approach of the electrophile and ArNi on the opposite terminals of the alkyne (D) towards the oxidative addition of ArNi (anti-addition, E). The resultant Ni(III) would then undergo reductive elimination to regenerate Ni(I).6m
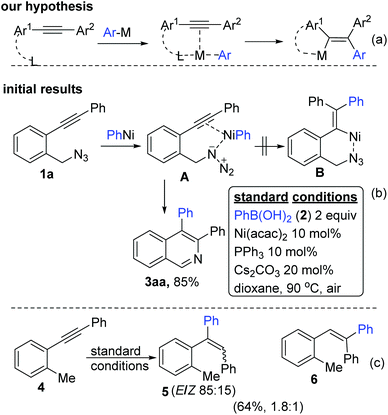
We initially hypothesized (Scheme 1a) that a prior co-ordination of the ArNi complex with a functional group in the substrate may incur the necessary bias for the regioselective arylnickelation of alkynes (in analogy to the above said chelation assisted regio- and syn-selective aryl metallation of unsymmetrical alkynes6). We first chose substrate 1a with azide tether at the ortho position to test our hypothesis under previously standardized conditions4a,e–g (Scheme 1b). We anticipated that PhNi (from PhB(OH)22a) would coordinate with both azide and alkyne moieties together as shown in A. PhNi now adds on to the alkyne in the syn fashion to give B which would then deliver the relevant end product. Surprisingly, A furnished 3aa as the only end product (in 85% yield) apparently through one of the aforementioned pathways (Fig. 1e). To demonstrate the necessity of an electrophile in this unusual regioselective aryl nickelation, we chose 4 excluding the N3 tether. Asserting the importance of the presence of an electrophile, it resulted in a mixture of stereo- and regio-isomers as shown in Scheme 1c.
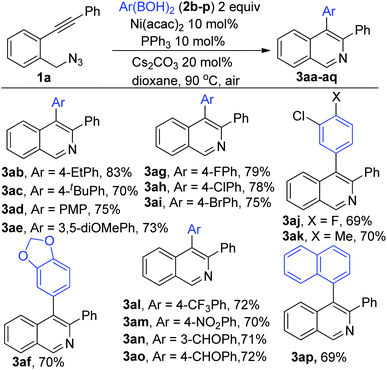
We next moved to examine the scope of the reaction (Scheme 2). The reaction was found to be highly general accommodating various Ar(BOH)2 with alkyl (2b–c), alkoxy (2d–f), halo (2g–l) and electron pulling groups (2m–p) to afford the corresponding adducts (3ab–ap) in excellent yields.
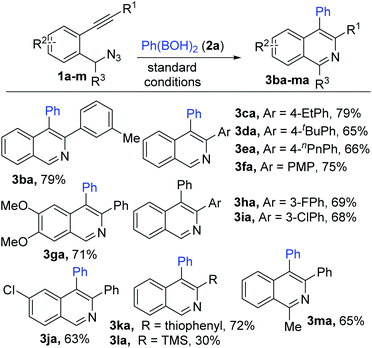
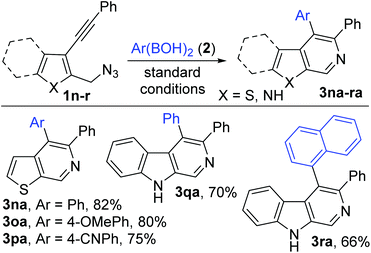
Substrates 1 (1b–m) with varying substitutions (alkyl, alkoxy and halo) both on the core aryl group as well as the pendant aryl group on the alkyne were successfully engaged in this transformation to afford the desired products (3ba–ka) in good to excellent yields (Scheme 3). 3-Silylated and 1-alkylated (methyl) adducts (3la–ma) could also be synthesized, but in lower yields. Furthermore, various heteroaryl (thiophene and indole) based substrates (1n–r) could be conveniently transformed to the expected products (thiophenopyridines 3na–pa and β-carbolines 3qa–ra) with equal ease (Scheme 4).
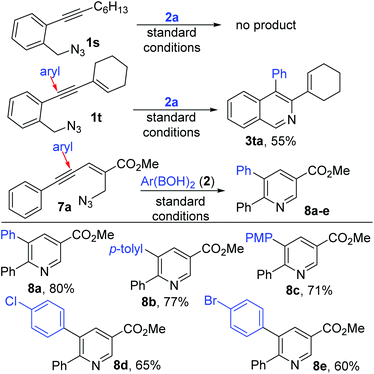
Surprisingly, alkyl substituted 1s did not give any expected product (but decomposed slowly) asserting the importance of the extended conjugation (Scheme 5). Supporting this, enyne 1t gave the anticipated adduct 3ta with the persistent installation of the aryl group proximal to the core aryl unit. In pleasing contrast, switching of the azidomethyl unit towards the olefin end of enyne as in 7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 also switched the position of arylation (distant to the core aryl unit) to afford the nicotinate derivative118a supporting our hypothesis of electrophile driven regioselective arylnickelation.
10 also switched the position of arylation (distant to the core aryl unit) to afford the nicotinate derivative118a supporting our hypothesis of electrophile driven regioselective arylnickelation.
This contrasts even the Cu-catalyzed conjugate addition (γ addition occurred instead of δ as in conjugate addition) of aryl boronic acids as reported by Yamamoto.3a,b This new pyridine product formation was observed to be general with various electronically different aryl boronic acids (8a–e).
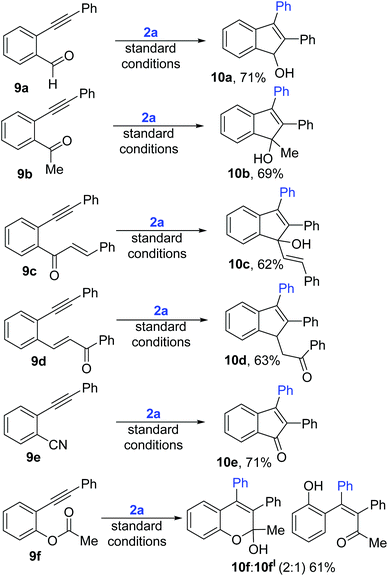
Next, to further expand the scope of this electrophile driven regioselective arylative cyclization, we synthesized diphenyl acetylenes with various other electrophilic but co-ordinating o-tethers (Scheme 6). 9a and 9b with weakly coordinating groups like aldehyde and ketone also cleanly underwent this regioselective arylative cyclization to afford indenols 10a–b in 69–71% yields. Substrates 9c and 9d with conjugated enones not only underwent the anticipated selective arylation but also selectively gave 5-membered indene adducts (10c–d) but not any trace of 7-membered products. Furthermore, diphenylacetylene with a CN handle (10e) also cleanly transformed to the desired indenone 10e. Interestingly, 11f with an acetoxy functional group underwent acyl migration following the anticipated regioselective arylation (10f).
In conclusion, a series of diaryl pyridine and indene derivatives are accessed from appropriately functionalized diarylacetylenes through a nickel catalyzed regioselective arylation followed by cyclization. The crucial regioselection is shown to have aroused from an electrophile driven polarization in alkynes. A wide range of electronically diverse substrates are generally converted to various biologically intriguing scaffolds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
MKRS thank CSIR, AN and VG thank the UGC and MR thanks CSIR for the fellowships. We thank Dr Chada Raji Reddy for some useful discussions, and thank analytical division CSIR-IICT for analytical support. We gratefully acknowledge the financial support by the DST (EMR/2017/0002413). We thank Director CSIR-IICT for constant support (IICT/Pubs./2019/395).Notes and references
- For reviews, see: (a) I. Marek, N. Chinkov and D. Banon-Tenne, Carbometallation Reactions, Wiley-VCH Verlag GmbH, Weinheim, 2008 Search PubMed; (b) A. Ding and H. Guo, in Comprehensive Organic Synthesis II, ed. P. Knochel, Elsevier, Amsterdam, 2nd edn, 2014, pp. 891–938 Search PubMed; (c) V. P. Boyarskiy, D. S. Ryabukhin, N. A. Bokach and A. V. Vasilyev, Chem. Rev., 2016, 116, 5894 CrossRef CAS; (d) Y. Yamamoto, Chem. Soc. Rev., 2014, 43, 1575 RSC; (e) J. Montgomery, Angew. Chem., Int. Ed., 2004, 43, 3890 CrossRef CAS; (f) A. G. Fallis and P. Forgione, Tetrahedron, 2001, 57, 5899 CrossRef CAS; (g) K. Tanaka and Y. Tajima, Eur. J. Org. Chem., 2012, 3715 CrossRef CAS.
- (a) N. Liu, J. Yao, L. Yin, T. Lu, Z. Tian and X. Dou, ACS Catal., 2019, 9, 6857 CrossRef CAS; (b) M. Callingham, B. M. Partridge, W. Lewis and H. W. Lam, Angew. Chem., Int. Ed., 2017, 56, 16352 CrossRef CAS; (c) Y. Li and M.-H. Xu, Org. Lett., 2014, 16, 2712 CrossRef CAS; (d) Z. Liu, J. Derosa and K. M. Engle, J. Am. Chem. Soc., 2016, 138, 13076 CrossRef CAS; (e) M. Jiang, T. Jiang and J.-E. Backvall, Org. Lett., 2012, 14, 3538 CrossRef CAS; (f) H. Tsukamoto and Y. Kondo, Angew. Chem., Int. Ed., 2008, 47, 4851 CrossRef CAS; (g) H. Tsukamoto, T. Ueno and Y. Kondo, J. Am. Chem. Soc., 2006, 128, 1406 CrossRef CAS.
- (a) Y. Yamamoto, E. Ohkubo and M. Shibuya, Green Chem., 2016, 18, 4628 RSC; (b) Y. Yamamoto, N. Kirai and Y. Harada, Chem. Commun., 2008, 2010 RSC; (c) L. Zhang, X. Xie, Z. Peng, L. Fu and Z. Zhang, Chem. Commun., 2013, 49, 8797 RSC; (d) M. Ueda, T. Ueno, Y. Suyama and I. Ryu, Chem. Commun., 2016, 52, 13237 RSC; (e) P. S. Lin, M. Jeganmohan and C. H. Cheng, Chem. – Eur. J., 2008, 14, 11296 CrossRef CAS; (f) R. E. Ruscoe, M. Callingham, J. A. Baker, S. E. Korkis and H. W. Lam, Chem. Commun., 2019, 55, 838 RSC; (g) B. M. Partridge, J. S. Gonzalez and H. W. Lam, Angew. Chem., Int. Ed., 2014, 53, 6523 CrossRef CAS.
- (a) X. Zhang, X. Xie and Y. Liu, Chem. Sci., 2016, 7, 5815 RSC; (b) C. Clarke, C. A. I. Pradillos and H. W. Lam, J. Am. Chem. Soc., 2016, 138, 8068 CrossRef CAS; (c) S. M. Gillbard, C.-H. Chung, S. N. Karad, H. Panchal, W. Lewis and H. W. Lam, Chem. Commun., 2018, 54, 11769 RSC; (d) S. N. Karad, H. Panchal, C. Clarke, W. Lewis and H. W. Lam, Angew. Chem., Int. Ed., 2018, 57, 9122 CrossRef CAS; (e) G. R. Kumar, R. Kumar, M. Rajesh and M. S. Reddy, Chem. Commun., 2018, 54, 759 RSC; (f) M. Rajesh, M. K. R. Singam, S. Puri, B. Sridhar and M. S. Reddy, J. Org. Chem., 2018, 83, 15361 CrossRef CAS; (g) M. H. Babu, G. R. Kumar, R. Kant and M. S. Reddy, Chem. Commun., 2017, 53, 3894 RSC; (h) Z. Li, A. Garica-Dominguez and C. Nevado, Angew. Chem., Int. Ed., 2016, 55, 6938 CrossRef CAS.
- (a) J. Keilitz, S. G. Newman and M. Lautens, Org. Lett., 2013, 15, 1148 CrossRef CAS; (b) S. A. Murthy, S. Donikela, C. S. Reddy and R. Chegondi, J. Org. Chem., 2015, 80, 5566 CrossRef; (c) M. Lautens and M. Yoshida, J. Org. Chem., 2003, 68, 762 CrossRef CAS; (d) S.-I. Ikeda, S. Kaori and K. Odashima, Chem. Commun., 2006, 457 RSC; (e) M. Nie, W. Fu, Z. Cao and W. Tang, Org. Chem. Front., 2015, 2, 1322 RSC.
- (a) J. Montgomery, Acc. Chem. Res., 2000, 33, 467 CrossRef CAS; (b) E. Oblinger and J. Montgomery, J. Am. Chem. Soc., 1997, 119, 9065 CrossRef CAS; (c) G. Liu, W. Fu, X. Mu, T. Wu, M. Nie, K. Li, X. Xu and W. Tang, Commun. Chem., 2018, 1, 90 CrossRef; (d) R. Shintani, K. Okamoto, Y. Otomaru, K. Ueyama and T. Hayashi, J. Am. Chem. Soc., 2005, 127, 54 CrossRef CAS; (e) T. Miura, M. Shimada and M. Murakami, J. Am. Chem. Soc., 2005, 127, 1094 CrossRef CAS PubMed; (f) T. Matsuda, M. Makino and M. Murakami, Angew. Chem., Int. Ed., 2005, 44, 4608 CrossRef CAS; (g) T. Miura, H. Nakazawa and M. Murakami, Chem. Commun., 2005, 2855 RSC; (h) T. Miura, Y. Takahashi and Y. Murakami, Org. Lett., 2007, 9, 5075 CrossRef CAS; (i) J. Song, Q. Shen, F. Xu and X. Lu, Org. Lett., 2007, 9, 2947 CrossRef CAS; (j) H. Tsukamoto, T. Suzuki, T. Uchiyama and Y. Kondo, Tetrahedron Lett., 2008, 49, 4174 CrossRef CAS; (k) X. Han and X. Lu, Org. Lett., 2010, 12, 108 CrossRef CAS; (l) K. Shen, X. Han and X. Lu, Org. Lett., 2012, 14, 1756 CrossRef CAS; (m) K. W. Shimkin and J. Motagomery, J. Am. Chem. Soc., 2018, 140, 7074 CrossRef CAS.
- (a) T. Miura, T. Toyoshima, Y. Takahashi and M. Murakami, Org. Lett., 2008, 10, 4887 CrossRef CAS; (b) H. Tsukamoto, T. Ueno and Y. Kondo, Org. Lett., 2007, 9, 3033 CrossRef CAS.
- (a) M. H. Babu, V. Dwivedi, R. Kant and M. S. Reddy, Angew. Chem., Int. Ed., 2015, 54, 3783 CrossRef; (b) V. Dwivedi, M. H. Babu, R. Kant and M. S. Reddy, Chem. Commun., 2015, 51, 14996 RSC; (c) M. Rajesh, S. Puri, R. Kant and M. S. Reddy, Org. Lett., 2016, 18, 4332 CrossRef CAS; (d) M. Rajesh, N. Thirupathi, T. J. Reddy, S. Kanojiya and M. S. Reddy, J. Org. Chem., 2015, 80, 12311 CrossRef CAS PubMed; (e) N. Thirupathi, S. Puri, R. Kant and M. S. Reddy, Adv. Synth. Catal., 2016, 358, 303 CrossRef CAS; (f) M. Rajesh, S. Puri, R. Kant and M. S. Reddy, J. Org. Chem., 2017, 82, 5169 CrossRef CAS PubMed; (g) N. Thirupathi, M. H. Babu, V. Dwivedi and M. S. Reddy, Org. Lett., 2014, 16, 2908 CrossRef CAS PubMed; (h) N. Thirupathi, Y. K. Kumar, R. Kant and M. S. Reddy, Adv. Synth. Catal., 2014, 356, 1823 CrossRef CAS.
- (a) Y. Zhang, Z. Yang, L. Guo, W. Li, X. Cheng, X. Wang, Q. Wang, L. Hai and Y. Wu, Org. Chem. Front., 2018, 5, 1604 RSC; (b) M. Chrzanowska, A. Grajewska and M. D. Rozwadowska, Chem. Rev., 2016, 116, 12369 CrossRef CAS; (c) M. Wan, H. Lou and L. Liu, Chem. Commun., 2015, 51, 13953 RSC; (d) P. Giri and G. S. Kumar, Mini-Rev. Med. Chem., 2010, 10, 568 CrossRef CAS; (e) M. Chrzanowska and M. D. Rozwadowska, Chem. Rev., 2004, 104, 3341 CrossRef CAS; (f) E. Prade, C. Barucker, R. Sarkar, G. Althoff-Ospelt, D. A. Lopez, M. Juan, S. Hossain, Y. Zhong, G. Multhaup and B. Reif, Biochemistry, 2016, 55, 1839 CrossRef CAS; (g) J. Recio, A. Perez-Redondo, J. Alverez-Builla and C. Burgos, Org. Chem. Front., 2019, 6, 3300 RSC; (h) B. G. Das, A. Chirila, M. Tromp, J. N. H. Reek and B. D. Bruin, J. Am. Chem. Soc., 2016, 138, 8968 CrossRef CAS; (i) A. P. Femia, P. V. Soares, C. Luceri, M. Lodovici, A. Giannini and G. Caderni, BMC Cancer, 2015, 15, 1 CrossRef; (j) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS; (k) Z. Feng, Z. Yuan, X. Zhao, Y. Huang and H. Yao, Org. Chem. Front., 2019, 6, 3535 RSC.
- S. P. Park, S.-H. Ahn and K.-J. Lee, Tetrahedron, 2010, 66, 3490 CrossRef CAS.
- (a) C. R. Reddy, K. S. Prajapati and R. Rajan, Org. Lett., 2018, 10, 3128 CrossRef; (b) D. E. Shaw, F. Baig, I. Bruce, S. Chamoin, S. P. Collingwood, S. Cross, S. Dayal, P. Drückes, P. Furet and V. Furminger, et al. , J. Med. Chem., 2016, 59, 7901 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qo01266d |
| This journal is © the Partner Organisations 2020 |