Open Access Article
Open Access ArticleA flower-like CoS2/MoS2 heteronanosheet array as an active and stable electrocatalyst toward the hydrogen evolution reaction in alkaline media†
Mengtong Shiab,
Yang Zhang *bc,
Yaxing Zhub,
Wei Wangb,
Changzheng Wang
*bc,
Yaxing Zhub,
Wei Wangb,
Changzheng Wang *a,
Aifang Yubc,
Xiong Pu
*a,
Aifang Yubc,
Xiong Pu bc and
Junyi Zhai
bc and
Junyi Zhai *bc
*bc
aKey Laboratory of Urban Stormwater System and Water Environment, Ministry of Education, Beijing University of Civil Engineering and Architecture, Beijing 100044, China. E-mail: changzhwang@163.com
bBeijing Institute of Nanoenergy and Nanosystems, Chinese Academy of Sciences, Beijing 100083, China. E-mail: zhangyang@binn.cas.cn; jyzhai@binn.cas.cn
cSchool of Nanoscience and Technology, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 3rd March 2020
Abstract
CoS2/MoS2 heteronanosheet arrays (HNSAs) with vertically aligned flower-like architectures are fabricated through in situ topotactic sulfurization of CoMoO4 nanosheet array (NSA) precursors on conductive Ni foam. CoMoO4 NSAs are prepared by a self-template hydrothermal method without using any hard template and surfactant. Benefiting from a 3D flower-like architecture constituted by ultrathin nanosheets with abundant exposed heterointerfaces as highly active sites and predesigned void spaces, the as-synthesized CoS2/MoS2 HNSAs exhibit an excellent hydrogen evolution reaction (HER) performance with a low overpotential of 50 mV at 10 mA cm−2, and a small Tafel slope of 76 mV dec−1 in 1.0 M KOH, which outperforms most previously reported CoS2 and MoS2 based electrocatalysts with compositional or morphological similarity. This work demonstrates the great potential in developing high-efficiency and earth-abundant electrocatalysts for alkaline HER through heterointerface engineering and morphological design by utilizing transition metal molybdate as a promising platform.
1. Introduction
Water splitting has been widely regarded as a promising approach towards sustainable hydrogen generation from aqueous solution. Development of hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) electrocatalysts with high-efficiency, long-term durability comparable to precious-metal based electrocatalysts is one of the key building blocks for any future renewable energy system.1 However, electrochemical water splitting suffers severely from sluggish HER kinetics in high concentration alkaline solutions. Considering the high price and scarcity of Pt, a variety of transition metal based compounds and composites have been recently investigated for alkaline HER, including metal alloys,2,3 sulfides,4,5 carbides,6,7 nitrides8,9 and phosphides10,11 due to their abundance, high reactivity, electrochemical stability. In particular, transition metal sulfides are among the most investigated noble-metal-free candidates and have recently attracted substantial research interest as low-cost and active alternative electrocatalysts for alkaline HER.12–14 The previous works revealed that both morphological control and interface engineering played indispensable roles in boosting the eletrocatalytic performance of nanostructured transition metal dichalcogenide composites. Compared to mono-metal sulfides, such as NiS2 and CoS2, bi-metallic or mixed-metal sulfides (e.g., Ni0.33Co0.67S2 and NiCo2S4) demonstrated a higher HER electrocatalytic activity mainly due to the exposure of electrochemically active surface edge sites.15,16 Moreover, chemical doping and incorporation approaches played an essential role in boosting electrocatalytic performance through the modulation of intrinsic activity and electronic structures, such as N-doped Ni3S2 nanosheet arrays, Co-doped mesoporous MoS2 foam and O-incorporated MoS2 nanosheets.17–20 Transition metal dichalcogenide based nanocomposites were investigated as well, demonstrating enhanced electrocatalytic performance due to the heterointerfaces acting as accessible and stable active sites. For instance, various electrocatalysts with core/shell nanostructures have been recently investigated, such as CoS2/MoS2 nanowires, Ni3S2/MoS2 nanorods, Ni3S2/MoS2 nanoparticles and MoC/Mo2C nanowires, etc.6,21–23 Those synergistically enhanced electrocatalytic performances of transition metal dichalcogenides or carbides could pave a pathway for achieving sustainable energy conversion.The development of environmentally-friendly and facile synthetic routes for electrocatalysts with tunable morphology and composition to maximize the number of active surface sites still remains a challenging task. The fabrication of active electrocatalysts on a conductive substrate, such as Ni foam/foil, carbon fiber/cloth or Ti foil, could provide the efficient pathways for charge transport and open channels for rapid release of H2 bubbles during HER process.24 Among different approaches for synthesizing transition metal sulfides electrocatalysts, synergistic effects of morphological control and heterointerface engineering strongly significantly depend on the appropriate choice of precursors. Transition metal oxides and hydroxides with various nanostructures have been employed as precursors to synthesize transition metal sulfide based composites.21,25,26 A transformation process of transition metal oxides or hydroxides to transition metal sulfides would occur in a sulfur vapor atmosphere or ions solutions. According to previous works, metal molybdate (AMoO4) comprising molybdenum and second transition metals (A = Fe, Co and Ni, etc.) could be prepared by several convenient synthetic approaches.27 Although diverse metal molybdates have been reported to exhibit excellent performance in supercapacitor devices, those metal molybdates were found to be nearly inactive towards the HER.4,28–33 Recent studies shed light on the role of metal molybdates as potential precursors towards the rational design of transition metal dichalcogenides heterointerface through the simultaneous sulfurization of corresponding molybdenum and second transition metal. For instance, Yu et al. reported that NiMoO4 nanowires were employed as the precursor for the preparation of bi-metal sulfide NiS2/MoS2 heteronanowires, which exhibiting an improved HER performance in comparison with NiS2 and MoS2.4 In order to further improve the electrocatalytic performance, great endeavors have been made to overcome the bottlenecks associated with the use of insulating binders and inactive conductive agent due to the serious concerns on the reduced number of exposed active edge sites and the increased resistance between the electrocatalyst and electrode.24 It should be highlighted that some of metal molybdates can be directly grown on the conducting substrates through a facile one-step hydrothermal approach.32,34 Recently, Zheng et al. recently reported the synthesis of NiMoO4 nanowire array on the Ti foil and the conversion to NiS2/MoS2 heteronanowire array with enhanced HER performance.35 Due to their inherent characteristics, numerous molybdate compounds could be considered as prospective precursors for the synthesis of transition metal dichalcogenides, aiming at taking the advantages of synergistic effect through the optimization of precursor morphology and heterointerface engineering. In fact, the development of 3D integrated electrodes fabricated by electroactive nanomaterials are appealing not only in electrochemical water splitting but other electrochemical energy conversion and storage systems as well.
Herein, we developed a facile strategy to synthesize 3D flower-like CoS2/MoS2 heteronanosheet arrays (HNSAs) on conductive Ni foam by in situ topotactic conversion of CoMoO4 nanosheet arrays (NSAs) precursor. The simultaneous sulfurization of Co and Mo in CoMoO4 precursor led to the formation of CoS2/MoS2 nanocomposite while the structural and morphological characteristic of ultrathin nanosheets was well retained during the vapor phase sulfurization process. The as-prepared CoS2/MoS2 HNSAs demonstrated an excellent HER activity with a low overpotential of 50 mV at current density of 10 mA cm−2 and a small Tafel slope of 76 mV dec−1 in 1.0 KOH. The superior HER performance of CoS2/MoS2 HNSAs could be attributed to the following three aspects: (i) the abundant accessible CoS2/MoS2 heterointerface served as highly active catalytic sites after in situ topotactic sulfurization; (ii) 3D flower-like CoS2/MoS2 HNSAs integrating directly on the conductive Ni foam facilitated fast electron transport; (iii) the 3D network structure of CoS2/MoS2 HNSAs offered the internal void spaces between neighboring heteronanosheets, which were suitable for both the diffusion of electrolyte and release of gas bubble process. This work serves as a proof of concept for the heterointerface engineering of transition metal dichalcogenides nanocomposite as efficient electrocatalysts through rational compositional and morphological control.
2. Experimental section
2.1 Chemicals
All of the chemicals were purchased from Alfa Aesar and used as received without further purification, including sublimed S powder, Na2MoO4·2H2O and Co(NO3)2·6H2O. The porous Ni foams were obtained from Kunshan Company.2.2 Materials synthesis
The Ni foams (1.0 cm × 1.5 cm) were cleaned in acetone, absolute ethanol, HCl solution for 30 min, respectively. The preparation of 3D flower-like CoMoO4 NSAs was conducted as follow, 1.6 mM Na2MoO4·2H2O and 1.6 mM Co(NO3)2·6H2O was dissolved under continuous stirring in 20 mL deionized water. The pretreated Ni foams were then immersed into the mixed solution and kept at 90 °C in an electrical oven for 48 h. After cooling to the room temperature, the as-prepared CoMoO4 NSAs on Ni foam were washed with the assistance of sonication to remove surface residues and then dried at 60 °C in a vacuum oven for overnight. The in situ topotactic sulfurization was conducted by a vapor phase sulfurization procedure. CoMoO4 NSAs precursor was converted to CoS2/MoS2 HNSAs at 370 °C for 45 min. Mass loading of CoS2/MoS2 HNSAs on Ni foam was 0.9 mg cm−2.2.3 Materials characterization
The morphology of the as-prepared 3D flower-like CoMoO4 NSAs and CoS2/MoS2 HNSAs were characterized by field emission scanning electron microscopy (SEM, SU8020, Hitachi), transmission electron microscopy (TEM, F20 and Talos F200S, FEI) with an energy-dispersive X-ray spectroscopy (EDS) detector. The crystal structure of all samples was characterized by X-ray diffraction (XRD, X'Pert3 Powder diffractometer, Cu Kα radiation (λ = 1.5418 nm)). The chemical composition and valence state of CoMoO4 NSAs and CoS2/MoS2 HNSAs was investigated by X-ray photoelectron spectroscopy (XPS, ThermoFisher Scientific ESCALAB 250Xi).2.4 Electrochemical characterization
The electrochemical measurements of 3D flower-like CoS2/MoS2 HNSAs were conducted in a three-electrode system using a CHI 660E electrochemical workstation (CH Instruments, Shanghai, China). The 3D flower-like CoS2/MoS2 HNSAs were used as working electrode with a fixed geometric area of 1 cm × 1 cm, while Hg|HgO and graphite rod was used as the reference electrode and counter electrode, respectively. In this work, the HER activity of 3D flower-like CoS2/MoS2 HNSAs was performed in 1.0 M KOH solution while all applied potentials were converted with respect to reversible hydrogen electrode (RHE). Linear sweep voltammetry (LSV) with a scan rate of 5 mV s−1 was conducted without iR compensation. The electrochemical double-layer capacitance (Cdl) was measured basing on the data of cyclic voltammetry (CV) at various scan rates (10, 20, 30, 40, 50 and 60 mV s−1) in range between 0.1 and 0.2 V (versus RHE). The long-term stability was conducted by chronoamperometry and 2000 cycles continuously in 1.0 M KOH, respectively. Electrochemical impedance spectroscopy (EIS) measurements were carried out at an overpotential of 150 mV in a frequency range from 100 kHz to 0.01 Hz. The KOH solution was purged with high-purity N2 to remove dissolved oxygen before each electrochemical measurement.3. Results and discussion
Inspired by recent studies of in situ synthesis of transition metal dichalcogenides based electrocatalysts on conductive substrate, the high density and uniformly distributed nanoarray architectures, such as 1D nanowire/rod and 2D nanosheet arrays, can significantly improve the HER catalytic activity and stability. The fabrication of 3D flower-like CoS2/MoS2 HNAS on conductive Ni foam were facilely conducted by a two-step procedure without using any surfactant and hard template. The synthetic process and composition evolution of CoS2/MoS2 HNSAs is schematically illustrated in Fig. 1.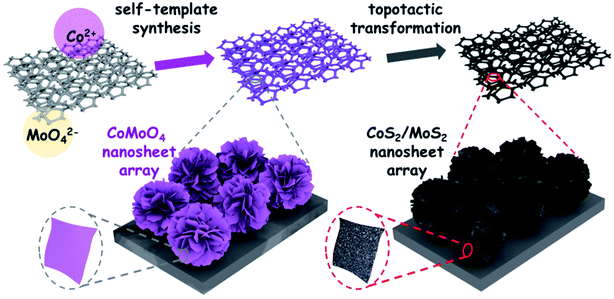 | ||
| Fig. 1 Schematic illustration for the self-template synthesis of 3D flower-like CoMoO4 NSAs and in situ topotactic sulfurization to CoS2/MoS2 HNSAs. | ||
The flower-like CoMoO4 NSAs were synthesized on porous Ni foam via a self-template method (see Experimental section for more details).36,37 As shown in Fig. 2a and b, the low-magnification scanning electron microscopy (SEM) images demonstrate that the 3D network structure of flower-like CoMoO4 NSAs anchored on Ni foam. The self-assembled CoMoO4 nanosheets with lateral sizes of about 2 μm were grown uniformly on Ni foam surface with a 3D flower-like micro/nanostructure (Fig. 2c). It can be explained by the large amount of nanosized CoMoO4 nucleation clusters were formed in the solution for the self-assembly of 2D nanosheets, which further led to the formation of flower-like morphology. The high-magnification SEM image exhibits the as-synthesized CoMoO4 nanosheets with thickness in the range of 10–20 nm are interconnected with each other (Fig. 2d), providing a large number of internal void spaces between adjacent nanosheets. It can be also observed some CoMoO4 nanosheets with slightly curved surface and crumpled edges due to the larger lateral dimension than the thickness. Transmission electron microscopy (TEM) characterizations were conducted to investigate the structure of CoMoO4 nanosheets. Fig. 2e and f presents that 3D flower-like CoMoO4 micro/nanostructure is made up of interconnected nanosheets with a lateral size of several hundred nanometers. It is found that the CoMoO4 nanosheets are slightly folded in some regions. For instance, the observation of dark strips resulted from the curled edges or wrinkles of the CoMoO4 nanosheets which is consistent with the SEM observation. Fig. 2g shows the enlarged TEM image of CoMoO4 nanosheet edge, revealing the existence of slight disorder. From high resolution TEM (HRTEM) image, the lattice spacing is 0.33 nm corresponding well to (002) plane of monoclinic CoMoO4 (Fig. 2i).34,38 The high-angle annular dark-field scanning TEM (HAADF-STEM) and corresponding energy-dispersive X-ray spectroscopy (EDS) characterization (Fig. 2i–l) confirms the uniform distributions of Co, Mo and O elements throughout the 3D flower-like nanosheets. XRD pattern shows no obvious characteristic peak of the CoMoO4 NSAs, indicating the polycrystalline nature of self-assembled nanosheets through the low temperature hydrothermal method.32
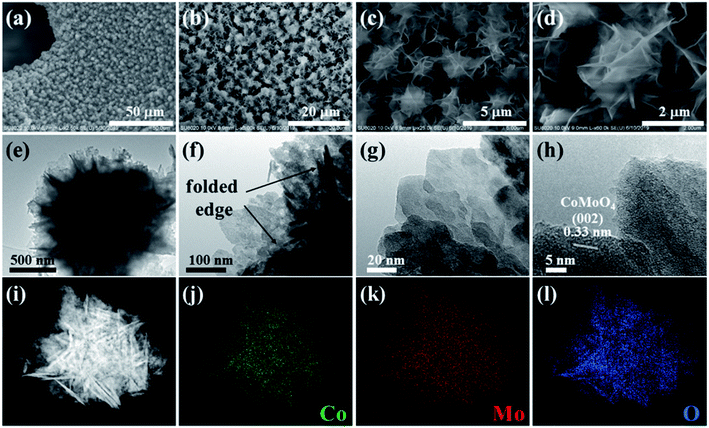 | ||
| Fig. 2 (a–d) SEM images, (e–h) TEM images, (i) HAADF-STEM and (j–l) EDS mapping images of 3D flower-like CoMoO4 nanosheets. | ||
Owing to the morphological features of ultrathin CoMoO4 nanosheets, the in situ topotactic conversion to CoS2/MoS2 nanosheets was successfully achieved by the vapor phase sulfurization process. Fig. 3a–d demonstrates the dense and uniform coverage of flower-like CoS2/MoS2 HNSAs. Compared to CoMoO4 nanosheets with a relative smooth surface, the high-magnification SEM image undoubtedly reveals that the coarse surface of CoS2/MoS2 heteronanosheets after sulfurization (Fig. 3d). Basing on the morphology evolution, it can be seen that the flower-like CoMoO4 NSAs acted as a self-template for the topotactic conversion to CoS2/MoS2 HNSAs. Similar to the case of CoMoO4 nanosheets, CoS2/MoS2 heteronanosheets with transparent feature indicated the ultrathin nature was also retained after the in situ conversion (Fig. 3e and f). As shown in Fig. 3g, the enlarged TEM image demonstrates that heteronanosheet integrated with CoS2 and MoS2 species after sulfurization. The HRTEM image reveals lattice fringes of 0.62 and 0.24 nm, corresponding to the (002) plane of MoS2 and (210) plane of CoS2, respectively (Fig. 3h). The observation of lattice discontinuities in CoS2/MoS2 heteronanosheet indicates the existence of the slight disordered structure. On the one hand, a large number of heterointerface forming between CoS2 and MoS2 can be undoubtedly identified. On the other hand, the intimate contact of CoS2 and MoS2 can be ascribed to the simultaneous sulfurization of Co and Mo in CoMoO4 precursor. It should be noted that the as-obtained heteronanosheets demonstrated a relatively uniform distribution of CoS2 and MoS2 species. Furthermore, the elemental distributions of 3D flower-like CoS2/MoS2 nanosheets were characterized by HAADF-STEM and the corresponding EDS characterizations, revealing the homogeneous distribution of Co, Mo and S throughout the whole heteronanosheets (Fig. 3i–l). As shown in Fig. S1 (ESI†), the EDS spectra analysis further confirmed CoMoO4 NSAs were transformed to CoS2/MoS2 HNSAs after in situ topotactic sulfurization due to the disappearance of O signal and appearance of S signal. The overlapping Co, Mo and S signals indicates the uniform formation of CoS2 and MoS2 nanocomposites while the predesigned void spaces can still be observed between neighboring heteronanosheets. Attributed to the good preservation of 3D network structure of CoMoO4 precursor after in situ topotactic conversion, 3D flower-like CoS2/MoS2 HNSAs exhibit porous structures which is essential to increase the accessible surface areas and facilitate the effective contact between electrocatalyst and electrolyte ions.
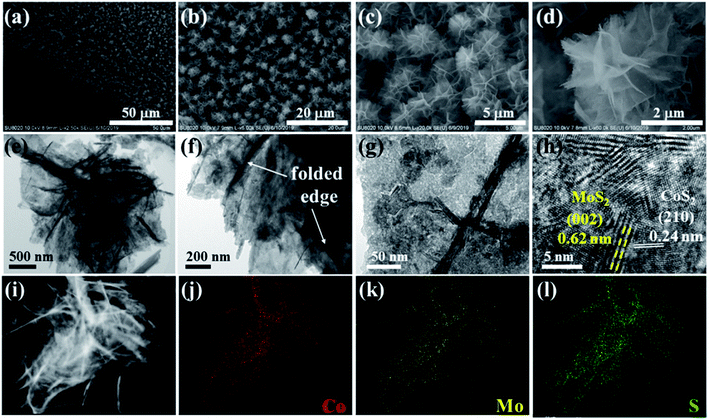 | ||
| Fig. 3 (a–d) SEM images, (e–h) TEM images, (i) HAADF-STEM and (j–l) EDS mapping images of 3D flower-like CoS2/MoS2 nanosheets. | ||
To confirm the composition and valence states of the as-prepared flower-like CoMoO4 NSAs and CoS2/MoS2 HNSAs, X-ray photoelectron spectroscopy (XPS) characterization were conducted. For the CoMoO4 NSAs, the Co 2p spectrum exhibits two main peaks at 780.8 and 797.1 eV (Fig. 4a), corresponding to the low energy band of Co 2p3/2 and high energy band of Co 2p1/2, respectively, as well as their satellite peaks (denoted as “sat.”).39 In the Mo 3d spectrum of CoMoO4 precursor, two significant peaks at 232.1 and 235.3 eV are associated with Mo 3d5/2 and Mo 3d3/2 electronic configurations, respectively, indicating a +6 oxidation state of Mo in the CoMoO4 NSAs (Fig. 4b). As shown in Fig. 4c, the O 1s spectrum of CoMoO4 precursor can be deconvoluted into four peaks at 530.2, 530.9, 531.8 and 533.0 eV, corresponding to the Co–O bonds, hydroxyl groups in the hydrated sample, oxygen ions in low coordination and physic- and/or chemisorbed water, respectively.40 After sulfurization, the XPS spectrum of Co 2p3/2 and Co 2p1/2 signals are found to be 778.9 and 793.9 eV, respectively. The obvious shifts of Co 2p3/2 and Co 2p1/2 binding energy indicate the formation of CoS2 after in situ sulfurization because sulfur has a lower electronegativity than oxygen (see Fig. 4d). As shown in Fig. 4e, two characteristic peaks at the binding energies of 228.8 and 232.0 eV are ascribed to Mo 3d5/2 and Mo 3d3/2, indicating a +4 oxidation state of Mo and therefore confirming the conversion of MoS2 after in situ topotactic sulfurization.21 It should be noted the observation of broad S 2s peaks around 226.0 eV suggests the formation of Co–S and Mo–S bonding. The analysis of the S 2p spectrum reveals the characteristic peaks at 161.6 and 162.6 eV are assigned to S 2p3/2 due to the formation of Co–S and Mo–S bonding while the broad peak around 163.2 and 164.2 eV is attributed to bridging S22− between CoS2 and MoS2 (Fig. 4f), which were identified as active HER sites.41–43 Therefore, it can be concluded that the formation of CoS2/MoS2 nanocomposite via in situ topotactic conversion.
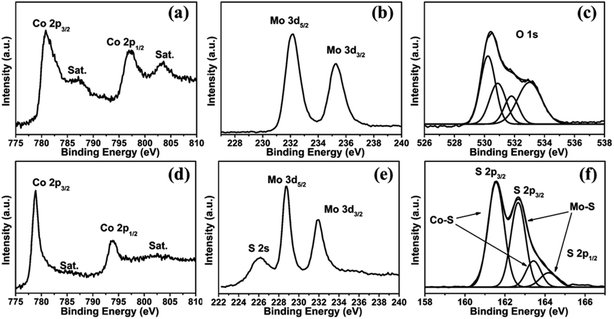 | ||
| Fig. 4 High-resolution XPS profiles of (a) Co 2p, (b) Mo 3d and (c) O 1s in CoMoO4 NSAs; (d) Co 2p, (e) Mo 3d and S 2s, and (f) S 2p in CoS2/MoS2 HNSAs. | ||
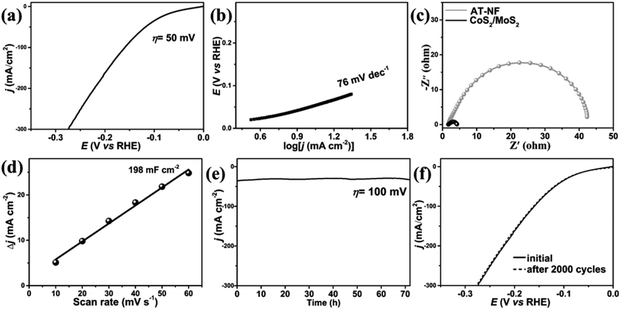
To evaluate the electrocatalytic activity for HER, 3D flower-like CoS2/MoS2 HNSAs was tested in a 1.0 M KOH solution using a typical three-electrode configuration. Fig. 5a demonstrates the polarization curves without iR correction. The overpotential for 3D flower-like CoS2/MoS2 HNSAs to achieve a current density of 10 mA cm−2 (η10) is 50 mV (vs. RHE) while the conductive Ni foam demonstrated negligible HER activity. In comparison with the previously reported transition metal sulfides based electrocatalysts (Table S1 in ESI†), the η10 of 3D flower-like CoS2/MoS2 HNSAs is much lower those of the reported NiCo2S4 nanowire arrays (210 mV),15 Ni0.33Co0.67S2 nanowire arrays (88 mV),16 Ni–Mo–S nanowire networks (210 mV),44 MoS2 nanosheet arrays (200 mV),45 Ni3S2 nanosheet arrays (223 mV),46 CoS2 nanosheet arrays (192 mV),47 FeS2 mesoporous nanoparticles (96 mV),48 Ni3S2 nanorod arrays (200 mV),49 Na-doped 1T-MoS2 nanosheets (183 mV)50 and 1T-MoSe2 nanosheets (152 mV).51 In particular, the electrocatalytic performance of 3D flower-like CoS2/MoS2 HNSAs is also comparable to the noble metal doped or decorated transition metal sulfides, such as Pt–MoS2 nanosheet clusters (60 mV),52 Pt–MoS2 nanoparticles (58 mV)53 and Rh–MoS2 nanosheets (47 mV).54 Via further comparison with other Co-, Mo- and Ni-based heterostructured electrocatalysts (Table S1, ESI†), the electroactivity of 3D flower-like CoS2/MoS2 HNSAs is better than those of CoS2/MoS2 nanosheet composites (154 mV),55 MoS2/CoS2 nanowire arrays (87 mV),21 NiS2/CoS2/C carnation-like nanosheets (165 mV),56 Co9S8/MoS2 core/shell nanocrystals (97 mV),57 MoS2/NiS/MoO3 nanowire arrays (90 mV),58 NiS2/MoS2 nanowires (204 mV),4 MoS2/Ni3S2 nanorod arrays (98 mV),22 CoS2@MoS2/RGO composites (98 mV),59 MoS2/Ni3S2 nanosheets/particles (110 mV),23 oxygenated-CoS2/MoS2 nanosheet arrays (97 mV),60 CoS2/MoS2 nanowire arrays (97 mV)61 CoS2–C@MoS2 nanofibers (173 mV)62 and Ag2S/MoS2/RGO composites (190 mV).63 In particular, previous report demonstrated CoS2/MoS2 nanosheet composites were synthesized through a one-step hydrothermal method.55 However, the CoS2/MoS2 HNSAs demonstrated a remarkable alkaline HER activity, which can be attributed to the formation of heteronanosheet-based flower-like microarchitecture anchored on the Ni foam. It should be highlighted that the Ni foam with porous and conductive characteristics played essential roles in providing more pathways for charge transport and offering open channels for the release of gas bubbles.
Volmer reaction
| H2O + M* + e− → M–H* + OH− | (1) |
Heyrovsky reaction
| M–H* + H2O + e− → H2 + OH− + M* | (2) |
Tafel reaction
| M–H* + M–H* → H2 + 2M* | (3) |
According to the previous reports, three principal steps were proposed for hydrogen evolution: (1) Volmer reaction with a Tafel slope of ∼120 mV dec−1, (2) Heyrovsky reaction with a Tafel slope of ∼40 mV dec−1, and (3) Tafel reaction with a Tafel slope of ∼30 mV dec−1. It should be noted that the asterisk indicates the active site for HER while M–H* represents the hydrogen atom bound to the active site on electrocatalyst. In fact, the determination of the specific mechanism cannot be achieved by the direct evaluation of the Tafel slope for most of electrocatalysts. As shown in Fig. 5b, the relatively small slope of 3D flower-like CoS2/MoS2 HNSAs (76 mV dec−1) falls within the range of 40–120 mV dec−1, which is comparable to those of abovementioned composites, such as CoS2/MoS2 nanosheets (61 mV dec−1),55 MoS2/CoS2 nanowire array (73.4 mV dec−1),21 and Co9S8/MoS2 core/shell nanocrystals (71 mV dec−1),57 Co9S8@MoS2 nanoparticles (110 mV dec−1),43 NiS2/MoS2 nanowires (65 mV dec−1),4 MoS2/Ni3S2 nanorod arrays (61 mV dec−1),22 MoS2/Ni3S2 nanosheets/particles (88 mV dec−1),23 oxygenated-CoS2/MoS2 nanosheet arrays (83.1 mV dec−1),60 CoS2/MoS2 nanowire arrays (78.7 mV dec−1)61 and CoS2–C@MoS2 nanofibers (61 mV dec−1).62 The small Tafel slope of 3D flower-like CoS2/MoS2 HNSAs unveils a fast increase of hydrogen generation rate with increasing applied overpotential. It can concluded that the considerably promoted Volmer step in HER kinetics indicates that the Heyrovsky mechanism is dominant for alkaline HER on 3D flower-like CoS2/MoS2 HNSAs.64 Furthermore, the electrochemical impedance spectroscopy (EIS) measurements were conducted to investigate the electrochemical behavior during the HER process (Fig. 5c). The Nyquist plot of 3D flower-like CoS2/MoS2 HNSAs demonstrates the low charge transfer resistance, indicating the fast electron transfer at the solution electrode interfaces. We further investigate the double-layer capacitance (Cdl) of as-fabricated 3D flower-like CoS2/MoS2 HNSAs, which is known to be proportional to the electrochemical surface area (Fig. S2, ESI†). Fig. 5d plots the capacitive current densities of CoS2/MoS2 HNSAs (Δj = ja − jb, where ja and jb denotes current densities in CV at 0.15 V (vs. RHE)), the Cdl value is estimated to be 198 mF cm−2, which is comparable to the previously reported transition metal based electrocatalysts.26 The large Cdl value indicates the enriched active sites for alkaline HER, resulting from a large number of heterointerfaces between CoS2 and MoS2 after in situ sulfurization. The long-term stability in high concentration alkaline solutions is an essential issue for transition metal dichalcogenides electrocatalysts to be practically applicable.65 As presented in Fig. 5e, the stability test was conducted for 70 h, there was no apparent change in the current density at η = 100 mV. As demonstrated in Fig. 5f, the stability test of 3D flower-like CoS2/MoS2 HNSAs was also conducted for 2000 CV cycles, indicating the good durability in alkaline media. In comparison with previously reported alkaline HER electrocatalysts, the considerable enhancement in alkaline HER performance of 3D flower-like CoS2/MoS2 HNSAs will be discussed in more details below. Basing on the morphological evolution, flower-like CoMoO4 NSAs were employed as precursor for the topotactic conversion of CoS2/MoS2 HNSAs with well retained 3D network structure. After sulfurization, the coarse nanosheets surface indicated the homogeneous distribution of CoS2 and MoS2 species through the whole nanosheets as well as abundant heterointerfaces. On the one hand, the high conductivity of porous Ni foam enabled the efficient electron transport to 3D flower-like heteronanosheets. On the other hand, the intimate integration of CoS2 and MoS2 play a key role in facilitating the faster charge transport through the whole heteronanosheets and then promoted the hydrogen generation. The formation of 3D flower-like heteronanosheet array morphology significantly increased the electroactive surface areas through the exposed active sites and well-exposed heterointerface not only from the nanosheet edges but also the nanosheet surfaces, which considerably facilitated efficient electron and ion transportation as well as the release of as-produced H2 gas bubbles during the HER process. According to previous studies, multi-layered MoS2 have rarely shown superior HER performance without surface modification and/or heterointerface engineering because the highly active sites of multilayer MoS2 are mainly located on the exposed edges instead of the basal plane.5 The heterointerface between CoS2 and MoS2 facilitated electron transfer between Co and Mo through bridging S22− species which simultaneously bonding to Co and Mo atoms, leading to the creation of latent active sites.43 Theoretical studies also indicated that H* preferably adsorbs on these transition metal-site at the localized heterointerface region due to a lower chemisorption free energy, therefore resulting in the remarkably enhanced HER performance.4 For these reasons, 2D ultrathin heteronanosheets composed of interconnected CoS2 and MoS2 species would be highly advantages. Previous studies unveiled that the well-exposed transition metal related active sites could act as an exceptional H2O dissociation center while the Mo-related active sites demonstrate superior adsorption capability toward H.4,13,57,66 The formation of well-exposed CoS2/MoS2 heterointerfaces may simultaneously promote the adsorption towards H in alkaline media and strengthen the dissociation of H2O molecules, which require further theoretical investigation.67,68
4. Conclusion
In summary, 3D flower-like CoS2/MoS2 heteronanosheets array was fabricated on conductive Ni foam through a self-template hydrothermal synthesis and subsequent in situ topotactic sulfurization. The morphological design of CoMoO4 nanosheet array as precursor allow the well-retained 3D network structure with large exposed surface areas after sulfurization. CoS2/MoS2 heteronanosheet array as efficient electrocatalyst for alkaline HER with a low overpotential of 50 mV at current density of 10 mA cm−2 and small Tafel slope of 76 mV dec−1, which is comparable to those of benchmark transition metal based electrocatalysts. Basing on our experimental characterizations, we demonstrated that the enhanced electrocatalytic performance can be mainly attributed to the synergistic effect of rational morphological design of 3D interconnected network structure and the formation of abundant well-exposed CoS2/MoS2 heterointerfaces. Out synthetic strategy can possibly be extended to the preparation of other traditional metal dichalcogenide nanocomposite by the rational design of metal molybdate precursor, including substituting Co with other transition metal (such as Fe, Ni or Cu) or tuning their morphology from 2D heteronanosheet to 1D heteronanowire arrays. Further improvement in electrochemical energy conversion and storage systems basing on low-cost and earth-abundant transition metal dichalcogenides can be expected by utilizing the various molybdate precursors to increase the intrinsic activity of non-precious electrocatalysts via morphological control and heterointerface engineering.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (NSFC 21603014 and 51872031), and BUCEA Post Graduate Innovation Project (PG2019037).References
- W. J. Zhou, J. Jia, J. Lu, L. J. Yang, D. M. Hou, G. Q. Li and S. W. Chen, Nano Energy, 2016, 28, 29–43 CrossRef CAS.
- Q. Zhang, P. Li, D. Zhou, Z. Chang, Y. Kuang and X. Sun, Small, 2017, 13, 1701648 CrossRef PubMed.
- D. Gao, J. Guo, X. Cui, L. Yang, Y. Yang, H. He, P. Xiao and Y. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 22420–22431 CrossRef CAS PubMed.
- P. Kuang, T. Tong, K. Fan and J. Yu, ACS Catal., 2017, 7, 6179–6187 CrossRef CAS.
- J. Xie, H. Zhang, S. Li, R. Wang, X. Sun, M. Zhou, J. Zhou, X. W. Lou and Y. Xie, Adv. Mater., 2013, 25, 5807–5813 CrossRef CAS PubMed.
- H. Lin, Z. Shi, S. He, X. Yu, S. Wang, Q. Gao and Y. Tang, Chem. Sci., 2016, 7, 3399–3405 RSC.
- L. Ma, L. R. L. Ting, V. Molinari, C. Giordano and B. S. Yeo, J. Mater. Chem. A, 2015, 3, 8361–8368 RSC.
- M. Zhou, Q. Weng, Z. I. Popov, Y. Yang, L. Y. Antipina, P. B. Sorokin, X. Wang, Y. Bando and D. Golberg, ACS Nano, 2018, 12, 4148–4155 CrossRef CAS PubMed.
- B. Liu, B. He, H. Q. Peng, Y. Zhao, J. Cheng, J. Xia, J. Shen, T. W. Ng, X. Meng, C. S. Lee and W. Zhang, Adv. Sci., 2018, 5, 1800406 CrossRef PubMed.
- G. Chen, T. Ma, Z. Liu, N. Li, Y. Su, K. Davey and S. Qiao, Adv. Funct. Mater., 2016, 26, 3314–3323 CrossRef CAS.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- P. D. Tran, T. V. Tran, M. Orio, S. Torelli, Q. D. Truong, K. Nayuki, Y. Sasaki, S. Y. Chiam, R. Yi, I. Honma, J. Barber and V. Artero, Nat. Mater., 2016, 15, 640–646 CrossRef CAS PubMed.
- D. S. Kong, J. J. Cha, H. T. Wang, H. R. Lee and Y. Cui, Energy Environ. Sci., 2013, 6, 3553–3558 RSC.
- A. Sivanantham, P. Ganesan and S. Shanmugam, Adv. Funct. Mater., 2016, 26, 4661–4672 CrossRef CAS.
- Z. Peng, D. S. Jia, A. M. Al-Enizi, A. A. Elzatahry and G. F. Zheng, Adv. Energy Mater., 2015, 5, 1402031 CrossRef.
- J. Deng, H. Li, S. Wang, D. Ding, M. Chen, C. Liu, Z. Tian, K. S. Novoselov, C. Ma, D. Deng and X. Bao, Nat. Commun., 2017, 8, 14430 CrossRef CAS PubMed.
- J. Xie, J. Zhang, S. Li, F. Grote, X. Zhang, H. Zhang, R. Wang, Y. Lei, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 17881–17888 CrossRef CAS PubMed.
- T. Kou, T. Smart, B. Yao, I. Chen, D. Thota, Y. Ping and Y. Li, Adv. Energy Mater., 2018, 8, 1703538 CrossRef.
- J. Zhou, G. Fang, A. Pan and S. Liang, ACS Appl. Mater. Interfaces, 2016, 8, 33681–33689 CrossRef CAS PubMed.
- J. Huang, D. Hou, Y. Zhou, W. Zhou, G. Li, Z. Tang, L. Li and S. Chen, J. Mater. Chem. A, 2015, 3, 22886–22891 RSC.
- Y. Yang, K. Zhang, H. Ling, X. Li, H. Chan, L. Yang and Q. Gao, ACS Catal., 2017, 7, 2357–2366 CrossRef CAS.
- J. Zhang, T. Wang, D. Pohl, B. Rellinghaus, R. Dong, S. Liu, X. Zhuang and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 6702–6707 CrossRef CAS PubMed.
- N. K. Chaudhari, H. Jin, B. Kim and K. Lee, Nanoscale, 2017, 9, 12231–12247 RSC.
- X. Q. Du, Q. B. Wang and X. S. Zhang, New J. Chem., 2018, 42, 18201–18207 RSC.
- Q. Ma, C. Hu, K. Liu, S. Hung, D. Ou, H. Chen, G. Fu and N. Zheng, Nano Energy, 2017, 41, 148–153 CrossRef CAS.
- W. Xiao, J. S. Chen, C. M. Li, R. Xu and X. W. Lou, Chem. Mater., 2010, 22, 746–754 CrossRef CAS.
- Z. X. Yin, Y. J. Chen, Y. Zhao, C. Y. Li, C. L. Zhu and X. T. Zhang, J. Mater. Chem. A, 2015, 3, 22750–22758 RSC.
- D. Cai, B. Liu, D. Wang, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li and T. Wang, J. Mater. Chem. A, 2014, 2, 4954–4960 RSC.
- L. Q. Mai, F. Yang, Y. L. Zhao, X. Xu, L. Xu and Y. Z. Luo, Nat. Commun., 2011, 2, 381 CrossRef PubMed.
- L. Zhang, S. Zheng, L. Wang, H. Tang, H. Xue, G. Wang and H. Pang, Small, 2017, 13, 1700917 CrossRef PubMed.
- S. J. Peng, L. L. Li, H. B. Wu, S. Madhavi and X. W. Lou, Adv. Energy Mater., 2015, 5, 1401172 CrossRef.
- Z. Yin, S. Zhang, Y. Chen, P. Gao, C. Zhu, P. Yang and L. Qi, J. Mater. Chem. A, 2015, 3, 739–745 RSC.
- J. Wang, X. Zhang, Q. Wei, H. Lv, Y. Tian, Z. Tong, X. Liu, J. Hao, H. Qu, J. Zhao, Y. Li and L. Mai, Nano Energy, 2016, 19, 222–233 CrossRef CAS.
- T. C. An, Y. Wang, J. Tang, W. Wei, X. Q. Cui, A. M. Alenizi, L. J. Zhang and G. F. Zheng, J. Mater. Chem. A, 2016, 4, 13439–13443 RSC.
- D. Guo, H. Zhang, X. Yu, M. Zhang, P. Zhang, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 7247–7254 RSC.
- J. Wang, S. K. Liu, X. Zhang, X. S. Liu, X. X. Liu, N. Li, J. P. Zhao and Y. Li, Electrochim. Acta, 2016, 213, 663–671 CrossRef CAS.
- I. Hussain, A. Ali, C. Lamiel, S. G. Mohamed, S. Sahoo and J. J. Shim, Dalton Trans., 2019, 48, 3853–3861 RSC.
- F. Wang, J. Zhao, W. Tian, Z. Hu, X. Lv, H. Zhang, H. Yue, Y. Zhang, J. Ji and W. Jiang, RSC Adv., 2019, 9, 1562–1569 RSC.
- L. Huang, J. W. Xiang, W. Zhang, C. J. Chen, H. H. Xu and Y. H. Huang, J. Mater. Chem. A, 2015, 3, 22081–22087 RSC.
- H. Vrubel, D. Merki and X. Hu, Energy Environ. Sci., 2012, 5, 6136–6144 RSC.
- J. Jiang, M. Gao, W. Sheng and Y. Yan, Angew. Chem., Int. Ed., 2016, 55, 15240–15245 CrossRef CAS PubMed.
- H. Zhu, J. Zhang, R. Yanzhang, M. Du, Q. Wang, G. Gao, J. Wu, G. Wu, M. Zhang, B. Liu, J. Yao and X. Zhang, Adv. Mater., 2015, 27, 4752–4759 CrossRef CAS PubMed.
- Z. Ma, H. Meng, M. Wang, B. Tang, J. Li and X. Wang, ChemElectroChem, 2018, 5, 335–342 CrossRef CAS.
- F. Z. Wang, M. J. Zheng, B. Zhang, C. Q. Zhu, Q. Li, L. Ma and W. Z. Shen, Sci. Rep., 2016, 6, 31092 CrossRef CAS PubMed.
- L. L. Feng, G. Yu, Y. Wu, G. D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS PubMed.
- X. Han, X. Wu, Y. Deng, J. Liu, J. Lu, C. Zhong and W. Hu, Adv. Energy Mater., 2018, 8, 1800935 CrossRef.
- R. Miao, B. Dutta, S. Sahoo, J. He, W. Zhong, S. A. Cetegen, T. Jiang, S. P. Alpay and S. L. Suib, J. Am. Chem. Soc., 2017, 139, 13604–13607 CrossRef CAS PubMed.
- C. Ouyang, X. Wang, C. Wang, X. Zhang, J. Wu, Z. Ma, S. Dou and S. Wang, Electrochim. Acta, 2015, 174, 297–301 CrossRef CAS.
- N. H. Attanayake, A. C. Thenuwara, A. Patra, Y. V. Aulin, T. M. Tran, H. Chakraborty, E. Borguet, M. L. Klein, J. P. Perdew and D. R. Strongin, ACS Energy Lett., 2018, 3, 7–13 CrossRef CAS.
- Y. Yin, Y. Zhang, T. Gao, T. Yao, X. Zhang, J. Han, X. Wang, Z. Zhang, P. Xu, P. Zhang, X. Cao, B. Song and S. Jin, Adv. Mater., 2017, 29, 1700311 CrossRef PubMed.
- J. Deng, H. Li, J. Xiao, Y. Tu, D. Deng, H. Yang, H. Tian, J. Li, P. Ren and X. Bao, Energy Environ. Sci., 2015, 8, 1594–1601 RSC.
- J. Li, J. Kang, Q. Cai, W. Hong, C. Jian, W. Liu and K. Banerjee, Adv. Mater. Interfaces, 2017, 4, 1700303 CrossRef.
- Y. Cheng, S. K. Lu, F. Liao, L. Liu, Y. Li and M. Shao, Adv. Funct. Mater., 2017, 27, 1700359 CrossRef.
- L. L. Chen, W. X. Yang, X. J. Liu and J. B. Jia, Int. J. Hydrogen Energy, 2017, 42, 12246–12253 CrossRef CAS.
- W. Xin, W. J. Jiang, Y. Lian, H. Li, S. Hong, S. Xu, H. Yan and J. S. Hu, Chem. Commun., 2019, 55, 3781–3784 RSC.
- H. Zhu, G. Gao, M. Du, J. Zhou, K. Wang, W. Wu, X. Chen, Y. Li, P. Ma, W. Dong, F. Duan, M. Chen, G. Wu, J. Wu, H. Yang and S. Guo, Adv. Mater., 2018, 30, 1707301 CrossRef PubMed.
- C. Wang, B. Tian, M. Wu and J. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 7084–7090 CrossRef CAS PubMed.
- Y. Guo, L. Gan, C. Shang, E. Wang and J. Wang, Adv. Funct. Mater., 2017, 27, 1602699 CrossRef.
- J. Hou, B. Zhang, Z. Li, S. Cao, Y. Sun, Y. Wu, Z. Gao and L. Sun, ACS Catal., 2018, 8, 4612–4621 CrossRef CAS.
- N. Huang, Y. Ding, S. Yan, L. Yang, P. Sun, C. Huang and X. Sun, ACS Appl. Energy Mater., 2019, 2, 6751–6760 CrossRef CAS.
- Y. Zhu, L. F. Song, N. Song, M. X. Li, C. Wang and X. F. Lu, ACS Sustainable Chem. Eng., 2019, 7, 2899–2905 CrossRef CAS.
- G. Solomon, R. Mazzaro, S. You, M. M. Natile, V. Morandi, I. Concina and A. Vomiero, ACS Appl. Mater. Interfaces, 2019, 11, 22380–22389 CrossRef CAS PubMed.
- V. Vij, S. Sultan, A. M. Harzandi, A. Meena, J. N. Tiwari, W. G. Lee, T. Yoon and K. S. Kim, ACS Catal., 2017, 7, 7196–7225 CrossRef CAS.
- J. H. Wang, W. Cui, Q. Liu, Z. C. Xing, A. M. Asiri and X. P. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, H. Si, Q. Zhang, J. Wu, L. Gao, X. Wei, Y. Sun, Q. Liao, Z. Zhang, K. Ammarah, L. Gu, Z. Kang and Y. Zhang, J. Am. Chem. Soc., 2020 DOI:10.1021/jacs.9b12113.
- J. Zhang, T. Wang, P. Liu, S. Liu, R. Dong, X. Zhuang, M. Chen and X. Feng, Energy Environ. Sci., 2016, 9, 2789–2793 RSC.
- B. S. Tang, Z. G. Yu, Y. X. Zhang, C. H. Tang, H. L. Seng, Z. W. Seh, Y. W. Zhang, S. J. Pennycook, H. Gong and W. F. Yang, J. Mater. Chem. A, 2019, 7, 13339–13346 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: EDS spectra of CoMoO4 NSAs and CoS2/MoS2 HNSAs, and cyclic voltammograms of 3D flower-like CoS2/MoS2 HNSAs. See DOI: 10.1039/c9ra10963c |
| This journal is © The Royal Society of Chemistry 2020 |