Open Access Article
Open Access ArticleRetracted Article: Macrophage-derived exosomes mediate osteosarcoma cell behavior by activating AKT signaling
Bin Yan†
ab,
Qingbai Liu†ac,
Gang Liu†ad,
Xiaoyi Huangb,
Guangming Zhub,
Luoluo Gaob and
Yaozeng Xu *a
*a
aDepartment of Orthopaedics, The First Affiliated Hospital of Soochow University, 899 Pinghai Road, Suzhou, Jiangsu 215006, China. E-mail: xuyaozeng@163.com
bDepartment of Orthopaedics, Taixing People's Hospital, Taixing, Jiangsu 225400, China
cDepartment of Orthopaedics, The Affiliated Lianshui County People's Hospital of Kangda College of Nanjing Medical University, Huai'an, Jiangsu 223400, China
dDepartment of Orthopaedics, The Second Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu 221006, China
First published on 30th January 2020
Abstract
Osteosarcoma is the most common type of bone tumor, which severely threatens the health of adolescents and young adults. Tumor-infiltrating macrophages have been shown to mediate cancer progression via extracellular vesicles. However, their potential mechanisms in osteosarcoma progression and in drug-resistance are still not yet known. The macrophage cell line THP1 was stimulated by phorbol myristate acetate (PMA) to secrete exosomes. The exosomes isolated from THP1 were characterized via transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA) and by a western blot. Cell proliferation was determined using CCK-8. A transwell assay and flow cytometry were conducted to detect cell migration and apoptosis, respectively. The expression levels of AKT and its phosphorylation status were determined using a western blot. PMA-treated activated THP1 cells secreted an abundance of exosomes with the characteristics of being less than 200 nm in diameter, and showing the robust expression of exosome markers CD63 and CD81. The THP1-derived exosomes promoted cell proliferation, migration and drug-resistance to the chemical drug docetaxel in both osteosarcoma cell lines MG63 and 143B. The inhibition of the generation of exosomes by the knockdown of ALIX clearly suppressed the cell proliferation, migration and drug-resistance. Mechanistically, the THP1-derived exosomes activated AKT signaling by inducing the increased expression of the phosphorylated AKT at serine 473 (p-AKT). The AKT inhibitor MK2206 significantly abolished exosome-mediated cell proliferation and drug-resistance in osteosarcoma cells. In summary, our data demonstrated that macrophage-derived exosomes promoted osteosarcoma progression and drug-resistance by activating AKT signaling that could be used as a potential molecular target for osteosarcoma treatment.
Introduction
Osteosarcoma is the most common primary malignant bone tumor that is characterized by the production of osteoid. It has been reported that the highest incidence of osteosarcoma is in young adults and adolescents, and the second peak is observed in adults with an age of more than 50.1 Recently, due to the improvement in treatment strategies, such as gene target therapy, surgery and chemotherapy, the outcome has been clearly improved. Of note, the five-year survival rate is approximately 20%, but the overall survival rate is still poor in osteosarcoma patients with advanced stage and metastasis.2,3 In addition, treatment with chemotherapy contributes to drug-resistance, resulting in poor prognosis.4 For these reasons it is urgent to find novel administration modalities for osteosarcoma, especially for patients in advanced stages and for drug-resistance. In recent years, tumor-infiltrating macrophages have been shown to favor progression in various tumors.5,6 A previous study demonstrated that inhibition of M2-like macrophages prevents osteosarcoma cell initiation and stemness.7 Intriguingly, several studies confirmed that macrophages showed positive outcomes of osteosarcoma patients during diagnosis.8,9 However, the potential role and mechanism of macrophages on osteosarcoma progression are still not yet known.Macrophages are a kind of mononuclear phagocytic cell, and can originate from blood monocytes during inflammation stimulation. In addition, macrophages characterize functions in homeostasis, inflammatory response and immune regulation, as well as in wound healing and angiogenesis.8,10 A recently published study demonstrated that exosomes derived from macrophages affect the lung tissue microenvironment by influencing inflammatory signaling and the immune function.11 Furthermore, M2 macrophage-derived exosomes promote the progression of pancreatic ductal adenocarcinoma.12 From these cases, we inferred that the macrophage mediated osteosarcoma might relate to the exosomes, alternatively, the macrophages may influence osteosarcoma by directly releasing exosomes.
Exosomes are a class of extracellular vesicles with a natural size of 40–200 nm in diameter. It has been reported that exosomes can be released from all kind of cells and they are responsible for cellular communication under normal and pathological conditions. Exosomes are constituted by multiple molecules, such as proteins, DNA and RNA, as well as by cholesterol and sphingomyelin.13 Together with these characteristics, exosomes serve as important messengers in the interaction between tissues and organs. Intracellular signaling based on exosomes contributes to tumor microenvironment establishment and is involved in the progression of various tumors.14 However, little is known about the role of macrophage-derived exosomes on osteosarcoma progression and drug-resistance.
In the present study, we investigate the role of macrophages on osteosarcoma cell proliferation and migration, and we identify that exosomes derived from macrophages promote drug-resistance of osteosarcomas by activating AKT. These studies highlight novel evidence of macrophages during osteosarcoma progression and drug-resistance.
Materials and methods
Cell culture
Human macrophage THP1, and human osteosarcoma cell lines 143B and MG63 were all purchased from American Type Culture Collection (ATCC), and were cultured in the Dulbecco's Modified Eagles' Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and maintained in a humidified condition with 5% CO2 at 37 °C.Macrophage-derived exosome isolation and purification
For secretion of the exosomes, THP1 cells were planted in DMEM with a concentration of 2 × 105 cells per mL, and they were cultured with 0.5 nM phorbol myristate acetate (PMA) for 3 h to obtain the exosomes. After that, the cells were washed and planted in a 6-well plate for 24 h. After the macrophages arrived at the confluence of 80%, the supernatants were collected for isolating the exosomes using a centrifugation and filtration method. Briefly, after being centrifugated at 2000g for 40 min, the supernatants were filtered using a 0.22 μm sterilized filter to discard the cell debris and other impurities. Next, the supernatants were ultracentrifugated at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 2 h, followed by suspension in phosphate-buffered saline solution (PBS). The exosome-containing pellets were then ultracentrifugated again, and the obtained exosomes were resuspended in PBS for utilization. All the centrifugation steps were conducted at 4 °C.
000g for 2 h, followed by suspension in phosphate-buffered saline solution (PBS). The exosome-containing pellets were then ultracentrifugated again, and the obtained exosomes were resuspended in PBS for utilization. All the centrifugation steps were conducted at 4 °C.
Identification and characterization of the exosomes
Transmission electron microscopy (TEM) was performed to observe the morphology of the exosomes. Briefly, after isolation and purification, the exosomes were fixed by 2% paraformaldehyde for 30 min, followed by dropping on carbon coated copper grids. After that, the mixture was dried in air, and then stained twice using 1% uranyl acetate. The TEM images of the exosomes were taken using a Philips CM120 EM according to the manufacturer's instructions.For the nanoparticle tracking analysis (NTA), flow cytometric analysis was performed as described previously.15 In summary, the purified exosomes that were derived from THP1 cells were infiltrated within 10 mL of aldehyde/sulfate latex beads (Thermo Fisher Scientific) for 2 h. After blocking, the bead-bound exosomes were fixed, followed by incubating them with antibodies against CD81 and CD63, as indicated. The images were obtained using a Nanosight NS500, following the manufacturer's instructions.
siRNA-ALIX transfection
MG63 and 143B cells were planted in a 6-well plate for 24 h. The plasmids used in the present study were synthesized by the GENEWIZ Life Science company (Suzhou, China). siRNA targeting ALIX was synthesized by Sangon Biotech (Shanghai, China). The lipofectamine 2000 reagent (Invitrogen, USA) was used for the transfection. After 24 h, the stable transfected cells were collected for the following experiments.Western blot
The cells were prepared using the lysis buffer for 30 min on ice, and they were then centrifugated to obtain the protein. The extracted protein was separated by 10% SDS-PAGE and then equal amounts of protein were transferred onto polyvinylidene difluoride (PVDF) membranes and incubated with the primary antibodies against CD63, CD81, AKT, p-AKT and GAPDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, Abcam) at 4 °C for 24 h. Afterwards, the PVDF membrane was washed with TBST and incubated with the second antibody, namely horseradish peroxidase-conjugated antibody (Beyotime, Shanghai, China), at room temperature for 1 h. The protein bands were visualized using an Enhanced Chemiluminescence system. GAPDH served as an internal control.
1000, Abcam) at 4 °C for 24 h. Afterwards, the PVDF membrane was washed with TBST and incubated with the second antibody, namely horseradish peroxidase-conjugated antibody (Beyotime, Shanghai, China), at room temperature for 1 h. The protein bands were visualized using an Enhanced Chemiluminescence system. GAPDH served as an internal control.
Cell proliferation assays
Before detecting cell proliferation, both MG63 and 143B cells were grown in DMEM with a concentration of 1 × 104 cells per mL, and they then received the PMA treated THP1 as described above, or 5 μg exosomes with/without si-ALIX for 24, 48, and 72 h, respectively. Cell proliferation was carried out using Cell Counting Kit-8 (CCK-8). In summary, after carrying out the treatments as described above, the cells were seeded on a 96-well plate with 500 cells per well. A 10 μL CCK-8 solution was added to incubate the cells for 2 h, and the absorbance at OD = 450 nm was measured using a microplate reader.Analysis of cell apoptosis
The MG63 and 143B cells were cultured with 5 μg exosomes for 72 h and then incubated with docetaxel at the concentrations of 5, 10, and 20 nM, or transfected with si-ALIX for 24 h. The cells were resuspended with Annexin V binding buffer, followed by incubation with 5 μL Annexin V/FITC mix for 5 minutes. Flow cytometry was used for detection of the apoptotic cell numbers. The data were analyzed with CellQuest software (BD Biosciences).Transwell assays
Cell migration was determined using transwell assays. After obtaining 5 μg exosomes with/without si-ALIX transfection for 24 h, both MG63 and 143B cells were suspended in 200 μL serum-free medium in the upper chamber, while the lower chamber was filled with 400 μL medium with 10% FBS. After 24 h, the cells that migrated through the membrane were fixed and stained using crystal violet.Statistical analysis
All data are presented as mean ± SD and were analyzed using the SPSS 18.0 software. The Student's t-test was performed to analyze the statistical difference between two groups, whereas ANOVA was used to analyze the statistical difference among multi-groups. The statistical significance was set as p < 0.05.Results
Isolation and characterization of exosomes derived from activated macrophages
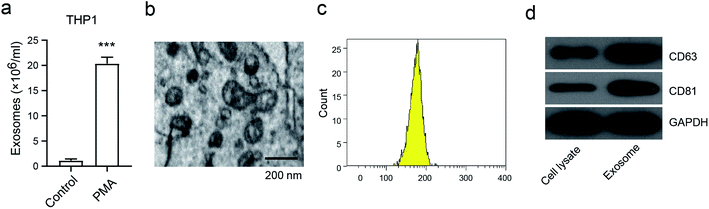
To investigate the generation of exosomes from macrophages, we first analyzed the exosome numbers in the macrophage cell line THP1, in the presence or absence of PMA, which is widely recognized as the activator to activate macrophages.16 We found that about 2 × 107 exosomes per cell were secreted in PMA-treated THP1, and this was markedly higher than in THP1 cells without PMA treatment (Fig. 1a). A representative exosome morphology was also observed by Transmission Electron Microscopy (TEM) (Fig. 1b). The nanoparticle tracking analysis (NTA) showed that the particle size was between 110–200 nm in diameter, with the peak diameter at 180 nm (Fig. 1c). Notably, the expressions of CD68 and CD81, which are considered to be the exosome markers, were enhanced in the exosomes compared to in THP1 without PMA treatment (Fig. 1d). These results confirmed that the release of exosomes was from activated macrophages, and not from inactivated macrophages.Macrophage-derived exosomes promote osteosarcoma cell proliferation
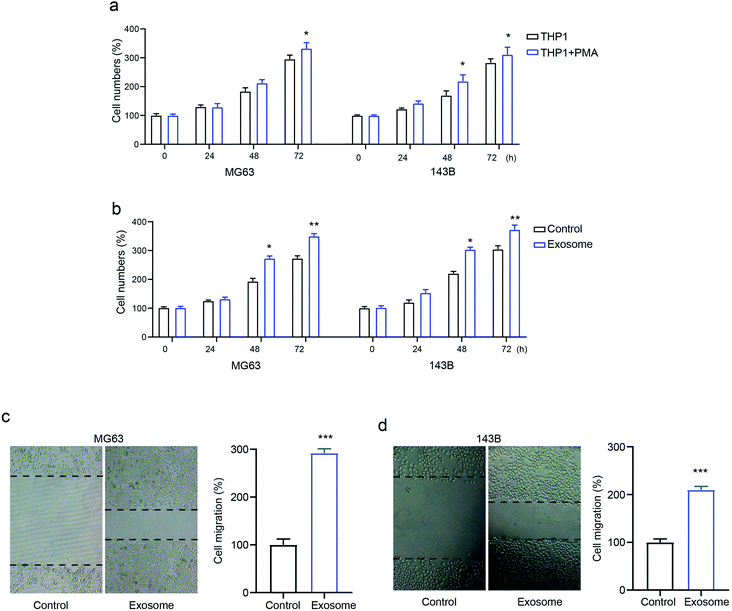
To identify the role of macrophage-derived exosomes on osteosarcomas, we first determined the proliferative ability of the osteosarcoma cell lines MG63 and 143B treated with macrophage THP1 in the presence or absence of PMA. The results revealed that activated THP1 dramatically enhanced cell numbers compared with the inactivated cells, in a time-dependent manner (Fig. 2a). Next, MG63 and 143B cells were incubated with or without exosomes isolated from activated THP1. This showed that the cell numbers were markedly elevated by treatment with exosomes (Fig. 2b), confirming the role of exosomes in osteosarcoma cell proliferation. Moreover, the transwell assay demonstrated that exosome treatment promoted cell migration in both MG63 and 143B cells compared with that of the control group (Fig. 2c and d). The data confirmed that exosomes secreted from activated macrophages promote osteosarcoma cell proliferation and migration.Macrophage-derived exosomes promote drug-resistance in osteosarcoma cells
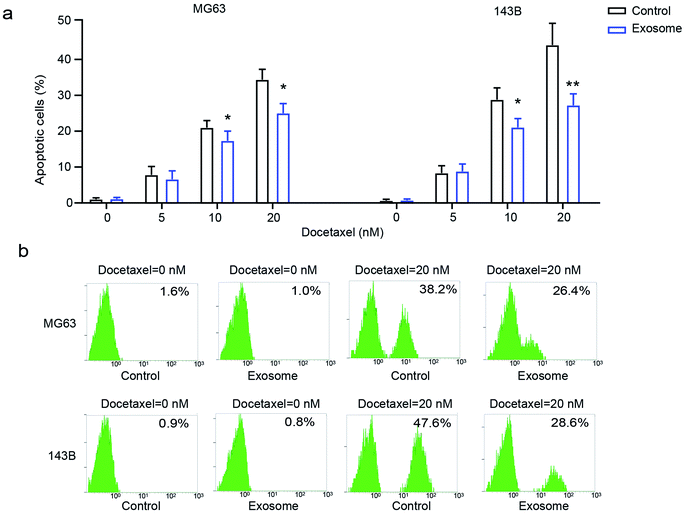
To explore the effect of exosomes secreted from macrophages on the drug-resistance of osteosarcoma cells, osteosarcoma cell lines MG63 and 143B were treated with various doses of docetaxel in the presence or absence of THP1-derived exosomes. We found that osteosarcoma cells had significantly decreased cell apoptosis in the presence of exosomes compared with the cells in the absence of exosomes (Fig. 3a and b), indicating that exosome treatment elicits drug-resistance in osteosarcoma cells. These results demonstrated that macrophage-derived exosomes significantly promote drug-resistance to docetaxel in osteosarcoma cells.Inhibition of exosome generation attenuates cell proliferation, migration and drug-resistance
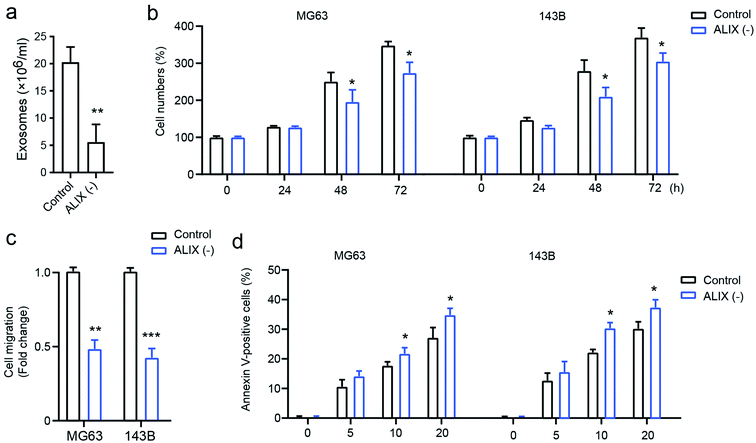
To confirm the effect of exosomes on OS cell progression, the apoptosis-linked gene 2-interacting protein X (ALIX), which is an important gene for exosome generation,17 was employed to study its role on exosome generation in THP1 cells. As presented in Fig. 4a, compared with the PMA-activated THP1 control, the knockdown of ALIX significantly inhibits exosome generation in THP1 cells in the presence of PMA compared with the control group. In addition, OS cells with the ALIX knockdown exhibited suppressed ability of cell proliferation (Fig. 4b), cell migration (Fig. 4c), and drug-resistance, and this is shown by increased cell apoptosis induced by docetaxel (Fig. 4d). The data demonstrated that macrophage-derived exosomes play a fundamental role in mediating OS cell proliferation, migration, and drug susceptibility.Exosomes upregulate AKT activation in osteosarcoma cells
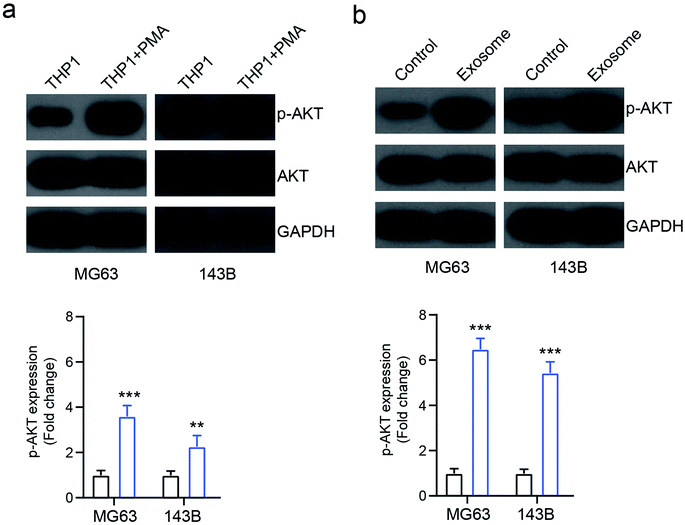
A previous study showed that AKT signaling is involved in exosome mediated cancer progression.18 To investigate the mechanism of exosome-mediated OS cell behavior, the activation of AKT signaling was investigated. We found that the PMA-treated activated THP1 cells increased the expression of phosphorylated AKT (p-AKT) in osteosarcoma cells MG63 and 143B, compared with the inactivated cells (Fig. 5a). Similarly, the exosomes isolated from activated THP1 also enhanced the expression of p-AKT compared with the control group (Fig. 5b), indicating that macrophage-derived exosomes affect the behavior of osteosarcoma cells by activating the AKT signaling pathway.Inhibition of AKT reverses exosome-mediated OS cell proliferation and apoptosis
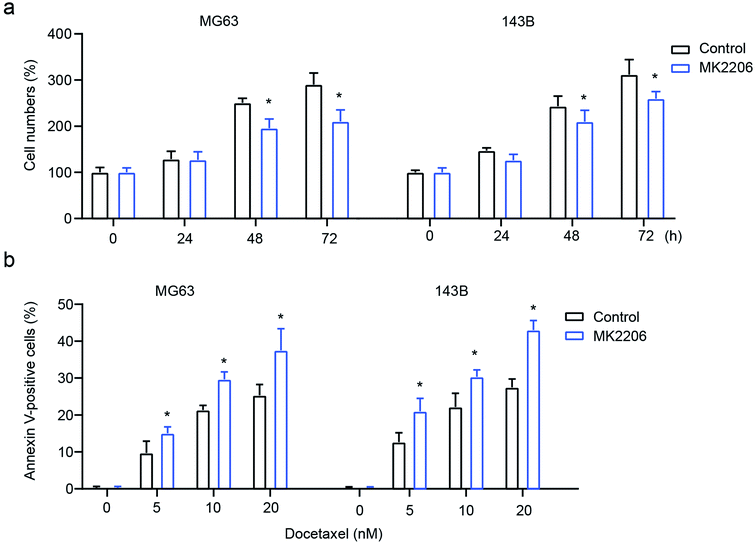
To confirm the role of AKT signaling in exosome-mediated OS cell behavior, the AKT inhibitor MK2206 was used to inhibit AKT. The results showed that the inhibition of AKT significantly attenuates exosome-mediated enhanced cell proliferation and drug-resistance, leading to decreased cell numbers and increased cell apoptosis in MG63 and 143B cells (Fig. 6a and b). All these results demonstrate that macrophage-derived exosomes mediate osteosarcoma cell behavior by modulating the activation of AKT.Discussion
The clinical treatment of osteosarcomas based on the combination of surgery and chemotherapy has clearly improved the survival rate of patients, however a serious problem of drug-resistance has occurred in response to chemotherapy, resulting in poor prognosis with local recurrence and metastasis. It has been reported that docetaxel, doxorubicin, cisplatin and methotrexate are widely used in osteosarcoma treatment, but the survival rate is low in patients who are resistant to these drugs.4,19 Therefore, it is essential to find a novel therapy method to improve osteosarcoma symptoms without resulting in drug-resistance.Macrophages belong to the component of tumor-infiltrating immune cells and possess great functions in host defense, as well as mediate solid tumor progression. A study reported that a high level of macrophages means poor prognosis in tumors.20 In the present study, we found that PMA activated THP1 promoted cell proliferation and the migration of osteosarcoma cells, indicating that PMA-activated THP1 promotes osteosarcoma progression. Phorbol myristate acetate (PMA) is widely used as a differentiated signal to stimulate macrophages,21 and is involved in the secretion of exosomes.22 Exosomes that are derived from macrophages have been reported to modulate tumor cell migration.23 To further determine whether macrophage-derived exosomes modulate osteosarcoma progression, we used THP1-derived exosomes on osteosarcoma cells. We found that both PMA-differentiated THP1 and the exosomes derived from PMA-differentiated THP1 showed the same effect on the promotion of cell proliferation of osteosarcoma cells, indicating that PMA stimulation promotes exosome secretion from macrophages, and this further promotes cell proliferation of osteosarcoma. We also tested the role of macrophage-derived exosomes on osteosarcoma cell migration. In agreement with cell proliferation, macrophage-derived exosomes clearly promoted cell migration. Exosomes mediate various physiologic processes by transmitting their contents, such as nucleic acids, proteins and lipids to recipient cells, therefore exerting their functions.25,26 For example, mouse colon cancer cell-derived exosomes carry Wnt1 to mediate autophagic flux and tumorigenic potential.24 miR-192-5p was shown within exosomes derived from lipotoxic hepatocyte to activate the macrophages.25 In the present work, macrophage-derived exosomes delivered their cargo content including nucleic acids, proteins and lipids into osteosarcoma cells, further promoting osteosarcoma progression and drug-resistance. Importantly, we observed that macrophage-derived exosomes suppressed docetaxel-induced cell apoptosis. The typical exosome marker ALIX is a transcription factor, and is involved in exosome biogenesis and secretion.26 Through the knockdown of ALIX, exosome production and secretion decreased. Our results revealed that ALIX knockdown altered the effect of macrophage-derived exosomes on cell proliferation, migration and drug-resistance. Our data strongly suggests that macrophages mediate osteosarcoma progression and drug-resistance by secreting exosomes. AKT signaling is a classical pathway that modulates various cellular processes, such as cell proliferation, apoptosis, autophagy, tumorigenesis, and drug-resistance.27 The activation of the AKT pathway demonstrates the poor outcome in various tumors.28,29 The increased expression of the phosphorylate-type AKT, that is the activation of AKT signaling, is observed in the osteosarcoma cells.30 It has been reported that the AKT-related signaling pathway mediates drug-resistance in various cancers, such as in gastric cancer31 and in breast cancer,32 as well as in osteosarcoma cells.33 In this research, we found that both PMA-differentiated THP1 and macrophage-derived exosomes significantly increased the expression of phosphorylated AKT, and that the AKT inhibitor MK2206 reversed the effect of the macrophage-derived exosomes, indicating that the macrophage-derived exosomes activated the AKT signaling pathway. Therefore, we can conclude that macrophage-derived exosomes promote osteosarcoma progression and drug-resistance through the activation of the AKT signaling pathway.
Overall, macrophage-derived exosomes were found to promote osteosarcoma progression by increasing cell proliferation and migration, and they augment osteosarcoma drug-resistance to docetaxel by activating the AKT signaling pathway. This suggests that they are a critical factor in modulating osteosarcoma progression and drug-resistance, providing a novel target for further osteosarcoma treatments.
Conflicts of interest
All authors declare that there are no conflicts of interest.References
- I. Zambo and K. Vesely, WHO classification of tumours of soft tissue and bone 2013: the main changes compared to the 3rd edition, Cesk. Patol., 2014, 50(2), 64–70 Search PubMed.
- C. L. Schwartz, et al., Multiple drug resistance in osteogenic sarcoma: INT0133 from the Children's Oncology Group, J. Clin. Oncol., 2007, 25(15), 2057–2062 CrossRef PubMed.
- K. Ando, et al., Current therapeutic strategies and novel approaches in osteosarcoma, Cancers, 2013, 5(2), 591–616 CrossRef CAS PubMed.
- X. Xiao, et al., HSP90AA1-mediated autophagy promotes drug resistance in osteosarcoma, J. Exp. Clin. Cancer Res., 2018, 37(1), 201 CrossRef PubMed.
- T. Hayashi, et al., Main Inflammatory Cells and Potentials of Anti-Inflammatory Agents in Prostate Cancer, Cancers, 2019, 11(8) DOI:10.3390/cancers11081153.
- V. Quaranta and M. C. Schmid, Macrophage-Mediated Subversion of Anti-Tumour Immunity, Cells, 2019, 8(7) DOI:10.3390/cells8070747.
- X. J. Shao, et al., Inhibition of M2-like macrophages by all-trans retinoic acid prevents cancer initiation and stemness in osteosarcoma cells, Acta Pharmacol. Sin., 2019, 40(10), 1343–1350 CrossRef CAS PubMed.
- E. P. Buddingh, et al., Tumor-infiltrating macrophages are associated with metastasis suppression in high-grade osteosarcoma: a rationale for treatment with macrophage activating agents, Clin. Cancer Res., 2011, 17(8), 2110–2119 CrossRef CAS PubMed.
- P. A. Meyers, et al., Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival—a report from the Children's Oncology Group, J. Clin. Oncol., 2008, 26(4), 633–638 CrossRef CAS PubMed.
- J. H. Pahl, et al., Macrophages inhibit human osteosarcoma cell growth after activation with the bacterial cell wall derivative liposomal muramyl tripeptide in combination with interferon-gamma, J. Exp. Clin. Cancer Res., 2014, 33, 27 CrossRef.
- Z. Yuan, et al., Macrophages exposed to HIV viral protein disrupt lung epithelial cell integrity and mitochondrial bioenergetics via exosomal microRNA shuttling, Cell Death Dis., 2019, 10(8), 580 CrossRef PubMed.
- Z. Yin, et al., Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-beta signaling pathway, J. Exp. Clin. Cancer Res., 2019, 38(1), 310 CrossRef PubMed.
- C. Thery, L. Zitvogel and S. Amigorena, Exosomes: composition, biogenesis and function, Nat. Rev. Immunol., 2002, 2(8), 569–579 CrossRef CAS PubMed.
- K. Nakamura, et al., Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target, Cancers, 2019, 11(8) DOI:10.3390/cancers11081147.
- H. Lee, et al., Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation, J. Immunol., 2018, 201(5), 1500–1509 CrossRef CAS.
- M. T. Shio, et al., PKC/ROS-Mediated NLRP3 Inflammasome Activation Is Attenuated by Leishmania Zinc-Metalloprotease during Infection, PLoS Neglected Trop. Dis., 2015, 9(6), e0003868 CrossRef.
- M. F. Baietti, et al., Syndecan-syntenin-ALIX regulates the biogenesis of exosomes, Nat. Cell Biol., 2012, 14(7), 677–685 CrossRef CAS.
- L. Wang, et al., Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway, Sci. Rep., 2017, 7(1), 5384 CrossRef PubMed.
- S. Ye, et al., NVP-TAE684 reverses multidrug resistance (MDR) in human osteosarcoma by inhibiting P-glycoprotein (PGP1) function, Br. J. Pharmacol., 2016, 173(3), 613–626 CrossRef CAS PubMed.
- B. Ruffell, N. I. Affara and L. M. Coussens, Differential macrophage programming in the tumor microenvironment, Trends Immunol., 2012, 33(3), 119–126 CrossRef CAS PubMed.
- A. K. Bolling, et al., Establishing a macrophage model with relevance for oral methacrylate monomer exposures: Attenuated Staphylococcus aureus-induced cytokine release from human macrophages, Dent. Mater., 2019, 35(10), e235–e248 CrossRef CAS PubMed.
- J. Madrigal-Matute, et al., Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis, J. Am. Heart Assoc., 2014, 3(4) DOI:10.1161/JAHA.114.000785.
- E. van der Pol, et al., Classification, functions, and clinical relevance of extracellular vesicles, Pharmacol. Rev., 2012, 64(3), 676–705 CrossRef CAS.
- M. Yeon, et al., CAGE-miR-140-5p-Wnt1 Axis Regulates Autophagic Flux, Tumorigenic Potential of Mouse Colon Cancer Cells and Cellular Interactions Mediated by Exosomes, Front. Oncol., 2019, 9, 1240 CrossRef.
- X. L. Liu, et al., Lipotoxic Hepatocyte-Derived Exosomal miR-192-5p Activates Macrophages via Rictor/Akt/FoxO1 Signaling in NAFLD, Hepatology, 2019 DOI:10.1002/hep.31050.
- N. Jabbari, M. Nawaz and J. Rezaie, Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy, Int. J. Mol. Sci., 2019, 20(15) DOI:10.3390/ijms20153649.
- G. S. Zhao, et al., TSSC3 promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR pathway to suppress tumorigenesis and metastasis in osteosarcoma, and predicts a favorable prognosis, J. Exp. Clin. Cancer Res., 2018, 37(1), 188 CrossRef PubMed.
- Z. Tang and Z. He, TIGAR promotes growth, survival and metastasis through oxidation resistance and AKT activation in glioblastoma, Oncol. Lett., 2019, 18(3), 2509–2517 Search PubMed.
- H. Pan, et al., EXOSC5 as a Novel Prognostic Marker Promotes Proliferation of Colorectal Cancer via Activating the ERK and AKT Pathways, Front. Oncol., 2019, 9, 643 CrossRef PubMed.
- H. Zhang, et al., Anti-tumor efficacy of phellamurin in osteosarcoma cells: Involvement of the PI3K/AKT/mTOR pathway, Eur. J. Pharmacol., 2019, 858, 172477 CrossRef PubMed.
- R. Lu, et al., Inhibition of CD133 Overcomes Cisplatin Resistance Through Inhibiting PI3K/AKT/mTOR Signaling Pathway and Autophagy in CD133-Positive Gastric Cancer Cells, Technol. Cancer Res. Treat., 2019, 18, 1533033819864311 CAS.
- J. He, et al., Multi-targeted kinase inhibition alleviates mTOR inhibitor resistance in triple-negative breast cancer, Breast Cancer Res. Treat., 2019, 178(2), 263–274 CrossRef CAS.
- Q. Zhou, T. Hu and Y. Xu, Anticancer potential of TUG1 knockdown in cisplatin-resistant osteosarcoma through inhibition of MET/Akt signalling, J. Drug Targeting, 2019, 1–8 Search PubMed.
Footnote |
| † Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |