Open Access Article
Open Access ArticleThree-dimensional microspheric g-C3N4 coupled by Broussonetia papyrifera biochar: facile sodium alginate immobilization and excellent photocatalytic Cr(IV) reduction
Qi Jina,
Guangyu Xieb,
Xiaoxi Caic,
Xinjiang Hu *b,
Hui Wangb,
Guoqiang Qiua,
Weixuan Wangd,
Daixi Zhoub,
Huiwen Huob,
Xiaofei Tan
*b,
Hui Wangb,
Guoqiang Qiua,
Weixuan Wangd,
Daixi Zhoub,
Huiwen Huob,
Xiaofei Tan ef and
Yunlin Zhao*ab
ef and
Yunlin Zhao*ab
aFaculty of Life Science and Technology, Central South University of Forestry and Technology, Changsha 410004, P.R. China. E-mail: zyl8291290@163.com; Tel: +8613548945666
bCollege of Environmental Science and Engineering, Central South University of Forestry and Technology, Changsha 410004, P.R. China. E-mail: huxinjiang@126.com; Tel: +8615243694564
cCollege of Art and Design, Hunan First Normal University, Changsha 410205, P.R. China
dCollege of Geography and Environmental Science, Northwest Normal University, Lanzhou 730070, P.R. China
eCollege of Environmental Science and Engineering, Hunan University, Changsha 410082, P.R. China
fKey Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, P.R. China
First published on 7th February 2020
Abstract
Photocatalysts comprising Broussonetia papyrifera biochar and g-C3N4 loaded on sodium alginate were prepared and characterized in terms of reusability and photocatalytic Cr(VI) reduction performance. The observed photocurrent responses as well as photoluminescence and UV-visible diffuse reflectance spectra showed that the best-performing catalyst featured the benefits of efficient photogenerated charge separation, superior electron conductance/transfer, and excellent light adsorption ability, which resulted in a higher photocatalytic Cr(VI) reduction performance compared to that of pure g-C3N4 powder. The prepared composite was shown to be reusable and well separable from the reaction mixture, thus being a promising material for the practical photocatalytic removal of Cr(VI) from wastewater. The trapping experiment and XPS spectra of catalysts after reactions confirm that the decontamination of Cr(VI) lies in the photocatalytic reduction of this species into low-toxicity Cr(III) by photoinduced electrons generated from g-C3N4, followed by the adsorption of Cr(III) on biochar or alginate with large specific areas.
1 Introduction
Cr is extensively used in tanning, electroplating, dyeing, and the manufacture of medicines and preservatives.1 However, the high redox potential and excellent solubility of this essential element can cause serious environmental pollution and threaten human health if Cr-contaminated wastewaters (mainly containing Cr(VI) and Cr(III)) are not properly treated.2 As the toxicity of the carcinogenic and mutagenic Cr(VI) exceeds that of Cr(III) more than 100-fold, Cr removal is mainly performed by electrolytic, chemical (with Fe0), or photocatalytic reduction of Cr(VI) to Cr(III) and the elimination of the latter as insoluble Cr(OH)3 in alkaline solutions.3,4 Among these methods, photocatalytic reduction features the advantages of operation simplicity, mild conditions, high cost-efficiency, catalyst recyclability, use of sustainable solar energy, non-toxicity, and technological maturity, and is therefore viewed as the method of choice.5,6Photocatalysis refers to the reaction of photogenerated charges and reactive free radicals with pollutants to realize decontamination, and the fabrication of high-efficiency photocatalysts is therefore vital for improving photocatalytic performance.7,8 TiO2 is a popular photocatalyst because of its nontoxicity, chemical stability, low cost, and impressive photoactivity;9 however, this material has a comparatively wide bandgap and, hence, a shortened optical response range.10,11 Consequently, photocatalysts active in the visible-light range are highly sought after. Among such catalysts, g-C3N4 (bandgap = 2.7 eV) is a novel metal-free photoactive material with a two-dimensional layered aromatic polycyclic structure comprising sp2-hybridized C and N atoms and featuring C–N bonds of uniform length.12–14 At present, g-C3N4 is used as a visible-light photocatalyst for the degradation of organic pollutants, semi-reaction of H2/O2 evolution, complete water splitting, CO2 reduction, and organic synthesis.15–19 However, its widespread application is hindered by the high rate of photogenerated electron–hole pair recombination.20
Biochar, a carbonaceous solid with excellent adsorption ability, is obtained by high-temperature anaerobic pyrolysis of biomass and is used in composites with g-C3N4 to realize more efficient visible light adsorption, faster electron transfer, slower electron–hole recombination, and higher surface area.21,22 Yan et al. ascribed the enhanced photocatalytic performance of carbon/g-C3N4 composites to the high electron conductivity of residual carbon, while Li et al. prepared biochar-coupled g-C3N4 from Camellia oleifera shells and melamine, revealing that these composites feature an increased Cr(VI) adsorption capacity and reduction ability due to their large specific surface area and superior electron conduction.23,24 Broussonetia papyrifera, a deciduous tree mainly found in Asian countries such as China and Japan, exhibits the advantages of fast growth, easy proliferation, and great disease resistance, which makes the utilization of its fallen leaves (e.g., by pyrolysis) a task of high practical importance.25,26 Biochar prepared from the fallen leaves of B. papyrifera features a pore structure and surface chemistry similar to those of activated carbon, possesses excellent adsorption ability, and is cheap to produce.27,28 Herein, B. papyrifera biochar was combined with g-C3N4 to enhance its photocatalytic performance by suppressing the recombination of photogenerated electron–hole pairs.
The practical application of numerous photocatalysts for water purification is hindered by their poor recyclability and reusability, especially in cases when these materials are applied as powders.29 Sodium alginate, a polysaccharide extracted from brown algae and also known as alginate gel, features the benefits of bioavailability, nontoxicity, and the ability to immobilize various substances.30 The exchange of Na+ in sodium alginate for divalent cations such as Ca2+, Cu2+, and Ba2+ results in the formation of stable biopolymers with a unique three-dimensional structure.31 Herein, g-C3N4 powder and biochar prepared from B. papyrifera fallen leaves were immobilized on sodium alginate to prepare photoactive biochar-coupled g-C3N4 (SABC), and the structure, morphology, and optical properties of this composite were probed by several instrumental techniques. The effects of electron conductivity and heterojunction charge separation were analyzed by photoluminescence spectroscopy and photocurrent density measurements, and Cr(VI) photoreduction experiments under visible-light illumination were carried out to demonstrate the excellent photocatalytic performance of the above heterojunction.
2 Materials and methods
2.1 Synthesis of SABC
Melamine (20 g) was loaded into a crucible and sequentially soaked by ultrapure water and ethanol and the supernatant were removed. The crucible was then heated at muffle furnace to 80 °C to evaporate ethanol, and further heated at 600 °C for 4 h to afford a light-yellow solid that was ground to obtain g-C3N4 powder.B. papyrifera leaves (harvested from a manganese mine in Xiangtan City, Hunan Province) were washed, dried, crushed, and sieved to obtain powders, which were then loaded into a corundum boat, placed into a tubular furnace, and pyrolyzed at 500 °C at a heating rate of 7 °C min−1 under a continuous flow of nitrogen. After 2 h pyrolysis, the furnace was cooled to 25 °C to obtain B. papyrifera biochar.
Sodium alginate (1.5 g) was dissolved in ultrapure water (100 mL) upon heating in a water bath, and the obtained solution was charged with different amounts of g-C3N4 and B. papyrifera biochar. The resulting mixtures were uniformly stirred, dropwise added to 4 wt% aqueous CaCl2 using injectors, and allowed to react for 4 h to obtain SABC microspheres, with SABC-1, SABC-2, and SABC-3 corresponding to biochar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g-C3N4 mass ratios of 1
g-C3N4 mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 2
2, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively (Scheme 1). Pure sodium alginate (SA), sodium alginate-biochar (SAB), and sodium alginate-g-C3N4 (SAC) samples were prepared for comparison.
1, respectively (Scheme 1). Pure sodium alginate (SA), sodium alginate-biochar (SAB), and sodium alginate-g-C3N4 (SAC) samples were prepared for comparison.
2.2 Characterization
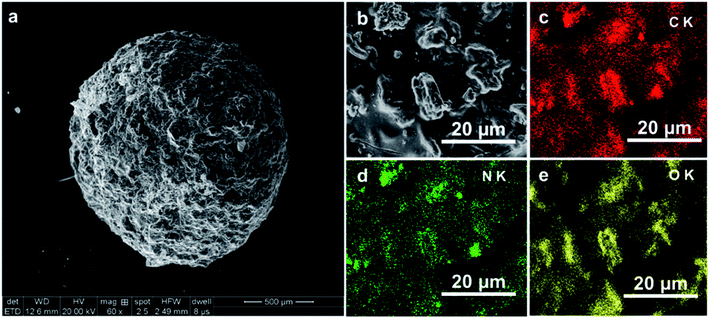
The Brunauer–Emmett–Teller (BET) surface area was measured with BET ratio surface and aperture analyzer (NOVA2000e, Quantachrome, USA). Scanning electron microscopy (SEM) and X-ray energy-dispersive spectroscopy (EDX) analyses were performed using a QUANTA250 scanning electron microscope (FEI, USA) equipped with an X-MAX-50 EDX module (INCA, UK). Crystal phase composition was probed by X-ray diffraction (XRD; D/max-2500 and Smartlab9K, Rigaku, Japan), and surface functional groups were identified by Fourier transform infrared (FTIR) spectroscopy (NICOLET 5700, Thermo Nicolet Corp., USA). The valence states of surface elements were determined by X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, Thermo Fisher Scientific, USA), and photoluminescence (PL) spectra were observed at an FLS 980 fluorescence spectrophotometer (Edinburgh Instruments, UK). Photocurrents were measured on a CHI760E electrochemical workstation, and light adsorption ability was probed by UV-vis spectrophotometry (U-4100, Hitachi, Japan). Electron signals generated in illuminated materials were detected by electron spin resonance (ESR; JES FA200, JEOL, Japan).2.3 Photoelectrochemical measurements
The photocurrent intensity of the materials was measured using a CHI760E electrochemical workstation via a three-electrode model. It consists of the Ag/AgCl electrode in the KCl solution, Pt electrode and a working electrode that were prepared by dropping 0.1 mL of sludge on a 1 × 2 cm FTO substrate. The sludge was made by dispersing 20 mg of materials in 2 mL 0.25% Nafion. The electrolyte solution used in this system is 0.5 M Na2SO4. The light source is the same as that in the photocatalytic experiments.2.4 Photocatalytic activity test
A Cr(VI) stock solution (1 g L−1) was obtained as follows. Potassium dichromate dried to constant mass (2.829 g) was dissolved in ultrapure water, and the solution was transferred into a volumetric flask and made up to 1000 mL. Variable-concentration solutions of Cr(VI) were prepared by stock solution dilution. The photocatalytic reduction of Cr(VI) by SABC microspheres was investigated under visible-light illumination using a 300 W Xe lamp (CEL-HXF300) with a UV cutoff filter as a visible light source. Typically, a solution of Cr(VI) (100 mL, 50 mg L−1) was placed in a beaker, treated with NaOH or HCl for pH adjustment (at 2.0), and charged with the photocatalyst under investigation (4 g wet weight). The mixture was magnetically stirred under visible light irradiation, and 4 mL aliquots were withdrawn every 30 min to measure the concentration of Cr(VI) by UV spectrophotometry at a wavelength of 540 nm.3 Results and discussion
3.1 Characterizations
| Composites | SA | SAB | SAC | SABC-1 | SABC-2 | SABC-3 |
|---|---|---|---|---|---|---|
| Surface area (m2 g−1) | 6.958 | 8.472 | 0.548 | 1.148 | 3.677 | 10.051 |
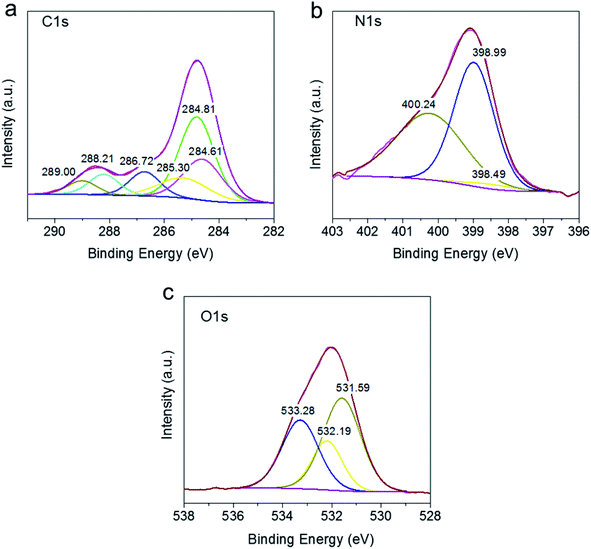
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration of g-C3N4, respectively.36–38 The similar peak patters in SABC spheres as SA, SAB, and SAC implies the successful preparation of g-C3N4 and biochar composite immobilized into alginate.
N stretching vibration of g-C3N4, respectively.36–38 The similar peak patters in SABC spheres as SA, SAB, and SAC implies the successful preparation of g-C3N4 and biochar composite immobilized into alginate.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and O–C
N and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O units, respectively.32,33 The N1s spectrum shown in Fig. 3b can be deconvoluted into peaks at 398.49, 398.99, and 400.24 eV, belonging to C–N
O units, respectively.32,33 The N1s spectrum shown in Fig. 3b can be deconvoluted into peaks at 398.49, 398.99, and 400.24 eV, belonging to C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and N-(C)3.34,40 From Fig. 3c, the O 1s spectrum featured the contributions of –OH (531.27 eV), C–O (532.19 eV), and O–C
C, C–N, and N-(C)3.34,40 From Fig. 3c, the O 1s spectrum featured the contributions of –OH (531.27 eV), C–O (532.19 eV), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (533.28 eV) moieties.41–43 The above results indicated that the composite possessed a sufficient number of active groups on its surface, which was expected to increase pollutant removal efficiency.
O (533.28 eV) moieties.41–43 The above results indicated that the composite possessed a sufficient number of active groups on its surface, which was expected to increase pollutant removal efficiency.
3.2 Charge separation and optical properties
PL spectroscopy probes the separation efficiency of photogenerated carriers in a given photocatalyst, with more intense PL signals indicating lower separation efficiency and more efficient carrier recombination.44 Fig. 4a shows that the PL signal of SAC was much more intense than that of SABC-3, indicating that biochar addition greatly suppressed electron–hole pair recombination. The peak of SABC-3 was slightly shifted relative to that of SAC, which indicated an interaction between g-C3N4 and biochar. Thus, biochar addition was concluded to facilitate charge transfer and diffusion in the composite to effectively inhibit the recombination of photogenerated electron–hole pairs and enhance photocatalytic performance.45 | ||
| Fig. 4 (a) PL spectra of SAC and SABC-3 microspheres; (b) Photocurrent response density of g-C3N4, SAB and SABC-3 microspheres; (c) UV-vis spectra of SAC and SABC microspheres. | ||
Fig. 4b presents the photocurrent responses of as-prepared materials, showing that g-C3N4, SAB, and SBC-3 featured stable photocurrents under visible-light illumination yet exhibited different photocurrent intensities. The lowest and highest photocurrents were observed for SAB and SABC-3, respectively, and the latter composite was therefore concluded to offer the highest photoelectric conversion efficiency, mainly due to the promotional effect of biochar on the separation of photogenerated carriers. These results further proved that the combination of alginate, g-C3N4, and B. papyrifera biochar improved photocatalytic performance.
The optical properties of photocatalytic materials determine their light absorption ability, which profoundly impacts photoelectrochemical properties and photocatalytic performance. Herein, the optical properties of SAC and SABC microspheres were probed by UV-vis diffuse reflectance spectroscopy (Fig. 4c). The slight red shift of the absorption band edge of SABC-3 (∼550 nm) compared to that of SAC (485 nm) indicated that the former material could efficiently absorb low-energy light and thus effectively utilize light energy to produce more electron–hole pairs and thus increase photocatalytic performance.
3.3 Photoreduction of Cr(VI)
3.4 Photocatalytic reaction mechanism
To study the contribution of different active species to the Cr(VI) photoreduction by SABC-3, photoreduction was performed in the presence of three typical scavengers (tert-butanol (TBA), K2S2O8, and EDTA-2Na, 0.01 M each) to quench ··OH, electrons, and holes, respectively.32 Fig. 7 shows that the Cr(VI) decontamination was inhibited in the presence of K2S2O8, indicating that electrons play an important role in the photocatalytic reduction process. The addition of TBA did not have a significant effect, i.e., hydroxyl radicals were not largely involved in the photocatalytic process. Notably, the reaction rate dramatically increased in the presence of EDTA-2Na, mainly because of the removal of holes to inhibit electron–hole recombination and allow more electrons to participate in Cr(VI) photoreduction process. The above results further illustrate that photogenerated e− are the main active substances in the photocatalytic reduction process. | ||
| Fig. 7 Trapping experiments for the photoreduction of Cr(VI) over SABC-3 microspheres under visible light irradiation: [Cr(VI)] = 50 mg L−1, m = 40 g L−1, pH = 2.0. | ||
Since photogenerated electrons are essential active species in the photocatalytic process, the ESR test was performed on SABC-3 to qualify the generation of electrons under visible-light irradiation according to the detected signal intensity and the result was shown in Fig. 8. The intensity of ESR signals represents the electron scavenger concentration, i.e., is negatively correlated with electron content. In the dark, the signal intensity was high (i.e., few electrons were generated), decreasing upon irradiation with visible light, i.e., upon the increase of the number of electrons in the reaction system. Thus, a large number of electrons could be generated in SABC-3 upon illumination with visible light to reduce Cr(VI) in the solution.
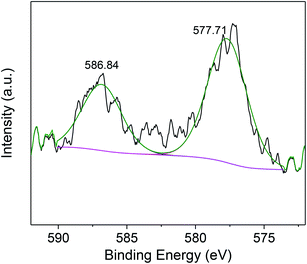
After the photocatalytic reaction, SABC-3 microspheres were probed by XPS (Fig. 9). The deconvoluted peaks at 577.71 and 586.84 eV were ascribed to Cr 2p3/2 and Cr 2p1/2 spectra of Cr(III) ions, which implied that the Cr(VI) is completely reduced to Cr(III) by electrons generated by g-C3N4.46,47 Finally, the produced Cr(III) species are captured by biochar or alginate on SABC-3 to achieve decontamination.
4 Conclusions
Biochar-coupled g-C3N4 microspheres (SABC) were prepared by immobilization of g-C3N4 and B. papyrifera biochar on alginate. The obtained microspheres featured excellent photocatalytic performance for Cr(VI) reduction, could be easily separated and recycled, and were therefore well suited for practical photocatalytic water purification. The mechanism of Cr(VI) reduction was elucidated by photoelectrochemical measurements and optical property tests, which revealed that g-C3N4 generates a sufficient amount of electron–hole pairs upon visible-light irradiation, while biochar accelerates the transfer of electrons and promotes their separation from holes. The composite with the largest biochar content (SABC-3) featured the fastest Cr(VI) adsorption and photocatalytic reduction rate, i.e., the addition of B. papyrifera biochar promoted adsorption and electron transmission. Cycling experiments revealed the high recyclability of the prepared composite microspheres. Photogenerated electrons were found to be the main active species in this photocatalytic system, and the reduced Cr(III) could be removed by adsorption on biochar or alginate.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Grant No. 51979294, 51608208), the Natural Science Foundation of Hunan Province (Grant No. 2018JJ3887, 2019JJ51005 and 2018JJ3096), the Research Foundation of Education Department of Hunan Province, China (Grant No. 17K105), the Training Program for Excellent Young Innovators of Changsha (Grant No. kq1905064), the Key Research and Development Program of Hunan Province, China (Grant No. 2019SK2191).References
- E. Malkoc, Y. Nuhoglu and Y. Abali, Chem. Eng. J., 2006, 119, 61–68 CrossRef CAS.
- S. Xu, J. Dai, J. Yang, J. You and J. Hao, Nanomaterials, 2018, 8, 472–489 CrossRef.
- M. A. Ahsan, S. K. Katla, M. T. Islam, J. A. Hernandez-Viezcas, L. M. Martinez, C. A. Díaz-Moreno, J. Lopez, S. R. Singamaneni, J. Banuelos, J. Gardea-Torresdey and J. C. Noveron, Environmental Technology & Innovation, 2018, 11, 23–40 Search PubMed.
- J. Ren, G. Zhang, D. Wang, D. Cai and Z. Wu, Bioresour. Technol., 2019, 291, 121856–121862 CrossRef.
- G. C. Zhang, J. Zhong, M. Xu, Y. Yang, Y. Li, Z. Fang, S. Tang, D. Yuan, B. Wen and J. Gu, Chem. Eng. J., 2019, 375, 122093–122102 CrossRef CAS.
- C. Xue, D. Li, Y. Li, N. Li, F. Zhang, Y. Wang, Q. Chang and S. Hu, Ceram. Int., 2019, 45, 17512–17520 CrossRef CAS.
- J. Li, P. Yan, K. Li, W. Cen, X. Yu, S. Yuan, Y. Chu and Z. Wang, Chin. J. Catal., 2018, 39, 1695–1703 CrossRef CAS.
- S. Wang, X. Zhang, L. Pan, F.-M. Zhao, J.-J. Zou, T. Zhang and L. Wang, Appl. Catal., B, 2015, 164, 234–240 CrossRef CAS.
- G. Shen, L. Pan, Z. Lü, C. Wang, F.-e. Aleem, X. Zhang and J.-J. Zou, Chin. J. Catal., 2018, 39, 920–928 CrossRef CAS.
- X. Zeng, Z. Wang, G. Wang, T. R. Gengenbach, D. T. McCarthy, A. Deletic, J. Yu and X. Zhang, Appl. Catal., B, 2017, 218, 163–173 CrossRef CAS.
- L. Ma, G. Wang, C. Jiang, H. Bao and Q. Xu, Appl. Surf. Sci., 2018, 430, 263–272 CrossRef CAS.
- S. C. Yan, Z. S. Li and Z. G. Zou, Langmuir, 2009, 25, 10397–10401 CrossRef CAS.
- Y. Li, H. Zhang, P. Liu, D. Wang, Y. Li and H. Zhao, Small, 2013, 9, 3336–3344 CAS.
- S. Sun, G. Shen, J. Jiang, W. Mi, X. Liu, L. Pan, X. Zhang and J. J. Zou, Adv. Energy Mater., 2019, 9, 1901505 CrossRef.
- O. Elbanna, M. Fujitsuka and T. Majima, ACS Appl. Mater. Interfaces, 2017, 9, 34844–34854 CrossRef CAS.
- L. Song, X. Kang and S. Zhang, Int. J. Energy Res., 2018, 42, 1649–1656 CrossRef CAS.
- G. Xu, H. Zhang, J. Wei, H. X. Zhang, X. Wu, Y. Li, C. Li, J. Zhang and J. Ye, ACS Nano, 2018, 5333–5340, DOI:10.1021/acsnano.8b00110.
- M. Ai, J.-W. Zhang, R. Gao, L. Pan, X. Zhang and J.-J. Zou, Appl. Catal., B, 2019, 256, 117805 CrossRef CAS.
- S. Sun, Y. Feng, L. Pan, X. Zhang and J.-J. Zou, Appl. Catal., B, 2019, 259, 118028 CrossRef CAS.
- P. Praus, J. Lang, A. Martaus, L. Svoboda, V. Matějka, M. Kormunda, M. Šihor, M. Reli and K. Kočí, J. Inorg. Organomet. Polym. Mater., 2019, 1219–1234, DOI:10.1007/s10904-019-01085-4.
- Y. Han, X. Cao, X. Ouyang, S. P. Sohi and J. Chen, Chemosphere, 2016, 145, 336–341 CrossRef CAS PubMed.
- X. Li, X. Qian, X. An and J. Huang, Appl. Surf. Sci., 2019, 487, 1262–1270 CrossRef CAS.
- Y. Li, C. Ji, Y. Lu, L. Wu, S. Sun, R. Qu, C. Sun, Y. Zhang and Z. Xue, Mater. Chem. Phys., 2018, 214, 28–33 CrossRef CAS.
- K. Li, Z. Huang, S. Zhu, S. Luo, L. Yan, Y. Dai, Y. Guo and Y. Yang, Appl. Catal., B, 2019, 243, 386–396 CrossRef CAS.
- X.-K. Ran, X.-T. Wang, P.-P. Liu, Y.-X. Chi, B.-J. Wang, D.-Q. Dou, T.-G. Kang and W. Xiong, Chin. J. Nat. Med., 2013, 11, 269–273 CAS.
- X. Peng, H. Liu, P. Chen, F. Tang, Y. Hu, F. Wang, Z. Pi, M. Zhao, N. Chen, H. Chen, X. Zhang, X. Yan, M. Liu, X. Fu, G. Zhao, P. Yao, L. Wang, H. Dai, X. Li, W. Xiong, W. Xu, H. Zheng, H. Yu and S. Shen, Mol. Plant, 2019, 661–677, DOI:10.1016/j.molp.2019.01.021.
- J. S. Cha, S. H. Park, S.-C. Jung, C. Ryu, J.-K. Jeon, M.-C. Shin and Y.-K. Park, J. Ind. Eng. Chem., 2016, 40, 1–15 CrossRef CAS.
- K. Weber and P. Quicker, Fuel, 2018, 217, 240–261 CrossRef CAS.
- V. S. Kosera, T. M. Cruz, E. S. Chaves and E. R. L. Tiburtius, J. Photochem. Photobiol., A, 2017, 344, 184–191 CrossRef CAS.
- Y.-g. Liu, X.-j. Hu, H. Wang, A.-w. Chen, S.-m. Liu, Y.-m. Guo, Y. He, X. Hu, J. Li, S.-h. Liu, Y.-q. Wang and L. Zhou, Chem. Eng. J., 2013, 226, 131–138 CrossRef CAS.
- Y. Ma, J. Wang, S. Xu, S. Feng and J. Wang, Appl. Surf. Sci., 2018, 430, 155–164 CrossRef CAS.
- X. Hu, W. Wang, G. Xie, H. Wang, X. Tan, Q. Jin, D. Zhou and Y. Zhao, Chemosphere, 2019, 216, 733–741 CrossRef CAS PubMed.
- G. Qiu, Y. Zhao, H. Wang, X. Tan, F. Chen and X. Hu, Environ. Sci. Pollut. Res., 2019, 26, 6565–6575 CrossRef CAS.
- D. Xiao, K. Dai, Y. Qu, Y. Yin and H. Chen, Appl. Surf. Sci., 2015, 358, 181–187 CrossRef CAS.
- S. Naeimi and H. Faghihian, J. Polym. Environ., 2019, 27, 1572–1583 CrossRef CAS.
- H. W. Kang, S. N. Lim, D. Song and S. B. Park, Int. J. Hydrogen Energy, 2012, 37, 11602–11610 CrossRef CAS.
- Y. Zhang, Q. Zhang, Q. Shi, Z. Cai and Z. Yang, Sep. Purif. Technol., 2015, 142, 251–257 CrossRef CAS.
- Y. Su, Y. Zhao, Y. Zhao, J. Lang, X. Xin and X. Wang, Appl. Surf. Sci., 2015, 358, 213–222 CrossRef CAS.
- Y. Shu, C. Tang, X. Hu, L. Jiang, X. Hu and Y. Zhao, Water, 2018, 10, 754–771 CrossRef.
- H. Wang, X. Yuan, H. Wang, X. Chen, Z. Wu, L. Jiang, W. Xiong and G. Zeng, Appl. Catal., B, 2016, 193, 36–46 CrossRef CAS.
- W. Dong, S. Chen, S. Jin, M. Chen, B. Yan and Y. Chen, Propellants, Explos., Pyrotech., 2019, 44, 413–422 CrossRef CAS.
- Y. Sun, D. Shao, C. Chen, S. Yang and X. Wang, Environ. Sci. Technol., 2013, 47, 9904–9910 CrossRef CAS.
- V. H. Tran Thi and B. K. Lee, J. Hazard. Mater., 2017, 324, 329–339 CrossRef PubMed.
- Y. Deng, L. Tang, G. Zeng, C. Feng, H. Dong, J. Wang, H. Feng, Y. Liu, Y. Zhou and Y. Pang, Environ. Sci.: Nano, 2017, 4, 1494–1511 RSC.
- Y. Liu, X. Yuan, H. Wang, X. Chen, S. Gu, Q. Jiang, Z. Wu, L. Jiang, Y. Wu and G. Zeng, Catal. Commun., 2015, 70, 17–20 CrossRef CAS.
- B. A. Manning, J. R. Kiser, H. Kwon and S. R. Kanel, Environ. Sci. Technol., 2007, 41, 586–592 CrossRef CAS PubMed.
- H. Nguyen Tran, Chem. Eng. J., 2019, 359, 810–812 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |