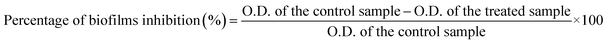Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nystatin-mediated bismuth oxide nano-drug synthesis using gamma rays for increasing the antimicrobial and antibiofilm activities against some pathogenic bacteria and Candida species
Ahmed I. El-Batal a,
Hanady G. Nada
a,
Hanady G. Nada *a,
Reham R. El-Beherya,
Mohamed Gobara
*a,
Reham R. El-Beherya,
Mohamed Gobara b and
Gharieb S. El-Sayyad
b and
Gharieb S. El-Sayyad *a
*a
aDrug Radiation Research Department, National Centre for Radiation Research and Technology (NCRRT), Egyptian Atomic Energy Authority (EAEA), P. O. Box 29, Nasr City, Cairo, Egypt. E-mail: Gharieb.Elsayyad@eaea.org.eg; hanady.nada@eaea.org.eg
bChemical Engineering Department, Military Technical College (MTC), Egyptian Armed Forces, Cairo, Egypt
First published on 5th March 2020
Abstract
The novelty of the present research is the synthesis of bismuth oxide nanoparticles (Bi2O3 NPs) loaded with the antifungal nystatin drug via gamma rays for increased synergistic antimicrobial potential against some pathogenic bacteria and Candida species. The full characterization of the synthesized Bi2O3 NPs-Nystatin was achieved by XRD, FT-IR, HR-TEM, and SEM/EDX mapping techniques in order to analyze the crystallinity, chemical functional groups, average particle size, morphology, and elemental structure, respectively. The antimicrobial activities of Bi2O3 NPs-Nystatin were examined against pathogenic bacteria and Candida species, including the zone of inhibition (ZOI), minimum inhibitory concentration (MIC), and antibiofilm activity. Additionally, the SEM/EDX method was performed to investigate the mode of action on the treated Candida cells. Our results revealed that Bi2O3 NPs-Nystatin possessed a well-crystallized semi-spherical shape with an average particle size of 27.97 nm. EDX elemental study of the synthesized Bi2O3 NPs-Nystatin indicated a high level of purity. Interestingly, the synthesized Bi2O3 NPs-Nystatin displayed encouraging antibacterial behavior against almost all the tested bacteria and a synergistic antifungal potential toward the investigated Candida species. Additionally, Bi2O3 NPs-Nystatin was found to be a promising antibiofilm agent, resulting in inhibition percentages of 94.15% and 84.85% against C. albicans (1) and E. coli, respectively. The present research provides a revolutionary nano-drug-based solution to address the increasing global resistance of pathogenic microbes at low concentrations, thus offering a new infectious disease treatment technique that is cost effective, eco-friendly, and works in an acceptable time frame.
Introduction
Like many microbes, some fungi live naturally as commensals inside human and animal bodies. However, fungal diseases happen when a pathogenic fungus invades a patient's body and affects their immune system.1 The most common fungi that possess the capability to create life-threatening infections include Candida albicans and Aspergillus fumigates.2 Candida species are classified as the fourth most common reason for clinical and systemic diseases in the USA, with aggressive death rates of up to 55%.3C. albicans is categorized as an opportunistic pathogen that can also be observed as a part of the normal microflora in the digestive systems of animals and humans.4 However, a slight change in the host protection system can support the conversion of C. albicans into a pathogen that is capable of producing diseases.5 They can create two major kinds of diseases in humans: surface diseases (oral or vaginal candidiasis) and life-endangering systemic diseases.6 There are some determinants willing to enhancing the virulence of Candida species such as the expanded usage of complete parenteral diet, intravenous catheters, broad-spectrum antibiotics, and cytotoxic chemotherapy.7
Tremendous progress in fungal diagnostics and antifungal medication has been made in the past 25 years; however, this has not been reflected in significant changes in antifungal production.8 The emergence of C. albicans has highlighted some real challenges because several species present different levels of resistance (acquired or natural), creating difficulties for the generally applied antifungal drugs to work effectively.9 The elevated number of drug-resistant fungal pathogens and the toxicity of the present antifungal composites have focused significant attention to the antimicrobial potential of biogenic nano-based composites.10
It must be noted that a small number of antifungal agents have been prepared for use in yeast treatment, most of which are considered as fungistatic. One of the key challenges associated with the treatment of bacterial and fungal infections with conventional drugs is that a tremendous resistance to antimicrobial drugs can occur, which is thus driving the research for alternative therapies.11 There are some articles in the literature12,13 regarding some examples of candidosis that were found to be clinically resistant to nystatin therapy, which makes the treatment of some Candida sp. by nystatin very difficult and requires nystatin to be incorporated into a nano-carrier.
Nanotechnology is an actively emerging discipline with great application potential in many fields, including pharmaceutics, chemistry, plant pathology, and biomedicine, and refers to materials with very small dimensions (at the atomic or molecular scales). In particular, in biomedical applications, a bio-nanotechnique is a proper method for eliminating or minimizing the attack of different pathogenic bacteria and fungi by administering a nano-drug.14 Many synthetic methods, like thermal reduction15 and biological synthesis,14 have been used to make metal oxide NPs.16
There is an increasing need for the green synthesis of metal oxide NPs for their application in pharmaceutical and biomedical fields due to their superior chemical, physical, and catalytic properties. Due to the encouraging characteristics of the prepared metal oxide NPs, they have found possible applications in biomedicine as anticancer and antimicrobial agents.17
However, the interactions between metal oxide NPs and biological systems depend on the cell type and uptake routes or the direction of different organelles. Despite the evolution of research in nanotechnology, there remain considerable challenges to overcome, including safety, scale-up production, decreasing costs, and understanding the biological activity.18 Nowadays, the safety concerns of using metal oxide NPs are considered one of the main future challenges for biomedical applications.18 Here, we tried to decrease the toxicity of the prepared metal oxide NPs by incorporation with a resistant-nystatin drug to increase the synergistic effect and decrease the necessary applied-dose in order to reduce the nanotoxicity, which means reducing the negative influence of the metal oxide NPs on the biological organisms.18,19
Bi2O3 NPs have a large surface area with various electrochemical balances and they have delivered important attention due to their potential applications for zinc sensing.20 Owing to their unique properties (non-toxic behavior, biocompatibility, and high chemical stability), they have been used as antibacterial and antifungal agents.21 There are some research reports on the antibacterial and antifungal activities of Bi2O3 NPs, including from Hernandez et al.,22 who investigated the fungicidal activity of Bi2O3 NPs against C. albicans as well as their antibiofilm capabilities, and showed that the Bi2O3 NPs displayed antimicrobial activity against C. albicans growth, reducing the colony size by 85%, and effected a complete inhibition of biofilm formation. Also, El-Batal et al.23 synthesized green Bi2O3 NPs from melanin pigment using gamma rays and examined their antimicrobial activity against some standard pathogenic bacteria and Candida sp. Their results indicated that the Bi2O3 NPs were active against Escherichia coli (13.0 mm ZOI), Staphylococcus epidermidis (23.0 mm ZOI), and C. albicans (20.0 mm ZOI).
Herein, we synthesized the nano-drug Bi2O3 NPs-Nystatin by gamma rays, which serves an eco-friendly and cost-efficient method. Full characterization techniques were performed to demonstrate the various properties of the synthesized Bi2O3 NPs-Nystatin. The synthesized Bi2O3 NPs-Nystatin possessed a small size, high crystallinity, complete elemental distribution, simplicity, and acceptable purity, which in turn led to elevated antimicrobial and antibiofilm activities. The significance of our results and findings is in the possible application of a new nano-drug synthesized by a green method at low concentration (to avoid the nanotoxicity and to reduce the used nystatin dose), which increases the synergistic potential of the nystatin drug against pathogenic microbes.
Materials and methods
Chemicals and reagents used
The media components were purchased from Hi-Media and Difco. The chemicals, such as bismuth nitrate, nystatin (NS), polyvinylpyrrolidone (PVP), dimethyl sulfoxide (DMSO), isopropyl alcohol, and other reagents used in the following tests (biological procedures) were utilized at the analytical grade as purchased from Sigma-Aldrich.Radiation source
The gamma-irradiation process was conducted at the NCRRT, Cairo, Egypt. The source of radiation was the 60Co-Gamma chamber 4000-A-India. The applied dose rate was fixed at 2.02 kGy h−1. Gamma rays produce a free radical and solvated electrons after water radiolysis.Synthesis and optimization of Bi2O3 NPs by the nystatin drug and gamma rays
Bi2O3 NPs were synthesized and incorporated the nystatin drug via applying gamma rays (as a reducing agent) in the presence of a capping polymer, like polyvinylpyrrolidone (PVP). Briefly, in a 10 ml test tube, a solution of 25.0 mg of bismuth nitrate and 1.0% PVP was mixed with 0.5 ml isopropyl alcohol and completed with distilled water to make up to a net volume of 9.0 ml aqueous solution (A). After that, 5.0 mg nystatin drug was dissolved in 1.0 ml dimethyl sulfoxide (DMSO) to form an aqueous solution (B). Finally, solutions A and B were mixed at room temperature (24.0 ± 2.0 °C) to a final ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (nystatin
5 (nystatin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bismuth nitrate; v/v).
bismuth nitrate; v/v).
The resulting mixtures were irradiated by different gamma-ray doses (1.0, 3.0, 5.0, 10.0, 15.0, 20.0, 25.0, and 30.0 kGy). The optical characterization of the synthesized Bi2O3 NPs-Nystatin was performed by UV-Vis spectroscopy (JASCO V-560-UV-Vis spectrophotometer) with respect to a negative control (the gamma-irradiated sample without bismuth nitrate).
A gamma-ray dose with high optical density (O.D.) was selected for further investigation. Additionally, the stability of the nystatin drug was examined after exposure to different gamma-rays doses (as mentioned-before).
A preliminary investigation was carried out to define the impact of the bismuth nitrate and nystatin drug concentrations regarding the Bi2O3 NPs production (after the exposure to the most-effective gamma-ray dose). The expected factorial investigation included two factors, i.e., bismuth nitrate and nystatin concentrations, over 12 levels (see Table 1). It must be noted that the main idea for the chosen factors used in the present study is that they had multiple significant influences on the Bi2O3 NPs production.
| Runs | Nystatin (NS) (mg/10 ml) | Bismuth nitrate (mg/10 ml) | Ratio (mg; w/w) | Absorption of Bi2O3 NPs (O.D.) | Wavelength of Bi2O3 NPs (λ nm) |
|---|---|---|---|---|---|
| a Control was used to autozero the results from the set of other experiments that did not contain one of the precursors used for Bi2O3 NPs-Nystatin synthesis.b Nil means there was no color change which confirms non-formation of Bi2O3 NPs-Nystatin. | |||||
| 1 | 5.0 | 0.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Controla | Controla |
| 2 | 5.0 | 5.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Nilb | Nilb |
| 3 | 5.0 | 12.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Nilb | Nilb |
| 4 | 5.0 | 25.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2.253 | 570.0 |
| 5 | 5.0 | 37.5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5 7.5 |
1.887 | 310.0 |
| 6 | 5.0 | 50.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1.462 | 315.0 |
| 7 | 0.0 | 25.0 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Controla | Controla |
| 8 | 2.5 | 25.0 | 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2.179 | 540.0 |
| 9 | 5.0 | 25.0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2.118 | 570.0 |
| 10 | 7.5 | 25.0 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.666 | 620.0 |
| 11 | 10.0 | 25.0 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Nilb | Nilb |
| 12 | 15.0 | 25.0 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Nilb | Nilb |
Bismuth nitrate solution (at different concentrations; see Table 1) was mixed with different concentrations of nystatin drug solution in addition to 0.2% isopropyl alcohol. The prepared solutions were stirred at room temperature (24.0 ± 2.0 °C) and finally exposed to varying gamma-ray doses (as determined from the last investigation).
Characterization of the synthesized Bi2O3 NPs-Nystatin
The crystallite sizes and the crystallinity of the synthesized Bi2O3 NPs-Nystatin were determined by XRD (XRD-6000, Shimadzu apparatus, SSI, Japan). The strength of the diffracted X-rays was recognized as per the diffraction angle 2θ.The common size and particle-size distribution of the Bi2O3 NPs-Nystatin were defined by dynamic light scattering (DLS-PSS-NICOMP 380-USA). Additionally, the average nano-structure and the particle size of the synthesized Bi2O3 NPs-Nystatin were determined by high-resolution transmission electron microscopy (HRTEM, JEM2100, Jeol, Japan).
The surface and morphological features were examined by scanning electron microscopy (SEM, ZEISS, EVO-MA10, Germany). Also, EDX spectrum examination (BRUKER, Nano GmbH, D-12489, 410-M, Germany) was used to estimate the elemental composition, purity, and the relationship of each metal. SEM/EDX mapping method was applied for obtaining further information regarding the structure/simplicity, relationships, and the position of the metals in the synthesized Bi2O3 NPs-Nystatin.
Finally, FT-IR spectroscopy was performed to provide important data about the chemical functional groups present on the nystatin drug. The analyses were carried out using a JASCO FT-IR 3600 infra-red spectrometer and by using the KBr pellet method. It was determined at a wavenumber scale from 4000 to 400 cm−1.
Antimicrobial activity of the synthesized Bi2O3 NPs-Nystatin
The antimicrobial activities of the synthesized Bi2O3 NPs-Nystatin, polyvinylpyrrolidone (PVP), dimethyl sulfoxide (DMSO), nystatin drug, and bismuth ions were tested against some selected Candida species and pathogenic bacteria using the agar disc distribution method.24The examined microbes were taken from the culture collections at the Drug Microbiology Laboratory, Drug Radiation Research Department, NCRRT, Cairo, Egypt. The tested unicellular fungi were Candida albicans (1), Candida tropicalis (1), Candida tropicalis (22), Candida albicans (25), and Candida albicans (33), while the pathogenic bacteria included Gram-positive (Bacillus cereus and Staphylococcus aureus; MRSA) and Gram-negative bacteria (Escherichia coli and Pseudomonas aeruginosa).
The tested bacterial inoculums were fixed with 0.5 McFarland (2–4) × 107 CFU ml−1, and all the examined C. albicans and C. tropicalis were fixed with 0.5 McFarland (3–5) ×108 CFU ml−1 after conducting UV-Vis spectrophotometry at 600 nm.25
Nystatin antifungal disc (NS 100; 100 μg ml−1) and amoxicillin/clavulanic acid (AMC; 20/10 μg ml−1) were examined as standard antibiotics. The growth restraint of the examined microbes was confirmed by the zone of inhibition (ZOI) after 24 h incubation.14
The minimum inhibitory concentration (MIC) was assessed referring to the lowest concentration of Bi2O3 NPs-Nystatin that inhibited 99.0% of the bacterial and yeast growth. For this, the serial dilution method of a Luria–Bertani (LB) broth medium containing the tested microbes was applied using the ELISA plate method.26 The inoculums were fixed as mentioned-before in the first antimicrobial screening.25 Broth medium, DMSO, and PVP were used as a negative control, while the standard antibiotics (AMC and NS) were used as a positive control. Finally, serial dilutions of Bi2O3 NPs-Nystatin (starting with a concentration of 250 μg ml−1) were used. All the plates were incubated for 24 h at 36.0 ± 1.0 °C and examined at 600 nm.14
Antibiofilm activity of the synthesized Bi2O3 NPs-Nystatin
A qualitative study of the biofilm inhibition was performed as described by Christensen et al.27 Biofilm formation across the tube walls in the absence and presence of the synthesized Bi2O3 NPs-Nystatin was investigated.The antibiofilm potential of the synthesized Bi2O3 NPs-Nystatin (at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) was examined against the most sensitive bacteria and Candida sp. with respect to the non-treated strain (control).
5 w/w) was examined against the most sensitive bacteria and Candida sp. with respect to the non-treated strain (control).
Briefly, 5.0 ml of the nutrient broth medium was poured in to the tubes and the tested pathogenic bacteria and yeast cells were inoculated with 0.5 McFarland adjusted from 1–3 × 107 CFU ml−1. Subsequently, they were incubated for 24 h at 35.0 ± 2.0 °C, and after that, the supernatant was discharged and all the tubes were mixed with phosphate buffer saline (PBS; pH 7.0) and finally dried.28
The biofilms around and at the bottom of the tube walls were rinsed with 3.0 ml sodium acetate (3.5%) for 15 min. and subsequently washed with de-ionized water. After that, 10.0 ml crystal violet (CV; 0.1%) was added to the biofilms that had developed inside the tubes (about 30 min.) for the staining and finally the tubes were cleaned with de-ionized water.29
It is worth mentioning that, for the semi-quantitative antibiofilm investigation (inhibition%), 3.0 ml of the absolute ethanol was connected to the tubes to separate the colored biofilms,30 and after that, the O.D. of CV was measured using a UV-Vis spectrophotometer at a fixed wavelength (570.0 nm).
The biofilms inhibition percentage was determined using the following equation:14,27,30
Reaction mechanism using SEM/EDX analysis of the control and treated microbial cells
The susceptible Candida sp. (from the antibiofilm results; Table 4) were mixed with PBS and maintained with 3.0 ml glutaraldehyde (3.0%). Additionally, they were washed regularly with PBS and dried by different ethanol solutions (30.0%, 50.0%, 70.0%, 80.0%, 95.0%, and 100.0%) for 15 min. at room temperature (24.0 ± 2.0 °C).30The prepared Candida cells were set up on aluminum stumps for the imaging process.28,30 The surface morphology of the non-treated and treated Candida cells with the synthesized Bi2O3 NPs-Nystatin was investigated by SEM to estimate the mode of action. The total elemental analysis of the tested Candida cells was estimated by EDX spectrum analysis.
Statistical analysis
The mathematical analysis of the data was performed by ONE WAY ANOVA (at p < 0.05), least significant differences (LSD), and Duncan's multiple systems.31 The effects and data were examined and analyzed through SPSS software (version 15).Results and discussion
Synthesis and optimization of Bi2O3 NPs by nystatin and gamma-rays
The synthesized Bi2O3 NPs-Nystatin solutions were a deep off-white color due to the surface plasmon resonance (SPR) phenomenon.32 Fig. 1A displays the optimum gamma-ray doses used for the Bi2O3 NPs synthesis, which was confirmed using a UV-Vis spectrophotometer and found to be 20.0 kGy with a high O.D. (2.751) at a wavelength of 465.0 nm.The synthesis of Bi2O3 NPs was assisted by the reduction of various conc. of bismuth nitrate solution (0.5, 12.5, 25.0, 37.5, and 50.0 mg/10 ml) in the presence of varying nystatin conc. (0.5, 2.5, 5.0, 7.5, 10.0, and 15.0 mg/10 ml) after mixing with PVP solution (0.2%) and exposure to 20.0 kGy gamma rays.
The results listed in Table 1 show that run (4) had the optimum concentrations (2.5 mg ml−1 bismuth nitrate and 0.5 mg ml−1 NS; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w), which was reflected by a high Bi2O3 NPs yield (2.253) at 570.0 nm.
5 w/w), which was reflected by a high Bi2O3 NPs yield (2.253) at 570.0 nm.
Table 1 shows that Bi2O3 NPs were not formed in runs 2 and 3, where the concentration of nystatin was set at 0.5 mg ml−1 and the concentration of bismuth nitrate was 0.5 and 1.25 mg ml−1, respectively which indicates that the concentration of bismuth nitrate was not sufficient for the Bi2O3 NPs production. In addition, there was a constant O.D. decrease from run 4 to run 6 when the nystatin concentration was set at 0.5 mg ml−1 and bismuth nitrate at 5.0 mg ml−1.
Also, Table 1 shows that there was a constant decline in the O.D. (runs 7 to 12) when the bismuth nitrate concentration was adjusted to 2.5 mg ml−1 and the nystatin concentration was changed from 0.25 to 1.5 mg ml−1, while both runs 11 and 12 involved a high concentration of nystatin, which antagonized the formation of Bi2O3 NPs.
The results confirmed that Bi2O3 NPs synthesis depended on the concentrations of bismuth nitrate and nystatin, although raising the concentration of both was ineffective. The optimum concentrations were 0.25 mg ml−1 of nystatin and 2.5 mg ml−1 of bismuth nitrate (run 8) and 0.50 mg ml−1 of nystatin and 2.5 mg ml−1 of bismuth nitrate (run 4).
On the other hand, the UV-Vis spectra of the starting materials (bismuth nitrate and nystatin) used in the Bi2O3 NPs synthesis are presented in Fig. 1B. The detectable wavelengths of both precursors were less than 300 nm, which assisted the formation of Bi2O3 NPs at the wavelength of 465.0 nm (Fig. 1A).
Fig. 1C shows the stability of the nystatin solution (0.5 mg ml; run 4) following exposure to the corresponding gamma-ray doses necessary for Bi2O3 NPs production. The results in Fig. 1C reveal that the nystatin was completely constant at all gamma-ray doses applied, which confirmed the opinion concerning the role of nystatin in the Bi2O3 NPs structure and stability. The common two peaks of nystatin appeared at 215.0 and 255.0 nm,33 and there was a slight decrease in the O.D. with the increase in gamma-ray doses.
This is in agreement with El-Sayyad et al.,34 who worked on the incorporation of selenium NPs with the gentamycin drug (CN) and stated that the production of Se NPs-CN nano-drug depended on the sodium selenite and CN concentrations.
Proposal reaction mechanism for Bi2O3 NPs synthesis
When the nystatin solution (0.5 mg ml−1) was mixed with (2.5 mg ml−1) bismuth nitrate solution (the common optimized state that resulted in a great Bi2O3 NPs yield; run 4 in Table 1) and exposed to 20.0 kGy gamma rays, it produced the free-radical species (OH˙, H˙, H2O2, and H3O+) and solvated electrons (eaq−) (eqn (1)).
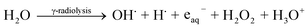 | (1) |
OH˙ radicals are powerful reducing agents and increase the oxidation of bismuth ions (Bi3+) to BiO3− (eqn (2)).
| 2Bi3+ + 3OH˙ → Bi2O3− + 3H+ | (2) |
The produced bismuth oxide is first dimerized while incorporated with the remaining Bi2O3− and/or Bi3+ (eqn (3) & (4)).
| Bi2O3− + Bi2O3− → 2Bi2O3− | (3) |
| Bi2O3− + Bi3+ → 2Bi2O3− | (4) |
The available radicals (OH˙ and H˙) are adequate to separate hydrogen atoms from nystatin to create nystatin free radicals (secondary radicals = 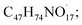 eqn (5)) then, the nystatin radicals react with Bi3+ to create Bi2O3 NPs and constant nystatin molecules (eqn (6)).
eqn (5)) then, the nystatin radicals react with Bi3+ to create Bi2O3 NPs and constant nystatin molecules (eqn (6)).
Finally, the formed nystatin can combine with the produced Bi0 (eqn (6)) though the oxygen atoms in the OH functional groups of the nystatin molecules to form more permanent Bi2O3 NPs, as displayed in eqn (7).
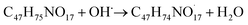 | (5) |
 | (6) |
 | (7) |
The overall reaction is related to the generation of free electrons and radical groups and the presence of notable stabilizing agents (nystatin) that drive the reduction of Bi3+ to Bi0.
Characterization of the synthesized Bi2O3 NPs-Nystatin
The HRTEM images showed round shapes with mono-dispersed Bi2O3 NPs-Nystatin (Fig. 2A) with a range from 18.40 to 34.99 nm and the average diameter was 27.97 nm as shown in the magnified image in Fig. 2B.
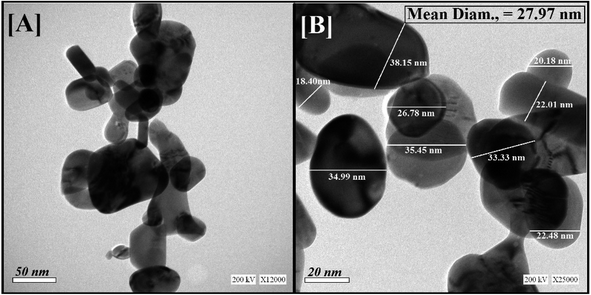 | ||
| Fig. 2 Shape, nano-structure, and mean particle-size determination by HR-TEM for Bi2O3 NPs-Nystatin, where [A] presents the low magnification (50 nm) and [B] the high magnification (20 nm) images. | ||
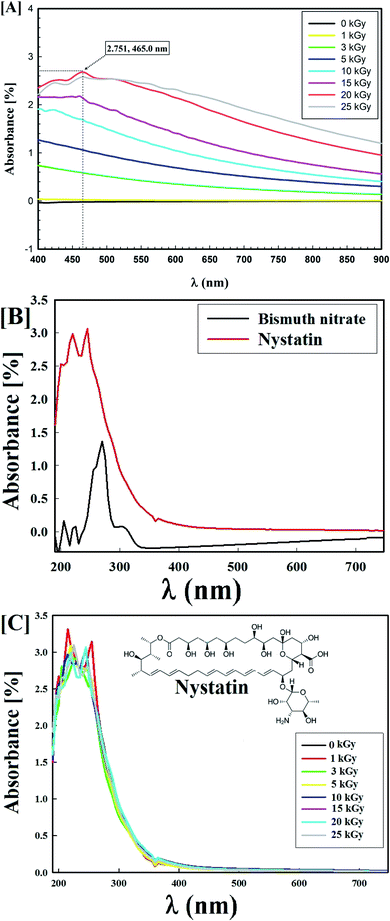
The average particle-size distribution was verified by the DLS method and determined as 40.74 nm in the Bi2O3 NPs incorporated with nystatin via applying 20.0 kGy gamma rays as shown in Fig. 3.
It was noted that the DLS size area of the incorporated Bi2O3 NPs-Nystatin was higher than the HRTEM size; this is because DLS considers the hydrodynamic diameter where the incorporated Bi2O3 NPs-Nystatin are enveloped by water molecules, leading to the greater sizes of the incorporated Bi2O3 NPs-Nystatin.36
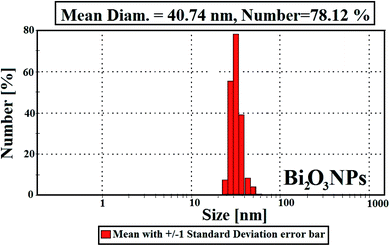
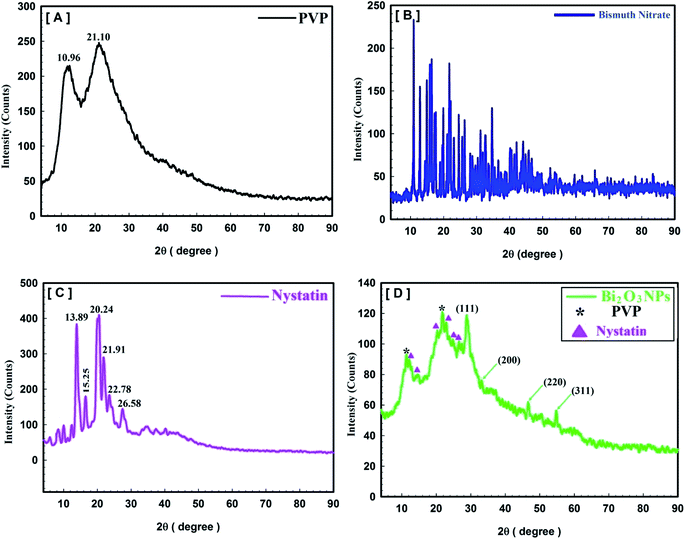
Fig. 4 describes the crystal and/or amorphous compositions of the starting primary materials (bismuth nitrate, PVP, and nystatin) and the synthesized Bi2O3 NPs. It must be noted that the XRD results for bismuth nitrate represent the crystal construction,38 as shown in Fig. 4B, and the 2θ at 10.96° and 21.10° were similar to the amorphous type of PVP (Fig. 4A).39 Additionally the 2θ was detected at 13.89°, 15.25°, 20.24°, 21.91°, 22.78°, and 26.58° (Fig. 4C).
The XRD data of the incorporated Bi2O3 NPs-Nystatin in Fig. 4D show the structure of the diffraction properties with 2θ at 28.15°, 33.65°, 46.17°, and 55.38°, which represent the Bragg's reflections at (111), (200), (220), and (311), respectively.
The presented peaks were similar with the Joint Committee on Powder Diffraction Standards (JCPDS) of Bi2O3 NPs (JCPDS file no 00-071-0465).40 This means that the incorporated Bi2O3 NPs-Nystatin stayed as a crystal and displayed the face-centered cubic (fcc) crystalline composition. There were additional amorphous peaks for PVP and nystatin (Fig. 4D) that was included in the assembly and stability of the Bi2O3 NPs, but their strength was less than that identified in Fig. 4A and C.
Additionally, the average crystallite size of the incorporated Bi2O3 NPs-Nystatin was defined by applying the Williamson–Hall (W–H) equation,41,42 and was observed to be 30.54 nm for Bi2O3 NPs produced by nystatin via 20.0 kGy gamma-ray application according to eqn (8).
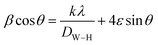 | (8) |
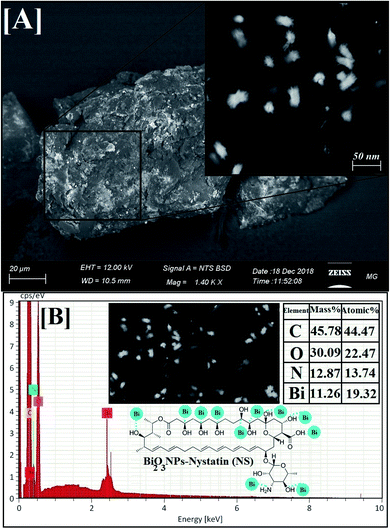 | ||
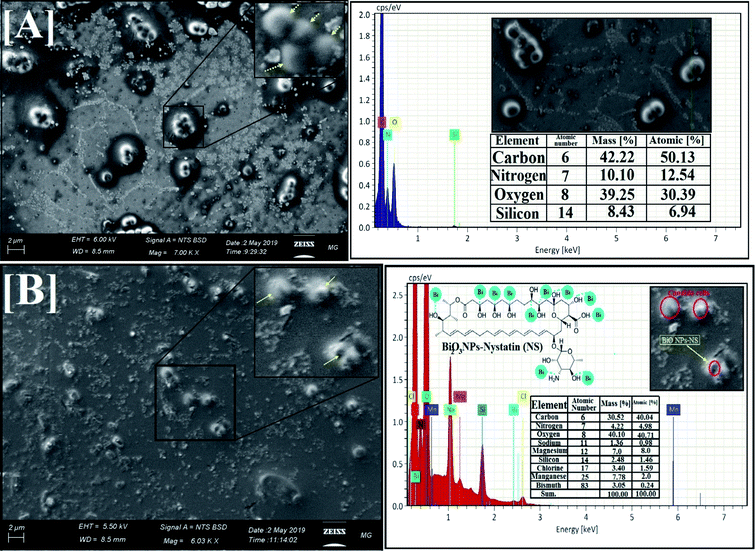
| Fig. 5 Surface morphology and elemental analysis of the synthesized Bi2O3 NPs-Nystatin, where [A] SEM image, [B] EDX elemental analysis. | ||
EDX is an analytical method used for the elemental investigation or the qualitative chemical validation of synthesized metal oxide NPs.37,42 EDX analysis was used to establish the elemental composition of the synthesized Bi2O3 NPs-Nystatin and its capacity for determining the purity of the synthesized Bi2O3 NPs as represented in Fig. 5B.
The synthesized Bi2O3 NPs exhibited notable absorption peaks related to the bismuth element at 0.25 and 2.35 keV. The lack of further elemental peaks and a large amount of bismuth in the spectra confirmed the purity of the bismuth element. The presence of carbon, oxygen, and nitrogen peaks in the examined samples was due to the presence of stabilizers or the capping factor (nystatin drug, Fig. 5B).
The displayed atoms were related to the distribution to Bi, C, O, and N atoms. Moreover, the C, O, and N atoms were in agreement with the nystatin drug. Subsequently according to the given image, the synthesized Bi2O3 NPs-Nystatin (bright red NPs) was developed regularly across the nystatin atoms (C, O, and N).
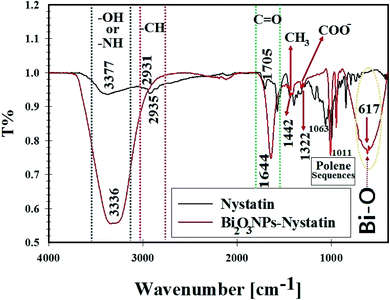 | ||
| Fig. 7 FT-IR spectra, surface bonding, and functional groups analysis for nystatin (NS) and the synthesized Bi2O3 NPs-Nystatin. | ||
The broad peak at 3336.16 cm−1 was assigned to the –OH of the hydroxyl group and –NH stretching, while the peak at 2931.52 cm−1 was attributed to the asymmetric and symmetric –CH vibrations of the –CH2 group. The peak at 1644.88 cm−1 was related to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester group.
O stretching of the ester group.
The peak located at 1442.55 cm−1 was designated to –CH3. A further band at 1322.0 cm−1 was related to –COO−. Also, the peaks located at 1011.08 cm−1 were due to polyene sequences. A definite peak at 617.44 cm−1 was identified in the FTIR of Bi2O3 NPs-Nystatin, which may be associated with the conjugation and attraction of Bi2O3 NPs beside the hydroxyl group in the nystatin drug as Bi–O.37 The FTIR results in the present study were similar to those in recently published research studies.23,43,44
According to the FTIR results in the present research, it was concluded that the intensity of all the detected peaks was reduced in the FTIR of Bi2O3 NPs-Nystatin. This may be because of the interaction of Bi2O3 NPs, the –OH, and other functional groups present in the nystatin drug.
It was noted that the nystatin drug may combine with Bi2O3 NPs either by the available amine residue and/or by the electrostatic attraction among the carboxylate groups, which hold a negative charge45 so they support the Bi2O3 NPs from aggregation through the influence of the O and/or N atoms present in the nystatin drug.
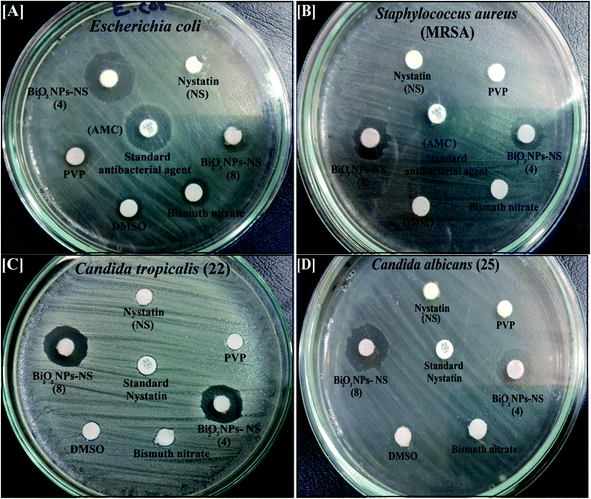 | ||
| Fig. 8 Antimicrobial activity of the synthesized Bi2O3 NPs-Nystatin (two different samples; see Table 2), nystatin, bismuth nitrate, DMSO, and PVP against [A] Escherichia coli, [B] Staphylococcus aureus, [C] Candida tropicalis (22), and [D] Candida albicans (25) as ZOI. | ||
| Pathogenic microbes | ZOI of Bi2O3 NPs-NS (run 4) (1 mg NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mg Bi) (mm) 5 mg Bi) (mm) |
MIC of Bi2O3 NPs-NS (run 4) (NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi; μg ml−1) Bi; μg ml−1) |
ZOI of Bi2O3 NPs-NS (run 8) (0.5 mg NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mg Bi) (mm) 5 mg Bi) (mm) |
ZOI of bismuth nitrate (mm) | ZOI of nystatin (mm) | ZOI of PVP (mm) | ZOI of DMSO (mm) | ⁃AMC or NS |
|---|---|---|---|---|---|---|---|---|
| a Values arethe mean ± SD (n = 3). Data within the groups were analyzed using one-way analysis of variance (ANOVA) followed by a–e Duncan's multiple range test (DMRT), LSD = least significant differences. •Nil means that no ZOI had been measured. ⁃AMC = amoxicillin/clavulanic acid (antibacterial standard), NS = nystatin (antifungal standard). | ||||||||
| Candida albicans (1) | 14.0ab ± 0.5773 | 1.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2 4.2 |
13.0de ± 0.5773 | Nil | Nil | Nil | Nil | Nil |
| Candida albicans (25) | 15.0c ± 0.5773 | 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.52 0.52 |
13.0bc ± 0.5773 | Nil | Nil | Nil | Nil | 7.0a ± 0.5000 |
| Candida albicans (33) | 14.0bc ± 0.5773 | 0.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05 |
12.0bc ± 0.2886 | Nil | Nil | Nil | Nil | Nil |
| Candida tropicalis (1) | 15.0bc ± 0.5773 | 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.52 0.52 |
13.0 cd ± 0.7637 | Nil | Nil | Nil | Nil | 7.0a ± 0.2886 |
| Candida tropicalis (22) | 15.0c ± 0.5773 | 0.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05 |
14.0e ± 1.1547 | Nil | 7.0 | 7.0 | Nil | 7.0a ± 0.7637 |
| Escherichia coli | 17.0d ± 0.5773 | 1.95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.2 4.2 |
12.0bc ± 0.5000 | 8.0 | Nil | Nil | 7.0 | 17.0c ± 0.2886 |
| Pseudomonas aeruginosa | •Nil | 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.4 8.4 |
Nil | Nil | Nil | Nil | Nil | Nil |
| Staphylococcus aureus; MRSA | 13.0a ± 0.5773 | 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.52 0.52 |
12.0b ± 0.6110 | Nil | Nil | Nil | Nil | 15.0b ± 0.5000 |
| Bacillus cereus | 13.0a ± 0.5773 | 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.52 0.52 |
10.0a ± 0.4509 | Nil | Nil | Nil | Nil | 7.0a ± 0.4618 |
| LSD | 1.00000 | — | 1.33333 | — | — | — | — | 1.83333 |
It worth noting that the antibacterial potency of the Bi2O3 NPs-Nystatin was significantly more powerful than bismuth nitrate, PVP, DMSO, the nystatin drug alone, and the standard antimicrobial agents (AMC).
It is also necessary to note that the synthesized Bi2O3 NPs-Nystatin were active against Gram-negative bacteria more than Gram-positive. Note, the cell wall constituents in Gram-negative bacteria contain principally little layers of lipopolysaccharide, lipid, and peptidoglycan. On the other hand, the cell wall of Gram-positive incorporate very solid peptidoglycan forms.46
Additionally, the synthesized Bi2O3 NPs-Nystatin were shown to incorporate promising antifungal factors as they exhibited tremendous antifungal efficiency against C. tropicalis (22) (15.0 mm ZOI; Fig. 8C) and C. albicans (25) (15.0 mm ZOI; Fig. 8D), as recorded in Table 2.
There is a relationship between the characteristics of the synthesized Bi2O3 NPs-Nystatin and the antimicrobial effects discussed. The Bi2O3 NPs-Nystatin were stable because of the PVP polymer applied, their reduced crystal size (30.54 nm; Fig. 4D), and separated spherical form with a particle size within the nano-scale (27.97 nm; Fig. 2), as well as their uniformity (EDX; Fig. 5B) and pattern of mono-dispersed highly-distributed NPs (40.74 nm; Fig. 3), which served as an essential objective for enhancing the antimicrobial potency of the Bi2O3 NPs-Nystatin at low concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w), against all the tested bacterial and Candida sp.
5 w/w), against all the tested bacterial and Candida sp.
The Bi2O3 NPs-Nystatin displayed individual physical and chemical properties better than the traditional organic and synthesized antimicrobial agents, such as decreased crystal sizes, reduced average particle size, more stability, and a higher potency for interaction with more pathogenic bacteria and Candida sp., thus consequently, increasing their antimicrobial potential.37
The MIC results of the Bi2O3 NPs-Nystatin against all the tested pathogenic bacteria and Candida sp. ranged from 3.9 μg ml−1 nystatin: 8.4 μg ml−1 Bi2O3 NPs, to 0.24 μg ml−1 nystatin: 0.52 μg ml−1 Bi2O3 NPs, as mentioned in Table 2. The Bi2O3 NPs-Nystatin possessed a promising MIC of 0.24 μg ml−1 nystatin: 0.52 μg ml−1 Bi2O3 NPs against S. aureus; MRSA, B. cerus, C. albicans (25), and C. tropicalis (1).
The Bi2O3 NPs-Nystatin's size was not the only parameter indicating the antimicrobial characteristics, but other features, such as their mono-dispersity, simplicity, stability, and their appearance, should be considered.
The results from similar studies23,47–49 of the antimicrobial behavior of the incorporated Bi2O3 NPs-Nystatin against some bacteria and fungi-causing infectious diseases are introduced in Table 3. The encouraging antimicrobial potential of the synthesized Bi2O3 NPs-Nystatin in our research was due to their small particle and/or crystal sizes, extensive purity, superior stability by using the PVP polymer, and the incorporation with the nystatin drug, which enhanced their synergistic impact.
| Methods of Bi2O3 NPs preparation | Average particle size (nm) | Starting concentration (μg ml−1) | Antimicrobial activity (ZOI; mm) and/or (MIC; μg ml−1) | References |
|---|---|---|---|---|
| Green synthesis using nystatin (NS) drug and gamma rays | 27.97 nm | 1 mg NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 mg Bi/ml for ZOI and MIC 5 mg Bi/ml for ZOI and MIC |
15.0 mm ZOI and 0.24 NS: 0.52 Bi μg ml−1 MIC | Our research |
| Biogenic synthesis by fungal melanin pigment and gamma rays | 29.82 nm | 0.8 μg ml−1 for ZOI and MIC | 20.0 mm ZOI and 0.4 μg ml−1 MIC | 23 |
| Chemical synthesis of bismuth dimercaptopropanol nanoparticles (BisBAL NPs) | 18.7 nm | 0.1–100 μM for ZOI and MIC | BisBAL NPs inhibited S. mutans and S. gordonii growth by more than 70% at 0.1 μM and, MIC of for S. mutans, S. gordonii was 5 μM, and C. albicans was 10 μM | 47 |
| Biological synthesis of bismuth nanoparticles by Delftia sp. | 40–120 nm | 1000 μg per disc for ZOI | 27.0 mm ZOI and 1280 μg ml−1 MIC | 48 |
| Biosynthesis of elemental bismuth oxide nanoparticles by Serratia marcescens | ≤5 nm | 40–140 μg ml−1 | 100 μg ml−1 MIC | 49 |
C. albicans (1) in the absence of Bi2O3 NPs-Nystatin formed a thick whitish-yellow matt across the air–liquid interface, which adhered entirely to the tube walls and gave a blue color when stained with crystal violet. Also, a dark blue suspension was formed following dissolving the CV by pure ethanol, as presented in Fig. 9A.
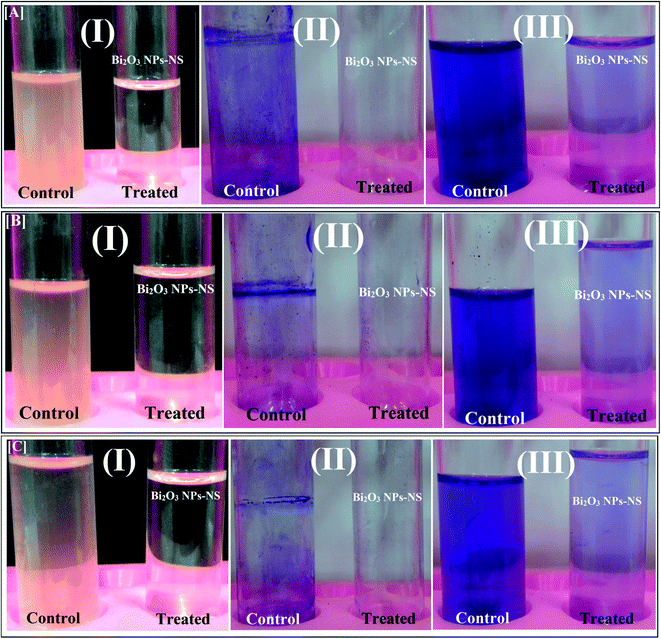 | ||
| Fig. 9 Antibiofilm activity of the synthesized Bi2O3 NPs-Nystatin using the tube method against [A] Candida albicans (1) [B] Escherichia coli, and [C] Bacillus cereus, where the steps are reported as follows: (I) growth of the bacterial and yeast cells and biofilm formation (rings) without treatment with the synthesized Bi2O3 NPs-Nystatin (NS) and the inhibition of bacterial and yeast growth after treatment with Bi2O3 NPs-Nystatin; (II) staining of the adherent bacterial and yeast cells with crystal violet and (III) removing and dissolving the adherent bacterial and yeast cells by ethanol for semi-quantitative biofilm inhibition (%) determination (as shown in Table 4). | ||
The C. albicans (1) tubes treated with Bi2O3 NPs-Nystatin (1.95 NS: 4.2 Bi; μg ml−1), revealed a negative biofilm formation. The color of the adherent cells was light blue after CV staining, as shown in Fig. 9A. The same conditions were described for the biofilm repression of E. coli and Bacillus cereus, as displayed in Fig. 9B and C, respectively.
To determine the inhibition percentage (%) of the biofilm created by the examined pathogens, a UV-Vis spectrophotometer (set at 570.0 nm) was applied. The O.D. estimated the subsequent dissolving of the stained biofilm through ethanol.29
Table 4 records the repression percentages of the biofilm generation by the tested bacteria and Candida sp. The highest restraint% was noted for C. albicans (1) (94.15%), followed by E. coli (84.85%), and B. cereus (84.79%) after treatment with Bi2O3 NPs-Nystatin (1.95 NS: 4.2 Bi; μg ml−1).
| Pathogenic microbes | O.D. of crystal violet staining at 570.0 nm (control) | O.D. of crystal violet staining at 570.0 nm (treated with Bi2O3 NPs-Nystatin; run 4) | Inhibition% |
|---|---|---|---|
| a Values are the mean ± SD (n = 3). Data within the groups were analyzed using one-way analysis of variance (ANOVA) followed by a–i Duncan's multiple range test (DMRT), and LSD = least significant differences. | |||
| Candida albicans (1) | 2.548h ± 0.0017 | 0.150c ± 0.0005 | 94.15% |
| Candida albicans (25) | 0.850e ± 0.0010 | 0.560g ± 0.0113 | 34.17% |
| Candida albicans (33) | 0.669c ± 0.0023 | 0.127a ± 0.0011 | 81.01% |
| Candida tropicalis (1) | 0.495a ± 0.0023 | 0.165d ± 0.0015 | 66.66% |
| Candida tropicalis (22) | 0.514b ± 0.0010 | 0.201f ± 0.0015 | 60.89% |
| Escherichia coli | 0.977f ± 0.0005 | 0.148c ± 0.0010 | 84.85% |
| Pseudomonas aeruginosa | 2.767i ± 0.0060 | 0.905h ± 0.0026 | 67.25% |
| Staphylococcus aureus; MRSA | 0.748d ± 0.0023 | 0.137b ± 0.0020 | 81.68% |
| Bacillus cereus | 1.217g ± 0.0015 | 0.185e ± 0.0010 | 84.79% |
| LSD | 0.01273 | 0.01267 | — |
The synthesized Bi2O3 NPs-Nystatin was applied to repress biofilm development in its constant adhesion step (also identified as the primary stage).50 However, the mechanistic behavior of the incorporated Bi2O3 NPs upon biofilm development has yet to be confirmed.
The difference in the inhibitory percentage may be described by many aspects, like antimicrobial potency, biosorption (due to the high surface area of the incorporated Bi2O3 NPs-Nystatin), physical features (Bi2O3 NPs-Nystatin size; 27.97 nm), penetration capabilities, and distinct chemical attributes that influence the relationship and synergy of the Bi2O3 NPs-Nystatin with the Candida biofilm.51
It was clear that the Bi2O3 NPs-Nystatin restrained C. albicans biofilm expansion by a factor of more than 98% at (1.95 NS: 4.2 Bi; μg ml−1) Bi2O3 NPs-Nystatin (MIC results; Table 2). The exopolysaccharide (the principal precursors of biofilm production) formation was hindered so C. albicans could not produce a biofilm.28 Our anti-biofilm research is comparable to the findings of Ashajyothi et al.,50 who stated that the synthesized ZnO NPs displayed a biofilm hindrance% of 10.7% against P. aeruginosa following 18 h incubation.
Initially, the yeast populations (control without Bi2O3 NPs-Nystatin treatment) were developed regularly and showed the definite normal cellular morphology with the usual cell surface, budding appearance, and a developed biofilm, as explained in Fig. 10A.
By comparison, the morphological modifications were recognized in C. albicans (1) cells following treatment with the incorporated Bi2O3 NPs-Nystatin at 1.95 NS: 4.2 Bi μg ml−1 (Fig. 10B). An obvious surface cell break and consequent deformation and failure to form budding properties of the treated C. albicans (1) upon Bi2O3 NPs-Nystatin addition were noted. Furthermore, the number of viable cells and biofilm production were repressed. SEM analysis revealed that the Bi2O3 NPs-Nystatin were directed to effect Candida cell wall depreciation (Fig. 10B).52 The EDX elemental study showed the appearance of Bi and O at the shrinking cell membrane and on the outside surface of the treated C. albicans (1), which confirmed Bi2O3 NPs-Nystatin action toward the yeast cells (Fig. 10B; inset).
One potential reason for Bi2O3 NPs-Nystatin action upon C. albicans (1) could be connected to the elevated surface area of the Bi2O3 NPs-Nystatin providing more reliable interactions among the negatively-charged Candida cell walls, as shown in Fig. 10B. Additional studies revealed that the metal oxide NPs combine with pathogens by their electrostatic potential and defeat bacteria by layer separation.53
A recent report examined the interaction between Bi2O3 NPs and the tested microbes and found that the attraction takes place by electrostatic attraction leading to the membrane leakages.53 Further research revealed that the metal oxide NPs attack the microbes and increase the oxidative pressure,54 which quickly changes the yeast cells due to the high level of ROS production. The free radicals generation is influenced by the prominent reduction in oxygen by the electron change above the oxygen atom through electron transport in the mitochondria.53
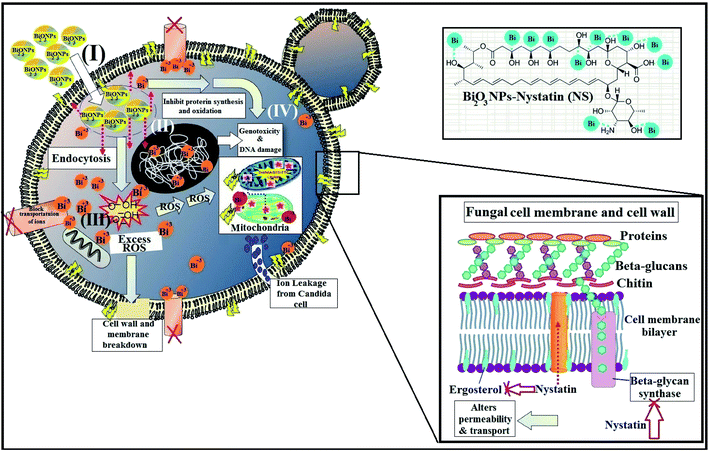
It must be mentioned that the suggested reaction mechanism of metal oxide NPs toward the pathogenic bacteria and Candida cells was explained in our earlier studies14 and is schematically drawn in Fig. 11.
The mode of action included four mechanisms that describe the effect of Bi2O3 NPs-Nystatin toward Candida cells. It begins with the adhesion of Bi2O3 NPs-Nystatin near the surface of the Candida cells. Following that, Bi3+ ions inside the Candida cells split the intracellular organic molecules (DNA and mitochondria). Then the cellular toxicity, like oxidative stress, is created by the formation of ROS. Finally, Bi2O3 NPs-Nystatin stimulates the Candida signal transduction pathways, in addition to the performance of nystatin which alters the action of beta-glycan synthase and ergosterol construction and subsequently alters the permeability of the cell membrane and the transportation of ions inside the Candida cells.55
The genotoxic effects of Bi2O3 NPs at various concentrations (12.5, 25.0, 50.0, 75.0, and 100.0 μg ml−1) were investigated on the root cells of Allium cepa. The results indicated that the exposure to Bi2O3 NPs considerably enhanced the mitotic index (except at 12.5 μg ml−1) and the total chromosomal differences, while confused anaphase–telophase, anaphase bridges, and stickiness chromosome laggards were recognized in anaphase–telophase cells. A notable improvement in DNA destruction was also recognized at all Bi2O3 NPs concentrations (except at 12.5 μg ml−1).57
In another study,56 the toxic impacts of Bi2O3 NPs on the liver (HepG2 cell), intestine (Caco-2 colorectal cell), kidney (NRK-52E epithelial cell), and lung (A549 lung cell) were studied. It was mentioned that Bi2O3 NPs reduced the cell viability by intruding on the mitochondrial and lysosomal purposes in HepG2, Caco-2, NRK-52E, and A549 cells in a dose-subject way. The IC50 values of Bi2O3 NPs were counted at 35.10–96.50 μg ml−1.
Furthermore, Kovriznych et al.58 stated that the acute toxic amount (LC50 evaluation) of Bi2O3 NPs was less than 1.6 μg ml−1 in adult fish and zebra fish eggs. The cytotoxicity of Bi2O3 NPs may be associated with several distinct agents, like oxidative destruction in living systems.
New studies report that Bi2O3 NPs influence oxidative stress by elevating reactive oxygen species, membrane lipid peroxidation, and reducing intracellular glutathione (GSH).59 Additionally, Yang Luo et al.60 reported that Bi NPs are non-toxic at a concentration of 0.5 nM, but at elevated concentration (50 nM) they induced cytotoxicity and killed about 45% of HeLa cells.
Our study assumed that the synthesized Bi2O3 NPs-Nystatin could serve as a bactericidal and strong fungicidal agent, and would not create any deadly impact on the human system. Animal kidney cells were exposed to 2 mM Bi2O3 NPs for one day and their cytotoxic influence was not verified.22 Although the Bi2O3 NPs represent a pleasant approach to reduce infections, more investigation is needed to ensure their proper application for human society.23
The interesting thing about inorganic NPs are their high surface-to-volume degrees, many structural advantages, several applications, and nano-scale size, which represent infinite dynamic forms to connect with living forms, like pathogenic bacteria and fungi. This is the significant difference between different NPs and traditional organic antimicrobial agents, which in turn could help minimize the risk of developing antimicrobial resistance.23
The results obtained in this study propose unique, efficient, low-cost, and broad-spectrum antimicrobial factors. The Bi2O3 NPs could be used at low concentration in foodstuff, pharmaceutical applications, dental supplements, labs, disinfectant, clinics, and geriatric and pediatric hospitals primarily for Candida sp. infection treatment.
Conclusion
In this study, the synergistic effect of Bi2O3 NPs and nystatin to govern “Candida” germination and biofilms creation was verified. The study also reported an encouraging method to restrain the pathogenic Candida sp. and some examined bacteria using Bi2O3 NPs-Nystatin. This planned method could not only dramatically reduce the administered nystatin dose but could also improve its potency. An eco-friendly and cost-efficient approach was applied to synthesize the Bi2O3 NPs using nystatin drug and polyvinylpyrrolidone (PVP) as stabilizing agents after exposure to 20.0 kGy gamma-ray irradiation. Joining Bi2O3 NPs with the nystatin antifungal agent is a modular approach that could be implemented as a strategy for enhancing the currently ineffective nystatin drug. The average particle size and surface morphology of the incorporated Bi2O3 NPs-Nystatin were exhibited to be mono-dispersed and rounded with an average size of 27.79 nm. EDX elemental analysis confirmed the growth of pure Bi2O3 NPs-Nystatin without any impurities. In addition, the antimicrobial potential in terms of ZOI and MIC toward different Candida sp. and some pathogenic bacteria was studied. The incorporated Bi2O3 NPs-Nystatin at low concentration (MIC = 0.24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.52; NS
0.52; NS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi μg ml−1) restrained the development and attack of C. albicans. The current study considers that the small crystal size (≈30 nm) and high purity and stability play the main roles in the success of the combination of Bi2O3 NPs-Nystatin, at reduced concentrations, toward all the examined bacteria and Candida cells. Furthermore, the antibiofilm potential of the incorporated Bi2O3 NPs-Nystatin was extremely encouraging (94.15% inhibition toward C. albicans (1)). The morphological modifications of the Candida cells after treatment with Bi2O3 NPs-Nystatin (at a ratio of 1
Bi μg ml−1) restrained the development and attack of C. albicans. The current study considers that the small crystal size (≈30 nm) and high purity and stability play the main roles in the success of the combination of Bi2O3 NPs-Nystatin, at reduced concentrations, toward all the examined bacteria and Candida cells. Furthermore, the antibiofilm potential of the incorporated Bi2O3 NPs-Nystatin was extremely encouraging (94.15% inhibition toward C. albicans (1)). The morphological modifications of the Candida cells after treatment with Bi2O3 NPs-Nystatin (at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w) were conceived as noticeable alterations in the cell hardness and visible surface cell brokenness. Also, the consequent deformation and loss of budding features of C. albicans (1) were limited after treatment with the synthesized Bi2O3 NPs-Nystatin. The novel synthetic process for the Bi2O3 NPs-Nystatin is a promising method for possible use in manufacturing, pharmaceutical, and biomedical purposes and for managing dangerous infections, particularly candidoses. However, future work in this area needs to continue to cover the safety of using these small-sized materials and their activity over the course of application. In addition, investigation of the mechanism of the interactions across the genetic level of this type of nano-drug with current and other types of bacteria and pathogenic fungal strains is an essential element needed to complete the work.
5 w/w) were conceived as noticeable alterations in the cell hardness and visible surface cell brokenness. Also, the consequent deformation and loss of budding features of C. albicans (1) were limited after treatment with the synthesized Bi2O3 NPs-Nystatin. The novel synthetic process for the Bi2O3 NPs-Nystatin is a promising method for possible use in manufacturing, pharmaceutical, and biomedical purposes and for managing dangerous infections, particularly candidoses. However, future work in this area needs to continue to cover the safety of using these small-sized materials and their activity over the course of application. In addition, investigation of the mechanism of the interactions across the genetic level of this type of nano-drug with current and other types of bacteria and pathogenic fungal strains is an essential element needed to complete the work.
Conflicts of interest
The authors declare that they have no conflict of interests.Acknowledgements
The authors would like to thank the Nanotechnology Research Unit (P. I. Prof. Dr Ahmed I. El-Batal), Drug Microbiology Lab., Drug Radiation Research Department, NCRRT, Egypt, for financing and supporting this study under the project “Nutraceuticals and Functional Foods Production by using Nano/Biotechnological and Irradiation Processes”. Also, the authors would like to thank the Chemical Engineering Department, Military Technical College, Egyptian Armed Forces, and Zeiss microscope team at Cairo, Egypt, for their invaluable support of this study.References
- J.-A. Van Burik, D. Myerson, R. W. Schreckhise and R. A. Bowden, J. Clin. Microbiol., 1998, 36, 1169–1175 CrossRef CAS PubMed.
- T. L. Dawson Jr, Cell Host Microbe, 2019, 25, 345–347 CrossRef PubMed.
- S. B. Wey, M. Mori, M. A. Pfaller, R. F. Woolson and R. P. Wenzel, Arch. Intern. Med., 1988, 148, 2642–2645 CrossRef CAS PubMed.
- M. Pfaller and D. Diekema, J. Clin. Microbiol., 2004, 42, 4419–4431 CrossRef CAS PubMed.
- L. de Repentigny, Curr. Opin. Microbiol., 2004, 7, 324–329 CrossRef PubMed.
- F. L. Mayer, D. Wilson and B. Hube, Virulence, 2013, 4, 119–128 CrossRef PubMed.
- S. C. Deorukhkar, S. Saini and S. Mathew, Int. J. Microbiol., 2014, 2014, 456878 Search PubMed.
- J. L. R. Tudela and D. W. Denning, Lancet Infect. Dis., 2017, 17, 1111–1113 CrossRef PubMed.
- S. Deorukhkar and S. Saini, Int. J. Med. Sci. Publ. Health, 2013, 2, 533–539 CrossRef.
- K. M. Rice, G. K. Ginjupalli, N. D. Manne, C. B. Jones and E. R. Blough, Nanotechnology, 2019, 30, 372001 CrossRef PubMed.
- C. Cavaleiro, E. Pinto, M. Gonçalves and L. Salgueiro, J. Appl. Microbiol., 2006, 100, 1333–1338 CrossRef CAS PubMed.
- M. Martin and R. Dinsdale, Br. J. Oral Surg., 1982, 20, 294–298 CrossRef CAS PubMed.
- G. Ritchie, A. Fletcher, D. Main and A. Prophet, J. Prosthet. Dent, 1969, 22, 185–200 CrossRef CAS PubMed.
- M. Abd Elkodous, G. S. El-Sayyad, I. Y. Abdelrahman, H. S. El-Bastawisy, A. E. Mohamed, F. M. Mosallam, H. A. Nasser, M. Gobara, A. Baraka, M. A. Elsayed and A. I. El-Batal, Colloids Surf., B, 2019, 180, 411–428 CrossRef CAS PubMed.
- N. A. Dhas, C. P. Raj and A. Gedanken, Chem. Mater., 1998, 10, 1446–1452 CrossRef CAS.
- G. S. El-Sayyad, F. M. Mosallam and A. I. El-Batal, Adv. Powder Technol., 2018, 29, 2616–2625 CrossRef CAS.
- K. Kanagamani, P. Muthukrishnan, K. Shankar, A. Kathiresan, H. Barabadi and M. Saravanan, J. Cluster Sci., 2019, 30, 1415–1424 CrossRef CAS.
- Y. M. Amin, A. M. Hawas, A. El-Batal and S. H. H. E. Elsayed, Br. J. Pharmacol. Toxicol., 2015, 6, 22–38 CrossRef CAS.
- K. L. Aillon, Y. Xie, N. El-Gendy, C. J. Berkland and M. L. Forrest, Adv. Drug Delivery Rev., 2009, 61, 457–466 CrossRef CAS PubMed.
- M. Khairy, R. O. Kadara, D. K. Kampouris and C. E. Banks, Anal. Methods, 2010, 2, 645–649 RSC.
- M. Chauhan, T. Jasrotia, G. Kaur, C. Prakash, R. Kumar, N. Dilbaghi, G. R. Chaudhary and S. Kumar, Environ. Res., 2020, 180, 108857 CrossRef PubMed.
- R. Hernandez-Delgadillo, D. Velasco-Arias, J. J. Martinez-Sanmiguel, D. Diaz, I. Zumeta-Dube, K. Arevalo-Niño and C. Cabral-Romero, Int. J. Nanomed., 2013, 8, 1645 Search PubMed.
- A. I. El-Batal, G. S. El-Sayyad, A. El-Ghamry, K. M. Agaypi, M. A. Elsayed and M. Gobara, J. Photochem. Photobiol., B, 2017, 173, 120–139 CrossRef CAS PubMed.
- S. Badger, S. Abraham, H. Stryhn, D. J. Trott, D. Jordan and C. G. Caraguel, Prev. Vet. Med., 2019, 172, 104782 CrossRef PubMed.
- M. Balouiri, M. Sadiki and S. K. Ibnsouda, J. Pharmaceut. Biomed. Anal., 2016, 6, 71–79 CrossRef PubMed.
- F. M. Mosallam, G. S. El-Sayyad, R. M. Fathy and A. I. El-Batal, Microb. Pathog., 2018, 122, 108–116 CrossRef CAS PubMed.
- G. D. Christensen, W. A. Simpson, A. L. Bisno and E. H. Beachey, Infect. Immun., 1982, 37, 318–326 CrossRef CAS PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, S. S. Cameotra and R. Pal, Appl. Nanosci., 2014, 4, 859–868 CrossRef CAS.
- G. S. El-Sayyad, M. A. Elkodous, A. M. El-Khawaga, M. A. Elsayed, A. I. El-Batal and M. Gobara, RSC Adv., 2020, 10, 5241–5259 RSC.
- M. A. Maksoud, G. S. El-Sayyad, A. Ashour, A. I. El-Batal, M. A. Elsayed, M. Gobara, A. M. El-Khawaga, E. Abdel-Khalek and M. El-Okr, Microb. Pathog., 2019, 127, 144–158 CrossRef CAS PubMed.
- K. Brownlee, Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, J. Am. Stat. Assoc., 1952, 47, 687–691 CrossRef.
- M. Składanowski, M. Wypij, D. Laskowski, P. Golińska, H. Dahm and M. Rai, J. Cluster Sci., 2017, 28, 59–79 CrossRef.
- P. Ramos, B. Pilawa, P. Pepliński, T. O. Sunmonu, F. B. Lewu, I. Koca, B. Tekguler, V. A. Yilmaz, S. Shoba and M. Sathiavelu, Indian J. Pharm. Educ. Res., 2019, 53, S186–S192 CrossRef CAS.
- G. S. El-Sayyad, H. S. El-Bastawisy, M. Gobara and A. I. El-Batal, Biol. Trace Elem. Res., 2019, 1–20, DOI:10.1007/s12011-019-01842-z.
- A. I. El-Batal, F. M. Mosallam, M. M. Ghorab, A. Hanora, M. Gobara, A. Baraka, M. A. Elsayed, K. Pal, R. M. Fathy, M. Abd Elkodous and G. S. El-Sayyad, Int. J. Biol. Macromol., 2019 DOI:10.1016/j.ijbiomac.2019.11.210.
- M. I. A. Abdel Maksoud, G. S. El-Sayyad, A. Abokhadra, L. I. Soliman, H. H. El-Bahnasawy and A. H. Ashour, J. Mater. Sci.: Mater. Electron., 2020 DOI:10.1007/s10854-019-02799-4.
- A. Ashour, A. I. El-Batal, M. A. Maksoud, G. S. El-Sayyad, S. Labib, E. Abdeltwab and M. El-Okr, Particuology, 2018, 40, 141–151 CrossRef CAS.
- C. Jadhav, P. K. Pagare, K. Inamdar and L. Kadam, Macromol. Symp., 2019, 387, 1800198, DOI:10.1002/masy.201800198.
- M. G. Naseri, E. Saion and N. K. Zadeh, Int. Nano Lett., 2013, 3, 19 CrossRef.
- D. Sánchez-Martínez, I. Juárez-Ramírez, L. M. Torres-Martínez and I. de León-Abarte, Ceram. Int., 2016, 42, 2013–2020 CrossRef.
- P. Belavi, G. Chavan, L. Naik, R. Somashekar and R. Kotnala, Mater. Chem. Phys., 2012, 132, 138–144 CrossRef CAS.
- M. A. Maksoud, G. S. El-Sayyad, A. Ashour, A. I. El-Batal, M. S. Abd-Elmonem, H. A. Hendawy, E. Abdel-Khalek, S. Labib, E. Abdeltwab and M. El-Okr, Mater. Sci. Eng., C, 2018, 92, 644–656 CrossRef PubMed.
- M. Gondal, T. A. Saleh and Q. Drmosh, Sci. Adv. Mater., 2012, 4, 507–510 CrossRef CAS.
- S. H. Hussein-Al-Ali, M. E. El Zowalaty, A. U. Kura, B. Geilich, S. Fakurazi, T. J. Webster and M. Z. Hussein, BioMed Res. Int., 2014, 2014, 651831, DOI:10.1155/2014/651831.
- E. R. Arakelova, S. G. Grigoryan, F. G. Arsenyan, N. S. Babayan, R. M. Grigoryan and N. K. Sarkisyan, Int. J. Med. Heal. Pharm. Biomed. Eng., 2014, 8, 33–38 Search PubMed.
- Z.-X. Tang and B.-F. Lv, Braz. J. Chem. Eng., 2014, 31, 591–601 CrossRef.
- A. R. Badireddy, R. Hernandez-Delgadillo, R. I. Sánchez-Nájera, S. Chellam and C. Cabral-Romero, J. Nanopart. Res., 2014, 16, 2456 CrossRef.
- M. Shakibaie, E. Hajighasemi, M. Adeli-Sardou, M. Doostmohammadi and H. Forootanfar, IET Nanobiotechnol., 2019, 13, 377–381 CrossRef PubMed.
- P. Nazari, R. Dowlatabadi-Bazaz, M. Mofid, M. Pourmand, N. Daryani, M. Faramarzi, Z. Sepehrizadeh and A. Shahverdi, Appl. Biochem. Biotechnol., 2014, 172, 570–579 CrossRef CAS PubMed.
- C. Ashajyothi, K. H. Harish, N. Dubey and R. K. Chandrakanth, J. Nanostruct. Chem., 2016, 6, 329–341 CrossRef CAS.
- H.-J. Park, H. Y. Kim, S. Cha, C. H. Ahn, J. Roh, S. Park, S. Kim, K. Choi, J. Yi and Y. Kim, Chemosphere, 2013, 92, 524–528 CrossRef CAS PubMed.
- S. Priyadarshini, A. Mainal, F. Sonsudin, R. Yahya, A. A. Alyousef and A. Mohammed, Res. Chem. Intermed., 2019, 46, 1077–1089 CrossRef.
- P. K. Stoimenov, R. L. Klinger, G. L. Marchin and K. J. Klabunde, Langmuir, 2002, 18, 6679–6686 CrossRef CAS.
- M. F. Khan, A. H. Ansari, M. Hameedullah, E. Ahmad, F. M. Husain, Q. Zia, U. Baig, M. R. Zaheer, M. M. Alam and A. M. Khan, Sci. Rep., 2016, 6, 27689 CrossRef CAS PubMed.
- W. Umbreit, J. Biol. Chem., 1949, 177, 703–714 CAS.
- M. Abudayyak, E. Öztaş, M. Arici and G. Özhan, Chemosphere, 2017, 169, 117–123 CrossRef CAS PubMed.
- R. Liman, Chemosphere, 2013, 93, 269–273 CrossRef CAS PubMed.
- J. A. Kovrižnych, R. Sotníková, D. Zeljenková, E. Rollerová, E. Szabová and S. Wimmerová, Interdiscip. Toxicol., 2013, 6, 67–73 Search PubMed.
- M. Ahamed, R. Posgai, T. J. Gorey, M. Nielsen, S. M. Hussain and J. J. Rowe, Toxicol. Appl. Pharmacol., 2010, 242, 263–269 CrossRef CAS PubMed.
- Y. Luo, C. Wang, Y. Qiao, M. Hossain, L. Ma and M. Su, J. Mater. Sci.: Mater. Med., 2012, 23, 2563–2573 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |