Open Access Article
Open Access ArticleLiubao Insect tea polyphenols prevent HCl/ethanol induced gastric damage through its antioxidant ability in mice
Jing Zhang†
ab,
De-Yun Lu†c,
Ying Yuana,
Jingxia Chena,
Sha Yia,
Benchou Chen*a and
Xin Zhao *b
*b
aEnvironment and Quality Inspection College, Chongqing Chemical Industry Vocational College, Chongqing 401228, China. E-mail: 712632367@qq.com; Tel: +86-23-6265-3650
bChongqing Collaborative Innovation Center for Functional Food, Chongqing University of Education, Chongqing 400067, China. E-mail: zhaoxin@cque.edu.cn
cDepartment of Gastroenterology, Chengdu First People's Hospital, Chengdu 610041, China
First published on 30th January 2020
Abstract
The aim of this study was to study the preventive effects of polyphenols extracted from Liubao Insect tea on gastric injury. The content of Liubao Insect tea polyphenols (LITP) was 72.36% by ion precipitation extraction method. HCl/ethanol-induced gastric injury in mice led to increased gastric juice volume and decreased pH. LITP increased the gastric juice pH value and reduced the gastric juice volume at slightly lower quantities than ranitidine. Visual observation of gastric tissue showed that LITP could effectively reduce the area of gastric injury, and higher concentrations of LITP had a greater effect. Pathological observation also confirmed that LITP can reduce the cell damage and inflammatory effects, and play a role in preventing gastric injury. Serum cytokine assays showed that LITP could reduce the levels of IL-6 (interleukin 6), TNF-α (tumor necrosis factor alpha) and IFN-γ (interferon gamma) induced by gastric injury, and the effects of higher concentration of LITP were similar to those of ranitidine. The results showed that LITP could increase SOD (superoxide dismutase) and GSH (glutathione) levels; decrease MDA (malondialdehyde) and MPO (myeloperoxidase) levels; up-regulate the expression of Cu/Zn-SOD (cuprozinc-superoxide dismutase), Mn-SOD (manganese superoxide dismutase), CAT (catalase), nNOS (neuronal nitric oxide synthase), eNOS (endothelial nitric oxide synthase); and down-regulate the expression of iNOS (inducible nitric oxide synthase), COX-2 (cyclooxygenase-2), TNF-α, and IL-1β (interleukin-1 beta) in mice with gastric injury, thus inhibiting gastric injury. We demonstrate that LITP is an active substance which could prevent gastric injury in experimental animals. With the increase of LITP concentration, its effects on preventing gastric injury were stronger and similar to those of ranitidine.
1 Introduction
Liubao Insect tea is a health or medicinal tea produced in Guangxi, China, made for the excreted droppings of insects that have eaten tea leaves.1 Droppings from Hydrillodes morosa Butler, Nodaria niphona Butler, Aglossa dimidiate Haworth., Herculia glaucinalis L., and Fujimacia bicoloralis (Leech) that have fed on Trifolium pratense and other plants have been used to produce the tea. The products are processed after collection to produce insect tea products.2 Insect tea contains 17 minerals such as K, Mg, Ca, Na, Fe, Mn and Zn, including 10 trace elements essential for the human body. Quantities of Fe, Zn, Ca and Mg are higher in insect tea than in some famous tea varieties.3 In addition, insect tea is also rich in crude protein, crude fiber, fat, tea polyphenols, caffeine, sugar, vitamins, and amino acids.4,5 As a traditional Chinese medicinal drink, it has been reported to flush toxins out of the body, detoxify, strengthen the stomach and aid digestion. Beneficial effects on diarrhea, epistaxis, gingival bleeding and hemorrhoids bleeding have also been reported.6Alcoholic gastric injury, a mucosal injury caused by excessive alcohol consumption, often manifests clinically as gastritis.7 Ethanol directly damages gastric mucosal epithelial cells and submucosal vessels, making gastric mucosa vulnerable to various digestive enzymes, bile and gastric acid, and causing H+ antidiffusion. Additionally, ethanol can cause injury of the submucosal vascular endothelium, dilatation of blood vessels, rupture of small blood vessels, submucosal hemorrhage, and other changes, further destroying the mucosal barrier. As damage to the mucosal epithelium and vascular endothelium produces a large number of inflammatory mediators, this can lead to neutrophil infiltration, which further aggravates mucosal damage.8 Higher concentrations of ethanol have a strong stimulating effect on the gastric mucosa and can cause necrosis of mucosal epithelial cells.9 The oxidative stress produced by free radicals attacking cells is closely related to gastric injury. The increase of free radicals and the weakening of antioxidant mechanisms leads to cell damage, promotes cell inflammation, and triggers and aggravates gastric injury.10 Studies have shown that the polyphenols contained in insect tea inhibit gastric injury, have anti-oxidation, anti-cancer, anti-bacterial and anti-viral properties, lower blood sugar and blood pressure, reduce body fat and weight, and prevent cancer.11–13 Tea can effectively regulate the microecology and calcium status in the digestive system, thus regulating the immunity of the body and reducing inflammation. It may play a conservative role in treating inflammation and avoiding surgery.14–17
At present, there are few studies on Liubao Insect tea polyphenols (LITP), and little is understood about the active substances in this natural beverage. A mouse model of alcoholic gastric injury was established, the aim of this study was to the preventive effects of LITP on alcoholic gastric injury, and the mechanism of LITP's effects on gastric injury in mice was observed through biochemical and molecular analysis of serum and tissue, including inhibitory effects of LITP on proinflammatory factors and tissue damage induced by oxidative stress. The results of this study provide a theoretical basis for new applications of Liubao Insect tea, a food with biological activity. We also provide a protocol for LITP extraction. Although this study was conducted in experimental animals, a foundation is laid for further clinical research of LITP.
2 Materials and methods
2.1 Liubao Insect tea polyphenols extraction
Liubao Insect tea is a special drink made by insect larvae, which excretes fecal particles after eating Liubao tea (Camellia sinensis var. assamica). The Liubao Insect tea was purchased from Guangxi Wuzhou Shengyuan Tea Co., Ltd. (Wuzhou, Guangxi, China). Liubao Insect tea was crushed into powder, and 100 g was added to 150 mL of 45% (volume ratio) ethanol in water. The extract was heated in a constant temperature water bath (90 °C, 30 min). After performing a second, independent extraction as above, the two solutions were pooled. The pH of the extract was adjusted to 6.1 using 15% HCl. 160 mL of AlCl3 (6 g) and ZnCl2 (12 g) was added to induce precipitation. The mixture was centrifuged (3000 rpm, 10 min) and the precipitate was retained. 200 mL 12% HCl was added, and after an additional centrifugation, the supernatant was retained. Polyphenols were then extracted by adding 450 mL ethyl acetate, followed by rotating evaporation.182.2 Determination of polyphenols composition
The methanol was added (2 mL, chromatographic grade) separately to the following accurately weighed compounds: catechin, rutin, isoquercetin, isochlorogenic acid B, isochlorogenic acid A, quercetin, rhizocarpin, quercetin and kaempferol standards. Each standard substance was fully dissolved by oscillation to obtain the standard solution. The methanol (10 mL, chromatographic grade) was added to dried Liubao tea polyphenol and Liubao Insect tea polyphenol extracts (5 mg), and was dissolved using oscillation. Samples were filtered with the 0.22 μm microporous membrane to obtain the tea polyphenol solution. Component analysis was carried out under the following chromatographic conditions: mobile phase A was 0.5% acetic acid water; mobile phase B was acetonitrile; the flow rate was set at 0.5 mL min−1; chromatographic column was Accucore C18 (2.6 μm, 4.6 × 150 mm); the column temperature was 30 °C; wavelength was 359 nm; injection volume was 5 μL. The gradient elution conditions were pre-equilibrium, 10 min, 88% A–12% B; 0–30 min, 12–45% B; 30–35 min, 45–100% B, 35–40 min, 100% B. The chromatographic peak area of each component was recorded to analyze the content of each component (Ultimate 3000; Thermo Fisher Scientific, Inc., Waltham, MA, USA).192.3 Cytotoxic test
GES-1 human gastric epithelial cells were inoculated in DMEM liquid culture medium containing 10% inactivated calf serum to prepare cell suspension with concentration of 1 × 104 cells per mL. The GES-1 cells were inoculated with 200 μL per well in 96 well culture plate. After 24 h of culture in CO2 incubator, the upper liquid culture medium was discarded after the cells adhered to the wall, and then different concentrations of LITP were added into each well for 48 h. Finally, discard the upper liquid medium and add 200 μL liquid medium containing MTT reagent at a concentration of 5 mg mL−1 (weight concentration) into each hole for 4 h, and then add 200 μL DMSO into each hole for 30 min, measure the OD value of each hole at the wavelength of 570 nm and calculate the cell survival rate according to the formula: survival rate (%) = (OD value of treatment well/OD value of blank well) × 100.2.4 Induction of gastric injury in mice
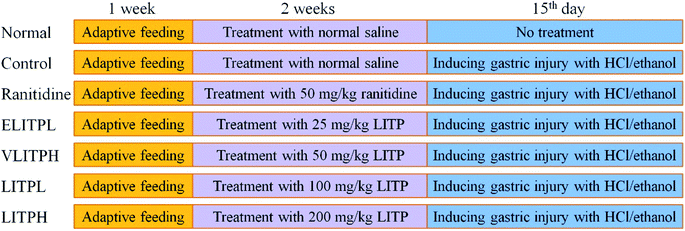
Fifty 6 week-old SPF Kunming mice (male, body weight: 20 ± 2 g, Chongqing Medical University. Chongqing, China) were maintained with free diet (basic feed) and drinking water, with pads changed every 2 days for one week at 25 °C and 60% relative humidity. The mice were divided with 10 mice each into eight groups: normal group, control group, extremely low concentration group (ELITPL), very low concentration group (VLITPL), low concentration group (LITPL), high concentration group (LITPH group), and ranitidine group. LITP was diluted with distilled water to different experimental concentrations. Normal group and control group were given normal saline, and ELITPL, VLITPL, LITPL and LITPH mice were given LITP at 25 mg kg−1, 50 mg kg−1, 100 mg kg−1 and 200 mg kg−1 respectively by gavage for 14 d. Ranitidine group mice were fed with ranitidine at a concentration of 50 mg kg−1 for 14 d (Fig. 1). On the 14th day, all mice were fasted for 24 h. On the 15th day, all mice except the normal group were given 0.1 mL ethanol mixture (60% ethanol, 40% 150 mmol L−1 hydrochloric acid) per 10 g body weight, 1 h after gastric administration.11 After 30 min of intragastric administration, the mice were killed by cervical disloca, the eyeball blood was collected and gastric tissue was dissected for use. Gastric juice volume and pH value were measured. The degree of gastric mucosal injury was naked eye observed, and the inhibition rate of gastric injury was calculated after taking pictures to judge the inhibition effect of gastric injury. The study was conducted in accordance with the protocol was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (201901002B, Chongqing, China).2.5 Pathological observation
The gastric tissues of 0.5 cm2 mice were fixed in 10% formalin solution for 48 h. The gastric tissues were dehydrated, clarified, waxed, embedded, sectioned, and stained with H&E. The morphological changes of the gastric tissues were observed under optical microscope (BX43, Olympus, Tokyo, Japan).2.6 Serum cytokine levels determination
After blood plasma centrifugation at 4000 rpm for 10 min, the upper serum was collected and the cytokine levels of IL-6, TNF-α and IFN-γ in the serum of mice were determined according to the kit instructions (Abcam, Cambridge, Massachusetts, USA).2.7 Serum and gastric tissue oxidation levels determination
The 9 mL of cold saline (4 °C) was added to 1 g of gastric tissue, and then 10% of the homogenate was prepared. Then the homogenate was centrifuged at 4000 rpm for 10 min. The supernatant was collected and the levels of SOD, GSH, MDA and MPO in serum and gastric tissue were determined according to the kit instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing City, China).2.8 sIgA levels of gastric and small intestinal mucosa determination
The gastric mucosa and small intestinal mucosa were cleaned with PBS, then 0.1 g mucosa was scraped with slide glass and put into centrifuge tube, 0.5 mL PBS was added and shaked and mixed evenly, then centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min in 4 °C, and detected with detection kit (Nanjing Jiancheng Bioengineering Institute).
000 rpm for 10 min in 4 °C, and detected with detection kit (Nanjing Jiancheng Bioengineering Institute).
2.9 Quantitative PCR (qPCR) assay
The gastric tissue of mice was crushed, and the general RNA in gastric tissue was extracted by RNAzol (Invitrogen, Carlsbad, CA, USA), then the total RNA concentration was diluted to 1 μg μL−1. Then the diluted general RNA solution of 5 μL was extracted and retrieved by reverse transcription kit to obtain the cDNA template. 2 μL of cDNA template were mixed with 10 μL of SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) and 1 μL upstream and downstream primers (1 μg μL−1) (Table 1). To react under 95 °C for 15 s, as well as 55 °C for 30 s, then 40 cycles under 72 °C for 35 s. Finally, under the conditions of 95 °C for 30 s, 55 °C for 35 s, the test was carried out. GAPDH was used as a control, and 2−ΔΔCt method was used to calculate the relative gene expression.20| Gene name | Sequence |
|---|---|
| a Cu/Zn-SOD: cuprozinc-superoxide dismutase; Mn-SOD: manganese superoxide dismutase; CAT: catalase; nNOS: neuronal nitric oxide synthase; eNOS: endothelial nitric oxide synthase; iNOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; TNF-α: tumor necrosis factor alpha; IL-1β: Interleukin-1 beta; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. | |
| Cu/Zn-SOD | Forward: 5′-AACCAGTTGTGTTGTCAGGAC-3′ |
| Reverse: 5′-CCACCATGTTTCTTAGAGTGAGG-3′ | |
| Mn-SOD | Forward: 5′-CAGACCTGCCTTACGACTATGG-3′ |
| Reverse: 5′-CTCGGTGGCGTTGAGATTGTT-3′ | |
| CAT | Forward: 5′-GGAGGCGGGAACCCAATAG-3′ |
| Reverse: 5′-GTGTGCCATCTCGTCAGTGAA-3′ | |
| iNOS | Forward: 5′-AGAGAGATCGGGTTCACA-3′ |
| Reverse: 5′-CACAGAACTGAGGGTACA-3′ | |
| nNOS | Forward: 5′-TCGTCCAACTTCTGGGCTCTT-3′ |
| Reverse: 5′-CCTTCTCTTCCTCCCCTCTCTTC-3′ | |
| eNOS | Forward: 5′-TCAGCCATCACAGTGTTCCC-3′ |
| Reverse: 5′-ATAGCCCGCATAGCGTATCAG-3′ | |
| COX-2 | Forward: 5′-GGTGCCTGGTCTGATGATG-3′ |
| Reverse: 5′-TGCTGGTTTGGAATAGTTGCT-3′ | |
| TNF-α | Forward: 5′-GACCCTCAGACTCAGATCATCCTTCT-3′ |
| Reverse: 5′-ACGCTGGCTCAGCCACTC-3′ | |
| IL-1β | Forward: 5′-CTCCATGAGCTTTGTACAAGG-3′ |
| Reverse: 5′-TGCTGATGTACCAGTTGGGG-3′ | |
| GAPDH (house-keeping gene, control gene) | Forward: 5′-AGGTCGGTGTGAACGGATTTG-3′ |
| Reverse: 5′-GGGGTCGTTGATGGCAACA-3′ | |
2.10 Statistical analysis
Three parallel experiments were carried out on the serum and tissue indexes of each mouse, and then the average values were taken. Then the data were analyzed by SAS 9.1 statistical software (SAS Institute Inc., Cary, NC, USA). The one-way ANOVA method and Duncan's test were used to analyze whether there were significant differences among the groups at the level of P < 0.05.3 Results
3.1 Composition analysis of Liubao tea polyphenols and Liubao Insect tea polyphenols
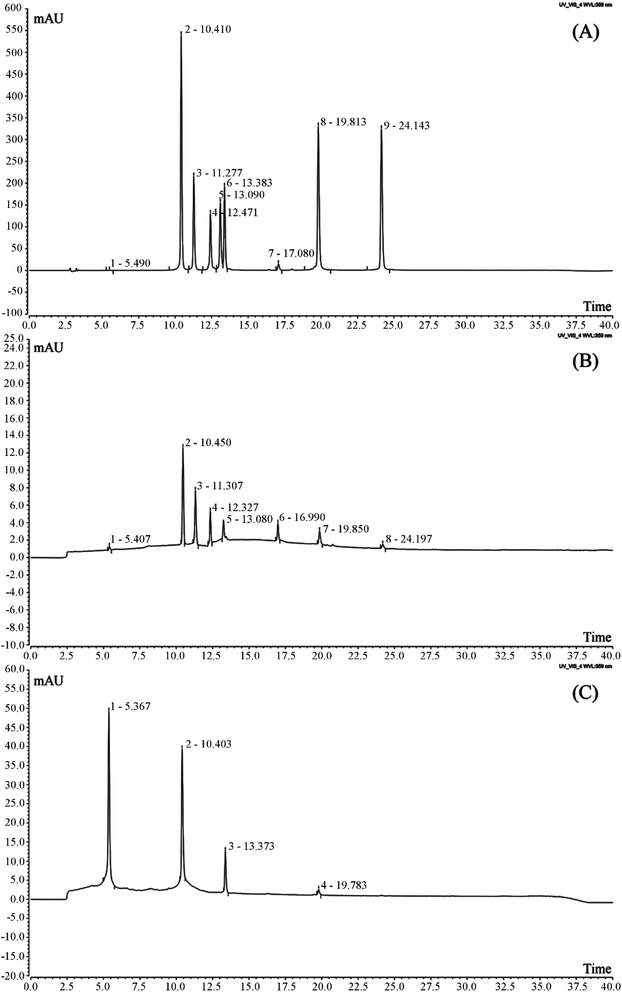
It was found that Liubao tea polyphenols contained catechin, rutin, isoquercetin, isochlorogenic acid B, isochlorogenic acid, quercitrin, rhizocarpin and quercetin by HPLC (Fig. 2, Table 2). After the process of insect metabolism, the polyphenols in Liubao Insect tea changed greatly from the raw material Liubao tea. It was found that there were only four polyphenols in Liubao Insect tea polyphenols (catechin, rutin, isoquercetin and isochlorogenic acid B), and the content of catechin was greatly increased.| Polyphenol | Liubao tea polyphenols (mg g−1) | Liubao Insect tea polyphenols (mg g−1) |
|---|---|---|
| Catechin | 342.207 ± 0.033 | 715.204 ± 0.026 |
| Rutin | 12.893 ± 0.012 | 6.887 ± 0.013 |
| Isoquercetin | 4.300 ± 0.012 | 1.428 ± 0.012 |
| Isochlorogenic acid B | 6.716 ± 0.015 | 0.081 ± 0.005 |
| Isochlorogenic acid A | 0.269 ± 0.006 | — |
| Quercetin | 288.754 ± 0.022 | — |
| Rhizocarpin | 0.537 ± 0.011 | — |
| Quercetin | 0.027 ± 0.002 | — |
3.2 Cytotoxicity of Liubao Insect tea polyphenols
As shown in Table 3, at the concentration of 0–4 mg mL−1 LITP, the growth of normal gastric mucosa cells GES-1 was not abnormal, and the proliferation of GES-1 cells was inhibited when the concentration was higher than 4 mg mL−1 LITP. According to the concentration conversion of animal experiment, the LITP had no toxic effect on gastric mucosa at the concentration of 0–200 mg kg−1.| Treatment | OD570 | Proliferation inhibition rate (%) |
|---|---|---|
| Untreated (0 mg mL−1) | 0.472 ± 0.004 | — |
| 1 mg mL−1 | 0.471 ± 0.006 | 0.2 ± 0.0 |
| 2 mg mL−1 | 0.469 ± 0.005 | 0.6 ± 0.1 |
| 3 mg mL−1 | 0.468 ± 0.005 | 0.8 ± 0.1 |
| 4 mg mL−1 | 0.463 ± 0.007 | 1.7 ± 0.2 |
| 5 mg mL−1 | 0.415 ± 0.009 | 12.1 ± 0.4 |
| 6 mg mL−1 | 0.378 ± 0.011 | 19.9 ± 0.6 |
| 7 mg mL−1 | 0.315 ± 0.010 | 33.3 ± 0.7 |
| 8 mg mL−1 | 0.245 ± 0.012 | 48.1 ± 1.1 |
| 9 mg mL−1 | 0.147 ± 0.009 | 68.9 ± 1.0 |
| 10 mg mL−1 | 0.112 ± 0.007 | 76.3 ± 0.9 |
3.3 Gastric juice volume and pH of gastric juice
As shown in Table 4, the control group's gastric juice had the largest volume and lowest pH, while the normal group's had the lowest volume and highest pH. Compared with the control group, both LITP concentrations and ranitidine reduced the gastric juice volume and increased the pH value in mice with gastric injury. LITPH had a similar effect to ranitidine, which produced gastric juice volume and pH closest to values found in the normal group. Compared with the control group, ELITPL and VLITPH did not significantly (P > 0.05) decrease the gastric juice volume and increase the pH of gastric juice.| Group | Gastric juice volume (mL) | pH of gastric juice |
|---|---|---|
| a Values presented are the mean ± standard deviation. a–eMean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; ELITPL group: 25 mg kg−1 b.w. Liubao Insect tea polyphenols dose; VLITPL group: 50 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | ||
| Normal | 0.12 ± 0.02e | 4.47 ± 0.35a |
| Control | 0.35 ± 0.03a | 1.89 ± 0.21d |
| Ranitidine | 0.19 ± 0.03d | 3.87 ± 0.23b |
| ELITPL | 0.34 ± 0.02a | 1.91 ± 0.20d |
| VLITPH | 0.31 ± 0.03a | 2.03 ± 0.22d |
| LITPL | 0.27 ± 0.03b | 2.42 ± 0.24c |
| LITPH | 0.22 ± 0.02c | 3.71 ± 0.31b |
3.4 Gastric injury inhibitory effect
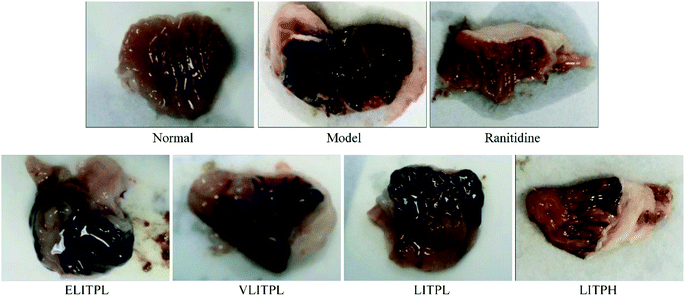
Fig. 3 and Table 5 showed that the gastric mucosa of normal mice was intact, normal color, smooth surface, and without bleeding, erosion, or other damage. The control group exhibited a large area of gastric mucosal injury. In the LITP and ranitidine groups, this area was significantly reduced (P < 0.05), and the reduction in the LITPH group was larger than that of the LITPL group, slightly lower than that of ranitidine. Thus, LITP could effectively reduce the area of alcohol damage to gastric mucosa, and reduce the degree of gastric injury. Compared with the control group, ELITPL and VLITPH did not obvious reduce the area of gastric injury. And the ED50 of LITP was 137.8 mg kg−1. Based on the above experimental results, we choose the concentrations of 100 and 200 mg kg−1 for further in-depth experiments.| Group | Gastric injury area (mm2) | Gastric injury inhibitory rate (%) |
|---|---|---|
| a Values presented are the mean ± standard deviation. a–g Mean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; ELITPL group: 25 mg kg−1 b.w. Liubao Insect tea polyphenols dose; VLITPL group: 50 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | ||
| Normal | 0.00 ± 0.00e | 100.00 ± 0.00a |
| Control | 22.57 ± 2.83a | 0.00 ± 0.00g |
| Ranitidine | 3.97 ± 0.41d | 82.41 ± 2.67b |
| ELITPL | 22.08 ± 2.16a | 2.17 ± 0.36f |
| VLITPH | 20.86 ± 2.61a | 7.58 ± 1.31e |
| LITPL | 14.39 ± 1.86b | 36.24 ± 1.89d |
| LITPH | 5.32 ± 0.51c | 76.43 ± 2.37c |
3.5 Pathological observation
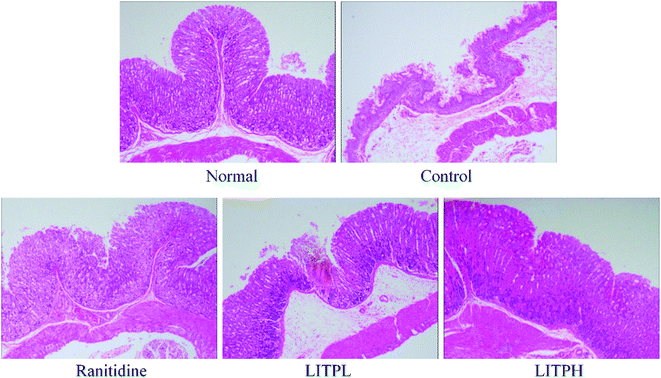
In mice from the normal group, the structure of gastric tissue is complete, the cells are arranged tightly and orderly, the size of the cells is the same, and the surface epithelium is intact and does not fall off (Fig. 4). In the control group, the structure of gastric tissue was incomplete, the number of cells decreased sharply, the arrangement of cells was completely disrupted, the upper epidermis was exfoliated, and serious hemorrhage occurred between cells and tissues. In the LITPL group, the cells were not uniform in size, arranged loosely, some of them were destroyed, there were hemorrhage and congestion among the cells, and some of the mucosa was exfoliated. The order and tightness of cells in LITPH group were more normal than those in LITPL group, the upper epidermis was more complete, and the phenomenon of congestion and hemorrhage was alleviated compared with that in LITPL group. In the ranitidine group, the gastric tissue cells were arranged closely, and only individual cells were scattered; the mucosal exfoliation was not obvious, and there was little congestion between the cells and tissues. These results showed that LITP could protect gastric tissue and avoid the damage caused by ethanol, and the effect of high dose was better than that of low dose.3.6 Cytokine levels of IL-6, TNF-α and IFN-γ in serum of mouse
As shown in Table 6, serum cytokine levels of IL-6, TNF-α and IFN-γ in mice of normal group were lowest, but these levels in mice of control group were highest. After treatment with LITP and ranitidine, the IL-6, TNF-α and IFN-γ levels in mice with gastric injury were reduced, and the LITPH treatment had higher reductive effect than LITPL, though not as high an effect as ranitidine.| Group | IL-6 (pg mL−1) | TNF-α (pg mL−1) | IFN-γ (pg mL−1) |
|---|---|---|---|
| a Values presented are the mean ± standard deviation. a–eMean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | |||
| Normal | 42.77 ± 3.52e | 36.79 ± 2.12e | 31.12 ± 1.86e |
| Control | 166.08 ± 8.32a | 128.35 ± 4.36a | 120.38 ± 3.97a |
| Ranitidine | 68.32 ± 3.20d | 49.30 ± 3.34d | 42.08 ± 2.91d |
| LITPL | 122.83 ± 5.39b | 97.28 ± 4.09b | 87.96 ± 4.80b |
| LITPH | 79.31 ± 2.88c | 58.17 ± 3.12c | 55.17 ± 2.08c |
3.7 SOD, GSH, MDA and MPO levels in serum and gastric tissue of mouse
Tables 7 and 8 showed that the levels of SOD and GSH were the lowest in the control group, while the levels of MDA and MPO were the highest. The gastric tissue of normal mice showed the opposite: the levels of SOD and GSH in gastric tissue were the highest, while the levels of MDA and MPO were the lowest. LITP could significantly increase SOD and GSH levels in gastric tissue of mice with gastric injury (P < 0.05) and decrease MDA and MPO levels (P < 0.05), making the indexes close to the normal group; the higher the concentration, the more obvious the effects; the higher the concentration of LITP, the closer the effects to ranitidine.| Group | SOD (U mL−1) | GSH (mg mL−1) | MDA (nmol mL−1) | MPO (μg mL−1) |
|---|---|---|---|---|
| a Values presented are the mean ± standard deviation. a–eMean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | ||||
| Normal | 144.70 ± 5.37a | 14.06 ± 1.39a | 12.39 ± 0.74e | 8.25 ± 0.35e |
| Control | 51.09 ± 3.25e | 3.42 ± 0.48e | 55.23 ± 2.61a | 15.31 ± 0.57a |
| Ranitidine | 118.91 ± 3.92b | 11.01 ± 1.26b | 23.79 ± 1.83d | 10.38 ± 0.22d |
| LITPL | 83.25 ± 2.69d | 7.24 ± 0.82d | 40.83 ± 2.63b | 13.71 ± 0.31b |
| LITPH | 107.36 ± 3.02c | 9.06 ± 0.55c | 28.36 ± 2.11c | 11.06 ± 0.23c |
| Group | SOD (U mgprot−1) | GSH (mg gprot−1) | MDA (nmol mgprot−1) | MPO (μg mgprot−1) |
|---|---|---|---|---|
| a Values presented are the mean ± standard deviation. a–eMean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | ||||
| Normal | 187.93 ± 7.36a | 6.32 ± 0.51a | 2.47 ± 0.25e | 2.02 ± 0.16e |
| Control | 102.52 ± 4.83e | 1.05 ± 0.19e | 9.82 ± 0.48a | 7.25 ± 0.31a |
| Ranitidine | 152.08 ± 5.44b | 4.83 ± 0.37b | 4.52 ± 0.38d | 3.19 ± 0.20d |
| LITPL | 129.36 ± 4.05d | 2.68 ± 0.26d | 7.05 ± 0.49b | 5.36 ± 0.25b |
| LITPH | 143.52 ± 4.21c | 4.01 ± 0.24c | 5.74 ± 0.21c | 3.92 ± 0.21c |
3.8 SOD, GSH, MDA and MPO levels in serum and gastric tissue of mouse
Table 9 showed that LITP could improve the levels of sIgA antibody in small intestine and gastric mucosa of mice with gastric injury, and the results also showed that the level of sIgA antibody in gastric mucosa was higher than that in small intestine mucosa. Meanwhile, with the increased of LITP concentration, the levels of sIgA antibody also increased.| Group | Gastric mucosa (μmol L−1) | Small intestinal mucosa (μmol L−1) |
|---|---|---|
| a Values presented are the mean ± standard deviation. a–eMean values with different letters in the same column are significantly different (P < 0.05) according to Duncan's new MRT. Ranitidine group: 50 mg kg−1 b.w. ranitidine treatment dose; LITPL group: 100 mg kg−1 b.w. Liubao Insect tea polyphenols dose; LITPH group: 200 mg kg−1 b.w. Liubao Insect tea polyphenols dose. | ||
| Normal | 6.33 ± 0.21a | 2.03 ± 0.12a |
| Control | 1.25 ± 0.17e | 0.47 ± 0.04e |
| Ranitidine | 4.82 ± 0.19b | 1.61 ± 0.08b |
| LITPL | 2.19 ± 0.20d | 0.72 ± 0.06d |
| LITPH | 4.37 ± 0.15c | 1.29 ± 0.07c |
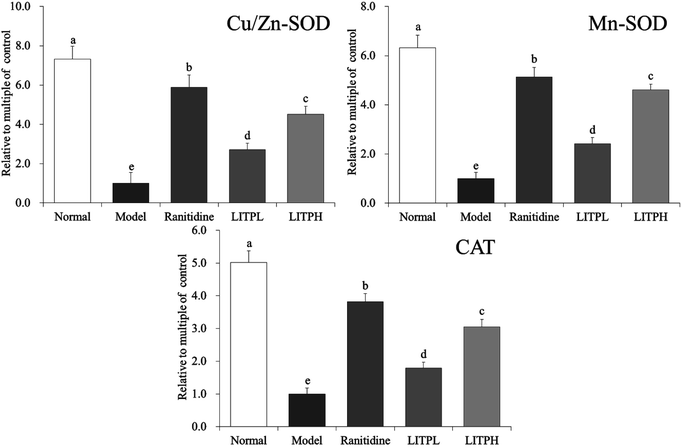
3.9 Cu/Zn-SOD, Mn-SOD and CAT mRNA expression in gastric tissue of mouse
From Fig. 5, it could be seen that the expression intensities of Cu/Zn-SOD, Mn-SOD and CAT in the control group were the lowest. The expression of Cu/Zn-SOD, Mn-SOD and CAT in mice with gastric injury were significantly increased after the action of LITP (P < 0.05). The effects of LITPH were stronger than those of LITPL, and the effects were close to those of ranitidine.3.10 nNOS, eNOS and iNOS mRNA expression in gastric tissue of mouse
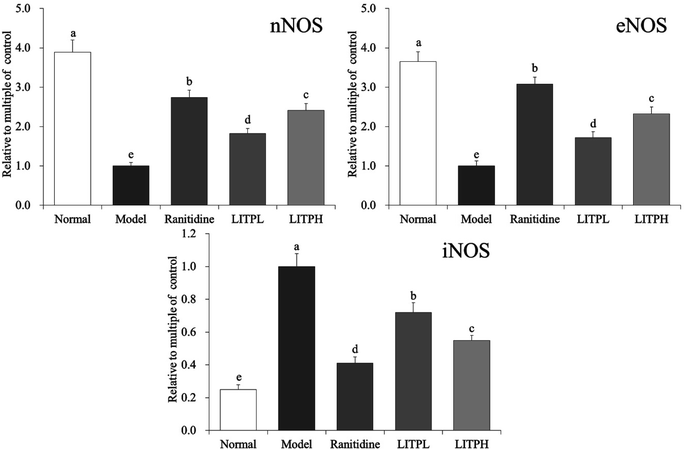
From Fig. 6, it could be seen that the expression of iNOS in gastric tissue of normal mice was significantly lower than those of other groups (P < 0.05), while the expression of nNOS and eNOS were significantly (P < 0.05) higher than those of other groups. The expression of nNOS, eNOS and iNOS in the control group showed the opposite trend to those of the normal group. The expression of nNOS and eNOS in gastric tissue of LITPH and ranitidine mice were lower than those of normal mice, while the expression of iNOS was higher than that of normal mice.3.11 COX-2, TNF-α and IL-1β mRNA expression in gastric tissue of mouse
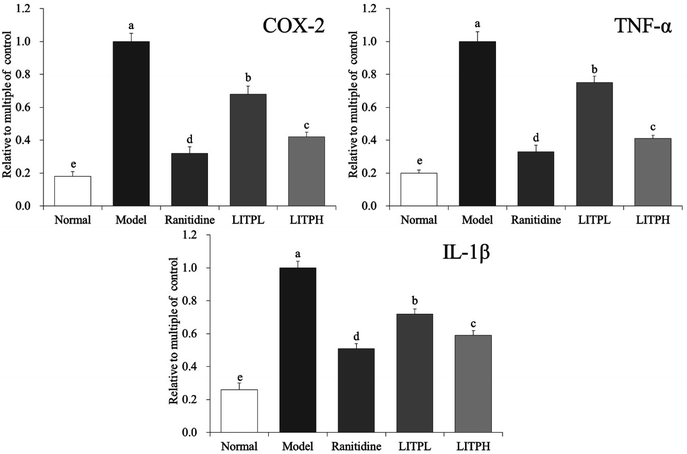
From Fig. 7, it could be seen that the mRNA expression of COX-2, TNF-α and IL-1β in gastric tissue of normal mice were significantly lower than those of other groups (P < 0.05). The expressions of COX-2, TNF-α and IL-1β in gastric tissue of control group mice were the strongest. The expression of COX-2, TNF-α and IL-1β in gastric damage mice were significantly reduced (P < 0.05) by LITP and ranitidine. And the expression of COX-2, TNF-α and IL-1β in gastric tissue of LITPH and ranitidine were similar to those in normal mice.4 Discussion
The maximum amount of tea per person per day is recommended to be 13 g of dry tea. According to WHO regulations, the harmful substances including heavy metals and rare earth in 13 grams of dried tea are lower than the dangerous value, and have no toxicity to human body. Therefore, this study calculated that the intake of tea polyphenols was not more than 200 mg kg−1 through recommended tea intake. Through oral acute toxicity test, cumulative toxicity test, thoracic bone marrow micronucleus test, sperm deformity test and Salmonella typhimurium/mammalian microsomal enzyme test, study had shown that insect tea had no toxic effect and could be used as a new food resource for development and utilization.21 Study had shown that insect tea was rich in amino acids, trace elements and vitamins, and also rich in polyphenols.22 In traditional Chinese culture and medicine, insect tea as a drink could nourish stomach and improve gastric function.23 Laboratory study had also shown that insect tea soup could prevent gastric injury.24 Through the above studies, we can preliminarily conclude that the effect of insect tea on gastric injury is to improve the body's gastric function and enhance the resistance of gastric tissue to chemical gastric injury, which is different from the effect of drug treatment on gastric injury. These factors may also contribute to the preventive effect of tea polyphenols from Liubao tea Insect on gastric injury. In this study, we aimed to study the preventive effect of Liubao Insect Tea as a food on gastric injury. After taking Liubao Insect tea polyphenols in advance, the gastric function of mice was strengthened, which may play a preventive role on chemical gastric injury. Alcohol-induced gastric injury is used to model human gastric acid secretion and imitate alcohol-induced acute digestive injury. This damage model is helpful to study whether potential bioactive substances have protective and antioxidant effects on gastric tissue. Ethanol/hydrochloric acid solution can be used to observe the ulceration of gastric mucosa in mice after gastric administration. The area of ulceration can be used to determine the degree of gastric injury directly, and other related indicators can be used to determine the degree of gastric injury.25 In this study, LITP also could reduce the area of ulceration as indicated in other literatures, LITP can be visually observed to reduce gastric injury.TNF-α is a polypeptide mediator with extensive biological activity, which mediates tissue injury caused by various factors. Increases of TNF-α levels are directly related to tissue injury.26 IL-6 can stimulate hepatocytes to synthesize acute phase proteins and participate in inflammation. IL-6 can also effectively promote cachexia induced by TNF and IL-1 and aggravate tissue damage.27 IFN-γ can mediate the anti-viral effect of non-target cell injury, and TNF-α participates in the injury response, promoting tissue injury and inflammation.28 This study demonstrated that LITP could prevent and alleviate gastric injury by reducing the inflammatory factors, such as IL-6, TNF-α and IFN-γ cytokines in mice.
sIgA is a protective antibody in the mucosal immune response. It is secreted into the intestinal cavity by the lamina propria cells of the mucosa. As a barrier of the body mucosa, sIgA can inhibit the invasion of foreign antigen molecules, neutralize the extracellular pathogenic microorganisms, and combine with antigens to form an immune complex. Beneficial substances can activate T and B cells of mucosa associated lymphoid tissue, stimulated by IFN-γ and other factors, B cells proliferated and transformed into B lymphoblasts with antigen-specific antigens such as IgA, IgG and IgE, Some of sIgA were directly distributed in the lamina propria of intestinal mucosa, most of sIgA returned to the gastrointestinal mucosa through mesentery and blood circulation, transformed into antibody forming cells under the stimulation of IL-6, secreted specific antibodies, and exerted local humoral immune response.29 It could be seen that the LITP could effectively stimulate immunity and reduce gastric injury.
Oxidative stress is the main characteristic of gastric mucosal injury. It has been shown that damage to gastric mucosal cells is aggravated by the decrease of antioxidants such as SOD and GSH after ethanol treatment. As a response to free radical accumulation, SOD and other cell antioxidant enzymes are considered as the first line of defense against oxidative damage. Similar effects are seen with GSH, which can clear free radicals in the body over time, keep the balance between oxidation and antioxidation, and protect the body from free radical damage. Therefore, the content of antioxidant enzymes can directly reflect the number of free radicals in the body.30,31 Decreased activity of antioxidant enzymes may trigger chain lipid peroxidation, which results in decreased fluidity and enhanced permeability of biofilms. MDA is an end product of lipid oxidation, so the MDA level is often used as a marker of lipid peroxidation.32 MPO is closely related to inflammation, and MPO-deficient neutrophils cause an oxidative reaction and produce a large number of superoxides and oxides, resulting in inflammatory cell damage.33 Our results showed that the SOD, GSH, MDA and MPO levels were all directly related to gastric injury, LITP could play a similar role to antioxidants and inhibit gastric injury by adjusting the levels of these markers to near normal values.
Cu/Zn-SOD and Mn-SOD are isomers of SOD in organisms. Mn-SOD is a free radical scavenger and active in mitochondria. Cu/Zn-SOD is a free radical scavenger of SOD and active in the cytoplasm to protect visceral tissues.34 Studies have shown that ethanol can induce oxidative stress in an organism and produce a large number of free radicals. Mn-SOD and Cu/Zn-SOD can inhibit free radicals and prevent gastric injury.35,36 CAT is an antioxidant enzyme, which can inhibit oxidative stress by clearing the body of hydrogen peroxide, slow down the oxidation caused by ethanol, and thus inhibit gastric injury.37 Based on the results of this study, LITP could enhance the expression of Cu/Zn-SOD, Mn-SOD and CAT in mice with gastric injury, thus playing a role in preventing gastric injury.
nNOS, eNOS and iNOS are the neurotype, endothelial type and inducible type of NOS, respectively.38 Under normal physiological conditions, the mechanism of NO production, release, diffusion and inactivation is precisely regulated in the nervous system, which is mainly achieved by regulating the activation and deactivation of nNOS.39 nNOS not only plays an important role in the nervous system, but is also distributed in gastric mucosal epithelial cells. The decrease of the nNOS level will lead to the aggravation of gastric tissue damage.40 The expression and activity of eNOS are relatively stable. eNOS is mainly involved in promoting epithelial repair, regulating gastric mucosal blood flow and adaptive cell protection, inhibiting gastric acid secretion, enhancing mucus barrier function and promoting vascular regeneration.41 eNOS can inhibit oxidative damage to blood vessels caused by oxidative stress, relax blood vessels, and protect them.42 iNOS activation lasts for a long time and produces large quantities of NO. Low concentration of NO can effectively resist gene mutation and activate the body's defensive ability, but high concentration of NO will inhibit control of gene mutation, stimulate gene mutation, and aggravate tissue damage.43 LITP could increase the expression of nNOS and eNOS in the gastric tissue of mice with gastric injury and reduce the expression of iNOS, thus inhibiting inflammatory response, protecting gastric mucosa, and inhibiting gastric injury.
Ethanol can lead to inflammation of the stomach, and causes the release of a large number of inflammatory factors. Inflammatory factor TNF-α is mainly secreted by monocyte–macrophage and binds to TNF-αR1 on hepatocyte membranes, resulting in apoptosis of stem cells and fragmentation of double-stranded DNA.44 Inflammatory factor COX-2 is not expressed in normal tissues. After gastric injury, Kupffer cells are activated. COX-2 is expressed and synthesized extensively, and the inflammatory damage of gastric tissue is aggravated.45 After gastric tissue damage, oxidative stress can lead to imbalance of TNF-α and IL-1β inflammatory factors, and increase the content of TNF-α and IL-1β inflammatory factors.46 Our results show that LITP can reduce the expression of COX-2, TNF-α and IL-1β in mice with gastric injury, thus playing a role in preventing gastric injury.
The composition of Liubao tea changed after insect metabolism. The kinds of active substances in the newly produced Liubao Insect tea polyphenols were less than those in the original Liubao tea, but the active ingredients were concentrated. The content of catechin accounted for nearly 99% of the extracted polyphenol active substances. It can be seen that catechin is the core active component of tea polyphenols from Liubao Insect tea. Catechins have anti-inflammatory, antimicrobial, antiviral and antioxidant effects. They can effectively balance free radicals in human body by scavenging reactive oxygen species (ROS), NO or reacting with ROS to form stable compounds.47 The antioxidant activity of catechin can effectively prevent and treat the tissue damage caused by oxidative stress.48 In this study, catechin could also play its role in inhibiting alcohol-induced oxidative stress injury of gastric mucosa and protecting gastric tissue. Rutin also has strong antioxidant activity, which can inhibit the formation of lipid peroxides and thus inhibit inflammation.49 Isoquercetin is also a bioactive substance, which can alleviate chronic bronchitis, coronary heart disease and hypertension.50 Isochlorogenic acid B has more phenolic hydroxyl groups and has good antioxidant activity. It can also inhibit inflammation through antioxidant effect.51 The combination of various antioxidants may play a better biological activity, enhance antioxidant activity and protect gastric tissue from injury.
Based on the results of this study, Liubao Insect tea may further reflect its clinical effect, which needs further study. This study preliminarily proved the preventive effect of LITP on acute gastric injury through animal experiments. The results can fully support the conclusion because of the use of molecular biology and other methods. However, the research is limited to animal experiments, and further clinical studies are needed to prove that this kind of active substance can be better applied in practice.
5 Conclusions
This study shows that LITP inhibits inflammation and oxidative stress in mice with gastric injury. Through gene expression level analysis, LITP is shown to regulate the degree of gastric injury, the level of inflammatory cytokines, and the oxidative stress in gastric tissue. We have demonstrated that LITP is a biological active class of compounds with gastric tissue protective activity, and has value for further development and utilization. In the future, in-depth human trials are needed to fully verify the clinical activity of LITP in humans.Abbreviations
| LITP | Liubao Insect tea polyphenols |
| IL-6 | Interleukin 6 |
| IL-1β | Interleukin-1 beta |
| TNF-α | Tumor necrosis factor alpha |
| IFN-γ | Interferon gamma |
| SOD | Superoxide dismutase |
| GSH | Glutathione |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| Cu/Zn-SOD | Cuprozinc-superoxide dismutase |
| Mn-SOD | Manganese superoxide dismutase |
| CAT | Catalase |
| nNOS | Neuronal nitric oxide synthase |
| eNOS | Endothelial nitric oxide synthase |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
Conflicts of interest
No conflicts of interest in this article.Acknowledgements
This research was supported by the Science and Technology Research Program of Chongqing Municipal Education Commission of China (KJQN201804504) and the Scientific Research Foundation for Returned Overseas Chinese Scholars, and the State Education Ministry [Jiaowaisiliu (2014)1685], China.References
- J. R. Li and L. T. Zhou, Acad. Period. Farm Prod. Proc., 2005, 34, 4–7 Search PubMed.
- S. J. Jiang, Spec. Econ. Anim. Plant, 2000, 5, 36 Search PubMed.
- L. C. Yang and Y. Yi, Sci. Technol. Food Ind., 2011, 32, 470–472 CAS.
- S. Y. Guo, W. X. Xu, L. Z. Wen, Y. M. Huang and F. Wang, Entomol. Knowl., 2008, 45, 128–132 CAS.
- Y. L. Zhou, X. Feng, K. Zhu and X. Zhao, Sci. Technol. Food Ind., 2015, 36, 339–342 CAS.
- X. Feng, M. Luo and X. Zhao, Mod. Food Sci. Technol., 2013, 29, 1898–1905 CAS.
- M. T. Monforte, F. Lanuzza, S. Pergolizzi, F. Mondello, O. Tzakou and E. M. Galati, Phytother. Res., 2012, 26, 839–844 CrossRef CAS PubMed.
- S. R. Hamad and H. R. Hamad Mohamed, Toxicol. Mech. Methods, 2018, 28, 130–139 CrossRef CAS PubMed.
- I. A. Ibrahim, S. W. Qader, M. A. Abdulla, A. R. Nimir, S. I. Abdelwahab and F. H. Al-Bayaty, Molecules, 2012, 17, 2796–2811 CrossRef PubMed.
- M. Xie, H. Chen, S. Nie, W. Tong, J. Yin and M. Xie, Chem.-Biol. Interact., 2017, 272, 125–134 CrossRef CAS PubMed.
- X. Zhao, P. Sun, G. Li, R. Yi, Y. Qian and K. Y. Park, Food Funct., 2018, 9, 1713–1725 RSC.
- Y. Qian, J. Zhang, X. Fu, R. Yi, P. Sun, M. Zou, X. Long and X. Zhao, Molecules, 2018, 23, 2848 CrossRef PubMed.
- S. Sur and C. K. Panda, Nutrition, 2017, 43–44, 8–15 CrossRef CAS PubMed.
- S. Zhang, T. Al-Maghout, R. Bissinger, N. Zeng, L. Pelzl, M. S. Salker, A. Cheng, Y. Singh and F. Lang, Oncotarget, 2017, 8, 89500–89514 Search PubMed.
- M. K. Walczak-Galezewska, D. Skrypnik, M. Szulinska, K. Skrypnik and P. Bogdanski, Medicine, 2018, 97, e11200 CrossRef PubMed.
- K. Skrypnik, P. Bogdański, I. Łoniewski, J. Reguła and J. Suliburska, Acta Sci. Pol., Technol. Aliment., 2018, 17, 185–192 CAS.
- C. Zhao, A. Y. Wu, X. Yu, Y. Gu, Y. Lu, X. Song, N. An and Y. Zhang, J. Physiol. Pharmacol., 2018, 69, 151–163 Search PubMed.
- Y. Pan, X. Long, R. Yi and X. Zhao, Nutrients, 2018, 10, 1280 CrossRef PubMed.
- B. Liu, Y. Fang, R. Yi and X. Zhao, Foods, 2019, 8, 48 CrossRef CAS PubMed.
- L. Chen, J. Zhang, H. Suo, W. Wang, H. Wang, Y. Zhang, Q. Hu, X. Zhao and J. Li, Foods, 2019, 8, 86 CrossRef CAS PubMed.
- L. Z. Wen, Z. R. Shen, X. B. Zang and Y. X. Hu, J. Chin. Inst. Food Sci. Technol., 2004, 4, 83–87 Search PubMed.
- S. Y. Guo, W. X. Xu, L. Z. Wen, Y. M. Huang and F. Wang, Chin. Bull. Entomol., 2008, 45, 128–132 CAS.
- L. J. Xu, W. Xiao, Y. Liu, Y. Peng, C. N. He and P. G. Xiao, Mod. Chin. Med., 2013, 15, 76–79 Search PubMed.
- X. X. Deng and X. Zhao, J. Beijing Union Univ., Nat. Sci., 2013, 27, 64–67 CAS.
- D. W. Mercer, J. M. Cross, G. S. Smith and T. A. Miller, Am. J. Physiol., 1997, 273, 365–373 Search PubMed.
- G. Zhao, Y. M. Yu, M. Kaneki, A. A. Bonab, R. G. Tompkins and A. J. Fischman, Ann. Surg., 2015, 261, 1006–1012 CrossRef PubMed.
- M. L. Orozco Morales, N. M. Marsit, O. D. McIntosh, A. Hopkinson and L. E. Sidney, World J. Stem Cell., 2019, 11, 84–99 CrossRef PubMed.
- D. Yee, K. M. Shah, M. C. Coles, T. V. Sharp and D. Lagos, J. Biol. Chem., 2017, 292, 20683–20693 CrossRef CAS PubMed.
- C. Q. Yu, Q. M. Zou, F. K. Wang, D. S. Lu and H. Zeng, J. Clin. Med., 2001, 26, 195–197 CAS.
- S. Kwiecien, K. Jasnos, M. Magierowski, Z. Sliwowski, R. Pajdo, B. Brzozowski, T. Mach, D. Wojcik and T. Brzozowski, J. Physiol. Pharmacol., 2014, 65, 613–622 CAS.
- Y. Yang, B. Yin, L. Lv, Z. Wang, J. He, Z. Chen, X. Wen, Y. Zhang, W. Sun, Y. Li and Y. Zhao, Life Sci., 2017, 189, 44–51 CrossRef CAS PubMed.
- J. Li, H. L. Tang, Y. Chen, Q. Fan, Y. T. Shao, M. Jia, J. C. Wang and C. M. Yang, Genet. Mol. Res., 2015, 14, 4361–4368 CrossRef CAS PubMed.
- K. Amirshahrokhi and A. R. Khalili, Eur. J. Pharmacol., 2017, 811, 240–248 CrossRef CAS PubMed.
- X. Lu, C. Wang and B. Liu, Fish Shellfish Immunol., 2015, 42, 58–65 CrossRef CAS.
- B. Liu, C. Zhang, J. Zhang and X. Zhao, Biomolecules, 2019, 9, 169 CrossRef PubMed.
- B. Liu, X. Feng, J. Zhang, Y. Wei and X. Zhao, Biomolecules, 2019, 9, 137 CrossRef CAS PubMed.
- D. A. Sayed, A. M. Soliman and S. R. Fahmy, Biomed. Pharmacother., 2018, 107, 90–95 CrossRef CAS PubMed.
- E. Bignon, S. Rizza, G. Filomeni and E. Papaleo, Comput. Struct. Biotechnol. J., 2019, 17, 415–429 CrossRef CAS PubMed.
- Q. Wei, N. A. Korejo, J. Jiang, M. Xu, K. Zheng, D. Mao and F. Shi, Tissue Cell, 2018, 54, 59–64 CrossRef CAS PubMed.
- X. Zhao, Q. Cheng, Y. Qian, R. Yi, L. Gu, S. Wang and J. L. Song, Exp. Ther. Med., 2017, 14, 5135–5142 CAS.
- L. T. Lucetti, R. O. Silva, A. P. Santana, B. de Melo Tavares, M. L. Vale, P. M. Soares, F. J. de Lima Júnior, P. J. Magalhães, F. de Queiroz Cunha, R. de Albuquerque Ribeiro, J. R. Medeiros and M. H. Souza, Dig. Dis. Sci., 2017, 62, 93–104 CrossRef CAS PubMed.
- F. Geyikoglu, E. G. Yilmaz, H. S. Erol, K. Koc, S. Cerig, N. S. Ozek and F. Aysin, Ann. Hepatol., 2018, 17, 980–991 CrossRef CAS.
- W. Liu, P. Shang, T. Liu, H. Xu, D. Ren, W. Zhou, A. Wen and Y. Ding, Chem.-Biol. Interact., 2017, 278, 1–8 CrossRef CAS PubMed.
- H. Zhang and W. Xiao, Cell Biol. Int., 2017, 41, 415–422 CrossRef CAS PubMed.
- R. F. Araújo Júnior, V. B. Garcia, R. F. Leitão, G. A. Brito, C. Miguel Ede, P. M. Guedes and A. A. de Araújo, PLoS One, 2016, 11, e0148868 CrossRef PubMed.
- J. Khan, N. Noboru, A. Young and D. Thomas, Pathophysiology, 2017, 24, 155–159 CrossRef CAS PubMed.
- J. Bernatoniene and D. M. Kopustinskiene, Molecules, 2018, 23, 965 CrossRef PubMed.
- G. Galati, A. Lin, A. M. Sultan and P. J. O'Brien, Free Radicals Biol. Med., 2005, 40, 570–580 CrossRef PubMed.
- R. Guo, P. Wei and W. Liu, J. Pharm. Biomed. Anal., 2007, 43, 1580–1586 CrossRef CAS.
- Y. Kim, S. Narayanan and K. O. Chang, Antiviral Res., 2010, 88, 227–235 CrossRef CAS.
- W. F. Du, D. J. Jiang, Y. Wu and B. C. Cai, Chin. J. Pharm. Anal., 2016, 36, 842–846 CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |