Open Access Article
Open Access ArticleHigh catalytic activity of CuY catalysts prepared by high temperature anhydrous interaction for the oxidative carbonylation of methanol
Yuchun Wang *a,
Zhaorong Liua,
Chao Tanb,
Hong Suna and
Zhong Li
*a,
Zhaorong Liua,
Chao Tanb,
Hong Suna and
Zhong Li c
c
aDepartment of Applied Chemistry, Yuncheng University, Fudan Street 1155, Yuncheng 044000, Shanxi, China. E-mail: wyc0104@126.com; Tel: +86 359 2513059
bKey Lab of Process Analysis and Control of Sichuan Universities, Yibin University, Yibin 644000, Sichuan, China
cKey Laboratory of Coal Science and Technology of Ministry of Education and Shanxi Province, Taiyuan University of Technology, Taiyuan 030024, Shanxi, China
First published on 20th January 2020
Abstract
CuY catalysts were prepared by high temperature anhydrous interaction between NH4Y zeolite and copper(II) acetylacetonate Cu(acac)2 and the activities were measured for the oxidative carbonylation of methanol to dimethyl carbonate under atmospheric pressure. The bulk and surface properties of the as-prepared catalyst were characterized by XRD, H2-TPR and XPS techniques. The activation atmosphere of the CuY catalyst and the testing temperature of the catalytic activity was systematically studied. During activation, nitrogen promotes the auto-reduction of Cu2+ to form the Cu+ active center, but deposited carbon on the surface of the CuY catalyst covers the active center, even plugging the channel, resulting in lower catalytic activity. Oxygen eliminates deposited carbon, but is not so good for the auto-reduction of Cu2+. Nitrogen doped with a small amount of oxygen not only eliminates the deposited carbon, but also promotes the auto-reduction of Cu2+ to form more Cu+ active centers. With the testing temperature increasing, the catalytic activity increases first and then decreases. When the testing temperature is 170 °C, the CuY catalyst with satisfactory activity and stability showed an excellent catalytic activity with 525.1 mg g−1 h−1 space time yield of DMC (STYDMC) and 18.9% methanol conversion. Then the longevity was investigated at 170 °C for 150 h. During the initial reaction period of 40 h, the STYDMC value was constant. In the next 20 h, the catalytic activity slightly decreased. But in the last 90 h, the catalytic performance is very stable and the STYDMC value remains 480 mg g−1 h−1. The main cause of deactivation is the growth of the particles.
Introduction
Recently, the global annual methanol production capacity has exceeded 100 million tons, but its demand was only around 65–70 million tons.1 Under this environment, to develop green technologies for the conversion of methanol to its downstream products is of great importance. The environmentally friendly dimethyl carbonate (DMC), as the downstream product of methanol, has been attracting wide attention.2–5 DMC is being considered as a component of reformulated fuels owing to its good blending properties and high oxygen content of 53%.6–8 Compared with the usual methyltert-butyl ether with 18% oxygen content, DMC not only significantly improves the fuel oxygenation but also displays a more valuable efficiency due to reducing the exhaust level of carbon monoxide, formaldehyde and unburned hydrocarbons, which reduces environmental pollution.9–11At present, the oxidative carbonylation of methanol to DMC has attracted increased attention due to its low-cost and green virtues.12,13 EniChem first commercialized a DMC production process based on a liquid phase oxidative carbonylation of methanol in the presence of cuprous chloride as a catalyst.14 This process, however, suffers from rapid deactivation of catalyst, corrosion of equipment and the separation of products from the catalyst. To overcome these drawbacks, vapor phase oxidative carbonylation processes have been proposed and was commercialized on the CuCl2/activated carbon (AC) by Dow Chemical Company.15
Some studies had compared copper-based catalyst with different support and found CuY catalyst is one of the effective catalysts. King16,17 showed that the activity and stability of CuY prepared by solid state ion exchange with helium purge is superior to that of the CuCl2/AC catalyst. Zhang et al.18 showed that, compared with Cu-ZSM-5 and Cu-MOR catalysts, the CuY catalyst had the higher activity and selectivity for the oxidative carbonylation of methanol to DMC. This is mainly because methanol was more easily absorbed on surface bound-Cu+ of the CuY catalyst to form Cu–OCH3 and CO inserted into Cu–OCH3 bonds, resulting in the formation of DMC. And many documents have recorded the Cu+ ion on the CuY catalyst is active center by a density functional theory.19,20
Research shows that the activation condition would determine the Cu+ content on the CuY catalyst, and then the catalytic activity for oxidative carbonylation of methanol to DMC. Richter et al.21 recently reported that NH4Y with copper(II) nitrate solution by incipient wetness impregnation and drying at 120 °C has no activity. After calcination at 400 °C in static air for 2 h, the samples revealed only limited activity. But a dry argon stream activation at 650 °C will improve the catalytic activity of CuY catalyst. Nam et al.22 prepared the Cu/Y-zeolite by precipitation sedimentation, after drying for 12 h at 120 °C, the sample exhibited a good catalytic activity with DMC yield of 49.9%. But after the sample was calcined at 550 °C for 2 h in an air, DMC yield decreased to 39.1%. All this proves that activation condition is a critical factor of affecting the catalytic activity of the CuY catalyst. Moreover, it was reported that nitrogen is the optimal activation atmosphere for the CuY catalyst, which is conducive to the reduction of Cu2+ and the formation of the Cu+ active center.23–25
Our preliminary investigations26–28 showed that NH4+ of NH4Y was ion-exchanged by Cu2+ of copper(II) acetylacetonate Cu(acac)2 under the condition of high temperature anhydrous interaction between NH4Y zeolite and Cu(acac)2. The ion-exchanged Cu2+ was reduced to Cu+ active center when the CuY catalyst was activated in a muffle furnace, and the as-prepared CuY catalyst has a good catalytic activity for oxidative carbonylation of methanol to DMC. But under an inert gas atmosphere the auto-reduction of Cu2+ is most likely to occur and form more Cu+ active centers. However, for the CuY catalysts prepared by high temperature anhydrous interaction between NH4Y and Cu(acac)2, we was surprised to find that the surface of the as-prepared CuY catalyst activated under nitrogen atmosphere was covered by a black material and the catalytic activity is poor. Then activation atmosphere of the CuY catalyst was investigated. Evaluation of catalytic activity for oxidative carbonylation of methanol to DMC and a detailed characterization of CuY catalysts were also performed. The major objectives are to elucidate the influence of activation atmosphere on copper species and catalytic activity by the characterization methods of X-ray, H2-TPR, XPS and TEM. In addition, the influence of oxidative carbonylation temperature on the selectivity of organic products and the space time yield of DMC was investigated. Finally, the catalyst life time was studied at 170 °C of oxidative carbonylation temperature.
Experimental section
Materials
NaY zeolite with a Si/Al molar ratio of 2.7 was purchased from Nankai Catalyst Factory. Reagents like NH4NO3 (AR, Tianjin Chemical Reagent No. 3 Factory), Cu(acac)2 (AR, Sinopharm Chemical Reagent Co. Ltd), CH3OH (AR, Tianjin Kemiou Chemical Reagent Company) as well as carbon monoxide (99.99%, Beijing Haipu Beifen Gas Industry Co., LTD), oxygen, nitrogen and hydrogen (99.99%, TISCO) were used.Catalyst preparation
Firstly, 30.002 g NaY zeolite (Si/Al = 2.7) was added to 300.00 mL 0.50 mol L−1 NH4NO3 aqueous solution and stirred to ion exchange for 4 h at 30 °C, and dried at 100 °C. Then the procedures were repeated and the final solid material was NH4Y zeolite.Typical preparation process of the CuY catalyst included two stages. In the first stage, a physical mixture with 2.454 g Cu(acac)2 and 5.001 g NH4Y zeolite was ground with a mortar and pestle to form an intimate gray-blue mixture. Then the mixture was placed in a crucible, and put in the muffle furnace. And the furnace temperature firstly increased from 25 °C to 250 °C at 3 °C min−1, and then was held constant at 250 °C for 4 h. The mixture turned brown and the final solid material was named as precursor. In the following activation stage, the precursor was placed in a tube furnace. And the furnace temperature was ramped at rate of 3 °C min−1 to 650 °C, held for 4 h and decreased slowly to room temperature. To investigate the effects of activation atmosphere, the CuY catalysts were activated using N2, O2, mixture of both gas with the ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10
1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 54
1, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at a flow rate of 120 mL min−1, the CuY catalysts are labeled CuY-1, CuY-2, CuY-3, CuY-4, CuY-5 respectively. The CuY-1 is black, and the light blue becomes shallow gradually from CuY-2 to CuY-4, CuY-5 is grey white. The as-prepared catalysts were stored in a drybox. Before catalytic measurements, the catalyst was pressed and sieved to 40–60 mesh.
1 at a flow rate of 120 mL min−1, the CuY catalysts are labeled CuY-1, CuY-2, CuY-3, CuY-4, CuY-5 respectively. The CuY-1 is black, and the light blue becomes shallow gradually from CuY-2 to CuY-4, CuY-5 is grey white. The as-prepared catalysts were stored in a drybox. Before catalytic measurements, the catalyst was pressed and sieved to 40–60 mesh.
Characterization techniques
X-ray diffraction (XRD) patters were obtained using CuKα radiation (λ = 0.154056 nm) on a Rigaku D/max 2500 diffractometer at 40 kV target voltage and 100 mA tube current in the 2θ range from 5° to 65° at a scanning rate of 8° min−1.Temperature-programmed reduction (H2-TPR) was performed on a Micromeritics Autochem II 2920 chemical adsorption instrument. Firstly, about 20 mg sample was loaded in a U-shaped quartz tube and degassed at a heating rate of 10 °C min−1 to 300 °C for 30 min at a N2 flow rate of 30 mL min−1. After cooling to room temperature, the gas was switched to 10 vol% H2 in argon flow, and the sample was heated at a heating rate of 10 °C min−1 to 1000 °C. The effluent gas was passed through a cold trap to remove water before detection, then the hydrogen consumption was monitored by a thermal conductivity.
X-ray photoelectron spectroscopy (XPS) data were collected on an AXIS ULTRA DLD electron spectrometer by using a 150 W monochromatic Al Kα radiation source operating at 1486.6 eV. The catalyst holder was placed into a fast entry air load-lock chamber without exposure to air and the pressure was maintained at 3 × 10−7 Pa overnight. Then the catalyst holder was transferred to the analysis chamber for XPS study. The binding energy and the Auger kinetic energy scales were referenced to the C1s line at 284.8 eV from adventitious carbon.
Transmission electron microscope (TEM) photographs were carried out using a JEOL JEM-2100F electron microscope operating at 200 kV. Exposition times were confined to about 2 s due to the beam sensitivity of the samples. The samples were prepared by dispersing the powdered products as slurry in ethanol, and then deposited on a carbon film coated on a copper grid.
Catalytic tests
Catalytic measurements were performed in a continuous fixed bed micro reactor (Φ8 mm × 500 mm) under atmospheric pressure with an on-line Agilent 6890 gas chromatograph (GC). 0.6006 g (about 1.50 mL) catalyst sample was packed in the tubular reactor and the reactor was positioned in the reactor furnace. 0.05 mL min−1 methanol was fed into by a constant-flux pump (2PB-05) and vaporized in the pre-heater. In the meantime, 55.0 mL min−1 carbon monoxide and 5.5 mL min−1 oxygen was introduced into the pre-heater and mixed well with vaporized methanol, and then the mixed reactants were fed into the catalyst bed to catalyze oxidative carbonylation of methanol. The activity testing time is 10 h and testing temperature is 140 °C, 150 °C, 160 °C, 170 °C, 180 °C respectively.The thermal gas products were analyzed by an on-line GC with three valves and four columns every 12 minutes. The products flowed through the HP-PLOT/Q capillary column (30 m × 0.53 μm × 40 μm), the organic products were hold back. And then the excurrent inorganic products flowed through the Porapak-Q packed column: CO2 firstly flowed out and entered into a thermal conductivity detector (TCD) and was detected; secondly, CO and O2 were separated by the HP-PLOT Molesieve/5A capillary column (30 m × 0.53 μm × 25 μm) and detected by TCD. The products flowed through the HP-INNOWax (30 m × 0.53 μm × 1 μm) capillary column and entered into a flame ionization detector (FID), thus MeOH, methyl formate (MF), dimethoxymethane (DMM), dimethyl ether (DME) and DMC were detected.
The catalyst longevity was studied at 170 °C of oxidative carbonylation temperature, and the thermal gas products were analyzed every 30 minutes.
Results and discussion
Characterization
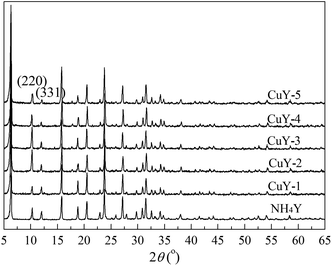
Fig. 1 shows the XRD pattern of NH4Y zeolite and the CuY catalysts with different activation atmosphere. As shown in Fig. 1, all the patterns show the characteristic diffraction peaks of Y zeolite, which proves that HAI between Cu(acac)2 and NH4Y zeolite does not affect the integrity of the Y zeolite crystal structure, even under the high temperature activation treatment at 650 °C. With the nitrogen content of the activation atmosphere, the peak strength of (220) and (331) lattice plane becomes gradually weaken and the ratio of I220/I331 gradual decreases, the value is 2.5, 2.0, 1.8 from CuY-3, CuY-4 to CuY-5 respectively. It indicates that after HAI between Cu(acac)2 and NH4Y zeolite and activation at high temperature, copper species enter into the pore of Y zeolite and the (220) and (331) lattice plane is mainly filled by disordered copper species. And the filling effect depends highly on activation atmosphere.And no obvious diffraction peaks corresponding to crystallized copper phase are observed in all the XRD patterns. Xie and Tang29 reported that some materials were dispersed spontaneously onto a highly specific surface of a suitable support after the heat treatment, which was identified by the disappearance of the crystalline phase of the matter from their XRD patterns. So it is concluded that copper species were well dispersed on the surface of Y zeolite.
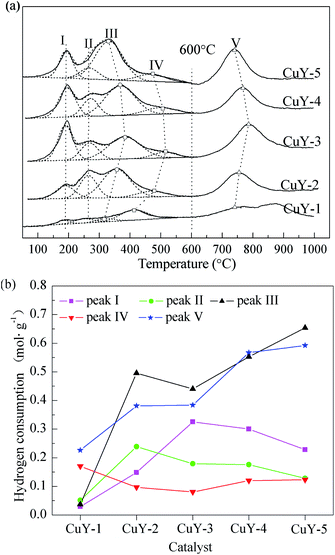
Fig. 2 presents the H2-TPR (a) of the CuY catalyst and hydrogen consumption of each reduction peak (b). According to the literatures,30–32 the reactions of ion-exchanged Cu2+ on zeolites involved in the reduction process occur by a two-step mechanism:
| Cu2+ + 0.5H2 → Cu+ + H+ | (1) |
| Cu+ + 0.5H2 → Cu0 + H+ | (2) |
The reduction of Cu2+ to Cu+ on the CuY catalyst is characterized by a H2-TPR peak below 500 °C, and the difficulty of reduction depends on the location of the cations, in the order of hexagonal prism > sodalite cage > supercage. The subsequent reduction step of Cu+ to Cu0 is observed at the higher temperatures of 700 °C.
The H2 consumption peak at about 230 °C corresponds to the reduction of CuO on the CuY catalyst proceeds in one step.
| CuO + H2 → Cu0 + H2O | (3) |
As mentioned above, the low temperature reduction peaks below 600 °C (LP) are defined peak I at 195 °C, peak III at about 380 °C, peak IV at 504 °C, peak II at about 260 °C, which is assigned to the reduction of Cu2+ to Cu+ in the supercage, sodalite cage, hexagonal prism and the reduction of CuO to Cu0 respectively. The high temperature reduction peaks above 600 °C (HP) is attributed to the reduction of Cu+ to Cu0.
For the CuY-1 catalyst activated under nitrogen atmosphere, there are weak LPs and broad HPs from 600 °C to 1000 °C, especially, the peak I and peak II are very weak. This shows that the reduction reaction of Cu2+ is easier to form Cu+ during activation of the CuY-1 catalyst under nitrogen atmosphere. In addition, it is clear that the total hydrogen consumption of the CuY-1 catalyst is low. Other four catalysts, with the increase the nitrogen content in activation atmosphere, the strength of the peak I increases at first and decreases afterwards. It illustrates that the ion-exchange reaction and the auto-reduction of Cu2+ to Cu+ are more likely to occur in relatively higher nitrogen content of activation atmosphere under the premise of no deposited carbon on the CuY catalyst. Besides the CuY-1 catalyst, the peak II becomes broad and the hydrogen consumption decreases gradually. The peak V shifts to low temperature from the CuY-3 to CuY-5 catalyst, and the hydrogen consumption is increased gradually, thus the Cu+ content also increases. So a small amount of oxygen ensures without detrimental deposited carbon to catalytic activity on the CuY catalyst. And in the activation atmosphere with a small amount of oxygen the Cu2+ is auto-reduced to Cu+ to the greatest extent, the content of the active center Cu+ reach to the maximum.
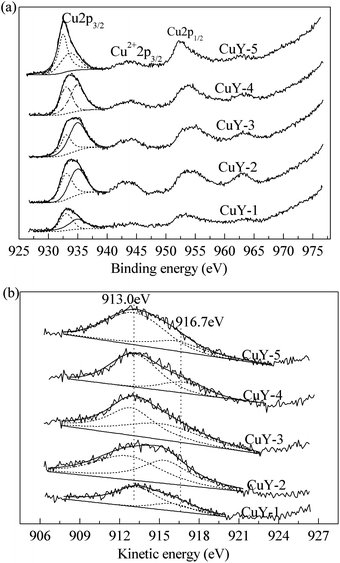
In order to study the surface composition of the catalysts and the nature of the surface copper species, XPS characterization was carried out. And Fig. 3 shows the XPS spectra for Cu2p and their curve-fitting results (a), Cu Auger spectrum (b) of all CuY catalysts activated under different activation atmosphere. The XPS peaks were different in shape as well as intensity. This indicates that the bonding type and the relative content of different copper species of all catalysts might be different. And the Auger analysis can complement the data obtained from XPS. Alonso et al.33 reported on Cu Auger spectrum, the kinetic energies at 918.2, 916.0 and 917.6 eV are assigned to Cu, Cu2O and CuO respectively. In addition, the kinetic energy for Cu+ in the CuY catalyst is 3 eV below that of Cu2O, at 913.0 eV.34 According to literature27 and the H2-TPR characterization (Fig. 2(a)), we determine that metallic copper is absent in the CuY catalyst. So the two peaks at 913.0 eV and 916.7 eV are assigned to monovalent copper species and divalent copper species respectively.
For XPS spectra of Cu2p, it is generally known that the overlapping peak from 930.0 eV to 940.0 eV can be resolved into two 2p3/2 peaks at 932.4 eV and 934.9 eV attributed to Cu+ and Cu2+ respectively.35–38 By means of XPS-peak-differentiating analysis, the relative content of copper species was determined and the results were listed in Table 1. As shown in Table 1, the total copper content and relative contents of Cu2+ and Cu+ vary with different activation atmosphere. The black CuY-1 catalyst, the total copper content is only 3.7 wt% due to deposited carbon on the surface. Therefore, the Cu+ content is also low, only 2.4 wt%. The CuY-2 catalyst, the total copper content reaches 7.7 wt%, but the Cu+ content is only 3.1 wt% due to oxidation of oxygen. The total copper content decreases gradually with the increase of the nitrogen content, when the mixture of nitrogen and oxygen used as activation atmosphere. And the Cu+ content increases from 3.1 to 3.5 wt%. The above analysis results show that nitrogen promotes migration of copper species from the surface to bulk, in addition, facilitates the formation of the Cu+ active center. The results are accord with that of H2-TPR.
| Catalyst | Peak area | Cu+/(Cu+ + Cu2+) (%) | Total Cu (wt%) | Cu+ (wt%) | |
|---|---|---|---|---|---|
| Cu+ peak | Cu2+ peak | ||||
| CuY-1 | 1582.5 | 921.4 | 63.2 | 3.7 | 2.4 |
| CuY-2 | 1882.9 | 2784.2 | 40.3 | 7.7 | 3.1 |
| CuY-3 | 2136.4 | 2205.9 | 49.2 | 6.4 | 3.1 |
| CuY-4 | 2509.4 | 1925.0 | 56.6 | 5.9 | 3.4 |
| CuY-5 | 2491.6 | 1520.0 | 62.1 | 5.6 | 3.5 |
Catalytic performance
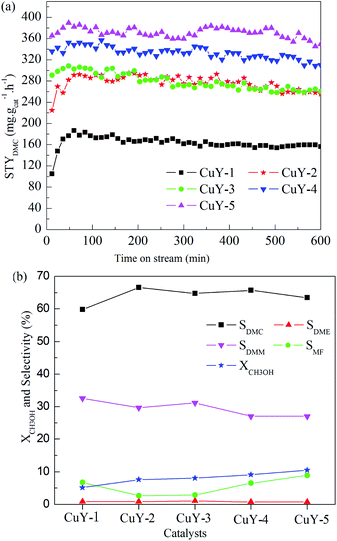
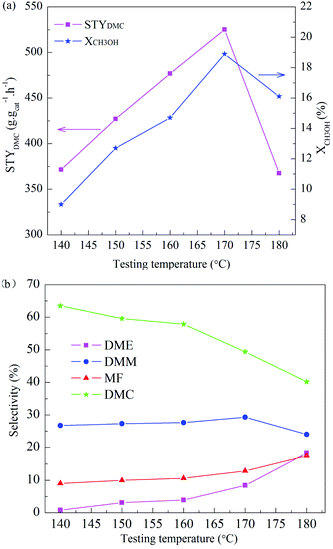
The effect of activation atmosphere on the catalytic activity is demonstrated for all CuY catalysts in the direct vapor oxidative carbonylation of methanol with a feed containing 62.5 vol% CO, 5.7 vol% O2, 31.8 vol% CH3OH, and gaseous hourly space velocity (GHSV) 3520 h−1, 10 h testing time at temperature 140 °C and under atmospheric pressure. Space time yield of DMC (STYDMC), conversion of CH3OH (XCH3OH) and selectivity of organic products DMC, DMM, DME, MF are shown in Fig. 4. All CuY catalysts prepared by HAI between NH4Y zeolite and Cu(acac)2 under different activation atmosphere exhibit different catalytic activity and no deactivation had taken place in 10 h testing time (Fig. 4(a)). From Fig. 4(a) and (b), it was found that STYDMC and XCH3OH shows the same pattern. When activated under nitrogen atmosphere, the black CuY-1 catalyst shows poor catalytic activity with 163.3 mg g−1 h−1 STYDMC, 5.1% XCH3OH and 59.8% DMC selectivity. The catalytic activity of the light blue CuY-2 catalyst activated in oxygen is better than that of the CuY-1 catalyst, although the CuY-2 catalyst has 40.3% Cu+ and the CuY-1 catalyst has 63.2% Cu+. It is likely to be because deposited carbon on the CuY-1 covers the Cu+ active centers, even plugs channel. Hence, for the CuY catalyst prepared by HAI between NH4Y zeolite and Cu(acac)2, activation atmosphere shows a significant influence on the activities of the CuY catalyst in DMC synthesis through oxidative carbonylation of methanol. For attaining the CuY catalyst with more Cu+ active centers and no deposited carbon on the surface of the CuY catalyst, the gas mixture of nitrogen and oxygen was used as activation atmosphere to prepare catalysts, the nitrogen content increasing sequence is as follow: CuY-3 < CuY-4 < CuY-5, and the STYDMC and XCH3OH also increase in the same order, and the selectivity is not changed obviously (see Fig. 4(b)). So the CuY-5 catalyst activated under the mixture of nitrogen with a small amount of oxygen (only 1.8 vol%) shows the best catalytic activity with STYDMC of 370.7 mg g−1 h−1, XCH3OH of 10.5% and DMC selectivity of 63.5%.In order to determine the optimal temperature of oxidative carbonylation of methanol to DMC, so the next work to study the testing temperature on the CuY-5 catalyst with other conditions fixed. The testing temperature was 140 °C, 150 °C, 160 °C, 170 °C, 180 °C respectively and testing time was 10 h. STYDMC and XCH3OH are showed in Fig. 5(a) and the selectivity of organic products are showed in Fig. 5(b). STYDMC and XCH3OH increase followed by a decrease with the increase of the testing temperature. However, the selectivity of DMC monotonically decreases with the increase of testing temperature and can hold about 50% at the testing temperature 170 °C. This is a decrease mirrored by a corresponding increase in DME and MF selectivity. The reason is the thermal decomposition of DMC can produce DME.39 Therefore, at 170 °C testing temperature, the CuY-5 catalyst has the optimal catalytic performance with STYDMC of 525.1 mg g−1 h−1, and XCH3OH reaches to 18.9%, far higher than the methanol conversion (5–12%) for a catalyst with 17.4 wt% Cu loading at the elevated pressure reported by Richter.21
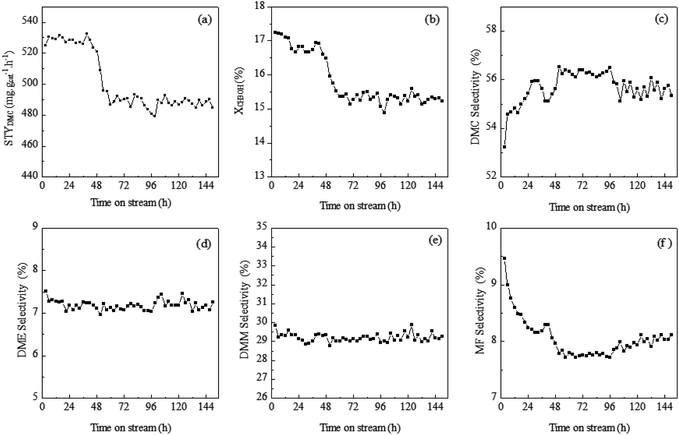
The long-term stability and activity of catalysts are vital for oxidative carbonylation of methanol to dimethyl carbonate from both academic and industrial viewpoints. The effects on catalyst longevity of vapor-phase oxidative carbonylation of methanol to DMC reactions have been investigated at 170 °C and the catalytic results of 150 h were presented in Fig. 6. In time on stream of 150 h, the selectivities of DMM and DME have been constant. However, during the initial reaction period of 40 h, the selectivity of MF is slowly falling which monitored by an increase of the CH3OH selectivity. But CH3OH conversion gradually increases. Finally, STYDMC have remained broadly stable. After a reaction period of 40 h, the CuY-5 catalytic activity decreased. In the next 20 h, the selectivities of DMC and MF also remains constant, but compared with the initial value, the deactivation degree of XCH3OH and STYDMC is 7.8% and 7.0% respectively. In the last 90 h, the catalytic activity with 480 mg g−1 h−1 of STYDMC remains basically unchanged.
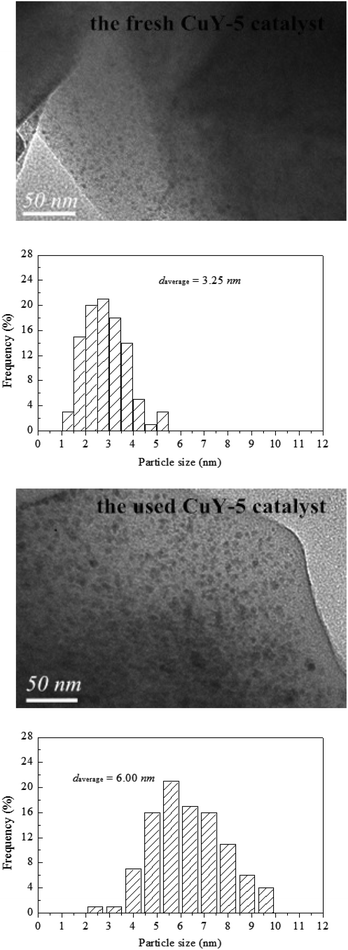
In order to investigate the reason for catalyst deactivation, TEM characterization was carried out and the TEM images and the size distribution of CuO particles for the fresh CuY-5 and the used CuY-5 catalyst showed in Fig. 7. The bigger particles about 4–9 nm are formed on the used CuY-2 compared with 1–5 nm of the fresh catalyst, which may be the main cause of activity decrease.
Conclusions
For the CuY catalysts prepared by HAI between NH4Y zeolite and Cu(acac)2, the activation atmosphere of the CuY catalyst show a significant influence on the activities of CuY catalysts in DMC synthesis through oxidative carbonylation of methanol. When the CuY catalyst is activated under pure nitrogen flow, there is deposited carbon on the catalyst. And the catalytic activity is inferior to other as-prepared catalysts because deposited carbon blocks the pores and covers the Cu+ active center, even causes the DMC selectivity decrease. Pure oxygen used as activation atmosphere, there is no deposited carbon on the as-prepared CuY-2 catalyst, but the formation of the Cu+ is limited, which is adverse to the catalytic activity. So nitrogen doped a small amount of oxygen used as activation atmosphere is the best choice. It not only assures no deposited carbon on the CuY catalyst, but also promotes the formation of the Cu+ active center.Additionally, the testing temperature of oxidative carbonylation of methanol to DMC is also a key factor that influence the catalytic activity of the CuY catalyst. As the testing temperature rises, STYDMC and XCH3OH increase at first and decrease afterwards, the DMC selectivity decrease gradually due to thermal decomposition of DMC to DME. At 170 °C testing temperature, the CuY catalyst exhibits the optimal catalytic activity and an excellent stability. At 170 °C the service life of the CuY-5 catalyst is very long and its catalytic activity with 480 mg g−1 h−1 of STYDMC remains basically unchanged for 150 h.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the Doctor Research Fund of Yuncheng University [YQ-2019024]; Applied Basic Research Programs of Foundation for Youths of Shanxi Province of China [201701D221045]; Subject Research Project of Foundation of Yuncheng University [XK-2019018] and the open Foundation of Key Lab of Process Analysis and Control of Sichuan Universities [Yibin University Grant No. 2015003].References
- K. Andersson and C. M. salazar, Methanol as a marine fuel report for Methanol Institute, FCBI Energy, 2015, http://www.methanol.org/Files/Homepage/FCBI-Methanol-Marine-Fuel-Report-Final.aspx?lang=en-US Search PubMed.
- M. Selva and A. Perosa, Green Chem., 2008, 10, 457–464 RSC.
- J. Bian, X. W. Wei, Y. R. Jin, L. Wang, D. C. Luan and Z. P. Guan, Chem. Eng. J., 2010, 165, 686–692 CrossRef CAS.
- A. H. Tamboli, A. A. Chaugule and H. Kim, Chem. Eng. J., 2017, 323, 530–544 CrossRef CAS.
- S. Huang, B. Yan, S. Wang and X. Ma, Chem. Soc. Rev., 2015, 44, 3079–3116 RSC.
- D. Stoian, F. Medina and A. Urakawa, ACS Catal., 2018, 8, 3181–3193 CrossRef CAS.
- J. O. Petunchi, G. Marcelin and W. K. Hall, J. Phys. Chem., 1992, 96, 9967–9975 CrossRef CAS.
- R. N. Shi, J. Wang, J. X. Zhao, S. S. Liu, P. P. Hao, Z. Li and J. Ren, Appl. Surf. Sci., 2018, 459, 707–715 CrossRef CAS.
- M. A. Pacheco and C. L. Marshall, Energy Fuels, 1997, 11, 2–29 CrossRef CAS.
- D. Zhu, F. Mei, L. Chen, T. Li, W. Mo and G. Li, Energy Fuels, 2009, 23, 2359–2363 CrossRef CAS.
- J. Bian, M. Xiao, S. J. Wang, X. J. Wang, Y. X. Lu and Y. Z. Meng, Chem. Eng. J., 2009, 147, 287–296 CrossRef CAS.
- D.-H. Lee, J. You, J.-M. Woo, J. Y. Seo, Y. C. Park, J.-S. Lee, H. Kim, J.-H. Moon and S. B. Park, Chin. J. Chem. Eng., 2018, 26, 1059–1063 CrossRef CAS.
- X. S. Ding, X. M. Dong, D. D. Kuang, S. F. Wang, X. Q. Zhao and Y. J. Wang, Chem. Eng. J., 2014, 240, 221–227 CrossRef CAS.
- U. Romano, R. Tesel, M. M. Mauri and P. Rebora, Ind. Eng. Chem. Prod. Res. Dev., 1980, 19, 396–403 CrossRef CAS.
- G. L. Curnutt, Catalytic vapor phase process for producing dihydrocarbyl carbonates, US Pat., US5004827, 1991.
- S. T. King, Catal. Today, 1997, 33, 173–182 CrossRef CAS.
- S. T. King, J. Catal., 1996, 161, 530–538 CrossRef CAS.
- Y. Zhang, D. N. Briggs, E. de Smit and A. T. Bell, J. Catal., 2007, 251, 443–452 CrossRef CAS.
- X. Zheng and A. T. Bell, J. Phys. Chem. C, 2008, 112, 5043–5047 CrossRef CAS.
- H. Zheng, J. Qi, R. Zhang, Z. Li, B. Wang and X. Ma, Fuel Process. Technol., 2014, 128, 310–318 CrossRef CAS.
- M. Richter, M. J. G. Fait, R. Eckelt, E. Schreier, M. Schneider, M. M. Pohl and R. Fricke, Appl. Catal., B, 2007, 73, 269–281 CrossRef CAS.
- J.-K. Nam, M.-J. Choi, D.-H. Cho, J.-K. Suh and S.-B. Kim, J. Mol. Catal. A: Chem., 2013, 370, 7–13 CrossRef CAS.
- Z. Li, R. Y. Wang, H. Y. Zheng and K. C. Xie, Fuel, 2010, 89, 1339–1343 CrossRef CAS.
- Z. Li, T. J. Fu and H. Y. Zheng, Chin. J. Inorg. Chem., 2011, 27, 1483–1490 CAS.
- T. J. Fu, H. Y. Zheng, Y. Y. Niu, R. Y. Wang and Z. Li, Acta Chim. Sin., 2011, 69, 1765–1772 CAS.
- Y. C. Wang, H. Y. Zheng, B. Liu, G. Q. Zhang and Z. Li, Chem. J. Chin. Univ., 2015, 36, 2540–2549 CAS.
- Y. C. Wang, H. Y. Zheng, Z. Li and K. C. Xie, RSC Adv., 2015, 5, 102323–102331 RSC.
- Y. C. Wang, H. Y. Zheng and Z. Li, Chin. J. Catal., 2016, 37, 1403–1412 CrossRef CAS.
- Y. C. Xie and Y. Q. Tang, Spontaneous Monolayer Dispersion of Oxides and Salts onto Surfaces of Supports: Applications to Heterogeneous Catalysis, in Advances In Catalysis, ed. H. P. D. D. Eley and B. W. Paul, Academic Press, 1990, pp. 1–43 Search PubMed.
- S. Kieger, G. Delahay, B. Coq and B. Neveu, J. Catal., 1999, 183, 267–280 CrossRef CAS.
- H. Song, X. Wan, M. Dai, J. J. Zhang, F. Li and H. L. Song, Fuel Process. Technol., 2013, 116, 52–62 CrossRef CAS.
- C. Torre-Abreu, C. Henriques, F. R. Ribeiro, G. Delahay and M. F. Ribeiro, Catal. Today, 1999, 54, 407–418 CrossRef CAS.
- F. Alonso, T. Melkonian, Y. Moglie and M. Yus, Eur. J. Org. Chem., 2011, 2524–2530 CrossRef CAS.
- Z. Li, T. J. Fu, R. Y. Wang, Y. Y. Niu and H. Y. Zheng, Chem. J. Chin. Univ., 2011, 1366–1372 CAS.
- J. M. Lázaro Martínez, E. Rodríguez-Castellón, R. M. T. Sánchez, L. R. Denaday, G. Y. Buldain and V. Campo Dall' Orto, J. Mol. Catal. A: Chem., 2011, 339, 43–51 CrossRef.
- Y. Yuan, W. Cao and W. Weng, J. Catal., 2004, 228, 311–320 CrossRef CAS.
- X. Li, X. Zhang and L. Lei, Sep. Purif. Technol., 2009, 64, 326–331 CrossRef CAS.
- M. Zahmakıran, F. Durap and S. Özkar, Int. J. Hydrogen Energy, 2010, 35, 187–197 CrossRef.
- S. A. Anderson, S. Manthata and T. W. Root, Appl. Catal., A, 2005, 280, 117–124 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |