Open Access Article
Open Access ArticleA coumarin-containing Schiff base fluorescent probe with AIE effect for the copper(II) ion†
Ying Wanga,
Xiaohui Hao*b,
Lixun Lianga,
Luyao Gaoa,
Xumin Rena,
Yonggang Wua and
Hongchi Zhao *a
*a
aCollege of Chemistry and Environmental Science, Hebei University, Baoding, 071002, P. R. China. E-mail: zhc@hbu.edu.cn
bCollege of Physics Science and Technology, Hebei University, Baoding, 071002, P. R. China. E-mail: haoxiaohui@hbu.edu.cn
First published on 7th February 2020
Abstract
A novel coumarin-derived Cu2+-selective Schiff base fluorescent “turn-off” chemosensor CTPE was successfully obtained, which showed an AIE effect. It could identify Cu2+ by quenching its fluorescence. The lower limit of detection was 0.36 μM. CTPE can act as a highly selective and sensitive fluorescence probe for detecting Cu2+.
The specific detection of transition metal ions has received considerable attention because of their significant roles in the fields of biological, chemical, medical and environmental processes.1 Among these metal ions, copper(II), acting as one of the most important micronutrients, is of particular interest due to its essential role in a variety of fundamental physiological processes. In spite of the fact that copper(II) is essential in living systems, accumulation of copper(II) can lead to serious environmental and health problems.2 For instance, the metabolic balance of Cu2+ in the body being destroyed can lead to neurodegenerative diseases, such as Alzheimer's, Parkinson's, Menkes, and Wilson's disease.3–8 Besides, free Cu2+ is regarded as a significant environmental pollutant.9,10 Therefore, an increasingly convenient and fast method to detect Cu2+ ion existing in environmental and biological resources is currently of considerably important. By far there existing many methods make it possible to detect and quantify Cu2+ ion, such as atomic absorption spectrometry,11,12 inductively coupled plasma mass spectrometry,13 inductively coupled plasma-atomic emission spectrometry14 and voltammetry,15,16 etc. However, these methods require tedious sample preparation procedures and complex instrumentation, which limit their prosperous applications. Alternatively, more and more attention has been attracted to the analytical methods based on fluorescent chemosensors for the detection of the metal ions owing to their high sensitivity, selectivity, and simplicity.17 As a result, various types of fluorescent probes such as organic dyes, magnetic nanoparticles (MNPs), semiconductor quantum dots (QDs), carbon dots (CDs), fluorescent metal nanoclusters (NCs), and fluorescent metal organic frameworks (MOFs) have been designed and prepared to determine Cu2+ ion.18–27 Since first reported in 2000, MNPs are extensively used in the field of nanotechnology for its nonhazardous feature, strong magnetization values, superparamagnetic property, active surface that can easily assembled of biological soluble structure and targeting, imaging, and therapeutic molecules.22 Unlike organic fluorescent dyes, QDs are semiconductor particles which exhibit high photochemical stability, excellent resistance to chemical degradation, outstanding photodegradation and extremely large Stokes shift.23 CDs is a comprehensive term for various nanosized carbon materials including graphene quantum dots (GQDs), carbon nanodots (CNDs), and polymer dots (PDs)24 which shows excellent properties including high stability, bio-compatibility, low photo bleaching and toxicity and so on.25 Despite successfully applying in detecting Cu2+, traditional fluorescent probes are still puzzled by the toxicity, poor solubility, “aggregation-caused quenching” (ACQ) effect, etc. Thus, preparing fluorescent probes that can compensate for these deficiencies is still imminently desired in the Cu2+ ion detection.
Coumarin derivatives are well known excellent fluorophores in the fluorescent probe design and synthesis with the advantages of high fluorescence intensity, excellent solubility, efficient cell permeation, high quantum yield and ease of preparation.28,29 As a result, an increasing number of papers concerning coumarin derivatives fluorescent chemosensors for copper(II) ion have emerged.30–33 In 2009, Lee Jin Yong and co-workers developed a novel coumarin-based fluorogenic probe, which can act as a fluorescent chemosensor with high selectivity and suitable affinity in biological systems toward Cu2+ (ref. 34). Besides, in 2019, Yin Jiqiu and co-workers obtained a fluorescent chemosensor concerning coumarin derivatives for copper(II) which exhibited good sensitivity, fast response time and high selectivity for Cu2+ ion in the presence of other important relevant metal ions.35
In 2001, Tang Benzhong and co-workers proposed the concept of “aggregation-induced emission” (AIE).36 Due to their effective in circumventing the ACQ effect, AIE-active materials provide a new path for the design and synthesis of fluorescent probes. Tetraphenylethene (TPE), one of the most commonly used aggregation-induced emission luminogens (AIEgens), has been widely favored by researchers.37,38 Owing to its simple structure, simple synthesis, easy modification, and obvious AIE effect, TPE is usually used as an ideal model for construction of various fluorescent sensors.39 In 2019, Zhao Feng and co-workers designed and synthesized a new tetraphenylethene-based Schiff base ligand with AIE effect, and it could be utilized as optical recording materials.40 The same year, Ni Zhonghai and co-workers obtained two different polymorphs of a new tetraphenylethene-based Schiff base, which exhibited totally different photochromic and fluorescence properties.41
Many Schiff base ligands have been synthesized for the detection of Cu2+, which have general fluorescence characteristics. To solve the ACQ problem, we are going to design and synthesize a Schiff base fluorescent probe with AIE effect. For this purpose, a Schiff base fluorescent probe (CTPE) incorporating the TPE group into coumarin framework has been designed and synthesized, which exhibits fast response time, simple synthetic step, lower cost and AIE effect. And it can rapidly recognize Cu2+ in a mixed THF/H2O system.
Scheme 1 was the synthetic route for CTPE. Other compounds and intermediates were labelled and displayed in Scheme S1 in the ESI.† As shown in Scheme 1, CTPE was prepared in high yield via Maillard reaction of 8-formyl-7-hydroxy-4-methylcoumarin (M1) and 1-(4′-aminophenyl)-1,2,2-triphenylethene (TPE-NH2) in absolute ethanol. M1, TPE-NH2 and CTPE were prepared according to the literatures.42,43 Their chemical structures were confirmed by nuclear magnetic resonance (NMR), high resolution mass spectrometry (HRMS) and Fourier transform infrared (FTIR) (Fig. S1–S10, ESI†). In Fig. S10,† compared with TPE-NH2 and M1, the spectrum of CTPE appeared an in-plane bending vibration absorption peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond at 1620 cm−1, and a C
N bond at 1620 cm−1, and a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond stretching vibration absorption peak on the aldehyde group at 1740 cm−1, indicating that CTPE has been successfully synthesized.
O bond stretching vibration absorption peak on the aldehyde group at 1740 cm−1, indicating that CTPE has been successfully synthesized.
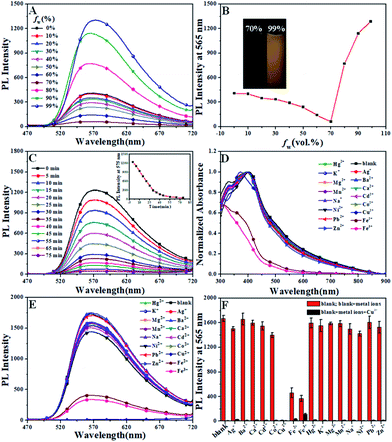
As an AIE-based fluorescent probe, the AIE effect of CTPE was confirmed by its fluorescence spectra in THF/H2O mixtures with various water contents. CTPE was in a good dispersion state in THF solvent, but CTPE gradually aggregated as the volume fraction of water (fw) increased. As shown in Fig. 1A and B, CTPE had good dispensability and exhibited weak luminescence in dilute THF solution (fw = 0%). The weak emissive nature of the luminogens in THF and aqueous mixtures with low fw values should be ascribed to active intramolecular rotations of the genuinely dissolved compounds, which effectively consumed the energy of the excitons non-radiatively.44 When fw increased from 10% to 70%, the fluorescence intensity of CTPE gradually decreased, which was attributed to the intramolecular charge transfer (ICT) effect with the increasing polarity of the solution for CTPE.40,45 When fw was above 70%, CTPE displayed a significantly sudden increase in the fluorescence intensity owing to molecular aggregation. The fluorescence intensity at 565 nm was about 60 in THF/H2O (30/70, v/v) mixtures, while it increased to about 1288 in THF/H2O (1/99, v/v) mixtures with about 21-fold enhancement. Simultaneously, and the maximum emission wavelength showed a slight red shift from 565 nm to 575 nm with the increase of fluorescence intensity. The fluorescence enhancement phenomenon could be ascribed to the restriction of the intramolecular rotations. In the aggregated state, intramolecular motion (mainly including C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and rotation of the benzene ring) was limited, and the attenuation of non-radiative energy was correspondingly blocked, so that a clear enhancement and a slight red shift of the fluorescence of CTPE were observed.40 The above results clearly implied that CTPE exhibited a significant AIE effect.
N isomerization and rotation of the benzene ring) was limited, and the attenuation of non-radiative energy was correspondingly blocked, so that a clear enhancement and a slight red shift of the fluorescence of CTPE were observed.40 The above results clearly implied that CTPE exhibited a significant AIE effect.
The Cu2+-specific binding of CTPE with various competitive metal ions was then investigated under the same experimental condition. Different from these AIE sensors that worked in the aggregated state, there was not complete fluorescence quenching even after about 1 h of adding 20 equivalent Cu2+ in THF/H2O (1/99, v/v) solution (shown in Fig. 1C). However, at the moment of adding Cu2+, the fluorescence was immediately quenched in THF/H2O (10/90, v/v) solution. Therefore the THF/H2O (10/90, v/v) system was adopted to study the selectivity of CTPE to all metal ions.
Metal ion selectivity studies were performed on absorption and fluorescence spectra. Absorption spectra of CTPE recorded after the addition of each metal ion (20 eq.) was shown in Fig. 1D. In Fig. 1D, upon addition of a constant amount (20 eq.) of Cu2+ ion to CTPE, a significant hypsochromic shift from 395 nm to 371 nm in absorption spectrum was observed. No other metal ion induced any change under the identical conditions except Fe3+ and Fe2+ ions. These results demonstrated the specificity of the CTPE for selective binding interaction with Cu2+ ion.
To further investigate the selectivity of CTPE to Cu2+, we also studied the fluorescence response of CTPE in THF/H2O (10/90, v/v) solution. The behaviour of fluorescence “turn off” merely shown by Cu2+ was observed in the Fig. 1E. Surprisingly, the fluorescence was immediately quenched in THF/H2O (10/90, v/v) solution at the moment of adding Cu2+. And the fluorescence intensities decreased after adding Fe3+ and Fe2+, respectively. No obvious changes were observed upon addition of 20 eq. other competitive metal ions. The interferences from the other metal ion with CTPE in its response to Cu2+ were performed. As shown in Fig. 1F, the red bars represent the emission changes of CTPE in the presence of metal ions of interest (all are 20 eq.). The black bars represent the changes of the emission that occurs upon the subsequent addition of Cu2+ to the above solution. The fluorescent intensity of CTPE in the presence of any of the other metal ions tested after adding Cu2+ was decreased significantly, demonstrating little interference from the other metal ions.
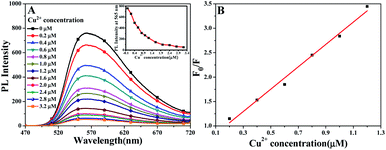
Fluorescent titration experiments of CTPE in THF/H2O (10/90, v/v) solution with Cu2+ ion were carried out and the changes in the fluorescence intensity at 565 nm of CTPE solution with the concentration of Cu2+ ion were measured and showed in Fig. 2A. It was found that the fluorescence intensity at 565 nm of CTPE solution decreased with the increase in the concentration of Cu2+ ion. As showed in Fig. 2B, F0 and F were the fluorescence intensities at 565 nm without adding Cu2+ and adding different concentrations of Cu2+, respectively. In addition, when the Cu2+ concentration in the solution to be tested was less than 1.2 μM, the F0/F of CTPE at 565 nm had a good linear relationship with the concentration of Cu2+.
By taking that change in fluorescence intensity in micro molar range we have calculated the lower limit of detection (LOD) from standard deviation and the slope of calibration plot (Fig. 2B) using the equation.46 It was deduced from the fluorescence titration profile that the LOD of CTPE toward Cu2+ ion reached 0.36 μM.
To gain a better understanding the sensing mechanism of CTPE towards Cu2+, 1H-NMR titration experiments were performed in DMSO-d6 at room temperature (Fig. 3A). The 1H-NMR spectra of CTPE showed considerable variation with the increasing of Cu2+ in DMSO-d6 solvent. Cu2+ is a paramagnetic ion that affects the NMR resonance frequency of protons that are close to the Cu2+ binding site.47 The downfield value of Ha (of –O![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ) at δ = 14.86 ppm in CTPE was due to the intramolecular hydrogen bond between the imine-N atom of CTPE with Ha forming a six-membered transition state.48 On addition of Cu2+ ion, the intramolecular hydrogen bonding was disturbed.49 As Cu2+ gradually increased, the proton signal of Ha almost disappeared. The proton signal of Hb (of –C
) at δ = 14.86 ppm in CTPE was due to the intramolecular hydrogen bond between the imine-N atom of CTPE with Ha forming a six-membered transition state.48 On addition of Cu2+ ion, the intramolecular hydrogen bonding was disturbed.49 As Cu2+ gradually increased, the proton signal of Ha almost disappeared. The proton signal of Hb (of –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) at δ = 9.16 ppm significantly decreased indicating the participation of the nitrogen atom in the binding with Cu2+ ion. The proton signals of the aromatic ring became broader and weaker with the increasing amount of Cu2+. These results confirmed that Cu2+ binded to the CTPE chemosensor through the N atom of the imine and the O atom of phenolic hydroxyl, which were directly connected to the aromatic rings.50 Thus, combined with the FTIR spectra of CTPE before and after the addition of Cu2+ (Fig. S11†), it was clearly illustrated that the fluorescence “turn off” of CTPE was attributed to the chelation between imine-N atom, phenolic hydroxyl-O atom and Cu2+ ion. The possible coordination modes of Cu2+ and CTPE were shown in Fig. 3B.
N–) at δ = 9.16 ppm significantly decreased indicating the participation of the nitrogen atom in the binding with Cu2+ ion. The proton signals of the aromatic ring became broader and weaker with the increasing amount of Cu2+. These results confirmed that Cu2+ binded to the CTPE chemosensor through the N atom of the imine and the O atom of phenolic hydroxyl, which were directly connected to the aromatic rings.50 Thus, combined with the FTIR spectra of CTPE before and after the addition of Cu2+ (Fig. S11†), it was clearly illustrated that the fluorescence “turn off” of CTPE was attributed to the chelation between imine-N atom, phenolic hydroxyl-O atom and Cu2+ ion. The possible coordination modes of Cu2+ and CTPE were shown in Fig. 3B.
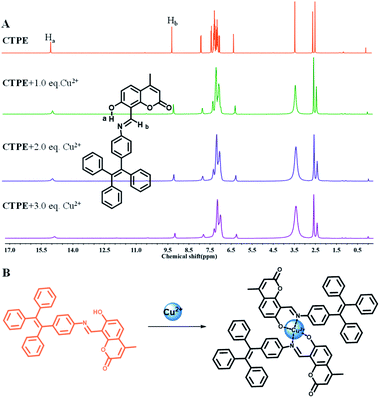 | ||
| Fig. 3 (A) 1H-NMR data of CTPE in DMSO-d6 solution in the absence and presence of Cu2+. (B) Proposed mechanism for CTPE upon addition of Cu2+. | ||
In summary, a novel Schiff base fluorescent probe CTPE based on coumarin and TPE showing an AIE effect has been successfully synthesized and characterized. The fluorescence of CTPE was rapidly quenched by Cu2+, but no significant influence was observed for other metal ions tested. CTPE can distinguish Cu2+ from other metal ions via the fluorescence “turn off”. The LOD of CTPE for Cu2+ can reach 0.36 μM. Recognition mechanism between CTPE and Cu2+ was given. Therefore, CTPE can act as a potential fluorescence probe to selectively and rapidly identify Cu2+.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the financial supports from the Natural Science Foundation of Hebei Province, China (B2018201281, B2019201337).Notes and references
- H. Tapiero, D. M. Townsend and K. D. Tew, Biomed. Pharmacother., 2003, 57, 386 CrossRef CAS.
- P. G. Welsh, J. Lipton, C. A. Mebane and J. C. A. Marr, Ecotoxicol. Environ. Saf., 2008, 69, 199 CrossRef CAS.
- E. Gaggelli, H. Kozlowski, D. Valensin and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS PubMed.
- X. X. He, J. Zhang, X. G. Liu, L. Dong, D. Li, H. Y. Qiu and S. C. Yin, Sens. Actuators, B, 2014, 192, 29 CrossRef CAS.
- Y. Gao, Y. Z. Li, X. P. Yang, F. F. He, J. M. Huang, M. H. Jiang, Z. H. Zhou and H. J. Chen, RSC Adv., 2015, 5, 80110 RSC.
- R. I. Khan and K. Pitchumani, RSC Adv., 2016, 6, 20269 RSC.
- L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 10 CrossRef CAS PubMed.
- E. L. Que and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 15942 CrossRef CAS PubMed.
- N. Narayanaswamy and T. Govindaraju, Sens. Actuators, B, 2012, 161, 304 CrossRef CAS.
- K. N. Buck, J. R. M. Ross, A. R. Flegal and K. W. Bruland, Environ. Res., 2007, 105, 5 CrossRef CAS PubMed.
- T. W. Lin and S. D. Huang, Anal. Chem., 2001, 73, 4319 CrossRef CAS PubMed.
- M. S. Chan and S. D. Huang, Talanta, 2000, 51, 373 CrossRef CAS.
- J. S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska and M. V. Zoriy, Anal. Chem., 2007, 79, 6074 CrossRef CAS PubMed.
- Y. Liu, P. Liang and L. Guo, Talanta, 2005, 68, 25 CrossRef CAS PubMed.
- M. Lin, M. Cho, W. S. Choe, Y. Son and Y. Lee, Electrochim. Acta, 2009, 54, 7012 CrossRef CAS.
- Y. Oztekin, A. Ramanaviciene and A. Ramanavicius, Sens. Actuators, B, 2011, 155, 612 CrossRef CAS.
- P. Pathirathna, Y. Y. Yang, K. Forzley, S. P. McElmurry and P. Hashemi, Anal. Chem., 2012, 84, 6298 CrossRef CAS PubMed.
- Y. B. Shi, Q. Y. Liu, W. Yuan, M. Xue, W. Feng and F. Y. Li, ACS Appl. Mater. Interfaces, 2019, 11, 430 CrossRef CAS PubMed.
- Y. E. Shi, X. M. Zhuang, L. L. Cao, S. Y. Gou, Y. Xiong, W. F. Lai, Z. G. Wang and A. L. Rogach, ChemNanoMat, 2019, 5, 110 CrossRef CAS.
- C. Fan, X. X. Lv, F. J. Liu, L. P. Feng, M. Liu, Y. Y. Cai, H. Liu, J. Y. Wang, Y. L. Yang and H. Wang, ACS Sens., 2018, 3, 441 CrossRef CAS PubMed.
- F. Yarur, J. Macairan and R. Naccache, Environ. Sci.: Nano, 2019, 6, 1121 RSC.
- Z. Chen, C. Wu, Z. F. Zhang, W. P. Wu, X. F. Wang and Z. Q. Yu, Chin. Chem. Lett., 2018, 29, 1601 CrossRef CAS.
- P. Zhao, Q. Xu, J. Tao, Z. W. Jin, Y. Pan, C. M. Yu and Z. Q. Yu, Wires Nanomed. Nanobi., 2018, 10, e1483 CrossRef PubMed.
- S. Y. Lu, L. Z. Sui, M. Wu, S. J. Zhu, X. Yong and B. Yang, Adv. Sci., 2019, 6, 1801192 CrossRef PubMed.
- S. J. Sun, Q. W. Guan, Y. Liu, B. Wei, Y. Y. Yang and Z. Q. Yu, Chin. Chem. Lett., 2019, 30, 1051 CrossRef CAS.
- J. Meng, S. E, X. Wei, X. W. Chen and J. H. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 21150 CrossRef CAS PubMed.
- H. Weng and B. Yan, Anal. Chim. Acta, 2017, 988, 89 CrossRef CAS PubMed.
- D. En, Y. Guo, B. T. Chen, B. Dong and M. J. Peng, RSC Adv., 2014, 4, 248 RSC.
- V. K. Gupta, N. Mergu, L. K. Kumawat and A. K. Singh, Sens. Actuators, B, 2015, 207, 216 CrossRef CAS.
- X. J. Meng, D. L. Cao, Z. Y. Hu, X. H. Han, Z. C. Li, D. Liang and W. B. Ma, Tetrahedron Lett., 2018, 59, 4299 CrossRef CAS.
- H. Q. Li, X. Q. Sun, T. Zheng, Z. X. Xu, Y. X. Song and X. H. Gu, Sens. Actuators, B, 2019, 279, 400 CrossRef CAS.
- K. S. Mani, R. Rajamanikandan, B. Murugesapandian, R. Shankar, G. Sivaraman, M. Ilanchelian and S. P. Rajendran, Spectrochim. Acta, Part A, 2019, 214, 170 CrossRef CAS PubMed.
- Y. Wang, Q. T. Meng, Q. Han, G. J. He, Y. Y. Hu, H. Feng, H. M. Jia, R. Zhang and Z. Q. Zhang, New J. Chem., 2018, 42, 15839 RSC.
- H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. H. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS PubMed.
- S. Feng, Q. M. Gao, X. Gao, J. Q. Yin and Y. Jiao, Inorg. Chem. Commun., 2019, 102, 51 CrossRef CAS.
- J. D. Luo, Z. L. Xie, J. W. Y. Lam, L. Cheng, H. Y. Chen, C. F. Qiu, H. S. Kwok, X. W. Zhan, Y. Q. Liu, D. B. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- C. C. Zhou, W. H. Xu, P. B. Zhang, M. J. Jiang, Y. C. Chen, R. T. K. Kwok, M. M. S. Lee, G. G. Shan, R. L. Qi, X. Zhou, J. W. Y. Lam, S. Wang and B. Z. Tang, Adv. Funct. Mater., 2019, 29, 1805986 CrossRef.
- T. F. Zhang, Y. Y. Li, Z. Zheng, R. Q. Ye, Y. R. Zhang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, J. Am. Chem. Soc., 2019, 141, 5612 CrossRef CAS PubMed.
- R. R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC.
- T. Sun, D. D. Cheng, Y. S. Chai, J. Gong, M. Y. Sun and F. Zhao, Dyes Pigm., 2019, 170, 107619 CrossRef CAS.
- H. Sun, S. S. Sun, F. F. Han, Z. H. Ni, R. Zhang and M. D. Li, J. Mater. Chem. C, 2019, 7, 7053 RSC.
- X. G. Zhou and M. S. Peng, Asian J. Chem., 2013, 25, 4509 CrossRef CAS.
- S. X. Chen, R. M. Qiu, Q. H. Yu, X. Y. Zhang, M. Wei and Z. Y. Dai, Tetrahedron Lett., 2018, 59, 2671 CrossRef CAS.
- H. Z. Gao, D. F. Xu, Y. H. Wang, Y. Wang, X. L. Liu, A. X. Han and C. Zhang, Dyes Pigm., 2018, 150, 59 CrossRef CAS.
- L. Zhou, D. F. Xu, H. Z. Gao, A. X. Han, X. L. Liu, C. Zhang, Z. Li and Y. Yang, Dyes Pigm., 2017, 137, 200 CrossRef CAS.
- V. Singh and A. K. Mishra, Sens. Actuators, B, 2016, 227, 467 CrossRef CAS.
- J. T. Yeh, W. C. Chen, S. R. Liu and S. P. Wu, New J. Chem., 2014, 38, 4434 RSC.
- U. N. Yadav, P. Pant, S. K. Sahoo and G. S. Shankarling, RSC Adv., 2014, 4, 42647 RSC.
- N. Mergu, M. Kim and Y. A. Son, Spectrochim. Acta, Part A, 2018, 188, 571 CrossRef CAS PubMed.
- S. Dalbera, S. Kulovi and S. Dalai, ChemistrySelect, 2018, 3, 6561 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10632d |
| This journal is © The Royal Society of Chemistry 2020 |