Open Access Article
Open Access ArticleIn situ synthesis of stretchable and highly stable multi-color carbon-dots/polyurethane composite films for light-emitting devices†
Fei Lianad,
Chuanxi Wang *a,
Qian Wub,
Minghui Yang*c,
Zhenyu Wang
*a,
Qian Wub,
Minghui Yang*c,
Zhenyu Wang a and
Chi Zhang
a and
Chi Zhang *be
*be
aInstitute of Environmental Processes and Pollution Control, School of Environmental and Civil Engineering, Jiangnan University, Wuxi, 214122, China. E-mail: wangcx2018@jiangnan.edu.cn
bInternational Joint Research Center for Photoresponsive Molecules and Materials, School of Chemical & Material Engineering, Jiangnan University, Wuxi 214122, P. R. China
cInstitute of New Energy Technology, Ningbo Institute of Industrial Technology, Chinese Academy of Sciences, Ningbo, 315201, P. R. China. E-mail: myang@nimte.ac.cn
dAgro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin 300191, China
eSchool of Chemical Science and Engineering, Tongji University, 1239 Siping Road, Shanghai 200092, P. R. China. E-mail: chizhang@tongji.edu.cn
First published on 7th January 2020
Abstract
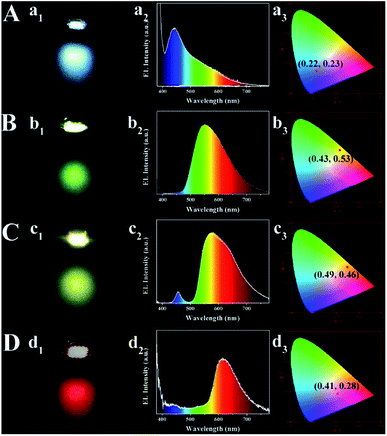
Multi-color-emissive fluorescent polymer nanocomposite films have potential applications in optoelectronic devices. Herein, stretchable, mechanically stable multi-color carbon-dots-based films are in situ fabricated by condensation and aging of carboxylated polyurethane in the presence of various carbon sources. As-prepared CDs/PU films emit different colors covering from blue (414 nm) to red (620 nm) by tuning reaction conditions. Moreover, CDs are fixed and have good dispersion in the PU matrix due to the interactions of amine groups from the carbon sources with the carboxylate group of PU. Thus, phase separation of composite films can be avoided. And, more than 90% of their emission intensity is preserved after soaking in water for 30 days, aging for up to 6 h at 100 °C, and subjecting to several cycles of stretching and natural recovery. These advantages are encouraging for the use of CDs/PU composite films in solid-state lighting applications. Remote multi-color LEDs have been fabricated by placing a down-conversion layer of CDs/PU films separated through coating them on the same chips (emission at 365 nm), with Commission Internationale de l'Eclairage color coordinates of (0.22, 0.23), (0.43, 0.53), (0.49, 0.46), and (0.41, 0.28), respectively.
Introduction
The development of phosphor-based light emitting diodes (LEDs) has been the subject of intense academic research for years, since LEDs achieve potential applications in multi-color display, low-cost back-lighting in liquid-crystal displays and next generation lighting sources for our daily life.1–3 To date, great progress has been made to design and fabricate high-performance phosphor-based LEDs.4,5 And various fluorescent nanomaterials including the rare earth nanomaterials,6 semiconductor quantum dots (CdSe, CdTe, and PbTe),7,8 perovskite nanocrystals (MAPbX3 or CsPbX3, MA = CH3NH3+, X = Cl, Br, I)9,10 and metal nanoclusters,11 have been considered as promising phosphors for LEDs due to high photoluminescence quantum yields (PLQYs), narrow full-width at haft-maximum and easily tunable band gap. However, rare earth-based phosphors are generally synthesized from expensive raw materials at high reaction temperature, which are detrimental to cost saving. The Cd and Pb-containing QDs/nanocrystals have severe toxicity. The nanoclusters and perovskite nanocrystals show poor stability. These problems have greatly hindered their practical application in LEDs.Carbon dots (CDs) as a novel luminescent material possess a number of distinct merits such as tunable and stable fluorescence, low cost, and especially for being environment-friendly, which appear to be an ideal alternative to Cd2+/Pb2+-based semiconductor QDs in LEDs.12–16 However, the aggregation-caused quenching of themselves limited the development of CDs-based solid-state phosphors. It should be noted that immobilizing CDs in solid matrix is a suitable way to overcome their emission quenching in solid state.17–19 For example, Zhou et al. prepared CDs@BaSO4 hybrid phosphors by assembling Ba2+ and SO42− onto the surface of CDs through electrostatic attraction, in which the CDs as the luminescence center.18 Rogach et al. reported CDs hybrids with polyhedral oligomeric silsesquioxane (POSS) as solid-state luminophore for white LEDs.19 Compared with BaSO4 and POSS, polymers-based nanocomposites are softness, machinability and suitable for roll-to-roll production.20,21 Our recent report confirmed poly-vinyl-alcohol (PVA) matrix prevent the aggregation-caused quenching of CDs and full-color CDs/PVA phosphors showed bright fluorescence, which was proved to be promising candidates for alternative light sources of LEDs.22 Moreover, Yang's group also found similar results and they reported full-color CDs/PVA phosphors for LEDs applications.23 Qu et al. prepared full-color inorganic CDs/polymers phosphors for white LEDs.24 However, most of these approaches require dispersion of pre-synthesized CDs powders within polymers matrix, which imposes several limitations, such as poor compatibility of components leading to undesirable phase separation and reabsorption of light. Therefore, it would be of great interest to obtain highly stable CDs/polymers phosphors by a straightforward and effective method. Thereby, new effective methods are highly desirable.
Herein, the one pot in situ technique is designed to prepare multi-color carbon-dots/polyurethane (CDs/PU) phosphors for LEDs. In this process, o-phenylenediamine (o-PD), m-phenylenediamine (m-PD), and 1,2,4-triphenylamine (1,2,4-3TD) were selected as carbon sources, respectively. The amino groups of them can react with carbonyl group of the water-borne polyurethane during the formation process of CDs. Thus, the CDs and PU form a uniform and stable unit, and phase separation would disappear. Moreover, as-prepared CDs/PU phosphors show high mechanically stable and stretchable. Besides, by changing various carbon precursors with different structures, multi-color emissive CDs/PU phosphors can be prepared and the emission wavelength is fine-tuned with the changes of operating temperature to obtain the full-color CDs/PU phosphors. The final in situ generated CDs/PU phosphors can be loaded on a UV LED chip to obtain blue, green, yellow, and red color LEDs.
Experimental
Materials
Analytical reagent grade of o-, m-phenylenediamines (o-, m-PDs), and acetone were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Analytical reagent of 1,2,4-triaminobenzene (1,2,4-3TD) was purchased from Shanghai Zaiqi Bio-Tech (China) Co, Ltd. Waterborne polyurethane (PU) was purchased from Shenzhen Jinxiu Waterproof Material Co., Ltd. (Guangdong, China). All chemical reagents were used as received without further purification. Deionized (DI) water was used throughout this study.Instruments
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were performed on a JEOL-2100 microscope (JEOL, Japan) at 200 kV. Fourier transform infrared (FT-IR) spectra were collected in the range from 4000 to 400 cm−1 on Agilent Cary660. Light transmittance spectra were carried out using the UV-vis spectrophotometer (Hitachi U-3900 Japan). X-ray photoelectron spectroscopy (XPS) were realized on AXIS ULTRA DLD (Shimadzu, Japan) spectrometer with Al Kα excitation (1486.6 eV). Binding energy calibration was based on C 1s at 284.8 eV. The emission and excitation spectra were measured on a FloroMax-4 spectrofluorimeter (HORIBA, France) at ambient conditions. PLQYs are measured on a QE-2100 quantum efficiency measurement system (Otsuka Electronics, Japan). Fluorescent lifetime was measured using Jobin-Yvon Spex Fluorolog 3-11 (HORIBA, France). Thermal gravimetric analysis (TGA) was performed on a Pyris Diamond TG/DTA instrument (PerkinElmer, USA) under the nitrogen gas atmosphere, by heating up to 500 °C with a heating rate of 10 °C min−1.In situ preparation of multi-color CDs/PU films
Fabrication of LEDs
Commercially available GaN LED chips (the emission centered at 365 nm) without phosphor coating are purchased from Advanced Optoelectronic Technology Inc. Then, the blue light-emitting CDs/PU films were fastened to the top of the GaN LED chip to achieve the fabrication of blue LEDs. The green LEDs, yellow LEDs, red LED are also prepared through the above method.Results and discussion
The one pot in situ synthesis of CDs/PU films was shown in Fig. 1. The o-, m-PDs and 1,2,4-3TDs were selected as carbon sources, respectively. These carbon sources have conjugated structures and heterogeneous N atoms, which is beneficial for preparing long-wavelength CDs.25,26 Then these carbon sources were mixed with PU together at room temperature. Then, the CDs can be in situ synthesized in PU matrix during increasing temperature and form CDs/PU composite films after aging. Through adjusting the carbon sources and reaction temperature, full-color and uniform CDs/PU composite films can be obtained. The m-PDs, o-PDs and 1,2,4-3TDs have been confirmed to prepare blue, yellow and red fluorescent CDs.27,28 Therefore, the m-PDs and 1,2,4-3TDs are mixed with PU for forming blue-emitting and red-emitting CDs/PU composite films, respectively. Moreover, the o-PDs can be used for preparing green-emitting and yellow-emitting CDs/PU composite films by changing reaction temperature. On the other hand, PU itself had good mechanical, thermal, and transparent property, which made resultant CDs/PU composite films show strong fluorescence, and high flexibility and stability as shown in Fig. 1.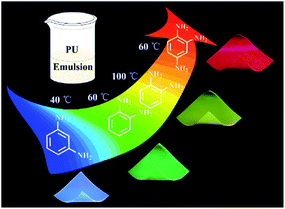 | ||
| Fig. 1 Schematic diagram of in situ synthesis of multi-color carbon dots/polyurethane composite films. | ||
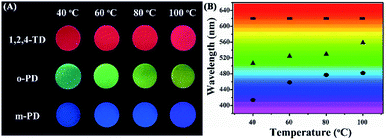
Fig. 2A shows the optical images of CDs/PU composite films prepared from different carbon sources under various temperatures. They emit different colors covering from blue to red emission indicating that the emission of CDs can be tuned by reaction conditions. Fig. 2B shows that the maximum emission peaks of the CDs/PU composite films vary with the reaction conditions such as carbon sources and reaction temperatures. It can be seen that the full color CDs/PU composite films can be formed through reaction conditions. In detail, it is noted that CDs/PU composite films prepared from m-PDs show blue-emitting and their emission peaks shift from 414 to 482 nm by increasing temperature from 40 to 100 °C (Fig. S1A† and 2B). Besides, the emission peaks of CDs/PU composite films prepared from o-PDs also showed red shift with increasing temperatures, which can be tunable from 507 to 560 nm (Fig. S1B† and 2B). As previous reports, the fluorescence of CDs depended on their graphitization, size and surface functionalization.20,29 In PU matrix, the surface functionalization of CDs prepared from o-PDs and m-PDs, is similar. Thus, the red shift of their emission peaks came from various graphitization causing different reaction temperatures. However, the emission peaks of CDs/PU composite films prepared from 1,2,4-3TDs are in red region (Fig. S1C† and 2B) and do not change with various temperatures since their surface property was fixed in PU matrix and no longer change with various temperatures. Herein, to investigate the structure and optical property of CDs/PU composite films, we selected blue emitting (prepared by m-PDs at 40 °C), green emitting (prepared by o-PDs at 60 °C), yellow emitting (prepared by o-PDs at 100 °C) and red emitting (prepared by 1,2,4-3TDs at 60 °C) CDs/PU composite. Their emission centers are 420, 530, 560 and 620 nm, and thus they are defended as B-, G-, Y- and R-CDs/PU films, respectively. Moreover, their PLQYs are 2.02%, 2.02%, 1.72% and 1.09% measured on a QE-2100 quantum efficiency measurement system. Their detailed optical properties are summarized in Table S1.†
CDs have various surface chemistry property and size distribution, thus they usually exhibit excitation dependent emission.30,31 However, the excitation independent emission of CDs prepared from aromatic compounds was usually observed since they had identical absorption structures and luminescent centers.27,28,32 For example, Lin et al. reported excitation independent blue-emitting and green-emitting CDs prepared from m-PDs and o-PDs, respectively;28 our recent report confirmed wavelength-independent fluorescence property of the yellow-emitting CDs prepared from o-PDs.27 Similar excitation independent behaviour is also observed in our selected samples. As shown in Fig. 3 and S2,† when changing the excitation wavelengths, the emission wavelengths of B-, G-, Y- and R-CDs/PU films did not shift. The amino groups of carbon source can react with carbonyl group of the water-borne polyurethane during increasing temperature. Thus, the CDs were fixed in the PU matrix. So surface chemistry property of CDs was same and their size distribution is narrow (Fig. 4). Thus, as-prepared CDs/PU showed excitation-independent PL. The optical uniformity of these samples facilitated a deep investigation of their luminescence mechanism based on a comparison of their compositions and structures.
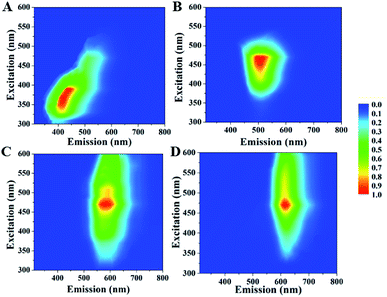 | ||
| Fig. 3 Excitation–emission properties for B-, G-, Y- and R-CDs/PU films. (A) B-CDs/PU films; (B) G-CDs/PU films; (C) Y-CDs/PU films; (D) R-CDs/PU films. | ||
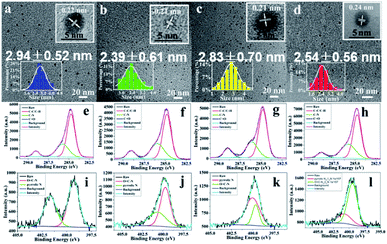
The transmission electron microscopy (TEM) images presented in Fig. 4a–d reveal that the as-fabricated composite films contain 1.5–4.0 nm CDs with good dispersion in PU matrix. The four kinds of CDs in CDs/PU films possess very similar particle size. The average particle sizes are 2.94 ± 0.52, 2.39 ± 0.61, 2.83 ± 0.70, and 2.54 ± 0.56 nm for B-, G-, Y- and R-CDs/PU films, respectively. Their size distribution is narrow (inset of Fig. 2a–d), which is one of reasons for their excitation independent emission. The high-resolution (HR) TEM images provided in the insets show that all of the CDs exhibited identical well-resolved lattice fringes with a spacing of 0.21–0.24 nm, corresponding to the (100) in-plane lattice of graphene.29,32,33
The Fourier transform infrared (FTIR) spectra shown in Fig. S3† reveal that all CDs/PU samples possessed same groups such as N–H (3350 cm−1), C–N (1730 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1457 cm−1) and C–O–C (1090 cm−1).32,34 The CDs are embedded in the PU matrix and the groups of carbon sources are overlapping with PU, thus it is hard to observe the difference of all CDs/PU samples in FTIR spectra. To further investigate the composition and valence state of these samples, X-ray photoelectron spectroscopy (XPS) was employed to characterize the all CDs/PU samples. The HR-XPS spectra presented in Fig. S4† and 4 show three typical peaks: C 1s (285 eV), N 1s (400 eV), and O 1s (531 eV). These findings indicated that the samples consisted of the same elements. In the HR-XPS spectra (Fig. 4e–l), the C 1s band can be deconvoluted into three peaks, corresponding to sp2 carbons (C–C, 284.7 eV), sp3 carbons (C–O/C–N, 285.8 eV), and carboxyl carbons (COOH, 289.0 eV).32 The N 1s band can be deconvoluted into two peaks at 399.3 and 400.8 eV, representing amino N and pyrrolic N, respectively.32 Moreover, XPS analysis reveals (Table S2†) that with the shift of blue emitting to red emitting, the C
O (1457 cm−1) and C–O–C (1090 cm−1).32,34 The CDs are embedded in the PU matrix and the groups of carbon sources are overlapping with PU, thus it is hard to observe the difference of all CDs/PU samples in FTIR spectra. To further investigate the composition and valence state of these samples, X-ray photoelectron spectroscopy (XPS) was employed to characterize the all CDs/PU samples. The HR-XPS spectra presented in Fig. S4† and 4 show three typical peaks: C 1s (285 eV), N 1s (400 eV), and O 1s (531 eV). These findings indicated that the samples consisted of the same elements. In the HR-XPS spectra (Fig. 4e–l), the C 1s band can be deconvoluted into three peaks, corresponding to sp2 carbons (C–C, 284.7 eV), sp3 carbons (C–O/C–N, 285.8 eV), and carboxyl carbons (COOH, 289.0 eV).32 The N 1s band can be deconvoluted into two peaks at 399.3 and 400.8 eV, representing amino N and pyrrolic N, respectively.32 Moreover, XPS analysis reveals (Table S2†) that with the shift of blue emitting to red emitting, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N content of CDs/PU samples increases significantly. The heterogeneous N atom doping would make the band gap be narrow, thus the emission would shift long wavelength.25,26,35 Indeed, most reported CDs with red emission are prepared based this way.16,26,28 However, as shown in Table S2,† no difference of C
N content of CDs/PU samples increases significantly. The heterogeneous N atom doping would make the band gap be narrow, thus the emission would shift long wavelength.25,26,35 Indeed, most reported CDs with red emission are prepared based this way.16,26,28 However, as shown in Table S2,† no difference of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N content is observed between G-CDs/PU and Y-CDs/PU films. We argue the reason for this shift is from increasing size (Fig. 4b and c). This means with the increase of pyrolysis temperature, the size of CDs became large, and caused red shift. This phenomenon is consist with previous researches.22,32 Therefore, luminescence mechanism of CDs in PU matrix is confuse; both the size and surface chemistry control their emission peak.
N content is observed between G-CDs/PU and Y-CDs/PU films. We argue the reason for this shift is from increasing size (Fig. 4b and c). This means with the increase of pyrolysis temperature, the size of CDs became large, and caused red shift. This phenomenon is consist with previous researches.22,32 Therefore, luminescence mechanism of CDs in PU matrix is confuse; both the size and surface chemistry control their emission peak.
Previous CDs/polymers composite films were usually prepared from pre-synthesized CDs and polymer, and the phase separation would appear.27 Interesting, this newly developed in situ technique can avoid this phenomenon. As shown in Fig. 5A, the green emitting CDs/PU films were soaked in water for long time (30 days), and their fluorescence was still observed and no fluorescence appeared in solution. Thus, as-prepared CDs/PU films showed high water stability since the reaction of carbonyl groups of the water-borne PU and the amino groups of carbon sources. This reaction not only makes the formation of CDs at low temperature but also makes CDs be good dispersion and high stable in the PU matrix. So the CDs were hard to move from the PU matrix and phase separation may not occur. Besides, the thermal stability of CDs/PU films is an important factor for their application. TGA curves taken in a nitrogen atmosphere (Fig. S5†) show the weight loss for the CDs/PU films heated to 200 °C is lower than 10%, which demonstrates their high thermal stability in the range of 30–200 °C. With the temperature increasing over 200 °C, a significant loss of weight is observed, which is caused by the decomposition of PU, as confirmed by the same trend for the TGA curve of the bare PU film shown in the same Fig. S5.† However, the weight loss of bare PU film is faster than CDs/PU films. This result pointed out the improved thermal stability of the CDs/PU films in this temperature range due to the interactions of amine groups from the carbon sources with carboxylate group of PU, where additional amide bonds formed contribute to the higher thermal stability of the composite film. Moreover, the thermal stability of CDs/PU films in air at different temperatures and for a different period of time was investigated. As shown in Fig. S6,† the PL intensity of CDs/PU films showed a minor change when they were thermal treatment in the temperature range from 40 to 160 °C for 1 h. In yet another thermal stability test, CDs/PU films were kept in air at an elevated temperature of 100 °C, and their PL intensity at different time intervals has been recorded as a function of time. As shown in Fig. S7,† there are no major changes in the relative PL intensity of CDs/PU films even after 6 h of treatment, with >90% of the PL intensity preserved. Stretchable and flexible luminescence materials become an extensively investigated topic because they offer the characteristics of luminescence properties and the ability to be stretched into arbitrary shapes.36 Polymer-based composite luminescent films have good mechanical properties and are an important sources of stretchable and flexible materials.20,21 The flexible CDs/PU films have been shown in Fig. 1, since the 3D network was formed by the carboxylated PU. Besides, as shown in Fig. 5B, the relative PL intensity of the CDs/PU films subjected to several cycles of stretching and natural recovery: there are only minor changes observed. Thus, the flexible and stretchable multi-color CDs-based polymer films are prepared.
Previously discussed data show that as-prepared multi-color CDs/PU films synthesized from this one pot in situ methods have many features, including tunable fluorescence, uniform surface, high water and thermal stability, stretchable and flexible films. These advantages are encouraging for the use of CDs/PU composite films in solid-state lighting applications. The monochrome blue, green, yellow and red down-conversion LED devices are prepared by coating CDs/PU films on the same chips (emission at 365 nm) (Fig. 6). Solid LEDs with various color emissions were fabricated with the Commission Internationale de L'Eclairage 1931 (CIE) coordinates of (0.22, 0.23), (0.43, 0.53), (0.49, 0.46), and (0.41, 0.28), respectively. For the performance parameters of blue, green, yellow, and red LEDs, the maximum external quantum efficiencies (EQEs) are determined to be 0.20% (blue LED), and the maximum luminous efficacy of optical radiation is 0.25 lm W−1 (blue LED). The relatively low EQE is attributed to the low quality of the commercially available 365 nm GaN LED chips. The performance of the LED devices is expected to improve further if better LED chips are applied. These results are encouraging for the use of CDs/PU composite films in solid-state lighting applications.
Conclusions
In summary, bright and multi-color CDs/PU films were prepared by a newly developed one pot in situ synthesis methods at low temperature. The carbon sources can be carbonized to form CDs and occur reaction with carbonyl groups of the water-borne PU in polymer matrix simultaneously. Thus, the CDs and PU form a unit, which avoids the phase separation of composite films. Moreover, the emission of CDs/PU films showed good water stability and thermal stability. Besides, the flexible and stretchable properties of CDs/PU films are observed. Furthermore, solid LEDs of various color emission from red to blue have been obtained. Therefore, this work provided a simple and cheap way to prepare mechanically stable and stretchable fluorescent composite film, which showed potential application for flexible solid-state lighting, displays, and other optoelectronic devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51432006, No. 41573127), Zhejiang Provincial Natural Science Foundation of China under Grant No. LY18E030010, Central Public-interest Scientific Institution Basal Research Fund (No. Y2017JC10). Open project of State Key Laboratory of Supramolecular Structure and Materials (sklssm2019026).Notes and references
- X. Wang, H. Tian, M. A. Mohammad, C. Li, C. Wu, Y. Yang and T.-L. Ren, Nat. Commun., 2015, 6, 7767 CrossRef CAS PubMed.
- J. Song, J. Li, L. Xu, J. Li, F. Zhang, B. Han, Q. Shan and H. Zeng, Adv. Mater., 2018, 1800764 CrossRef PubMed.
- H. E. Lee, J. H. Shin, J. H. Park, S. K. Hong, S. H. Park, S. H. Lee, J. H. Lee, I.-S. Kang and K. J. Lee, Adv. Funct. Mater., 2019, 1808075 CrossRef.
- Z. G. Wang, B. K. Chen and A. L. Rogach, Nanoscale Horiz., 2017, 2, 135 RSC.
- M. Zhang, B. Hu, L. Meng, R. Bian, S. Wang, Y. Wang, H. Liu and L. Jiang, J. Am. Chem. Soc., 2018, 140, 8690–8695 CrossRef CAS PubMed.
- C. Zhang, L. Yang, J. Zhao, B. Liu, M.-Y. Han and Z. Zhang, Angew. Chem., Int. Ed., 2015, 54, 11531–11535 CrossRef CAS PubMed.
- X. Yang, E. Mutlugun, C. Dang, K. Dev, Y. Gao, S. T. Tan, X. W. Sun and H. V. Demir, ACS Nano, 2014, 8, 8224–8231 CrossRef CAS PubMed.
- D. Zhou, H. Zou, M. Liu, K. Zhang, Y. Sheng, J. Cui, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 15830–15839 CrossRef CAS PubMed.
- H. Liu, Z. Wu, H. Gao, J. Shao, H. Zou, D. Yao, Y. Liu, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 42919–42927 CrossRef CAS PubMed.
- A. Pan, J. Wang, M. J. Jurow, M. Jia, Y. Liu, Y. Wu, Y. Zhang, L. He and Y. Liu, Chem. Mater., 2018, 30, 2771–2780 CrossRef CAS.
- J. Liu, Z. Wu, Y. Tian, Y. Li, L. Ai, T. Li, H. Zou, Y. Liu, X. Zhang, H. Zhang and B. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 24899–24907 CrossRef CAS PubMed.
- F. Yuan, S. Li, Z. Fan, X. Meng, L. Fan and S. Yang, Nano Today, 2016, 11, 565–586 CrossRef CAS.
- Y. Chen, M. Zheng, Y. Xiao, H. Dong, H. Zhang, J. Zhuang, H. Hu, B. Lei and Y. Liu, Adv. Mater., 2016, 28, 312–318 CrossRef CAS PubMed.
- C. Sun, Y. Zhang, K. Sun, C. Reckmeier, T. Zhang, X. Zhang, J. Zhao, C. Wu, W. W. Yu and A. L. Rogach, Nanoscale, 2015, 7, 12045–12050 RSC.
- F. Zhang, X. Feng, Y. Zhang, L. Yan, Y. Yang and X. Liu, Nanoscale, 2016, 8, 8618 RSC.
- F. Yuan, Z. Wang, Xi. Li, Y. Li, Z. Tan, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1604436 CrossRef PubMed.
- S. K. Bhunia, S. Nandi, R. Shikler and R. Jelinek, Nanoscale, 2016, 8, 3400–3406 RSC.
- D. Zhou, Y. Zhai, S. Qu, D. Li, P. Jing, W. Ji, D. Shen and A. L. Rogach, Small, 2017, 13, 1602055 CrossRef PubMed.
- Y. Wang, S. Kalytchuk, L. Wang, O. Zhovtiuk, K. Cepe, R. Zboril and A. L. Rogach, Chem. Commun., 2015, 51, 2950–2953 RSC.
- Q. Zhou, Z. Bai, W.-G. Lu, Y. Wang, B. Zou and H. Zhong, Adv. Mater., 2016, 28, 9163–9168 CrossRef CAS PubMed.
- Z. Wang, B. Chen, M. Zhu, S. V. Kershaw, C. Zhi, H. Zhong and A. L. Rogach, ACS Appl. Mater. Interfaces, 2016, 8, 33993–33998 CrossRef CAS PubMed.
- T. Hu, Z. Wen, C. Wang, T. Thomas, C. X. Wang, Q. Song and M. Yang, Nanoscale Adv., 2019, 1, 1413–1420 RSC.
- T. Feng, Q. Zeng, S. Lu, X. Yan, J. Liu, S. Tao, M. Yang and B. Yang, ACS Photonics, 2018, 5, 502–510 CrossRef CAS.
- Z. Tian, X. Zhang, D. Li, D. Zhou, P. Jing, D. Shen, S. Qu, R. Zboril and A. L. Rogach, Adv. Opt. Mater., 2017, 1700416 CrossRef.
- Z. Wang, F. Yuan, X. Li, Y. Li, H. Zhong, L. Fan and S. Yang, Adv. Mater., 2017, 29, 1702910 CrossRef PubMed.
- C. X. Wang, K. Jiang, Q. Wu, J. Wu and C. Zhang, Chem.–Eur. J., 2016, 22, 14475–14479 CrossRef CAS PubMed.
- Q. Wu, X. Wang, S. A. Rasaki, T. Thomas, C. X. Wang, C. Zhang and M. Yang, J. Mater. Chem. C, 2018, 6, 4508–4515 RSC.
- K. Jiang, S. Sun, L. Zhang, Y. Lu, A. Wu, C. Cai and H. Lin, Angew. Chem., Int. Ed., 2015, 54, 5360–5363 CrossRef CAS PubMed.
- X. Miao, D. Qu, D. Yang, B. Nie, Y. Zhao, H. Fan and Z. Sun, Adv. Mater., 2018, 30, 1704740 CrossRef PubMed.
- C. X. Wang, H. Lin, Z. Xu, Y. Huang, M. G. Humphrey and C. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 6621–6628 CrossRef CAS PubMed.
- C. X. Wang, Z. Xu, H. Cheng, H. Lin, M. G. Humphrey and C. Zhang, Carbon, 2015, 82, 87–95 CrossRef CAS.
- H. Ding, S.-B. Yu, J.-S. Wei and H.-M. Xiong, ACS Nano, 2016, 10, 484–491 CrossRef CAS PubMed.
- H. Ding, J.-S. Wei, P. Zhang, Z.-Y. Zhou, Q.-Y. Gao and H.-M. Xiong, Small, 2018, 1800612 CrossRef PubMed.
- C. Wang, T. Hu, T. Thomas, S. Song, Z. Wen, C. X. Wang, Q. Song and M. Yang, Nanoscale, 2018, 10, 21809 RSC.
- Q. Xu, T. Kuang, Y. Liu, L. Cai, X. Peng, T. S. Sreepcad, P. Zhao, Z. Yu and N. Li, J. Mater. Chem. B, 2016, 4, 7204–7219 RSC.
- Y. Wang, J. He, H. Chen, J. Chen, R. Zhu, P. Ma, A. Towers, Y. Lin, A. J. Gesquiere, S.-T. Wu and Y. Dong, Adv. Mater., 2016, 28, 10710–10717 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra06729a |
| This journal is © The Royal Society of Chemistry 2020 |