Open Access Article
Open Access ArticleThe structural composition of components contributes to the superiority of the geoherb Alisma orientale for “diuresis and diffusing dampness”
Ru Li†
a,
Zhi-li Li†a,
Ya-ping Chen†a,
Wei-quan Bub,
Wen-bo Dingb,
Bing Yangb,
Chun-fei Wangb,
Liang Ma*b,
Xiao-bin Jia*a and
Liang Feng *a
*a
aSchool of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, P.R.China. E-mail: wenmoxiushi@163.com; jiaxiaobin2015@163.com
bAffiliated Hospital of Integrated Traditional Chinese and Western Medicine, Third Clinical Medical College, Nanjing University of Chinese Medicine, Nanjing, Jiangsu 210028, P.R. China. E-mail: liangyu84@163.com
First published on 27th October 2020
Abstract
The purpose of this study is to explore the intrinsic reasons for the superiority of the salt-made geoherb Alisma orientale via comparing the content of various components of the salt-made geoherb Alisma orientale. The effects of “diuresis and diffusing dampness” using salt-made Alisma orientale from seven different origins were investigated through pharmacodynamic experiments in vivo and in vitro. The results indicated that salt-made Alisma orientale from different origins had diuretic efficacy; this was demonstrated by the significant increase in the volume of rat urine, the concentration of Na+, K+, and Cl− in the urine, and the significant decrease in the levels of AQP-2 in rat renal medulla and HK-2 cells. It was also revealed that the diuretic effect of salt-made Alisma orientale from Fujian Province is stronger than those from other provinces. Moreover, the main components and their proportions in the salt-made Alisma orientale samples were further analyzed via principal component analysis. The results showed that alisol A 24-acetate, alisol B, and 23-acetyl alisol B are the main components of salt-made Alisma orientale, and the optimal structural ratio of alisol A 24-acetate, alisol B, and 23-acetyl alisol B was found to be 5.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31 via optimizing the ratios of the three main components. It is worth noting that the optimal ratio of the three main components after optimization is the closest to the ratio of the three main components in salt-made Alisma orientale from Fujian Province. This paper reveals the “mystery” of the content ratio of the main active components and its effect on the efficacy, and showed that the proportional relationship between the content of multiple components is the key to their interactions. Therefore, this method of evaluating the quality of salt-made Alisma orientale is obviously reliable, and this study lays the foundations for quality evaluation of salt-made Alisma orientale and other herb slices.
11.31 via optimizing the ratios of the three main components. It is worth noting that the optimal ratio of the three main components after optimization is the closest to the ratio of the three main components in salt-made Alisma orientale from Fujian Province. This paper reveals the “mystery” of the content ratio of the main active components and its effect on the efficacy, and showed that the proportional relationship between the content of multiple components is the key to their interactions. Therefore, this method of evaluating the quality of salt-made Alisma orientale is obviously reliable, and this study lays the foundations for quality evaluation of salt-made Alisma orientale and other herb slices.
1 Introduction
As a traditional medicine in China, Chinese herbal slice has always been used to improve the health of our people. The quality of the herbal slice is the basis of ensuring the safety and effectiveness of traditional Chinese medicine. The quality evaluation of traditional Chinese herbal slice is still mainly based on the traditional method, the content of single or several components as the evaluation index. However, multi-component characteristics of traditional Chinese medicine have shown that the content of single or several components does not really represent the true quality and the true clinical efficacy of the herbal slice.1,2 Therefore, a method for quality evaluation of herbal slices needs to be developed urgently.3,4 In recent years, multi-dimensional chemical evaluation with statistical analysis and fingerprint analysis have been used to control the quality of herbal slices from a holistic perspective.5,6 However, these methods do not clearly explain the intrinsic reasons for the quality of the herbal slice. Our research group believes that the main property that effects the quality of a Chinese herbal slice is not the content of one or several components, but the specific proportional relationship of certain components within the herbal slice.Alisma orientale is a dried rhizoma of Alisma orientalis (sam.) Juzep., a perennial swamp herb of Alismataceae, which “promotes diuresis and eliminates dampness”.7–11 At present, Alisma orientale is mainly used for the treatment of dysuria, water swelling, diarrhea, scanty urine, heat stranguria, astringent pain and other diseases in China,12–17 and is mainly produced in Fujian, Jiangxi, Sichuan and other provinces and is divided into Jian Alisma orientale and Chuan Alisma orientale. It is generally believed that Fujian produces the best quality Alisma orientale, and the efficacy of the Jianzexie is better than that obtained from other areas, however, the relationship between the producing area and its pharmacodynamics has always been an unsolved mystery.
The multi-components characteristics of traditional Chinese medicines indicate that the efficacy of traditional Chinese medicine does not depend on the content of a certain component, but on the interaction of multiple components. Our research group believes that the proportional relationship between the content of multiple components is the key to affecting their interaction, and the advantages of Fujian Alisma orientale may be related to its unique proportional relationship between multiple components.
This paper compares the effect of “diuretic and dampness” in salt-made Alisma orientale from different producing origins and clarifies its efficacy and simple mechanism, the content of the active components in Alisma orientale was analyzed, as well as its proportional relationship, and this revealed the relationship between the content ratio of the main active ingredients in Alisma orientale and its efficacy.
This study lays the foundations for evaluation of the quality of salt-made Alisma orientale and other herb slices.
2 Experimental
2.1 Chemicals and materials
Furosemide tablets were purchased from Jiangsu Yabang Pharmaceutical Co., Ltd. (Jiangsu, China), a sodium ion detection kit, potassium ion detection kit and chloride ion detection kit were purchased from Nanjing Jiancheng Bioengineering Institute, Ltd. (Nanjing, China). The Total Protein Extraction Kit, Bradford protein content test kit, 5 × SDS-PAGE protein loading buffer, SDS-PAGE gel preparation kit, molecular weight of pre-dyed protein, AQP-2 antibody, EliVision plus kit, and DAB Kit were purchased from Fuzhou Maixin Biotechnology Co., Ltd. (Fuzhou, China). The ECL Test Kit, internal reference primary antibody (Rabbit β-actin), secondary antibodies (goat anti-mouse IgG) and secondary antibodies (rabbit anti-sheep IgG) were purchased from Nanjing KeyGEN Biotechnology Development Co., Ltd. (Nanjing, China).Standard material alisol A 24-acetate, alisol B and alisol B 23-acetate were supplied by China Food and Drug Administration (purity greater than 98%). Acetonitrile was purchased from TEDIA (USA, HPLC-grade). Prepared herbal medicine in small pieces ready for decoction (salt-made Alisma orientale) was collected from various companies in different provinces (Table 1) and identified as Alisma orientalis (sam.) Juzep. by Dr Long Wang from the Department of Traditional Chinese Medicine Resources, at China Pharmaceutical University.
| Origin | Company |
|---|---|
| Fujian Province | Anhui Jingquan Group Chinese Herbal Medicine Co., Ltd |
| Sichuan Province | Chengdu Zhongchuan Pharmaceutical Co., Ltd |
| Jiangxi Province | Jiangxi Heye Zexie Kunshan Sales Co., Ltd |
| Guangxi Province | Zhejiang Yingte Chinese Herbal Pieces Co., Ltd |
| Hubei Province | Hubei Yuancheng Saichuang Technology Co., Ltd |
| Henan Province | Nanyang Zhang Zhongjing Chinese Herbal Medicine Development Co., Ltd |
| Hebei Province | Hebei Dongsheng Yinghua Pharmaceutical Co., Ltd |
2.2 Preparation of Alisma orientale extract
Salt-made Alisma orientale (2 kg) from each origin was weighed, boiled and extracted twice with 10 times 80% ethanol (v/v) for 30 min. The extracts were combined and freeze-dried into a powder and stored at the School of Traditional Chinese Pharmacy, China Pharmaceutical University for further application (batch number: 20180103).2.3 Animal experiments
Eighty female SD rats (weighing 200 ± 20 g) were purchased from Shanghai SLAC Animal Center with certificate number SCXK (Shanghai). All the rats were kept in cages in the animal experimental center of the China Pharmaceutical University. After one week of adaptive feeding, these rats were intragastrically administrated with distilled water twice a day for 3 h, and the gastric perfusion volume was 20 mL kg−1. After 3 d of continuous gavage, the drug was administered after 30 min for the last gavage.18–20 These model rats were randomly divided into: the model group (distilled water 20 mL kg−1); the furosemide positive control group (20 mg kg−1) and salted Alisma orientale groups including Fujian, Sichuan, Jiangxi, Hebei, Hubei, Guangxi, and Henan (the extract of the high dose was equivalent to 4.0 g kg−1 of the crude drug and the low dose was equivalent to 0.9 g kg−1 relative to the crude drug). All animal procedures were performed in accordance with the guidelines approved by the Experimental Animal Research Committee of China Pharmaceutical University and approved by the Animal Ethics Committee of China Pharmaceutical University.2.4 Detection of biochemical indexes in rat urine
The urine from the rats was collected using metabolic cages for 24 h, and the volume of urine was measured accurately. The content of Na+, K+ and Cl− in the urine was detected according to manufacturer's protocols.2.5 Cell cultures and extract treatment
Human renal tubular epithelial cell line (HK-2) cells were supplied by Nanjing KeyGEN Biotechnology Development. Dulbecco's modified Eagle medium (DMEM) medium containing 10% fetal bovine serum was used as the cell culture medium, and the cells were cultured in a sterile cell incubator with 5% CO2 at 37 °C. HK-2 cells in logarithmic growth phase were seeded in a 6-well plate at a density of 1 × 107 cells/well and then treated using extracts of 1.6 × 10−2, 8 × 10−3, 4 × 10−4, 2 × 10−4, 1 × 10−4, 5 × 10−5, 2.5 × 10−5, 1.25 × 10−5, and 6.25 × 10−6 g mL−1 in DMEM high sugar F12 incomplete culture medium.2.6 MTT assays for cell viability
HK-2 cells in the logarithmic growth phase were seeded in a 96-well plate at a density of 1 × 105 cells per well and placed in a 37 °C, 5% CO2 incubator for 24 h. These cells were exposed to different concentrations of the extract of Fujian salt-made Alisma orientale for 24 h. Sequentially, 10 μL of MTT solution (5 mg mL−1) was added to each well and they were incubated for another 4 h. The medium was then removed and 100 μL of dimethyl sulfoxide (DMSO) was added into each well to dissolve the formazan. After shaking at 37 °C for 10 min the absorbance value of each well was measured using a full-automatic microplate reader. The percentage cell viability was calculated using the following formula, in which OD is the optical density: cell viability (%) = (OD value of compound group/OD value of the control group) × 100%.2.7 Western blotting
The kidney tissues of the rats or the cells were made into a homogenate with 200 μL of ice pre-cooling cracking buffer and were kept in an ice-bath for 30 min, vortexed and fully lysed. The supernatant was collected after being centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min at 4 °C, and the protein concentration was determined using the Bradford method. After denaturation of the protein, an equal amount of total protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to the membrane and blocked in 5% skim milk powder solution (4 °C, overnight). The blocking solution and the appropriate amount of primary antibody (1
000 rpm for 10 min at 4 °C, and the protein concentration was determined using the Bradford method. After denaturation of the protein, an equal amount of total protein was separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to the membrane and blocked in 5% skim milk powder solution (4 °C, overnight). The blocking solution and the appropriate amount of primary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were added in a shaker at 0.1 mL cm−2 (4 °C, overnight). After being washed with Tween-phosphate buffered solution (TPBS) four times, a secondary antibody (diluted at 1
1000) were added in a shaker at 0.1 mL cm−2 (4 °C, overnight). After being washed with Tween-phosphate buffered solution (TPBS) four times, a secondary antibody (diluted at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) was added and it was incubated for 2 h at room temperature, the membrane was then washed with TPBS four times. The developer (0.1 mL cm−2) was added onto the NC membrane and left for 1 min at room temperature. The film was wrapped with plastic wrap (to avoid bubbles as much as possible). The membrane protein was quickly exposed to X-ray film in a dark room, and developed and washed in a film processor.
5000) was added and it was incubated for 2 h at room temperature, the membrane was then washed with TPBS four times. The developer (0.1 mL cm−2) was added onto the NC membrane and left for 1 min at room temperature. The film was wrapped with plastic wrap (to avoid bubbles as much as possible). The membrane protein was quickly exposed to X-ray film in a dark room, and developed and washed in a film processor.
2.8 High performance liquid chromatography analysis
The extract, 0.2 g, was dissolved in 2 mL of methanol, and then treated twice (30 min each time) using an ultrasound machine. The solution was filtered and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. An Agilent 1100 series high performance liquid chromatograph with a quaternary pump and diode-array detector (DAD) was used for this analysis. An Alltima C18 (4.6 mm × 250 mm, 5 μm) column was used. The mobile phase consisted of acetonitrile–water and a gradient elution (0–5 min, 0–2% acetonitrile; 5–40 min, 2–35% acetonitrile; 40–115 min, 35–98% acetonitrile; 115–120 min, 98–100% acetonitrile) was used with a 1.0 mL min−1 flow rate. The column temperature was maintained at 25 °C and the detection wavelength was set at 210 nm. The injection volume was maintained at 10 μL.
