Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rationalization of the X-ray photoelectron spectroscopy of aluminium phosphates synthesized from different precursors†
Victoria Bemmer
 d,
Michael Bowker
d,
Michael Bowker a,
James H. Carter
a,
James H. Carter a,
Philip R. Davies
a,
Philip R. Davies *a,
Lee E. Edwards
*a,
Lee E. Edwards a,
Kenneth D. M. Harris
a,
Kenneth D. M. Harris b,
Colan E. Hughesb,
Fiona Robinson
b,
Colan E. Hughesb,
Fiona Robinson c,
David J. Morgan
c,
David J. Morgan a and
Matthew G. Thomasa
a and
Matthew G. Thomasa
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK. E-mail: daviespr@cardiff.ac.uk
bSchool of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK
cCogent Power Ltd, Newport NP19 0RB, UK
dDept. of Materials, Imperial College, South Kensington Campus, London SW7 2AZ, UK
First published on 26th February 2020
Abstract
The aim of this paper is to clarify the assignments of X-ray photoelectron spectra of aluminium phosphate materials prepared from the reaction of phosphoric acid with three different aluminium precursors [Al(OH)3, Al(NO3)3 and AlCl3] at different annealing temperatures. The materials prepared have been studied by X-ray photoelectron spectroscopy (XPS), powder X-ray diffraction (XRD), infrared spectroscopy and high-resolution solid-state 31P NMR spectroscopy. A progressive polymerization from orthophosphate to metaphosphates is observed by XRD, ATR-FTIR and solid state 31P NMR, and on this basis the oxygen states observed in the XP spectra at 532.3 eV and 533.7 eV are assigned to P–O–Al and P–O–P environments, respectively. The presence of cyclic polyphosphates at the surface of the samples is also evident.
Introduction
There are a wide array of applications of phosphate based materials, including biomedical (due to the natural occurrence of phosphates in physiology and their biocompatibility1–4), ceramics (due to their high strength and thermal stability) and refractory materials (in which phosphates are frequently used as binders5). Curing temperatures of phosphates are critical to their performance in the latter applications and as a result there has been a concerted effort to understand the thermal evolution of phosphate systems. Heating induces a progressive polymerization starting from orthophosphates (PO43−), which polymerize to form a series of polyphosphates such as the pyrophosphates (P2O74−) and finally metaphosphates, which are long range cross-linked networks (PO3−)n.6 This paper is focused on aluminium phosphates generated from a mixture of phosphoric acid and three different aluminium based precursors: Al(OH)3, Al(NO3)3 and AlCl3.The majority of studies on aluminium phosphates have focused on bulk analysis techniques,7 such as powder X-ray diffraction (XRD), solid-state 31P NMR, FTIR and thermogravimetric methods, but the surface properties of these materials are also of interest and one of the most commonly used techniques to investigate this aspect is X-ray photoelectron spectroscopy (XPS). A seminal study by Gresch et al.8 in 1979 on XPS of sodium phosphates provided well substantiated peak assignments for the oxygen region. This paper has since been extensively cited and used as a benchmark for XPS studies of phosphates. Gresch et al. proposed that the different states of oxygen created by crosslinking between phosphate units could be distinguished by their XP spectra, with the “bridging” oxygens appearing at higher binding energy (533.1–533.6 eV) than “non-bridging” oxygens (530.5–531.7 eV). This assignment was based on electronegativity arguments and spectra of model compounds. Concomitant with the shift in O(1s) binding energy, Gresch et al. reported that the P(2p) binding energy shifts from 132.5 eV to 134.5 eV as the degree of P–O–P bridging bonds increases.
More recently, Crobu et al.9 have also used the shift in O(1s) binding energy to assess the ratio of bridging to non-bridging oxygen in zinc polyphosphate glasses, correlating their results with secondary ion mass spectrometry measurements. They reported a shift from 532.2 eV to 534.0 eV as the reference material changed from an orthophosphate to a metaphosphate, with the P(2p) peak shifting from 134.0 eV to 134.8 eV.
Rotole and Sherwood, on the other hand, studied electrochemically deposited phosphate films on aluminium substrates.10 The XP spectra were referenced against data on aluminium orthophosphate11 and metaphosphate12 samples. They reported a constant P(2p) binding energy of 134.5 eV and a shift in the O(1s) peak from 531.4 eV for the orthophosphate to a broader peak at 531.8 eV for the metaphosphate. The latter peak clearly showed evidence for a second component at about 533.5 eV. However, the O(1s) data from the electrochemically treated surface were not so conclusive; the O(1s) spectrum of the “metaphosphate” was significantly broader than that of the orthophosphate and seemed to consist of two components. It is not clear whether the component due to bridging oxygens is at higher or lower binding energy than the component due to terminal oxygens.
In the present study, we have used solid-state 31P NMR, powder XRD and FTIR data to explore the structural changes that occur in aluminium phosphate materials synthesized from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures of phosphoric acid and an aluminium precursor (either aluminium hydroxide, aluminium nitrate or aluminium chloride), which throw light on the information available from the surface specific XPS technique. Our results show that the expected polymerization occurs in materials prepared from all three precursors, and largely confirm the assignments of XP spectra based on existing literature. However, we also find evidence for the presence, at the surface, of polyphosphate species that do not contain aluminium, which distorts the Al
1 mixtures of phosphoric acid and an aluminium precursor (either aluminium hydroxide, aluminium nitrate or aluminium chloride), which throw light on the information available from the surface specific XPS technique. Our results show that the expected polymerization occurs in materials prepared from all three precursors, and largely confirm the assignments of XP spectra based on existing literature. However, we also find evidence for the presence, at the surface, of polyphosphate species that do not contain aluminium, which distorts the Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios established from the XPS spectra.
P ratios established from the XPS spectra.
Experimental
Synthesis of aluminium phosphate powders
The aluminium precursor AlX3, where X = OH (Sigma-Aldrich, Reagent Grade), NO3 (Sigma-Aldrich, ACS Reagent, >98%) or Cl (Sigma-Aldrich, 99.99%), was added to water (50 ml) at 298 K and mixed until dissolved. H3PO4 (8.75 ml, Sigma-Aldrich, 85% wt, 99.99% purity) was added to the AlX3 solution to give a P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio of 3
Al ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the solution was allowed to mix for 30 min. The reaction mixture was then heated on a hotplate to evaporate the water, resulting in the formation of a viscous gel. Separate samples of the gel were heated in air in a furnace for a period of one hour at three different temperatures (300, 500 and 800 °C), and then allowed to cool to room temperature before analysis. In the case of the materials prepared from Al(OH)3 and Al(NO3)3, a slightly wet white powder was obtained on heating to 300 °C which was observed (by eye) to dry completely on heating at the higher temperatures. However, the samples prepared from AlCl3 remained gel-like at 300 °C. These samples were stable under vacuum and so could be analysed by XPS, but they had to be heated to 400 °C to obtain a solid that was suitable for analysis by solid-state NMR and ATR could be obtained. In the discussion below, the materials prepared from the Al(OH)3, Al(Cl)3 and Al(NO3)3 precursors are labelled AlPOH, AlPCl and AlPNO3, respectively.
1 and the solution was allowed to mix for 30 min. The reaction mixture was then heated on a hotplate to evaporate the water, resulting in the formation of a viscous gel. Separate samples of the gel were heated in air in a furnace for a period of one hour at three different temperatures (300, 500 and 800 °C), and then allowed to cool to room temperature before analysis. In the case of the materials prepared from Al(OH)3 and Al(NO3)3, a slightly wet white powder was obtained on heating to 300 °C which was observed (by eye) to dry completely on heating at the higher temperatures. However, the samples prepared from AlCl3 remained gel-like at 300 °C. These samples were stable under vacuum and so could be analysed by XPS, but they had to be heated to 400 °C to obtain a solid that was suitable for analysis by solid-state NMR and ATR could be obtained. In the discussion below, the materials prepared from the Al(OH)3, Al(Cl)3 and Al(NO3)3 precursors are labelled AlPOH, AlPCl and AlPNO3, respectively.
Materials characterization
XP spectra were recorded at room temperature on the powder samples using a Kratos Axis Ultra-DLD photoelectron spectrometer with a monochromatic Al Kα X-ray source in the “hybrid spectroscopy” mode resulting in an analysis area of 700 × 300 μm2 at a pass-energy of 40 eV for high-resolution scans and 160 eV for survey scans. The XPS data were analysed using CasaXPS13 with all binding energies referenced to the C(1s) peak at 284.7 eV with an uncertainty of ∼0.2 eV. Since intensities for powder samples are dependent on the surface area analysed, which is poorly reproducible between different powder samples, the XP spectra shown in the figures are normalized to the point of maximum intensity. Curve fits were made using Gaussian–Lorentzian (GL (30)) line-shapes.Powder X-ray diffraction (XRD) data were recorded at room temperature using a PANalytical X'Pert Pro diffractometer with a monochromatic Cu Kα source (λ = 0.154 nm) operating at 40 kV and 40 mA. The data were recorded over the 2θ range 10–80° with a step size of 0.016°.
High-resolution solid-state 31P NMR spectra were acquired at room temperature on a Chemagnetics Infinity Plus spectrometer (31P Larmor frequency, 121.50 MHz). The samples were contained in a 4 mm rotor with magic-angle spinning at 12 kHz. Methyldiphenylphosphine oxide (MDPPO) was used as a reference, with 31P chemical shift at 30.8 ppm.
FTIR spectra were recorded using a germanium crystal ATR on a Varian 3100 Excalibur system with Varian Resolutions Pro software.
Results
Bulk structural analysis using FTIR, XRD and solid-state 31P NMR
Throughout the discussion, we refer to each sample by the notation AlPOH(T), AlPCl(T) or AlPNO3(T), where the subscript identifies the precursor used in the synthesis and T denotes the annealing temperature. All the analysis was performed after cooling to room temperature.The crystalline phases present within each sample were investigated by powder XRD. Fig. 1, shows the XRD patterns for the AlPOH samples. For AlPOH(300) [i.e., the sample prepared from the Al(OH)3 precursor and annealed at 300 °C], only low intensity peaks are observed in the XRD data, and we have been unable to definitively match these peaks to a known structure. For the AlPOH(500) sample, the XRD data show sufficient crystallinity to allow Le Bail fitting, although Rietveld refinement was not possible. The Le Bail fitting confirms that two distinct phases are present: a cubic aluminium metaphosphate [Al(PO3)3] (ICSD: 26759) and an aluminium hexacyclophosphate (ICSD: 260723). For the AlPOH(800) sample, only the cubic aluminium metaphosphate is present. The results from Le Bail fitting are shown in Fig. S1 and S2 (in ESI†).
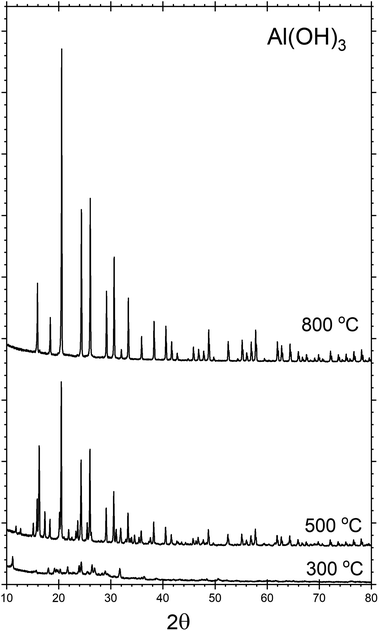 | ||
| Fig. 1 Powder XRD patterns recorded for the AlPOH materials, with annealing at different temperatures. The AlPOH(300) sample is mostly non-crystalline, but both cubic metaphosphate and hexacyclophosphate are present in the AlPOH(500) sample. The AlPOH(800) sample is a pure phase of the cubic metaphosphate. Data for the AlPCl and AlPNO3 materials are given in ESI.† | ||
d'Yvoire14,15 also reported a pure cubic phase for a material prepared from Al(OH)3 and annealed at 800 °C, with a second phase present in the material annealed at 500 °C. However, d'Yvoire assigned the second phase to a monoclinic structure rather than the aluminium hexacyclophosphate observed here.
XRD indicates that the AlPNO3(500) and AlPNO3(800) samples (Fig. S3 and S4†) are a mixture of the cubic aluminium metaphosphate and aluminium hexacyclophosphate phases [similar to AlPOH(500) but different from AlPOH(800)]. Among the samples prepared from the AlCl3 precursor, the only crystalline product was AlPCl(800), identified from XRD as pure cubic Al(PO3)3 (Fig. S5†).
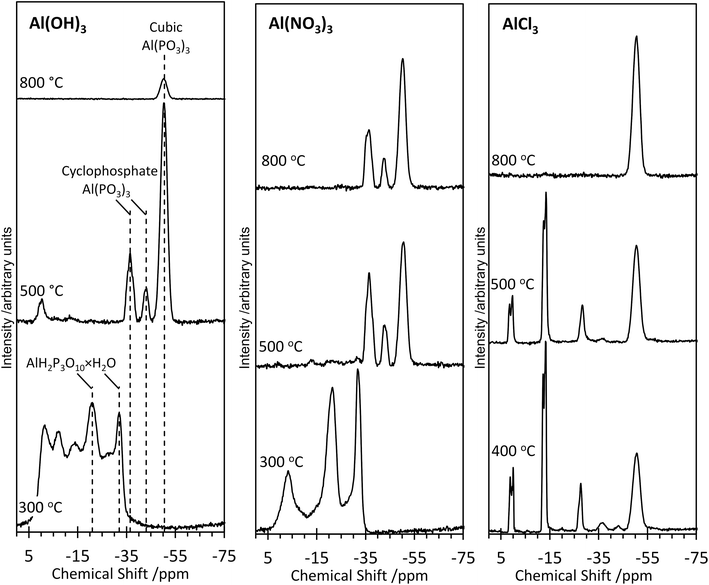
Further structural insights are obtained from high-resolution solid-state 31P NMR spectra (Fig. 2). For several of the samples, a peak at 0 ppm is present and assigned as the phosphoric acid starting material. As the annealing temperature increases, there is a trend towards increasingly negative 31P chemical shifts, attributed to polymerization.16 For AlPOH(300), a broad set of overlapping peaks is observed between 5 ppm and −40 ppm, possibly suggesting an amorphous structure. The peaks at −21 ppm and −32 ppm (which represent ca. 20% of the total signal) are assigned16 to aluminium tripolyphosphate (AlH2P3O10·H2O). These peaks are also observed for AlPNO3(300). For AlPOH(800), only one peak is observed (at 50.5 ppm) and is attributed unambiguously to cubic Al(PO3)3, consistent with the presence of a single phosphorus environment in this structure (Fig. 3). This peak is also present for the AlPOH(500) sample, together with peaks at −36.5 ppm and −43.0 ppm; the area ratio for these two peaks is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, consistent with the presence of three crystallographically distinct phosphorus environments in the aluminium hexacyclophosphate phase (Fig. 3). Monoclinic Al(PO3)3, on the other hand, has 9 distinct phosphorus environments (Fig. 3).
1, consistent with the presence of three crystallographically distinct phosphorus environments in the aluminium hexacyclophosphate phase (Fig. 3). Monoclinic Al(PO3)3, on the other hand, has 9 distinct phosphorus environments (Fig. 3).
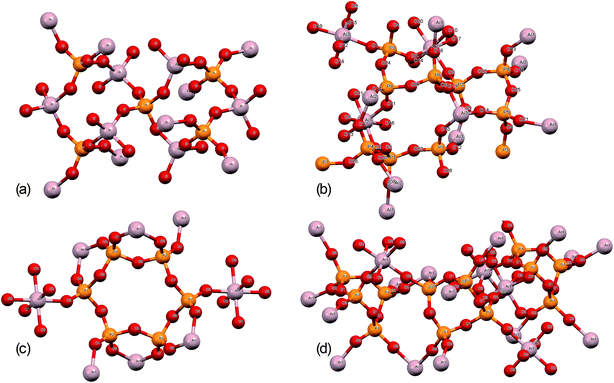 | ||
| Fig. 3 Structures of (a) aluminium orthophosphate (AlPO4), (b) monoclinic aluminium metaphosphate, (c) aluminium hexacyclophosphate, and (d) cubic aluminium metaphosphate. | ||
For the AlPNO3(300) sample, three peaks are observed between 5 ppm and −35 ppm [in contrast to the overlapping set of peaks observed in this region for AlPOH(300)], including peaks at −21 ppm and −32 ppm assigned to aluminium tripolyphosphate (AlH2P3O10·H2O), which represents ∼50% of the signal. The peak at −3.4 ppm is attributed to some remaining aluminium orthophosphate. For the AlPNO3(500) sample, the 31P NMR spectrum contains peaks characteristic of the hexacyclophosphate (43.3%) and cubic metaphosphate (52%). Annealing at a higher temperature does not complete the transformation to the cubic metaphosphate as the 31P NMR spectrum for the AlPNO3(800) sample clearly contains peaks due to the hexacyclophosphate phase (ca. 36% of the signal intensity).
The 31P NMR spectra for AlPCl(400) and AlPCl(500) are significantly different from those observed for the AlPOH and AlPNO3 materials. The major peaks are due to cubic metaphosphate (50.5 ppm), orthophosphate (1 ppm and 0 ppm), pyrophosphate (−12 ppm and −13.5 ppm) and polyphosphate (peaks in the range −20 ppm to −28 ppm), with only very weak peaks observed for hexacyclophosphate. In contrast, the AlPCl(800) sample is a pure phase of cubic Al(PO3)3. These observations suggest that the metaphosphate formed from the AlCl3 precursor may be produced via a slightly different pathway than from the Al(OH)3 and Al(NO3)3 precursors. We deduce that the stability of the Al–Cl bond hinders formation of aluminium phosphate from the Al(Cl)3 precursor at lower temperatures, leaving the phosphoric acid to react mostly with itself to form varying degrees of polyphosphates. However, at the higher annealing temperature of 800 °C, the phosphate transforms completely to cubic Al(PO3)3.
Surface analysis with XPS
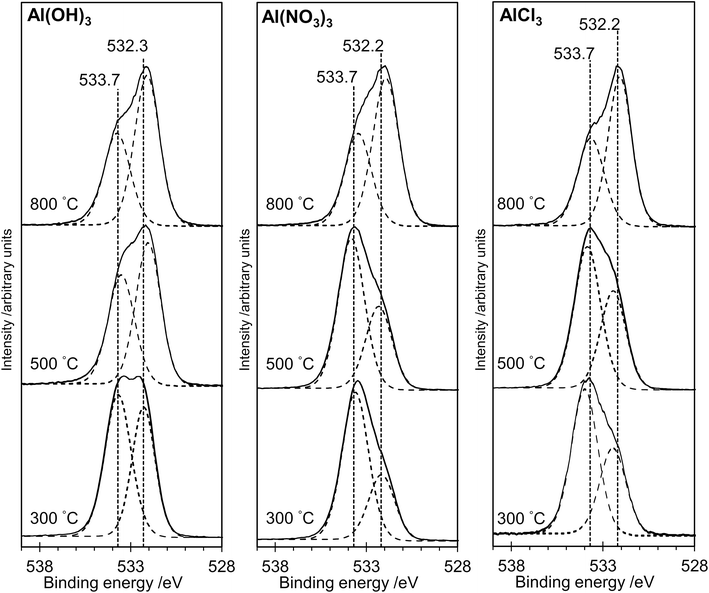
The XP spectra in Fig. 4 show the O(1s) data for all samples. For samples prepared from each of the three precursors, there is a general shift in peak position towards lower binding energy as the annealing temperature is increased. Curve fitting confirms that two distinguishable peaks are present at binding energies of ca. 533.7 eV and 532.3 eV in all spectra, with a transfer of intensity from the higher binding energy peak to the lower binding energy peak as the annealing temperature is increased (peak area ratios are given in Table 1). In particular, we note the close similarity between the spectra for the AlPOH(800), AlPNO3(800) and AlPCl(800) samples, all of which show an approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 intensity ratio (lower
1 intensity ratio (lower![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) higher binding energy peaks) and resemble the O(1s) spectrum of aluminium metaphosphate published by Rotole and Sherwood.12
higher binding energy peaks) and resemble the O(1s) spectrum of aluminium metaphosphate published by Rotole and Sherwood.12
| Reactant | Annealing temperature/°C | ||||||
|---|---|---|---|---|---|---|---|
| 300/400 | 500 | 800 | |||||
| a Binding energies are referenced to the C (1s) peak at 284.7 eV; curves are fitted with Gaussian–Lorentzian (GL (30)) line shapes. | |||||||
| Al(OH)3 | O(1s) binding energya | 533.7 | 532.3 | 533.5 | 532.0 | 533.8 | 532.1 |
| % of peak area | 55.5 | 44.5 | 44.6 | 55.4 | 39.0 | 61.0 | |
∼532![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 ratio 533 ratio |
0.8 | 1.2 | 1.6 | ||||
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio P ratio |
3.2 | 2.9 | 3.0 | ||||
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio Al ratio |
5.3 | 5 | 3.8 | ||||
| Al(NO3)3 | O(1s) binding energya | 533.6 | 532.2 | 533.8 | 532.3 | 533.7 | 532.1 |
| % of peak area | 69.1 | 30.9 | 64.4 | 35.6 | 38.5 | 61.5 | |
∼532![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 ratio 533 ratio |
0.43 | 0.55 | 1.6 | ||||
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio P ratio |
2.8 | 3 | 2.9 | ||||
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio Al ratio |
n/a | 10.2 | 3.6 | ||||
| AlCl3 | O(1s) binding energya | 534.0 | 532.5 | 533.9 | 532.4 | 533.7 | 532.1 |
∼532![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533 ratio 533 ratio |
62.4 | 37.6 | 59.8 | 40.2 | 39.6 | 60.4 | |
| Peak area ratio | 0.6 | 0.7 | 1.5 | ||||
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio P ratio |
3.3 | 3.1 | 2.8 | ||||
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio Al ratio |
n/a | n/a | 4.6 | ||||
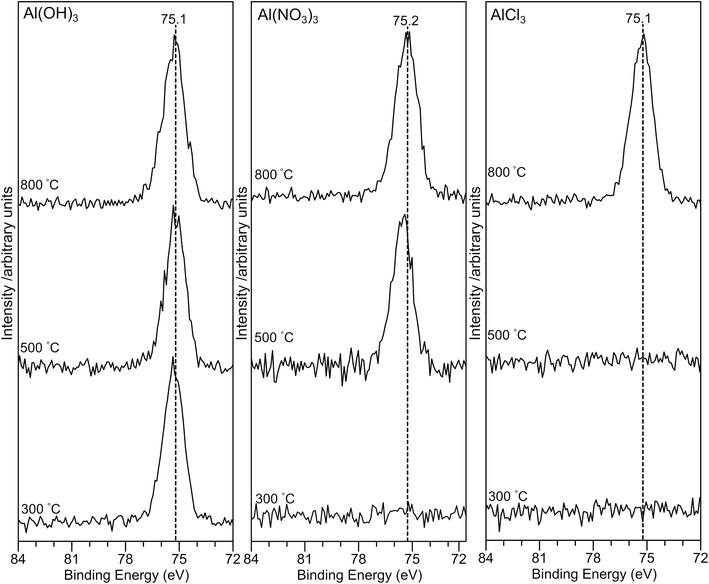
The Al(2p) XP spectra for all samples are shown in Fig. 5. For the AlPOH samples, a strong peak is present for all annealing temperatures at ∼75.3 eV for AlPOH(300) and shifting slightly to 75.1 eV for AlPOH(800). However, for the AlPNO3(300) sample, no peaks are observed in the Al(2p) XP spectrum, while a peak at ca. 75 eV is present for both AlPNO3(500) and AlPNO3(800). For the AlPCl samples, again no peaks are observed in the Al(2p) XP spectra for the AlPCl(300) and AlPCl(500) samples, while a peak is observed at ca. 75 eV for AlPCl(800). This difference in behaviour parallels the physical appearance of the AlPCl samples, for which both AlPCl(300) and AlPCl(500) are tacky but AlPCl(800) is not.
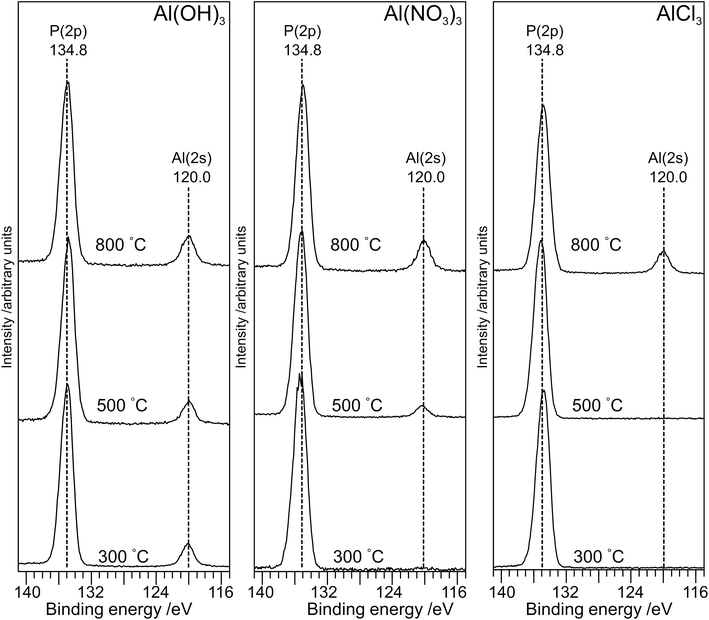
The XP spectra in the P(2p) region (Fig. 6) have a single peak at ∼134.8 eV for all samples with a small shift (∼0.2 eV) to lower binding energy as the annealing temperature is increased to 800 °C. The observed peak is consistent with the average peak position for metaphosphates in the NIST database17 (134.8 eV; σ = 0.5 eV) and with results of Rotole and Sherwood10,11 on aluminium phosphates. However, it is in marked contrast to sodium phosphates,8 for which the P(2p) binding energy shifts by 2 eV from the orthophosphate (132.5 eV) to the oxygen-bridged metaphosphate (134.5 eV).
The atomic ratios calculated from the XPS spectra (Table 1) are informative. The P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio is very close to 1
O ratio is very close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 for all samples but the P
3 for all samples but the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio is always higher than 3
Al ratio is always higher than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The fact that the XPS survey scans for materials prepared at lower annealing temperatures (Fig. S6†) do not contain any signal for aluminium or for any other cation suggests that the surface is dominated by hydrogen phosphates, with the consistent O
1. The fact that the XPS survey scans for materials prepared at lower annealing temperatures (Fig. S6†) do not contain any signal for aluminium or for any other cation suggests that the surface is dominated by hydrogen phosphates, with the consistent O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 3
P ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 indicating extensive polymerization at the surface. We also note that no XPS signal due to chlorine is observed for any of the samples (Fig. S7†). Following annealing at 800 °C, the samples from all precursors are highly crystalline, and the presence of the Al(2p) peak in the XP spectra suggests that the surface is now dominated by aluminium metaphosphate. However, the P
1 indicating extensive polymerization at the surface. We also note that no XPS signal due to chlorine is observed for any of the samples (Fig. S7†). Following annealing at 800 °C, the samples from all precursors are highly crystalline, and the presence of the Al(2p) peak in the XP spectra suggests that the surface is now dominated by aluminium metaphosphate. However, the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio remains higher than the expected 3
Al ratio remains higher than the expected 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, particularly for AlCl3(800), suggesting that some hydrogen polyphosphates are present at the surface.
1 ratio, particularly for AlCl3(800), suggesting that some hydrogen polyphosphates are present at the surface.
Surface analysis with ATR-FTIR
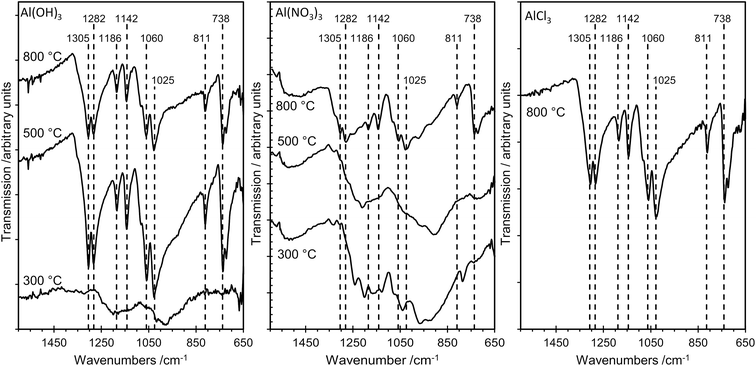
The sampling depth of ATR-FTIR spectroscopy, typically between 0.5–2 μm, is significantly larger than that for XPS, which detects only the top 2–4 nm of the surface for the elements studied here. Nevertheless, there is excellent agreement between the IR results and the results from the more surface sensitive XPS method, as illustrated in Fig. 7, which shows FTIR data for several samples. For AlPOH(300), broad, weak bands are present in the range ca. 1100–1250 cm−1, characteristic of an aluminium orthophosphate with some indication of P–O–P bond formation from the weak peak at 1025 cm−1.18 The spectra for AlPOH(500) and AlPOH(800) are dominated by strong bands assigned to metaphosphates. In particular, the peak at 738 cm−1 is assigned to Al–O–P, bands at 811, 1025, 1060 and 1070 cm−1 are assigned to P–O–P modes, and bands at 1282 and 1305 cm−1 are assigned to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the aluminium metaphosphate.
O bonds in the aluminium metaphosphate.
Discussion
The structures of aluminium orthophosphate, aluminium hexacyclophosphate and two aluminium metaphosphates are shown in Fig. 3, and can be used to rationalize the changes in the XP spectra observed for different annealing temperatures. Small shifts to lower binding energy are observed for the P(2p) and Al(2p) peaks as the annealing temperature is increased, but the most significant changes arise in the O(1s) spectra, which show a transfer of intensity from a higher binding energy peak at 533.7 eV to a lower binding energy peak at 532.3 eV. The best starting point to understand these changes is the O(1s) spectra for all samples annealed at 800 °C, as these spectra are all very similar to those reported by several other authors, including Gresch et al. for sodium metaphosphates,8 Crobu et al. for zinc phosphates,9 and Rotole and Sherwood for model aluminium phosphates.11,12 The FTIR, XRD and solid-state 31P NMR data indicate the presence of mainly cubic aluminium metaphosphate after annealing at 800 °C and we can therefore definitively assign the peaks in the O(1s) region at 533.7 eV and 532.3 eV, respectively, to oxygen atoms bridging between phosphorus atoms (P–O–P) and oxygen atoms bridging between phosphorus and aluminium atoms (P–O–Al), in agreement with Gresch et al.In the metaphosphate, these two bonding environments are expected to be present in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of P–O–Al to P–O–P. However, as shown in Table 1, quantification of the XPS data for the samples annealed at 800 °C gives a peak area ratio (532.3 eV
1 ratio of P–O–Al to P–O–P. However, as shown in Table 1, quantification of the XPS data for the samples annealed at 800 °C gives a peak area ratio (532.3 eV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 533.7 eV) of ca. 1.6
533.7 eV) of ca. 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas the expected ratio is 2
1, whereas the expected ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thus, the P
1. Thus, the P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio at the surface of these materials is higher than the expected 3
Al ratio at the surface of these materials is higher than the expected 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To understand these differences, we now consider the XP spectra recorded for samples annealed to lower temperatures.
1 ratio. To understand these differences, we now consider the XP spectra recorded for samples annealed to lower temperatures.
A key observation is that the AlPNO3(300) and AlPCl(300) samples show no evidence, in the XP spectra, for the presence of aluminium. The AlPNO3(500) sample does show evidence for aluminium, but the AlPCl(500) sample does not. The absence of aluminium indicates a purely hydrogen terminated phosphate material at the surface. Unreacted phosphoric acid can be ruled out based on the O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 3
P ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, but there is evidence from the solid-state 31P NMR results for hydrogen terminated or cyclic polyphosphates (giving peaks at −28 ppm and −32 ppm) which would have a 3
1, but there is evidence from the solid-state 31P NMR results for hydrogen terminated or cyclic polyphosphates (giving peaks at −28 ppm and −32 ppm) which would have a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. A cyclic polyphosphate such as P4O10 has a P–O–P to P
1 ratio. A cyclic polyphosphate such as P4O10 has a P–O–P to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond ratio of 1.5
O bond ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which could account for the XPS ratios if the oxygen in P
1, which could account for the XPS ratios if the oxygen in P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O has a binding energy of ∼532 eV, overlapping with the XPS peak for the oxygen in P–O–Al. This assignment would be in agreement with Gresch et al.8 Finally, the “wet” physical appearance of samples annealed at lower temperatures is also consistent with the presence of hydrogen polyphosphates which would be poorly crystalline.
O has a binding energy of ∼532 eV, overlapping with the XPS peak for the oxygen in P–O–Al. This assignment would be in agreement with Gresch et al.8 Finally, the “wet” physical appearance of samples annealed at lower temperatures is also consistent with the presence of hydrogen polyphosphates which would be poorly crystalline.
From the data presently available, we cannot determine whether annealing ultimately leads to sublimation or decomposition of the polymeric phosphates, or whether further reaction with unreacted aluminium precursor occurs. However, for samples annealed at 800 °C, the XP spectra are consistent with the presence of aluminium metaphosphates although the slightly higher P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio in the case of the material prepared from AlCl3 suggests the surface contains some hydrogen terminated polyphosphates.
Al ratio in the case of the material prepared from AlCl3 suggests the surface contains some hydrogen terminated polyphosphates.
Conclusions
Aluminium metaphosphate is formed from the reaction of phosphoric acid with three different aluminium compounds (Al(OH)3, Al(NO3)3 and AlCl3) followed by annealing in air. XRD, XPS and FTIR measurements of the resulting materials show almost identical behaviour from all three precursors, but the solid-state 31P NMR spectra are significantly different at the lower annealing temperatures (300 °C and 500 °C). The unique solid-state 31P NMR spectra of the materials annealed at lower temperatures indicates the presence of amorphous materials which would not be identified by XRD, but explains the lack of an Al(2p) signal in XP spectra of the materials prepared from the Al(NO3)3 and AlCl3 precursors at lower annealing temperatures. For all three precursors, a cubic metaphosphate is produced on annealing at 800 °C, with a hexacyclophosphate present at lower annealing temperatures in the case of the Al(NO3)3 and Al(OH)3 precursors. The XP spectra in the O(1s) region of the aluminium phosphate materials show two components at 532.3 eV and 533.7 eV, which are definitively assigned to the P–O–Al and P–O–P bonding environments, respectively. However, samples annealed at lower temperatures also exhibit surface species assigned as cyclic polyphosphates, with binding energies of 532.3 eV and 533.7 eV for the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and P–O–P bonding environments, respectively.
O and P–O–P bonding environments, respectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
VB, MT and LE are grateful for studentships funded by Cogent Power Ltd. XP spectra were recorded by the EPSRC National Facility for Photoelectron Spectroscopy (HarwellXPS), operated by Cardiff University and UCL under contract number PR16195. The UK 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MHz solid-state NMR Facility used in this research was funded by EPSRC and BBSRC (contract reference PR140003), as well as the University of Warwick including via part funding through Birmingham Science City Advanced Materials Projects 1 and 2 supported by Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF).
MHz solid-state NMR Facility used in this research was funded by EPSRC and BBSRC (contract reference PR140003), as well as the University of Warwick including via part funding through Birmingham Science City Advanced Materials Projects 1 and 2 supported by Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF).
Notes and references
- S. V. Dorozhkin and M. Epple, Angew. Chem., Int. Ed., 2002, 41, 3130–3146 CrossRef CAS PubMed.
- M. Nabiyouni, T. Brueckner, H. Zhou, U. Gbureck and S. B. Bhaduri, Acta Biomater., 2018, 66, 23–43 CrossRef CAS.
- M. Nabiyouni, Y. Ren and S. B. Bhaduri, Mater. Sci. Eng., C, 2015, 52, 11–17 CrossRef CAS PubMed.
- X. Yu, C. Qian and X. Wang, Sci. Adv. Mater., 2015, 7, 1730–1733 CrossRef CAS.
- Y. N. Trepalina, V. A. Doroganov and E. I. Evtushenko, Refract. Ind. Ceram., 2011, 52, 291–293 CrossRef CAS.
- F. Rashchi and J. A. Finch, Miner. Eng., 2000, 13, 1019–1035 CrossRef CAS.
- R. N. Panda, M. F. Hsieh, R. J. Chung and T. S. Chin, J. Phys. Chem. Solids, 2003, 64, 193–199 CrossRef CAS.
- R. Gresch, W. Muller-Warmuth and H. Dutz, J. Non-Cryst. Solids, 1979, 34, 127–136 CrossRef CAS.
- M. Crobu, A. Rossi, F. Mangolini and N. D. Spencer, Anal. Bioanal. Chem., 2012, 403, 1415–1432 CrossRef CAS PubMed.
- J. A. Rotole and P. M. A. Sherwood, Chem. Mater., 2001, 13, 3933–3942 CrossRef CAS.
- J. A. Rotole and P. M. A. Sherwood, Surf. Sci. Spectra, 1998, 5, 60–66 CrossRef CAS.
- J. A. Rotole and P. M. A. Sherwood, Surf. Sci. Spectra, 1998, 5, 67–74 CrossRef CAS.
- N. Fairley, CasaXPS Manual: 2.3.15 Spectroscopy, Casa Software Ltd, 2009 Search PubMed.
- F. d'Yvoire, Bull. Soc. Chim. Fr., 1961, 1762 Search PubMed.
- F. d'Yvoire, Bull. Soc. Chim. Fr., 1961, 2277 Search PubMed.
- G. Tricot, D. Coillot, E. Creton and L. Montagne, J. Eur. Ceram. Soc., 2008, 28, 1135–1141 CrossRef CAS.
- NIST X-ray Photoelectron Spectroscopy Database, Version 4.1, National Institute of Standards and Technology, Gaithersburg, 2012, http://srdata.nist.gov/xps/ Search PubMed.
- D. Wang, Y. Xu, L. Yang, F. Wang, A. M. Asiri and K. A. Alamry, J. Mol. Liq., 2018, 258, 327–334 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08738a |
| ‡ Current address: Dept. of Materials, Imperial College, South Kensington Campus, London SW7 2AZ, UK. |
| This journal is © The Royal Society of Chemistry 2020 |