Metal-free insertion of sulfur dioxide with aryl iodides under ultraviolet irradiation: direct access to sulfonated cyclic compounds†
Shengqing
Ye‡
a,
Kaida
Zhou‡
b,
Pornchai
Rojsitthisak
 c and
Jie
Wu
c and
Jie
Wu
 *ad
*ad
aSchool of Pharmaceutical and Materials Engineering & Institute for Advanced Studies, Taizhou University, 1139 Shifu Avenue, Taizhou 318000, China. E-mail: jie_wu@fudan.edu.cn
bDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai 200438, China
cDepartment of Food and Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Chulalongkorn University, 254 Phayathai Road, Patumwan, Bangkok 10330, Thailand
dState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China
First published on 6th November 2019
Abstract
Metal-free insertion of sulfur dioxide with aryl iodides and silyl enolates or allylic bromide under ultraviolet irradiation at room temperature is accomplished. This protocol provides a convenient route to sulfonated cyclic compounds under mild conditions. Not only N-(2-iodophenyl)-N-methylmethacrylamides but also 1-iodo-2-allenoxybenzene is workable. A plausible mechanism is proposed, which shows that during the reaction process, aryl radicals formed in situ from aryl iodides under ultraviolet irradiation undergo intramolecular 5-exo-cyclization, with subsequent sulfonylation via insertion of sulfur dioxide. The resulting sulfonyl radicals are further trapped by silyl enolates or allylic bromide giving rise to sulfonated cyclic compounds.
As part of a program for the generation of sulfonyl-containing drugs and related compounds,1 we are interested in method development with the insertion of sulfur dioxide by using the sulfur dioxide surrogates of DABCO·(SO2)2 (1,4-diazabicyclo[2.2.2]octane-sulfur dioxide) or potassium/sodium metabisulfite.2,3 Recently, we focused on the radical processes with the insertion of sulfur dioxide, due to the advantages of mild conditions and excellent selectivity.4 So far, various radical precursors have been utilized in the transformation, including aryldiazonium tetrafluoroborates, arylhydrazines, aryl iodides/bromides, diaryliodonium salts, 4-substituted Hantzsch esters, and potassium alkyltrifluoroborates.4 Among these compounds, aryl iodides are attractive, as they are cheap and easily available.
Recently, rapid progress in the photoinduced reactions of aryl iodides under visible light or ultraviolet irradiation has been witnessed.5,6 In some cases, the transformations could proceed under metal-free conditions under ultraviolet irradiation.7 For instance, Larionov and co-workers described the synthesis of arylboronic acids through a metal- and additive-free, photoinduced borylation of haloarenes under ultraviolet irradiation.7a Encouraged by these results, we conceived that sulfonylation of aryl halides with the insertion of sulfur dioxide under photoinduced metal-free conditions would be feasible as well. Considering the importance of heterocycles and sulfonyl compounds in pharmaceutical and agrochemical molecules8 and prompted by the recent advances in the metal-free coupling of aryl halides under ultraviolet irradiation,7 we conceived that the construction of sulfonated cyclic compounds might be achieved via a tandem process through the insertion of sulfur dioxide from aryl iodides under photoinduced metal-free conditions. A proposed synthetic route is designed and presented in Scheme 1.
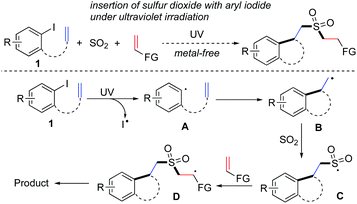 | ||
| Scheme 1 Synthesis of sulfonated cyclic compounds via a tandem process through the insertion of sulfur dioxide under photoinduced metal-free conditions. | ||
We envisioned that the C–I bond in compound 1 would be dissociated under ultraviolet irradiation, thus providing aryl radical intermediate A. Consequently, an intramolecular 5-exo radical cyclization and subsequent insertion of sulfur dioxide would occur leading to sulfonyl radical C. Functionalized alkene was designed to trap the resulting sulfonyl radical, affording another carbon radical intermediate D. This carbon radical intermediate D would undergo further transformation giving rise to the final outcome. Although this proposed route seemed feasible under suitable conditions, several competitive pathways involving the direct sulfonylation of aryl halide might be inevitable. Additionally, the resulting aryl radical or sulfonyl radical would be possibly captured by another alkene as well. To verify the practicability of this hypothesis in Scheme 1, we therefore started to explore the feasibility for the synthesis of sulfonated cyclic compounds via a tandem process through the insertion of sulfur dioxide under photoinduced metal-free conditions.
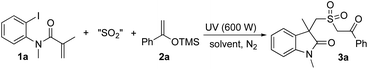
At the outset, the reaction of N-(2-iodophenyl)-N-methylmethacrylamide 1a, DABCO·(SO2)2, and trimethyl((1-phenylvinyl)oxy)silane 2a was selected for method development. At the beginning, the reaction was performed in MeCN at room temperature under ultraviolet irradiation (600 W, Table 1, entry 1). To our delight, the corresponding product 3a was generated in 26% yield. However, further screening of solvents could not provide an improved result. The yield was decreased to 6% when the reaction occurred in 1,4-dioxane (Table 1, entry 2). No reaction took place when THF or DMSO was used instead (Table 1, entries 3 and 4). Only a trace amount of the product was observed when the reaction was performed in DMF (Table 1, entry 5). The desired product 3a was afforded in 13% yield when toluene was utilized as the solvent (Table 1, entry 6). We also explored the reaction by using potassium metabisulfite or sodium metabisulfite as the source of sulfur dioxide (Table 1, entries 7 and 8). However, the results were inferior. With the addition of tetrabutylammonium iodide (TBAI) in the reaction system, the yield was increased to 45% (Table 1, entry 9). Changing the concentration of the mixture would result in the formation of compound 3a with higher yields (Table 1, entries 10 and 11). No better results were obtained when other additives such as TBAB, KI (Table 1, entries 12 and 13), NaI and I2 (data not shown in Table 1) were used. Although the exact role of TBAI in this reaction was not clear, the presence of TBAI would improve the solubility of DABCO·(SO2)2 and thus increase the transmission rate. The reaction was hampered when the light conditions were changed to white 65 W LED light irradiation (Table 1, entry 14). Additionally, only a trace amount of the product was observed when aryl bromide was used as a replacement.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Solvent | Additive | “SO2” | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: N-(2-Iodophenyl)-N-methylmethacrylamide 1a (0.2 mmol), DABCO·(SO2)2 (0.2 mmol), trimethyl((1-phenylvinyl)oxy)silane 2a (0.3 mmol), solvent (4.0 mL), rt, N2. b Isolated yield based on N-(2-iodophenyl)-N-methylmethacrylamide 1a. c In the presence of MeCN (6.0 mL). d In the presence of MeCN (8.0 mL). e Under irradiation with white 65 W LED light. | ||||
| 1 | MeCN | DABCO·(SO2)2 | 26 | |
| 2 | 1,4-Dioxane | DABCO·(SO2)2 | 6 | |
| 3 | THF | DABCO·(SO2)2 | NR | |
| 4 | DMSO | DABCO·(SO2)2 | NR | |
| 5 | DMF | DABCO·(SO2)2 | Trace | |
| 6 | Toluene | DABCO·(SO2)2 | 13 | |
| 7 | MeCN | K2S2O5 | 17 | |
| 8 | MeCN | Na2S2O5 | 19 | |
| 9 | MeCN | TBAI | DABCO·(SO2)2 | 45 |
| 10c | MeCN | TBAI | DABCO·(SO2)2 | 64 |
| 11d | MeCN | TBAI | DABCO·(SO2)2 | 81 |
| 12d | MeCN | TBAB | DABCO·(SO2)2 | 31 |
| 13d | MeCN | KI | DABCO·(SO2)2 | 21 |
| 14e | MeCN | TBAI | DABCO·(SO2)2 | NR |
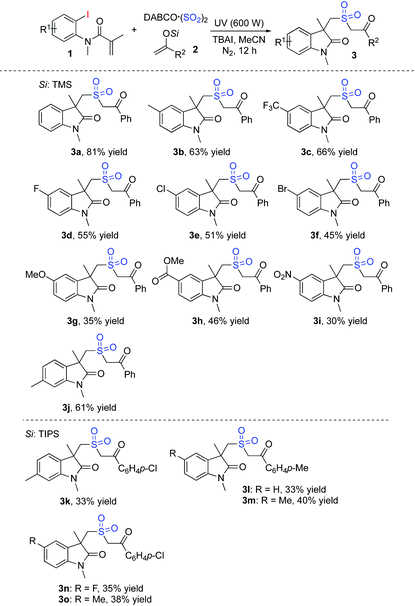
To explore the scope generality of this method, the metal-free reaction of (2-iodophenyl)-N-methylmethacrylamides 1, DABCO·(SO2)2 and silyl enolates under the above optimized conditions was then examined. As shown in Table 2, various (2-iodophenyl)-N-methylmethacrylamides 1 were workable with the insertion of sulfur dioxide, leading to the corresponding products. Although different functional groups were compatible under ultraviolet irradiation, the yields were not satisfactory in most cases. For instance, the ester-substituted product 3h was afforded in 46% yield, and the nitro-containing product 3i was produced in 30% yield. Although these results were not as good as expected, they also provided a convenient route to sulfonated cyclic compounds under mild conditions. The structure of compound 3b was confirmed by X-ray diffraction analysis (see the ESI†),9 which showed excellent chemoselectivity during the reaction process.
| a Isolated yield based on N-(2-iodoaryl)acrylamides 1. |
|---|
|
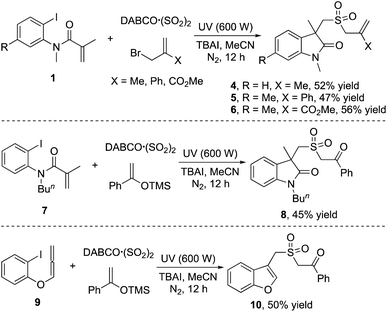
We further explored the reaction of (2-iodophenyl)-N-methylmethacrylamides 1, DABCO·(SO2)2 and allylic bromide under the standard conditions (Scheme 2). Interestingly, the corresponding products 4, 5 and 6 were afforded in 52%, 47% and 56% yields, respectively. Furthermore, the reaction of N-butyl-N-(2-iodophenyl)methacrylamide 7 with DABCO·(SO2)2 and trimethyl((1-phenylvinyl)oxy)silane 2a was examined, which afforded the corresponding 1-butyl-3-methyl-3-(((2-oxo-2-phenylethyl)sulfonyl)methyl)indolin-2-one 8 in 45% yield. However, no reaction occurred when N-benzyl-N-(2-iodophenyl)methacrylamide was employed under the conditions (data not shown in Scheme 2). Interestingly, 1-iodo-2-allenoxybenzene 9 could be used as a replacement for (2-iodophenyl)-N-methylmethacrylamide 1a in the reaction of DABCO·(SO2)2 and trimethyl((1-phenylvinyl)oxy)silane. This transformation was effective, giving rise to the expected benzofuran 10 in 50% yield (Scheme 2). Since we hypothesized that this route might be a radical process, we then examined the model reaction in Table 1 with the addition of 3.0 equivalents of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) under the standard conditions (data not shown in Scheme 2). Consequently, only a trace amount of the product 3a was observed. Additionally, the related product could be obtained in 31% yield (determined by NMR) when ethene-1,1-diyldibenzene was used as the radical trapper.
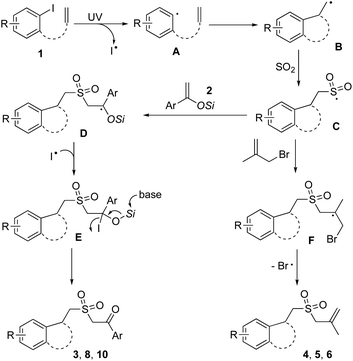
As suggested by the previous reports7 and the above experimental results, we postulated a plausible route for the outcomes, as shown in Scheme 3. We reasoned that under ultraviolet irradiation, aryl radical A would be formed from aryl iodide via the cleavage of the C–I bond, which prefers to undergo intramolecular 5-exo-cyclization leading to alkyl radical intermediate B. Subsequently, sulfonylation would occur through the insertion of sulfur dioxide to provide sulfonyl radical intermediate C. In the presence of silyl enolate or allylic bromide, sulfonyl radical C would be trapped by the double bond, giving rise to intermediate D or F respectively. Radical intermediate D would then couple with the iodo radical, affording compound E. However, other possible routes could not be excluded. For example, intermediate E would be formed by trapping intermediate D with iodine generated in situ from two iodo radicals. Alternatively, in the presence of iodine, intermediate D would be oxidized to a cation. This cation would be subsequently trapped with iodide to afford compound E. Further desilylation would take place to produce the corresponding product 3, 8 or 10. For the radical intermediate F, the sulfonated cyclic compound 4–6 would be furnished with the release of a bromo radical.
In conclusion, we have reported a metal-free process for the insertion of sulfur dioxide with aryl iodides and silyl enolates or allylic bromide under ultraviolet irradiation at room temperature. This protocol provides a convenient route to sulfonated cyclic compounds under mild conditions. Not only N-(2-iodophenyl)-N-methylmethacrylamides but also 1-iodo-2-allenoxybenzene is workable. This pathway is highly selective, and several competitive routes are not observed. A plausible mechanism is proposed, which shows that during the reaction process, aryl radicals formed in situ from aryl iodides under ultraviolet irradiation undergo intramolecular 5-exo-cyclization, with subsequent sulfonylation via insertion of sulfur dioxide. The resulting sulfonyl radicals are further trapped by silyl enolates or allylic bromide giving rise to sulfonated cyclic compounds.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21672037 and 21532001) is gratefully acknowledged.Notes and references
- (a) Y. Harrak, G. Casula, J. Basset, G. Rosell, S. Plescia, D. Raffa, M. G. Cusimano, R. Pouplana and M. D. Pujol, J. Med. Chem., 2010, 53, 6560 CrossRef CAS; (b) D. A. Smith and R. M. Jones, Curr. Opin. Drug Discovery Dev., 2008, 11, 72 CAS; (c) T. Guo, Y. Liu, Y.-H. Zhao, P.-K. Zhang, S.-L. Han and H.-M. Liu, Tetrahedron Lett., 2016, 57, 4629 CrossRef CAS; (d) W. Xie, Y. Wu, J. Zhang, Q. Mei, Y. Zhang, N. Zhu, R. Liu and H. Zhang, Eur. J. Med. Chem., 2018, 145, 35 CrossRef CAS; (e) W. Xie, S. Xie, Y. Zhou, X. Tang, J. Liu, W. Yang and M. Qiu, Eur. J. Med. Chem., 2014, 81, 22 CrossRef CAS; (f) W. Xie, H. Zhang, J. He, J. Zhang, Q. Yu, C. Luo and S. Li, Bioorg. Med. Chem. Lett., 2017, 27, 530 CrossRef CAS; (g) T. Guo, Y. Liu, Y.-H. Zhao, P.-K. Zhang, S.-L. Han and H.-M. Liu, Tetrahedron Lett., 2016, 57, 3920 CrossRef CAS; (h) Y. Zheng, M. Liu, G. Qiu, W. Xie and J. Wu, Tetrahedron, 2019, 75, 1663 CrossRef CAS.
- For reviews, see: (a) G. Qiu, K. Zhou, L. Gao and J. Wu, Org. Chem. Front., 2018, 5, 691 RSC; (b) K. Hofman, N. Liu and G. Manolikakes, Chem. – Eur. J., 2018, 24, 11852 CrossRef CAS; (c) D. Zheng and J. Wu, Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017 CrossRef; (d) G. Liu, C. Fan and J. Wu, Org. Biomol. Chem., 2015, 13, 1592 RSC; (e) E. J. Emmett and M. C. Willis, Asian J. Org. Chem., 2015, 4, 602 CrossRef CAS; (f) S. Ye, G. Qiu and J. Wu, Chem. Commun., 2019, 55, 1013 RSC; (g) P. Bisseret and N. Blanchard, Org. Biomol. Chem., 2013, 11, 5393 RSC; (h) G. Qiu, L. Lai, J. Cheng and J. Wu, Chem. Commun., 2018, 54, 10405 RSC; (i) G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561 RSC.
- For recent selected examples, see: (a) Y. Liu, Q. Lin, Z. Xiao, C.-G. Zheng, Y. Guo, Q.-Y. Chen and C. Liu, Chem. – Eur. J., 2019, 25, 1824 CrossRef CAS; (b) Y. Chen, P. R. D. Murray, A. T. Davies and M. C. Willis, J. Am. Chem. Soc., 2018, 140, 8781 CrossRef CAS; (c) D. Sun, K. Yin and R. Zhang, Chem. Commun., 2018, 54, 1335 RSC; (d) Z. Chen, N.-W. Liu, M. Bolte, H. Ren and G. Manolikakes, Green Chem., 2018, 20, 3059 RSC; (e) Q. Lin, Y. Liu, Z. Xiao, L. Zheng, X. Zhou, Y. Guo, Q.-Y. Chen, C. Zheng and C. Liu, Org. Chem. Front., 2019, 6, 447 RSC; (f) X. Y. Qin, L. He, J. Li, W. J. Hao, S. J. Tu and B. Jiang, Chem. Commun., 2019, 55, 3227 RSC; (g) R. J. Reddy, A. H. Kumari, J. J. Kumar and J. B. Nanubolu, Adv. Synth. Catal., 2019, 361, 1587 CrossRef CAS; (h) L. Lu, C. Luo, H. Peng, H. Jiang, M. Lei and B. Yin, Org. Lett., 2019, 21, 2602 CrossRef CAS; (i) X. Wang, M. Yang, W. Xie, X. Fan and J. Wu, Chem. Commun., 2019, 55, 6010 RSC; (j) F.-S. He, X. Gong, P. Rojsitthisak and J. Wu, J. Org. Chem., 2019, 84, 13159 CrossRef CAS PubMed.
- For recent examples, see: (a) X. Gong, M. Wang, S. Ye and J. Wu, Org. Lett., 2019, 21, 1156 CrossRef CAS PubMed; (b) X. Wang, M. Yang, W. Xie, X. Fan and J. Wu, Chem. Commun., 2019, 55, 6010 RSC; (c) S. Ye, D. Zheng, J. Wu and G. Qiu, Chem. Commun., 2019, 55, 2214 RSC; (d) S. Ye, Y. Li, J. Wu and Z. Li, Chem. Commun., 2019, 55, 2489 RSC; (e) X. Gong, X. Li, W. Xie, J. Wu and S. Ye, Org. Chem. Front., 2019, 6, 1863 RSC; (f) J. Zhang, W. Xie, S. Ye and J. Wu, Org. Chem. Front., 2019, 6, 2254 RSC; (g) S. Ye, T. Xiang, X. Li and J. Wu, Org. Chem. Front., 2019, 6, 2183 RSC; (h) S. Ye, X. Li, W. Xie and J. Wu, Asian J. Org. Chem., 2019, 8, 893 CrossRef CAS; (i) S. Ye, X. Li, W. Xie and J. Wu, Eur. J. Org. Chem., 2019 DOI:10.1002/ejoc.201900396; (j) J. Zhang, X. Li, W. Xie, S. Ye and J. Wu, Org. Lett., 2019, 21, 4950 CrossRef CAS; (k) Y. Zong, Y. Lang, M. Yang, X. Li, X. Fan and J. Wu, Org. Lett., 2019, 21, 1935 CrossRef CAS; (l) F.-S. He, Y. Wu, X. Li, H. Xia and J. Wu, Org. Chem. Front., 2019, 6, 1873 RSC.
- For selected examples, see: (a) S. E. Creutz, K. J. Lotito, G. C. Fu and J. C. Peters, Science, 2012, 338, 647 CrossRef CAS; (b) D. T. Ziegler, J. Choi, J. M. Muñoz-Molina, A. C. Bissember, J. C. Petersm and G. C. Fu, J. Am. Chem. Soc., 2013, 135, 13107 CrossRef CAS; (c) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS; (d) Y. C. Tan, J. M. Munoz-Molina, G. C. Fu and J. C. Peters, Chem. Sci., 2014, 5, 2831 RSC; (e) J. A. Terrett, J. D. Cuthbertson, V. W. Shurtleff and D. W. C. MacMillan, Nature, 2015, 524, 330 CrossRef CAS; (f) Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2013, 339, 1593 CrossRef; (g) Z. Zuo, D. T. Ahneman, L. Chu, J. A. Terrett, A. G. Doyle and D. W. C. MacMillan, Science, 2014, 345, 437 CrossRef CAS; (h) X. Gong, J. Chen, X. Li, W. Xie and J. Wu, Chem. – Asian J., 2018, 13, 2543 CrossRef CAS; (i) Z. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu and D. W. C. MacMillan, J. Am. Chem. Soc., 2016, 138, 1832 CrossRef CAS; (j) L. Chu, J. M. Lipshultz and D. W. C. MacMillan, Angew. Chem., Int. Ed., 2015, 54, 7929 CrossRef CAS.
- (a) X. Fu, Y. Meng, X. Li, M. Stepień and P. J. Chmielewski, Chem. Commun., 2018, 54, 2510 RSC; (b) X. Li, Y. Meng, P. Yi, M. Stepień and P. J. Chmielewski, Angew. Chem., Int. Ed., 2017, 56, 10810 CrossRef CAS; (c) B. Liu, T. Yoshida, X. Li, M. Stepień, H. Shinokubo and P. J. Chmielewski, Angew. Chem., Int. Ed., 2016, 55, 13142 CrossRef CAS; (d) Z. Deng, X. Li, M. Stepień and P. J. Chmielewski, Chem. – Eur. J., 2016, 22, 4231 CrossRef CAS; (e) K. Deng, X. Li and H. Huang, Electrochim. Acta, 2016, 204, 84 CrossRef CAS; (f) B. Liu, X. Li, M. Stepień and P. J. Chmielewski, Chem. – Eur. J., 2015, 21, 7790 CrossRef CAS; (g) B. Liu, H. Fang, X. Li, W. Cai, L. Bao, M. Rudolf, F. Plass, L. Fan, X. Lu and D. M. Guldi, Chem. – Eur. J., 2015, 21, 746 CrossRef CAS; (h) B. Liu, X. Li, J. Maciołek, M. Stepień and P. J. Chmielewski, J. Org. Chem., 2014, 79, 3129 CrossRef CAS; (i) B. Liu, X. Li, X. Xu, M. Stepień and P. J. Chmielewski, J. Org. Chem., 2013, 78, 1354 CrossRef CAS; (j) X. Li, B. Liu, P. J. Chmielewski and X. Xu, J. Org. Chem., 2012, 77, 8206 CrossRef CAS; (k) X. Li, B. Liu, X. Yu, P. Yi, R. Yi and P. J. Chmielewski, J. Org. Chem., 2012, 77, 2431 CrossRef CAS; (l) X. Li, B. Liu, P. Yi, R. Yi, X. Yu and P. J. Chmielewski, J. Org. Chem., 2011, 76, 2345 CrossRef CAS.
- (a) A. M. Mfuh, J. D. Doyle, B. Chhetri, H. D. Arman and O. V. Larionov, J. Am. Chem. Soc., 2016, 138, 2985 CrossRef CAS; (b) G. Qiu, Y. Li and J. Wu, Org. Chem. Front., 2016, 3, 1011 RSC; (c) W. Liu, L. Li and C.-J. Li, Nat. Commun., 2015, 6, 6526 CrossRef CAS; (d) L. Li, W. Liu, H. Zeng, X. Mu, G. Cosa, Z. Mi and C.-J. Li, J. Am. Chem. Soc., 2015, 137, 8328 CrossRef CAS; (e) Y. Li, D. Zheng, Z. Li and J. Wu, Org. Chem. Front., 2016, 3, 574 RSC; (f) X. Gong, Y. Ding, X. Fan and J. Wu, Adv. Synth. Catal., 2017, 359, 2999 CrossRef CAS; (g) P. Kamal Walia, M. Kumar and V. Bhalla, ACS Omega, 2018, 3, 1983 CrossRef; (h) X. Yang, W. Liu, L. Li, W. Wei and C.-J. Li, Chem. – Eur. J., 2016, 22, 15252 CrossRef CAS.
- (a) C. Wu, L.-H. Lu, A.-Z. Peng, G.-K. Jia, C. Peng, Z. Cao, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20, 3683 RSC; (b) L.-H. Lu, S.-J. Zhou, M. Sun, J.-L. Chen, W. Xia, X. Yu, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2019, 7, 1574 CrossRef CAS; (c) K.-J. Liu, S. Jiang, L.-H. Lu, L.-L. Tang, S.-S. Tang, H.-S. Tang, Z. Tang, W.-M. He and X. Xu, Green Chem., 2018, 20, 3038 RSC; (d) L.-Y. Xie, S. Peng, J.-X. Tan, R.-X. Sun, X. Yu, N.-N. Dai, Z.-L. Tang, X. Xu and W.-M. He, ACS Sustainable Chem. Eng., 2018, 6, 16976 CrossRef CAS; (e) L.-Y. Xie, S. Peng, L.-L. Jiang, X. Peng, W. Xia, X. Yu, X.-X. Wang, Z. Cao and W.-M. He, Org. Chem. Front., 2019, 6, 167 RSC; (f) L.-Y. Xie, S. Peng, F. Liu, G.-R. Chen, W. Xia, X. Yu, W.-F. Li, Z. Cao and W.-M. He, Org. Chem. Front., 2018, 5, 2604 RSC.
- CCDC 1938512 contains the supplementary crystallographic data (compound 3b) for this paper.†.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1938512. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qo01274e |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2020 |