000 rpm for 10 min. An Agilent 1100 series high performance liquid chromatograph with a quaternary pump and diode-array detector (DAD) was used for this analysis. An Alltima C18 (4.6 mm × 250 mm, 5 μm) column was used. The mobile phase consisted of acetonitrile–water and a gradient elution (0–5 min, 0–2% acetonitrile; 5–40 min, 2–35% acetonitrile; 40–115 min, 35–98% acetonitrile; 115–120 min, 98–100% acetonitrile) was used with a 1.0 mL min−1 flow rate. The column temperature was maintained at 25 °C and the detection wavelength was set at 210 nm. The injection volume was maintained at 10 μL.
2.9 Composition ratio of terpenoids in Alisma orientale
The three main component terpenoids of salt-made Alisma orientale were optimized in the structural composition ratio. The specific scheme is shown in Table 2. According to the proportion of alisol A 24-acetate, alisol B and 23-acetyl alisol B in the above described optimization scheme, different media were prepared. The content ratio is the same as that shown in Table 2.24A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23B 23B |
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23B 23B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24A 24A |
23B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24A 24A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B B |
|---|---|---|
| a “24A”, “B”, and “23B” respectively represent “alisol A 24-acetate”, “alisol B”, and “23-acetyl alisol B”. | ||
(I-1) 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.43 11.43 |
(II-1) 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.48 11.48 |
(III-1) 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.25 14.25 |
(I-2) 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.08 8.08 |
(II-2) 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.38 5.38 |
(III-2) 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.06 9.06 |
(I-3) 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.01 6.01 |
(II-3) 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64 2.64 |
(III-3) 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.84 8.84 |
(I-4) 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.23 5.23 |
(II-4) 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.20 2.20 |
(III-4) 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.85 7.85 |
2.10 Statistical analysis
The high-performance liquid chromatography (HPLC) chromatograms of seven batches of Alisma orientale were imported into the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2012) composed by the Chinese Pharmacopoeia Committee. The common peaks of Alisma orientale from different producing area were calculated.The statistical data are presented as means ± standard deviation (SD). SPSS16.0 data statistical software was used for the principal component analysis and variance analysis. The T test method was used for comparison between groups. P < 0.05 indicates that the difference has statistical significance.
3 Results
3.1 Effect of Alisma orientale extract on urine volume and concentrations of Na+, K+, and Cl− in the urine of water-loaded rats
Compared with the blank group, the urine volume and concentration of Na+, K+ and Cl− in the positive group of furosemide were significantly increased (P < 0.01), and the urine volume and concentration of Na+, K+ and Cl− in the salt-made Alisma orientale group from different origins were also significantly increased (P < 0.05 or P < 0.01) (Table 4), but there was no significant difference between the high and low doses from the same origin. Using urine volume as the main index, the urine ratio of rats in the high-dose salt-made Alisma orientale group from different producing areas followed the order Fujian > Jiangxi > Sichuan > Hubei > Guangxi > Henan > Hebei, while the urine ratio of rats in the low-dose salt-made Alisma orientale group from different producing areas was Fujian > Jiangxi > Sichuan > Hubei > Guangxi > Henan > Hebei. The experimental results show that Alisma orientale in Fujian can obviously increase the urine output of water-loaded rats, and its diuretic effect is stronger than that of other producing areas.Principal component analysis was performed to determine the urine volume and concentration of Na+, K+ and Cl− in the urine from water-loaded rats treated with salt-made Alisma orientale from different origins. The results showed that the cumulative contribution rate of the first principal component is 87.49% (Table 3), and the first principal component is the urine volume through correlation principal component matrix analysis. Therefore, the urine volume of the principal component is taken as the main index for comparison of the drug efficacy between different origins in the later period.
| Component | Initial characteristic value | Extraction sums of squared loadings | ||||
|---|---|---|---|---|---|---|
| Characteristic value | % of variance | Cumulative% | Total | % of variance | Cumulative% | |
| 1 | 3.500 | 87.490 | 87.490 | 3.500 | 87.490 | 87.490 |
| 2 | 0.395 | 9.865 | 97.354 | |||
| 3 | 0.080 | 2.001 | 99.355 | |||
| 4 | 0.026 | 0.645 | 100.000 | |||
| Normal adult required quantity (g d−1) | Dosage in rats relative to crude drug (g kg−1 d−1) | Urine volume (mL) | Na+ (mmol L−1) | K+ (mmol L−1) | Cl− (mmol L−1) | |
|---|---|---|---|---|---|---|
| a Compared with the blank group, *P < 0.05, **P < 0.01. | ||||||
| Blank control group | — | — | 4.70 ± 0.25 | 51.48 ± 23.09 | 38.96 ± 0.75 | 109.83 ± 13.95 |
| Furosemide positive control group | — | 0.02 | 20.00 ± 1.06** | 174.95 ± 38.79** | 58.49 ± 0.47** | 207.55 ± 7.25** |
| Fujian Alisma orientale high-dose group | 45 | 4.0 | 13.80 ± 0.25** | 94.74 ± 25.43** | 57.08 ± 2.81** | 194.35 ± 16.74** |
| Fujian Alisma orientale low-dose group | 10 | 0.9 | 6.20 ± 0.14* | 79.15 ± 39.73* | 47.41 ± 3.94* | 158.95 ± 27.68* |
| Jiangxi Alisma orientale high-dose group | 45 | 4.0 | 12.90 ± 0.10** | 95.12 ± 27.62** | 51.59 ± 1.40** | 183.83 ± 26.62** |
| Jiangxi Alisma orientale low-dose group | 10 | 0.9 | 6.10 ± 0.11* | 67.34 ± 15.81* | 43.00 ± 0.85* | 143.79 ± 11.39* |
| Sichuan Alisma orientale high-dose group | 45 | 4.0 | 12.80 ± 0.50** | 94.77 ± 24.76** | 49.44 ± 0.63** | 189.86 ± 6.69** |
| Sichuan Alisma orientale low-dose group | 10 | 0.9 | 6.00 ± 0.05* | 57.29 ± 22.72* | 41.37 ± 1.74* | 141.79 ± 18.24* |
| Guangxi Alisma orientale high-dose group | 45 | 4.0 | 11.20 ± 0.95* | 95.52 ± 10.15* | 56.36 ± 2.92* | 191.75 ± 20.71* |
| Guangxi Alisma orientale low-dose group | 10 | 0.9 | 5.60 ± 0.40* | 71.42 ± 8.45* | 49.90 ± 1.52* | 152.81 ± 16.51* |
| Hubei Alisma orientale high-dose group | 45 | 4.0 | 11.90 ± 0.85* | 90.15 ± 18.65* | 54.13 ± 0.78* | 181.32 ± 9.47* |
| Hubei Alisma orientale low-dose group | 10 | 0.9 | 5.80 ± 0.60* | 67.11 ± 11.89* | 45.36 ± 1.80* | 155.38 ± 14.13* |
| Henan Alisma orientale high-dose group | 45 | 4.0 | 11.7 ± 0.40* | 93.43 ± 22.23* | 53.58 ± 2.55* | 184.11 ± 12.24* |
| Henan Alisma orientale low-dose group | 10 | 0.9 | 5.70 ± 0.15* | 66.30 ± 36.79* | 50.27 ± 1.11* | 127.09 ± 21.80* |
| Hebei Alisma orientale high-dose group | 45 | 4.0 | 10.50 ± 1.20* | 91.60 ± 23.50* | 54.85 ± 2.70* | 180.79 ± 6.25* |
| Hebei Alisma orientale low-dose group | 10 | 0.9 | 5.40 ± 1.13* | 76.30 ± 22.69* | 44.29 ± 2.38* | 124.76 ± 16.68* |
The male SD water loaded rat model has been used in previous literature reports.21 In this experiment, the feasibility of the female SD rat water load model was investigated, it can be seen that the female SD rat model has a good sensitivity and is consistent with the in vitro cell experiment results.
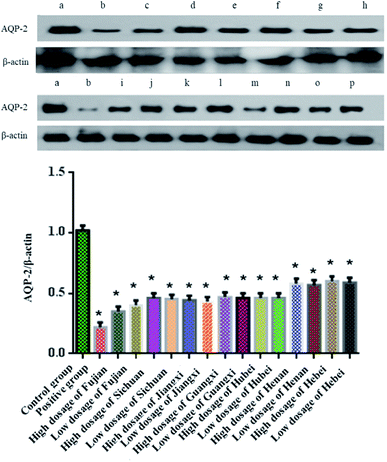
3.2 Effect of Alisma orientale alcohol extract on AQP-2 in rat kidneys
As shown in Fig. 1, compared with the blank group, the expression of AQP-2 in the renal medulla of rats in the furosemide group was significantly reduced (P < 0.05), while the expression of AQP-2 in the renal medulla of rats in the salt-made Alisma orientale group from different origins was also significantly reduced (P < 0.05), but there was no significant difference between the high and low doses from different origins. The expression comparison of AQP-2 in the kidney medulla of rats in the high dose group of salt-made Alisma orientale from different origins followed the order Fujian < Jiangxi < Sichuan < Hubei < Guangxi < Henan < Hebei, and the expression comparison of AQP-2 in the kidney medulla of rats in the low dose group was Fujian < Jiangxi < Sichuan < Hubei < Guangxi < Henan < Hebei. The ability of Fujian Alisma orientale to reduce AQP-2 expression in rat renal medulla is higher than that of other producing areas, which further indicates that Fujian Alisma orientale has a stronger diuretic effect than that from other origins.3.3 Effect of salt-made Alisma orientale on HK-2 cell viability
The MTT results show that the cell viability is 95.36 ± 1.54% when the concentration of the extract of salt-made Alisma orientale is 3.125 × 10−5 g mL−1. There was no significant difference in the cell viability in the control group, which indicates that these extracts have no obvious toxicity to the cells at this concentration. Therefore, 3.125 × 10−5 g mL−1 and 1.5625 × 10−5 g mL−1 were used for subsequent experimental studies (Table 5).| Group | Dosage (g mL−1) | Cell survival rate (%) |
|---|---|---|
| a Compared with the blank group, *P < 0.05. | ||
| Blank | — | 100 ± 1.15 |
| Alcohol extract of Fujian salt-made Alisma orientale | 1.0 × 10−3 | 45.06 ± 1.12* |
| 5.0 × 10−4 | 49.08 ± 1.45* | |
| 2.5 × 10−4 | 56.18 ± 1.28* | |
| 1.25 × 10−4 | 67.30 ± 1.53* | |
| 6.25 × 10−5 | 83.52 ± 1.70* | |
| 3.125 × 10−5 | 95.36 ± 1.54 | |
| 1.5625 × 10−5 | 96.37 ± 1.49 | |
| 7.8125 × 10−6 | 98.33 ± 1.62 | |
| 3.90625 × 10−6 | 99.67 ± 1.31 | |
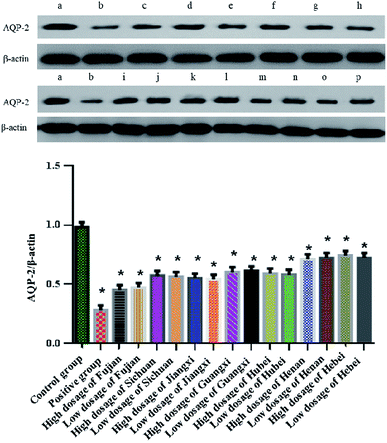
3.4 Effect of Alisma orientale extract on AQP-2 expression in HK-2 cells
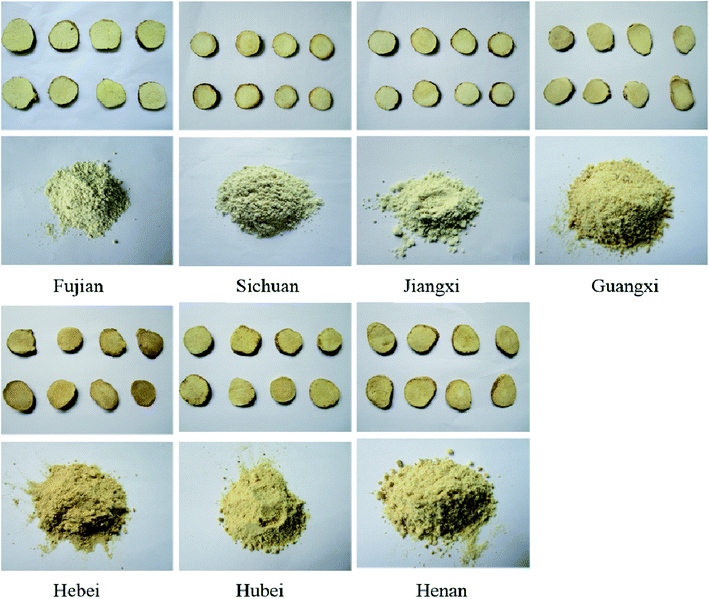
As shown in Fig. 2, compared with the blank group, the expression of AQP-2 in HK-2 cells in the furosemide group was significantly reduced (P < 0.05), while the expression of AQP-2 in HK-2 cells in the salt-made Alisma orientale group from different origins was also significantly reduced (P < 0.05), but there was no significant difference between the high and low dose groups from different origins. The AQP-2 expression of HK-2 cells in the high-dose group of salt-made Alisma orientale from different origins followed the order Fujian < Jiangxi < Sichuan < Hubei < Guangxi < Henan < Hebei, while the AQP-2 expression of HK-2 cells in the low-dose group was Fujian < Jiangxi < Sichuan < Hubei < Guangxi < Henan < Hebei. The ability of Fujian Alisma orientale to reduce AQP-2 expression in HK-2 cells is higher than that in other producing areas. The results of the in vitro experiments also show that Fujian Alisma orientale has a stronger diuretic effect than those from other origins.3.5 Comparison of the description of salt-made Alisma orientale from different origins
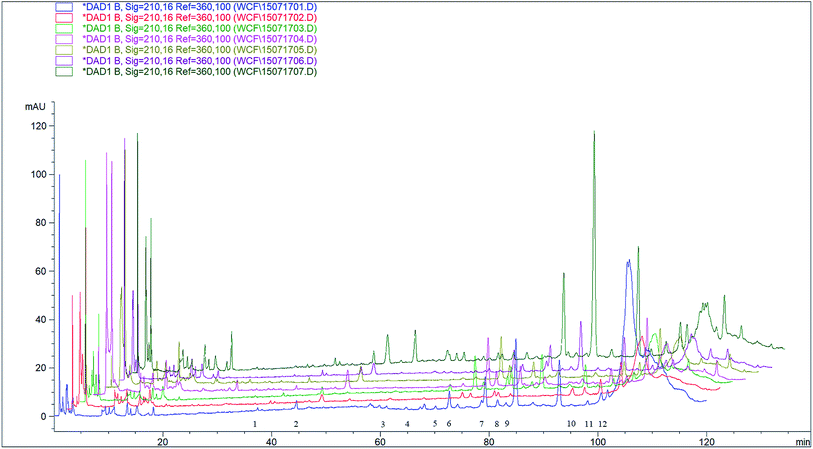
As can be seen from Fig. 3, the cross section of salt-made Alisma orientale from different origins is yellowish white, and the powder is powdery. The powder of salt-made Alisma orientale from Guangxi, Hebei, Hubei and Henan producing areas is yellowish, which may be related to the origin of salt-made Alisma orientale or a difference in the processing technology.3.6 Comparison of chromatograms of Alisma orientale from different origins
HPLC was used to analyze the compounds of the salt-made Alisma orientale from different origins at 210 nm (Fig. 4). Peak area statistics and comparisons were carried out on the peaks of the 12 components in the methanol ultrasonic extract of Alisma orientale from different origins (Table 6).| Peak no. | Fujian | Jiangxi | Sichuan | Guangxi | Hubei | Hebei | Henan |
|---|---|---|---|---|---|---|---|
| 1 | 34.1 | 184.8 | 144.9 | 31.7 | 317.7 | 346.8 | 222.0 |
| 2 | 117.0 | 163.0 | 111.7 | 143.2 | 287.5 | 60.6 | 152.9 |
| 3 | 9.4 | 213.8 | 24.2 | 761.3 | 496.2 | 780.2 | 965.3 |
| 4 | 20.3 | 40.3 | 26.3 | 18.5 | 15.7 | 44.9 | 25.7 |
| 5 | 357.3 | 121.4 | 777.1 | 1433.1 | 695.9 | 151.9 | 659.3 |
| 6 | 687.6 | 160.5 | 516.8 | 58.4 | 921.0 | 1868.5 | 653.5 |
| 7 | 189.4 | 111.8 | 516.8 | 321.5 | 107.5 | 112.1 | 75.2 |
| 8 | 176.4 | 160.5 | 338.8 | 610.2 | 376.4 | 51.8 | 275.0 |
| 9 | 70.9 | 105.9 | 190.4 | 438.6 | 219.1 | 37.8 | 225.1 |
| 10 | 23.0 | 205.3 | 579.1 | 453.0 | 45.6 | 153.0 | 38.1 |
| 11 | 89.2 | 295.6 | 154.4 | 183.0 | 143.9 | 252.7 | 133.2 |
| 12 | 10.6 | 592.9 | 225.8 | 45.6 | 227.9 | 711.2 | 178.5 |
3.7 Principal component analysis of the components of salt-made Alisma orientale
We analyzed the peak areas of 12 common components of salt-made Alisma orientale from nine different origins, we set 12 components as variables X1–X12 respectively, and established chromatographic peak area data for the seven origins. The results showed that the cumulative contribution rate of the first principal component to the fourth principal component can reach 90.63%, indicating that these four representative components are the main components of salt-made Alisma orientale. Finally, the peak numbers for the four components were determined to be 5, 8, 9 and 10 respectively, of which peaks 8, 9 and 10 are alisol A 24-acetate, alisol B and alisol B 23-acetate respectively (Tables 7 and 8).| Component | Initial characteristic value | Extraction sums of squared loadings | ||||
|---|---|---|---|---|---|---|
| Characteristic value | % of variance | Cumulative% | Total | % of variance | Cumulative% | |
| 1 | 4.864 | 40.533 | 40.533 | 4.864 | 40.533 | 40.533 |
| 2 | 2.831 | 23.589 | 64.122 | 2.831 | 23.589 | 64.122 |
| 3 | 2.078 | 17.315 | 81.437 | 2.078 | 17.315 | 81.437 |
| 4 | 1.103 | 9.192 | 90.629 | 1.103 | 9.192 | 90.629 |
| 5 | 0.682 | 5.679 | 96.308 | |||
| 6 | 0.311 | 2.594 | 98.903 | |||
| 7 | 0.078 | 0.646 | 99.549 | |||
| 8 | 0.054 | 0.451 | 100.000 | |||
| 9 | 4.631 × 10−16 | 3.859 × 10−15 | 100.000 | |||
| 10 | 1.870 × 10−16 | 1.558 × 10−15 | 100.000 | |||
| 11 | 1.970 × 10−17 | 1.641 × 10−16 | 100.000 | |||
| 12 | −2.272 × 10−16 | −1.893 × 10−15 | 100.000 | |||
| Component | Principal component | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| X1 | −0.070 | 0.714 | 0.530 | −0.215 |
| X2 | 0.237 | −0.405 | 0.568 | −0.603 |
| X3 | 0.015 | −0.108 | 0.713 | 0.676 |
| X4 | −0.718 | 0.481 | −0.199 | 0.236 |
| X5 | 0.955 | −0.071 | 0.132 | 0.206 |
| X6 | −0.760 | 0.068 | 0.140 | 0.255 |
| X7 | 0.655 | 0.216 | −0.655 | 0.009 |
| X8 | 0.973 | −0.121 | 0.156 | 0.045 |
| X9 | 0.932 | −0.061 | 0.281 | 0.202 |
| X10 | 0.695 | 0.451 | −0.488 | 0.114 |
| X11 | 0.204 | 0.915 | 0.164 | −0.095 |
| X12 | 0.163 | 0.894 | 0.305 | −0.092 |
The results of the principal component analysis showed that peaks 5, 8, 9 and 10 were the principal components of salt-made Alisma orientale prepared from different origins. Peaks 8, 9 and 10 are alisol A 24-acetate, alisol B and alisol B 23-acetate, respectively. Therefore, the three components are representative components of salt-made Alisma orientale. According to the pharmacodynamic results mentioned above, the peak area ratio of alisol A 24-acetate, alisol B and alisol B 23-acetate is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40
0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 which represents the best effect of the drug. This ratio is the characteristic feature of geoherb Alisma orientale and enables the excellent “diuresis and diffusing dampness” performance (Table 9).
0.13 which represents the best effect of the drug. This ratio is the characteristic feature of geoherb Alisma orientale and enables the excellent “diuresis and diffusing dampness” performance (Table 9).
| Origin | Peak area | Structural characteristics (peak area ratio) | ||
|---|---|---|---|---|
| Alisol A 24-acetate | Alisol B | Alisol B 23-acetate | ||
| Fujian | 176.4 | 70.9 | 23.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40 0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.13 0.13 |
| Jiangxi | 160.5 | 105.9 | 205.3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.66 0.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.28 1.28 |
| Sichuan | 338.8 | 190.4 | 579.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.56 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.71 1.71 |
| Guangxi | 610.2 | 438.6 | 453.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.72 0.72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.74 0.74 |
| Hubei | 376.4 | 219.1 | 45.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.58 0.58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.12 0.12 |
| Hebei | 51.8 | 37.7 | 153.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.73 0.73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.95 2.95 |
| Henan | 275.0 | 225.1 | 38.1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.82 0.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.14 0.14 |
3.8 Effects of different structural optimizations of terpenoids on the expression of AQP-2 and Kim-1 in HK-2 cells
From the experimental results described in the previous section, it can be seen that the diuretic effect of salt-made Alisma orientale is related to a significant reduction in the expression of the AQP-2 protein, and the nephrotoxicity is manifested by a significant enhancement in the Kim-1 protein expression. As shown in Fig. 5 and Table 8, compared with the blank group, when the component structure of alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B
alisol B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B 23-acetate is 2.99
alisol B 23-acetate is 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.43 to 2.99
11.43 to 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.01 (i.e. I-1–I-3), the expression of the AQP-2 protein is significantly reduced (P < 0.05), and at the same time, the level of the Kim-1 protein is not significantly different from the blank group when I-2–I-4 (P > 0.05). To summarize, the structural ratios of alisol A 24-acetate
6.01 (i.e. I-1–I-3), the expression of the AQP-2 protein is significantly reduced (P < 0.05), and at the same time, the level of the Kim-1 protein is not significantly different from the blank group when I-2–I-4 (P > 0.05). To summarize, the structural ratios of alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B
alisol B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B 23-acetate are 2.99
alisol B 23-acetate are 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.08 and 2.99
8.08 and 2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.01, and the components are safe and effective. With the decrease in the 24A structural ratio, the expression of the AQP-2 protein decreased gradually, and the expression of the Kim-1 protein also decreased gradually. When alisol B
6.01, and the components are safe and effective. With the decrease in the 24A structural ratio, the expression of the AQP-2 protein decreased gradually, and the expression of the Kim-1 protein also decreased gradually. When alisol B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B 23-acetate
alisol B 23-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol A 24-acetate is 14.34
alisol A 24-acetate is 14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31
11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.38 (i.e. II-2), the expression of the terpene component AQP-2 protein decreased significantly (P < 0.05) and its toxicity was relatively low compared with the blank group. Compared with the blank group, when the structural ratio of alisol B 23-acetate
5.38 (i.e. II-2), the expression of the terpene component AQP-2 protein decreased significantly (P < 0.05) and its toxicity was relatively low compared with the blank group. Compared with the blank group, when the structural ratio of alisol B 23-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol A 24-acetate
alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B is 11.31
alisol B is 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99
2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.25 to 11.31
14.25 to 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99
2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.84 (i.e. III-1–III-3), the AQP-2 protein expression is significantly reduced (P < 0.05), but when the structural ratio of them is 11.31
8.84 (i.e. III-1–III-3), the AQP-2 protein expression is significantly reduced (P < 0.05), but when the structural ratio of them is 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99
2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.25, it is revealed that the HK-2 cells are toxic (P > 0.05). Therefore, when the structural ratios of alisol B 23-acetate
14.25, it is revealed that the HK-2 cells are toxic (P > 0.05). Therefore, when the structural ratios of alisol B 23-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol A 24-acetate
alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B are 11.31
alisol B are 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99
2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.84 and 11.31
8.84 and 11.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.99
2.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.06, the components are safe and effective. To summarize, the high efficiency and low toxicity of Alisma orientale were fully analyzed, and the terpenoid components of Alisma orientale were preliminarily confirmed as alisol A 24-acetate
9.06, the components are safe and effective. To summarize, the high efficiency and low toxicity of Alisma orientale were fully analyzed, and the terpenoid components of Alisma orientale were preliminarily confirmed as alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B
alisol B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B 23-acetate and their structural ratio is 5.38
alisol B 23-acetate and their structural ratio is 5.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31.
11.31.
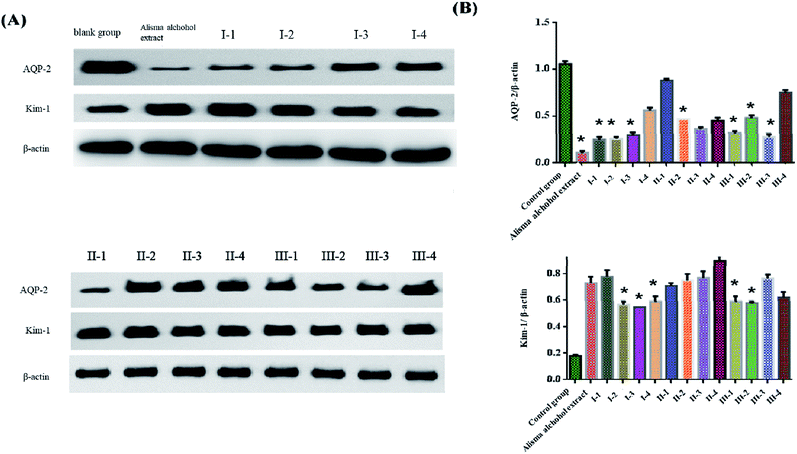 | ||
| Fig. 5 The protein expressions of AQP-2 and Kim-1 were determined using western blot. *: P < 0.05, compared with the control group. | ||
4 Discussion
It is very common that the same kind of traditional Chinese medicine produced in different areas shows differences in the quality of medicinal materials, owing to the significant difference in the climatic conditions and soil environment in China. Therefore, an accurate quality evaluation of traditional Chinese medicine from different regions is the key to preventing and treating diseases. In order to explore the intrinsic reasons for the superiority of geoherb salt-made Alisma orientale, a pharmacodynamic experiment was carried out to verify the superiority of Fujian Alisma. HPLC and principal component analysis were carried out and they revealed the structural composition of the components of salt-made Alisma orientale from the Fujian Province. Finally, in order to study the toxicity of Alisma orientale, an experiment was performed to determine the structural optimization and showed the safe and effective range of the ratio of components in Alisma orientale.“Diuresis and diffusing dampness” have been regarded as the traditional effect of Alisma orientale. Aquaporin is the target molecule for diuretic traditional Chinese medicine to exerts its efficacy. AQP-2 is the main target molecule of the antidiuretic hormone for the regulation of water permeability of the collector tube, and it plays an important role in adjusting urine concentration.22 Diuretic effects are closely related to the excretion of Na+, K+ and Cl− in the body and the regulation of aquaporins.23,24 Therefore, the urinary Na+, K+ and Cl− levels and the expression of AQP-2 were used to study the efficacy of diuresis and diffusing dampness. In this paper, the diuretic effects of Alisma orientale were investigated in vivo and in vitro, and the efficacy of salted Alisma orientale from seven different producing areas was compared. The results showed that the effect of Alisma orientale was mainly the increase in urine volume and the level of Na+, K+ and Cl− in urine, and it is also related to the regulation of AQP-2 expression. By analyzing the diuretic effect of Alisma orientale from different producing areas, we found that the effect of Alisma orientale from Fujian on increasing urine volume and reducing AQP-2 expression was the most significant, which suggests that Fujian Salted Alisma orientale has a better “diuresis and diffusing dampness” effect than those from other producing areas, which can be used as a preliminary indication of its “superiority”.
In our research, we evaluated the nephrotoxicity of Alisma orientale. Kim-1, a type I transmembrane protein, is highly expressed in the proximal tubule cells after ischemia and renal toxicity injury, but is rarely expressed in normal renal tissue. Previous studies have shown that Kim-1 is a highly sensitive and specific biomarker of renal tubular toxicity.25 Traditional Chinese medicine as a whole consists of a certain ratio of components and its efficacy can be exerted through the synergistic action of each component. Toxicity also has a regulatory mode when the ratio of components is beyond a certain balance range. Therefore, the structural optimization of the components of Alisma orientale was determined in order to obtain the safe ratio range of the components. The results showed that when the ratio of alisol A 24-acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B
alisol B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alisol B 23-acetate is 5.38
alisol B 23-acetate is 5.38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14.34
14.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11.31, we can ensure the efficacy of Alisma orientale and reduce the nephrotoxicity caused by it.
11.31, we can ensure the efficacy of Alisma orientale and reduce the nephrotoxicity caused by it.
The multi-target and multi-path action mode of traditional Chinese medicine suggests that the effectiveness of traditional Chinese medicine depends not on the role of a certain component, but on the result of the synergy of multiple components within/between components. Principal component analysis is used to convert multiple indicators into a few comprehensive indicators, that is, the main component, and the use of comprehensive indicators to analyze problems is widely used in the field of traditional Chinese medicine. Principal component analysis was carried out and three main components were obtained, alisol A 24-acetate, alisol B and alisol B 23-acetate, and the peak area ratio of the main three components in Fujian Alisma orientale was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, which is close to the ratio of the three components after optimization with better efficacy and no obvious nephrotoxicity. Judging from the three main component peak areas of salt-made Alisma orientale in various producing areas, Fujian salt-made Alisma orientale did not show any advantages in terms of the contents of the individual components. In contrast, the peaks area of these three components in Fujian salt-formed Alisma orientale are much smaller than that of several other producing areas, which further indicates that studying the quality evaluation of traditional Chinese medicine by determining the contents of several components is unreliable. There is a synergy between the three main components of Alisma orientale. This synergy is not a simple superposition of components, but a difference in the contribution of each component to the overall drug effect. According to the pharmacodynamic experiments, we can see that when the component ratio of the different areas producing Alisma orientale approaches the optimal ratio, the efficacy was improved.
1.3, which is close to the ratio of the three components after optimization with better efficacy and no obvious nephrotoxicity. Judging from the three main component peak areas of salt-made Alisma orientale in various producing areas, Fujian salt-made Alisma orientale did not show any advantages in terms of the contents of the individual components. In contrast, the peaks area of these three components in Fujian salt-formed Alisma orientale are much smaller than that of several other producing areas, which further indicates that studying the quality evaluation of traditional Chinese medicine by determining the contents of several components is unreliable. There is a synergy between the three main components of Alisma orientale. This synergy is not a simple superposition of components, but a difference in the contribution of each component to the overall drug effect. According to the pharmacodynamic experiments, we can see that when the component ratio of the different areas producing Alisma orientale approaches the optimal ratio, the efficacy was improved.
It is worth noting that there was no significant difference between the high and low doses from different origins. This suggests that the relationship between the Alisma orientale dose and diuretic efficacy may not be linear. A similar phenomenon can be seen in other studies and the results suggest that Alisma orientale may contain both diuretic and antidiuretic components. The antidiuretic effect of Alisma orientale might be exerted by promoting the sodium-chloride co-transporter in the distal tubule.26,27 In addition, many kinds of traditional Chinese medicine have been found to have a bidirectional regulation effect through different targets.28 Alisma orientale may also have this effect. The relationship between the dose of Alisma orientale and the diuretic efficacy requires further research.
5 Conclusions
The “Structural Composition of Components Theory” proposed by our research group clearly indicates that the proportional relationships between components29 are the material basis of traditional Chinese medicine. This study found that Fujian salt-made Alisma orientale exhibits the best diuretic effect and the optimal structural composition of the components of salt-made Alisma orientale has a ratio of alisol A 24-acetate, alisol B, and alisol B 23-acetate of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3. The quality evaluation of traditional Chinese medicine based on the “Structural Composition of multi-Components Theory” can effectively avoid differences in the levels of components caused by the environment, and lays the foundation for the quality evaluation of salt-made Alisma orientale and other herb slices.
1.3. The quality evaluation of traditional Chinese medicine based on the “Structural Composition of multi-Components Theory” can effectively avoid differences in the levels of components caused by the environment, and lays the foundation for the quality evaluation of salt-made Alisma orientale and other herb slices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation Committee of P. R. China (No. 81603382, 81703775), “Double First-Class” University project of China Pharmaceutical University (CPU2018GF07, CPU2018PZQ19), and Key research projects on modernization of traditional Chinese medicine (2018YFC1706900).References
- G. Yu, Z. Luo, W. Wang, Y. Li, Y. Zhou and Y. Shi, Front. Pharmacol., 2019, 10, 799 CrossRef CAS.
- M. S. Tsang, P. C. Shaw, I. M. Chu, L. Cheng, E. C. Wong, D. T. Lau, C. W. Lam and C. K. Wong, Molecules, 2019, 24, 2880 CrossRef CAS.
- W. K. Zheng, L. Zhang and S. J. Zan, Zhongguo Zhongyao Zazhi, 2019, 44, 624–628 Search PubMed.
- P. Zhang, M. H. Li, Y. Shi, X. L. Cheng, H. Yan, W. Liu, F. Wei and S. C. Ma, Zhongguo Zhongyao Zazhi, 2018, 43, 4198–4202 Search PubMed.
- L. X. Zhu, J. Xu, Y. Wu, L. F. Su, K. Y. C. Lam, L. Ching, E. R. Qi, X. P. Dong, H. B. Chen, Y. D. Liu and Z. Z. Zhao, J. Food Drug Anal., 2019, 27, 766–777 CrossRef CAS.
- Y. X. Li, X. H. Gong, M. C. Liu, C. Peng, P. Li and Y. T. Wang, J. Evidence-Based Complementary Altern. Med., 2017, 2017, 6238464 Search PubMed.
- Chinese Pharmacopoeia Commission of China, Ch. P., Beijing, 2015, p. 229 Search PubMed.
- A. C. Zhou, C. F. Zhang and M. Zhang, Chin. J. Nat. Med., 2008, 6, 109–111 CrossRef CAS.
- X. Y. Hu, Y. Q. Guo, W. Y. Gao and T. J. Zhang, Chin. Chem. Lett., 2008, 19, 438–440 CrossRef CAS.
- X. Liu, S. L. Li and H. X. Xu, J. Mass Spectrom., 2010, 24, 1514–1522 CAS.
- X. Y. Hu, Y. Q. Guo and H. X. Chen, Sep. Purif. Technol., 2008, 10, 481–484 Search PubMed.
- Y. L. Feng, H. Chen and R. C. Lin, J. Ethnopharmacol., 2014, 154, 386–390 CrossRef.
- D. Q. Chen, Y. L. Feng and R. C. Lin, J. Ethnopharmacol., 2014, 157, 114–118 CrossRef CAS.
- T. Tian, H. Chen and Y. Y. Zhao, J. Ethnopharmacol., 2014, 158, 373–387 CrossRef CAS.
- Z. Shu, J. Pu, L. Chen, Y. Zhang, K. Rahman, L. Qin and C. Zheng, Am. J. Chin. Med., 2016, 44, 227–251 CrossRef CAS.
- L. L. Zhang, W. Xu, Y. L. Xu, X. Chen, M. Huang and J. J. Lu, Ann. N. Y. Acad. Sci., 2017, 1401, 90–101 CrossRef CAS.
- H. S. Lee, I. H. Hwang and J. A. Kim, Bull. Korean Chem. Soc., 2011, 32, 697–700 CrossRef CAS.
- W. X. Zhao, N. Cui, H. Q. Jiang, X. M. Ji, X. C. Han, B. B. Han, T. Wang and S. J. Wang, J. Evidence-Based Complementary Altern. Med., 2017, 2017, 4946031 Search PubMed.
- Y. Y. Zhao, R. M. Xie, X. Chao, Y. Zhang, R. C. Lin and W. J. Sun, J. Ethnopharmacol., 2009, 126, 184–187 CrossRef CAS.
- P. L. Jørgensen, J. Petersen and W. D Rees, Biochim. Biophys. Acta, Biomembr., 1984, 775, 105–110 CrossRef.
- X. Zhang, X. Y. Li, N. Lin, W. L. Zhao, X. Q. Huang, Y. Chen, M. Q. Huang, W. Xu and S. S. Wu, Molecules, 2017, 22, 1459 CrossRef.
- J. He, L. Zeng, R. Wei, G. Zhong, Y. Zhu, T. Xu and L. Yang, J. Ethnopharmacol., 2019, 231, 446–452 CrossRef CAS.
- E. Moreno, J. A. Gayosso, J. R. Montejano, G. Almaguer, N. Vázquez, C. Cruz, A. Mercado, N. A. Bobadilla, G. Gamba, A. Sierra and V. Ramírez, Am. J. Physiol.: Renal Physiol., 2017, 314, F240–F250 CrossRef.
- L. Santucci, G. Candiano, M. Bruschi, M. Bodria, C. Murtas, A. Petretto and G. M. Ghiggeri, Am. J. Nephrol., 2013, 26, 610–616 CrossRef CAS.
- L. Yang, C. R. Brooks, S. Xiao, V. Sabbisetti, M. Y. Yeung, L. L. Hsiao, T. Ichimura, V. Kuchroo and J. V. Bonventre, J. Clin. Invest., 2015, 125(4), 1620–1636 CrossRef.
- D. Q. Chen, Y. L. Feng, T. Tian, H. Chen, L. Yin, Y. Y. Zhao and R. C. Lin, J. Ethnopharmacol., 2014, 157, 114–118 CrossRef CAS.
- Y. L. Feng, H. Chen, T. Tian, D. Q. Chen, Y. Y. Zhao and R. C. Lin, J. Ethnopharmacol., 2014, 154(2), 386–390 CrossRef.
- H. J. Wang, J. J. Wu, Q. Xue, J. F. Zhang and X. Y. Xu, China J. Chin. Mater. Med., 2017, 11, 2200–2207 Search PubMed.
- X. B. Jia, B. Yang, L. Feng, X. H. Shi, H. Wang and L. G. Liu, Acta Pharmacol. Sin., 2018, 53, 1943–1953 Search PubMed.
Footnote |
| † Ru Li, Zhi-li Li, and Ya-ping Chen contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |