Open Access Article
Open Access ArticleSonochemical degradation of pesticides in aqueous solution: investigation on the influence of operating parameters and degradation pathway – a systematic review
Meghdad Pirsaheb and
Negin Moradi *
*
Research Center for Environmental Determinants of Health, Department of Environmental Health Engineering, School of Public Health, Kermanshah University of Medical Sciences, Kermanshah, Iran. E-mail: negin.moradi724@yahoo.com; Tel: +989188867003
First published on 19th February 2020
Abstract
Along with the wide production, consumption and disposal of pesticides in the world, the concerns over their human and environmental health impacts are rapidly growing. Among developing treatment technologies, sonochemistry as an emerging and promising technology for the removal of pesticides in the aqueous environment has attracted the attention of many researchers in recent years. This systematic review presents an extensive study of sonochemical degradation of different types of pesticides from aqueous solution. The influence of various parameters including reactor configurations, initial concentration of pesticide, ultrasonic frequency, intensity of irradiation, bulk solution temperature, operational pH and sonication time on the degradation efficiency has been analyzed. The mechanism of ultrasonic degradation has been discussed, and recommendations for optimum operating conditions have been reported for maximizing degradation efficiency. Additionally, the intensification of ultrasonic cavitation by combining with oxidation processes was overviewed and the main advantages and disadvantages were pointed out, in order to address future studies and promote efficient large-scale operations. As a conclusion, it appears that ultrasonic irradiation can be effectively used for intensification of the degradation of pesticides from aqueous solution.
1. Introduction
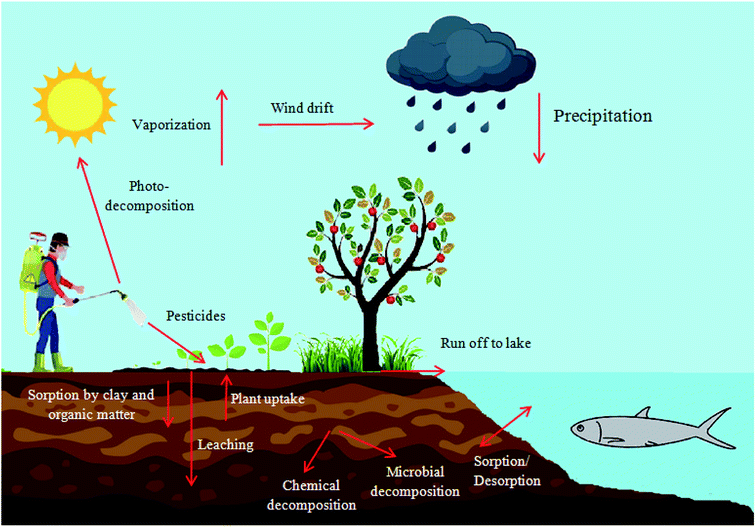
Pesticides are agrochemicals widely used worldwide in agriculture and forestry, on sports fields, public urban green areas, industrial sites, educational facilities, etc.1,2 Pesticides are typically applied in order to protect plants from pests, diseases, overgrowth by weeds and humans from vector-borne diseases.3 Based on function and the target pest organism pesticides can be classified into: insecticides (insects), fungicides (fungi), bactericides (bacteria), herbicides (weed), acaricides (mites), rodenticides (mice and other rodents), algaecides (algae), larvicides (larvae) and repellents. Others include: desiccants, ovicides (insects and mites), virucides (viruses), molluscicides (slugs and snails), nematicides (nematode), avicides (birds), moth balls (mold or moth larvae), lampricides (lampreys), piscicides (fishes), silvicides (woody vegetation) and termiticides (termites).4,5 Depending on the chemical composition and nature of active ingredients, pesticides are classified into four main groups namely; organochlorines, organophosphorus, carbamates and synthetic pyrethroids.6,7Despite the noticeable benefits of using pesticides in agricultural production and the help in mitigating food scarcity and controlling infectious diseases, their use also poses serious threats to ecosystems.8,9 Their fate in the environment is of great concern, since many sources like agricultural runoffs and industrial sewage spread them into soil and surface water or groundwater. Pesticides cause serious health hazards to human health due to direct exposure or through residues in food and drinking water.10 A typical pesticide cycle in an ecosystem is schematically represented in Fig. 1. Pesticides hold a unique position among environmental contaminants of today's world due to their toxicity, high biological activity, long-term stability and bioaccumulative.11 Therefore, it is imperative to develop efficient treatment approaches for the removal of residual pesticides.
So far, several treatment techniques such as biological, physical, chemical and physicochemical methods have been investigated for removal of pesticides from different types of matrices, such as water and soil.6 The biological remediation process depends on numerous factors, such as pH, temperature, soil moisture content, nutrient availability and oxygen level.12,13 Physical methods including nanofiltration and adsorption using various materials such as clays, zeolite, carbon nanotubes, activated carbon, and polymeric materials are very common for eliminating pesticides from water. However, major limitations of these processes are slow kinetics, dependency on the mobility and polarity of pesticides sites, high sorbent costs, capital investment and the high processing temperatures.14 In addition, physical processes such as adsorption merely transfer pollutants from one phase to another phase creating problems of secondary pollution. Also, utilization of chemical methods is not efficient but because of high consumption of chemicals, high treatment cost, incomplete removal, and time consuming.15 The physicochemical methods based on the production and use of hydroxyl radicals, named advanced oxidation processes (AOPs) have shown to be very efficient to remove different organic compounds from aqueous solutions.16,17
Among the different existing AOPs, ultrasound (US) irradiation has been recently attracting considerable attention for the degradation of organic and inorganic pollutants in water and wastewater such as pesticides.18 Ultrasound, as a newly developed treatment technology, has unique advantages over conventional treatment methods.16,19 Ultrasound operates at ambient temperature and pressure conditions. Moreover, ultrasound technology has been considered as an environmental protection method, as it produces no secondary pollutants.16,20 Ultrasound is no need for extra chemicals; therefore, the operating cost will be reduced. This technique is not only appropriate from the economic viewpoint, but it is also easy to implement. Furthermore, it has lesser safety issues and faster remediation rate compared to other existing technologies. In addition, sonolysis is not affected by the biodegradability and toxicity of the compounds.14 On the other hand, the sonochemical process also has the advantage of being compatible and attachable to other biological or physical processes.21
A goal of this review was to judge the potential use of ultrasound in the removal of pesticides from aqueous solutions and evaluate the applicability of ultrasound process in water treatment. The first part describes an overview of ultrasound, induced phenomena, mechanisms of ultrasound in degradation of pesticides. Then, a detailed analysis of the existing literature related in the specific area of ultrasonic treatment of pesticide-containing water has also been presented. In the next part, the combinatorial treatment schemes based on the use of sonication have been examined which can further intensify the degradation process and led to an economical operation even at commercial scale operations. Finally, the future perspectives of ultrasound applications in removal of pesticides will be discussed as well. This paper is aimed at providing the fundamental background information and outline research directions to those who are involved or about to be involved in this field.
2. Theory of ultrasound
Ultrasound is an acoustic wave with frequencies above the realms of human hearing (i.e., 20 kHz).22 The propagation of ultrasonic waves through the liquid medium, cause acoustic cavitation phenomenon which consists of formation, growth during the rarefaction cycle (negative pressure period) and transient collapse of the bubble during the compression phase (positive pressure period).23 The acoustic cavitation can be classified into stable and transient cavitation. At high frequencies, stable cavitation is dominant, where bubbles only oscillate and do not implode. In this type of cavitation, the motion of cavitation bubbles lead to micro-streaming which can provide micro agitation in their surrounding area. On the other hand, low frequency ultrasound primarily generates transient cavitation bubbles which live for only a few acoustic cycles and grow rapidly over a period of a few cycles to a critical size until implode very violently.24 Fig. 2a illustrates schematic of stable and transient cavitation.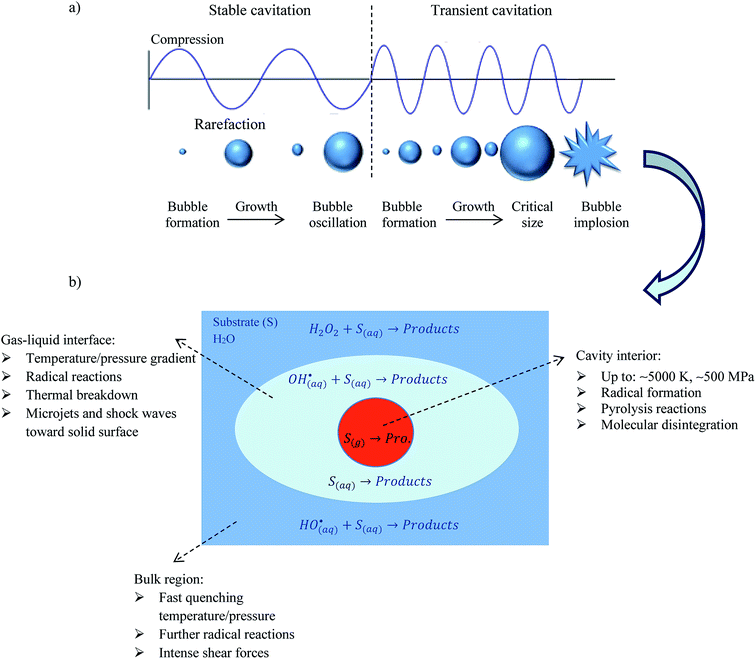 | ||
| Fig. 2 Schematic representation of (a) cavitation bubbles displaying stable and transient cavitation and (b) reaction zones in cavitation process. | ||
The collapse of transient bubble causes some hydrodynamic phenomena such as shock waves, shear forces and high-velocity micro-jets can easily disruption of cell walls or breakdown of polymer chains.25–27 In addition, transient collapse of cavitation bubbles creates causes a high local temperature (up to 5000 K) and pressure (up to 100 MPa) which lead to breakdown and pyrolytic decomposition of volatile substance and organic pollutants inside the cavitation bubbles.24,28 Inside the cavitation bubble, water vapor and oxygen molecules undergo thermal dissociation to produce different highly reactive radicals (H˙, 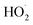 and HO˙). These primary radicals of sonolysis react with dissolved organic and inorganic compounds leading to hydroxylation and oxidation reactions.29,30 A simple mechanism for radical formation, during sonolysis of water is described below (reactions (1)–(5)):31
and HO˙). These primary radicals of sonolysis react with dissolved organic and inorganic compounds leading to hydroxylation and oxidation reactions.29,30 A simple mechanism for radical formation, during sonolysis of water is described below (reactions (1)–(5)):31
| H2O + )))US → H˙ + HO˙ | (1) |
| O2 + )))US → 2O˙ | (2) |
| O˙ + H2O → 2HO˙ | (3) |
| H˙ + O2 → HO˙ + O | (4) |
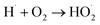 | (5) |
Hydroxyl radical is among the strongest oxidants that can react non-selectively with almost all types of organic and inorganic compounds. The trapped organic compounds in the bubble, either undergoes pyrolysis or reacts with the hydroxyl radical.32 At the interface of liquid–gas bubbles, high temperature gradient leads to locally condense HO˙ and the degradation reaction occurs in the aqueous phase. Though the temperature in this region is lower than that in the bubble core, there is an adequately high temperature to thermal decomposition of the substrate. Moreover, hydrogen peroxide (H2O2) can be generated by recombination hydroxyl radicals during sonolysis a diluted aqueous solution, which does not usually play a crucial role in oxidizing organic species and the amount may be too small to be significant (reactions (6) and (7)).33–36
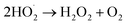 | (6) |
| 2HO˙ → H2O2 | (7) |
Generally, there are two mechanisms responsible for the oxidation/degradation of pesticides by ultrasound which is decided on the basis of physical and chemical properties of the pesticides. The first mechanism is pyrolysis inside the cavitation bubbles which is expected to be the main reaction path for the degradation of hydrophobic or apolar and more volatile compounds. The second mechanism is the formation of hydroxyl radicals in the cavitation bubbles, which subsequently are thrown out in the bulk liquid on cavity collapse and oxidise the organic compounds which are hydrophilic or polar and non-volatile compounds.37 In bulk liquid, the reactions are basically between the substrate and radicals that migrate from the interface. In the bulk phase, shear forces, turbulence and micro-streaming help radical reaction to proceed more quickly.38 Most of the hydrophobic and volatile compounds react inside and at the interface of cavities, inside the cavitation bubble whereas hydrophilic and non-volatile compounds react at bulk water that contains insufficient OH radicals.39 Fig. 2b depicts the schematic illustration of the sonochemical reaction zones.
3. Methods
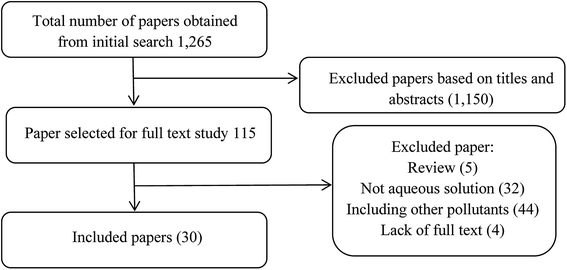
Initially, an electronic literature search in the international databases (Google Scholar, Web of Science, Science Direct, Scopus and PubMed) was done to find the published studies and reports associated with the subject of this study from 2000 to 2019. Our systematic search was performed using English keywords with any possible combinations of keywords such as ultrasound, sonolysis, pesticide, removal, degradation, aqueous, advanced oxidation process, ozonation, Fenton, photo-Fenton, sono-photo-Fenton, photocatalysis, sonophotocatalysis additives, hydrogen peroxide, carbon tetrachloride and titanium dioxide. Besides, a manual search of the bibliographies of eligible papers was done to identify additional relevant publications which were missed by online searches.The “AND” and “OR” operators were used to make our outcome of search inclusive and restrictive. Study selection procedure was including of title-reading, abstract-reading, and full-text-reading steps. In total, 1265 documentaries were found on international databases, then a large number of search results were eliminated by reviewing their titles or abstracts, and only 115 documents were selected for evaluation. Papers that mention other pollutants in their titles were excluded from the study. The remaining papers were carefully reviewed and relevant papers were selected and 30 papers were finally analyzed. Fig. 3 illustrates the search process.
4. Results and discussion
4.1. Influence of the operational variables
Operation parameters such as reactor configurations, initial concentration of pesticide, ultrasonic frequency, intensity of irradiation, bulk solution temperature, operational pH and sonication time have been studied widely because of their significant effect on the performance of sonochemical processes for the degradation of pesticides. Therefore, these parameters need to be optimized for achieving the best removal efficiency and the lowest economic costs. In this part, some optimization rules for these parameters are systematically outlined based on the exhaustive analysis of the existing literature. Information on some studies conducted in recent years for the degradation of pesticides using ultrasound is presented in Table 1.| No. | Pesticides | Chemical structure | Initial conc. | Sonochemical conditions | Other experimental cond. | Degradation intermediates/products | Results | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Chlorpyrifos, M (g mol−1) = 350.57, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.96 P: 4.96 |
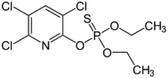 |
2–3 ppm | A probe system: 20 kHz and 0–1.2 kW | Temperature 15, 25 and 35 °C; pH 5–8; sonication time 1–120 min | Chlorpyrifos oxon and TCP | • Optimum degradation occurred at 900 W, 35 °C and pH 7 | 40 |
| • Hydrolysis and oxidation are the degradation pathway of chlorpyrifos | ||||||||
| • The toxicity decreased for chlorpyrifos solution after ultrasonic irradiation due to chlorpyrifos oxon formation | ||||||||
| 2 | Chlorpyrifos | 1 and 2 ppm | An ultrasonic bath: 35 and 130 kHz, 300 and 500 W | Temperature 25 °C; pH 4, 7 and 9; sonication time 20, 40 and 60 min | Not reported | • 98.96% degradation was occurred under optimal conditions (pH 9, pesticide concentration 1 ppm, frequency 130 kHz, ultrasonic 500 W and sonication time 20 min) | 16 | |
| • The polynomial equations satisfactorily described the behavior of ultrasonic treatment | ||||||||
| • According to the absolute effects of the independent variables, the initial concentration of chlorpyrifos had more importance over other variables for the derived first-order polynomial models | ||||||||
| 3 | DDT, M (g mol−1) = 354.5, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 6.91 P: 6.91 |
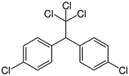 |
8 ppm | A probe system: 1.6 MHz and 20 W (150 W L−1) | pH 7.0; sonication time 90 min | DDD (C14H10Cl4), DDE (C14H8Cl4) and DDMU (C14H9Cl3) | • Low power high frequency ultrasound with operating costs much lower than low frequency is effective for the degradation of non-polar pollutant DDT | 20 |
| • 90% degradation occurred by ultrasound after 90 min | ||||||||
| • Combination of ultrasound and FeSO4 increased degradation rate of DDT | ||||||||
| 4 | Diazinon, M (g mol−1) = 304.4, pKa = 2.6, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.69 P: 3.69 |
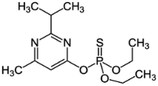 |
7.82, 32.52 and 65.19 μM | A probe system: 25 kHz and 0–650 W | Temperature 15 °C; sonication time 15–120 min | IMP, diazoxon, hydroxydiazinon, 2-hydroxydiazinon | • Pseudo-first-order kinetics; 90% degradation occurred by ultrasound after 2 h, ultrasonic intensity 500 W at initial concentration of 7.82 μmol L−1 | 41 |
| 5 | Diazinon | 800, 1200, and 1800 ppm | 1.7 MHz and 9.5 W | Temperature 20 °C; sonication time 600 s; solution volumes 40, 50, and 60 mL | Not reported | • Pseudo-first-order kinetics; 70% degradation occurred for 1200 ppm as initial concentration and 50 ml solution volume | 42 | |
| 6 | Diazinon | 2–3 ppm | A probe system: 20 kHz and 0–1.2 kW | Temperature 15, 25 and 35 °C; pH 5–8; sonication time 1–120 min | IMP, diazoxon, hydroxydiazinon, 2-hydroxydiazinon, diazinon methyl ketone | • Optimum degradation occurred at 900 W, 35 °C and pH 7 | 40 | |
| • Hydrolysis, hydroxylation, dehydration and oxidation are the degradation pathway of diazinon | ||||||||
| • The toxicity of diazinon solution declined after ultrasonic irradiation | ||||||||
| 7 | Dicofol, M (g mol−1) = 370.5, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.3 P: 4.3 |
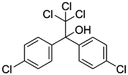 |
5.4 to 54 μM | A probe system: 20 kHz and 150–450 W | Temperature 10–40 °C; pH 3–7; sonication time 60 min | 3,3′-Dichlorobenzophenone, 4-chlorobenzophenone and benzophenone | • Pseudo-first-order kinetic model | 43 |
| • The optimum condition: acoustic power of 375 W, temperature of 20 °C, pH of 3 and an initial dicofol concentration of 27 μM | ||||||||
| • Addition of H2O2 (5000 μM) during the sonication led to a slight increase in degradation rate | ||||||||
| • Thermal decomposition along with radical attack at bubble–vapor interphase is the degradation pathway of dicofol | ||||||||
| 8 | Dichlorvos, M (g mol−1) = 221, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 1.43 P: 1.43 |
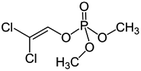 |
20 ppm | A probe system: 20 kHz and 0–270 W | Temperature 15–45 °C; pH 2–8; sonication time 120 min | Not reported | • Maximum extent of degradation of dichlorvosis as obtained at pH of 3 and temperature of 25 °C | 44 |
| • H2O2 and CCL4 enhanced the reaction by producing of the oxidizing species | ||||||||
| • The free radical attack is the controlling mechanism for the degradation of dichlorvos under sonication | ||||||||
| 9 | Dichlorvos | 5.0 × 10−4 M | A probe system: 500 kHz and 86–161 W | Temperature 20 °C; pH 3.3; sonication time 140 min | Dimethyl phosphate, formate, carbon dioxide, chloride and phosphate | • Increasing ultrasonic power from 86 to 161 W led to the enhancement of the rate constant from 0.018 ± 0.001 min−1 to 0.037 ± 0.002 min−1 | 45 | |
| • Mixture of the argon and oxygen gases (Ar/O2: 60/40% v/v) with flow rate flow rate 100 mL min−1 during sonication at a power of 161 W resulted in the highest rate constant (0.079 ± 0.005 min−1) | ||||||||
| 10 | Malathion, M (g mol−1) = 330.4, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.75 P: 2.75 |
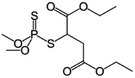 |
2, 4 and 8 ppm | A batch reactor: 130 kHz and 300 W, 400 W, 500 W | Temperature 18–20 °C; pH 6.8–7; sonication time 20–120 min | Not reported | • The extent of malathion degradation decreased by increase of initial malathion concentration and decrease of ultrasonic power | 46 |
| • The results showed that sonication time and temperature had no significant effect on degradation of malathion (p > 0.05) | ||||||||
| • Radial attack at bubble–vapor interphase is the dominant pathway decomposition of malathion insecticide by ultrasound | ||||||||
| 11 | Organochlorine pesticides (OCPs) | 20 and 40 μg L−1 | A probe system: 20 kHz and 200 W | Temperature 20 °C; pH 3, 7 and 11; treatment time 5–60 min | Not reported | • 2.3% degradation was occurred by ultrasound under follows condition: 40 μg L−1 concentration for each pesticide, pH 7 and sonication time 60 min | 47 | |
| • Combined ultrasound/H2O2 process was less effective than using ultrasonic waves alone which reflected inappropriacy of the H2O2 dose, pH or time | ||||||||
| • The decomposition mechanism of pesticide under ultrasound can be described by OH• radical reaction with pesticide by double-bond addition or hydrogen abstraction | ||||||||
| 12 | Parathion, M (g mol−1) = 291.3, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.83 P: 3.83 |
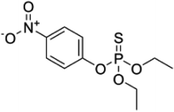 |
0.8, 2.9 and 5.2 μM | 200, 400, 600 and 800 kHz; 17.4, 37.8 and 55.2 W | Temperature 25 °C; pH 7.0; sonication time 30 min | 4-Nitrophenol, 2,4-dinitrophenol and paraoxon | • Pseudo-first-order kinetics | 48 |
| • The optimal frequency for parathion degradation was 600 kHz | ||||||||
| • The extent of parathion degradation rate of parathion decreased with increasing initial concentration and decreasing ultrasonic power. The N2 in air takes part in the parathion degradation through the formation of ˙NO2 during sonication | ||||||||
| • The gas–solution interfacial regions are predominately the reaction zones for sonochemical degradation of parathion. The gas/liquid heterogeneous reaction obeys pseudo-first-order-kinetic model based on Langmuir–Hinshelwood model | ||||||||
| 13 | Pentachlorophenol, M (g mol−1) = 266.3, pKa = 4.73, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.32 P: 3.32 |
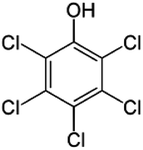 |
20 and 60 μM | A probe system: 20 kHz and 66.54 W; a tube resonator: 20 kHz and 466 W; an orthoreactor: 500 kHz and 48.3 W | Temperature 30 °C; pH 7.3; sonication time 5–150 min | Tetrachloro-o-benzoquinone (o-chloranil tetrachlorocatechol (TCC), oxalate, and chloride | • Pseudo-first-order kinetics | 30 |
| • Lower frequency and PCP concentration resulted in more rapid rates | ||||||||
| 14 | Pentachlorophenol | 0.1 mM | Dual-frequency (20 kHz/40 kHz, 22.73 W; 20 kHz/530 kHz, 22.97 W; 20 kHz/800 kHz, 20.39 W; 20 kHz/1040 kHz, 18.32 W) | Temperature 30 °C; natural pH; sonication time 5–120 min | Not reported | • Pseudo-first-order kinetics | 18 | |
| • Rate of pentachlorophenol degradation at dual-frequency irradiation is the highest compared to single low frequency | ||||||||
| • Order of dual-frequency systems for PCP degradation at 20 kHz is as follows: 530 kHz > 800 kHz > 40 kHz > 1040 kHz |
As shown in Table 2, Bagal and Gogate8 studied sonochemical degradation of alachlor using ultrasonic horn (20 kHz, 100 W) and ultrasonic bath (20 kHz, 80 W) reactors. They observed a decrease in the extent of degradation in the case of bath reactor as compared to horn reactor which could be explained by the lower operating power density in the case of the bath as compared to horn.
| No. | Pesticides | Chemical structure | Initial conc. | Sonochemical conditions | Other technologies | Other experimental cond. | Degradation intermediates/products | Results | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Acephate, M (g Mol) = 183.2, pKa = 8.35, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: −0.85 P: −0.85 |
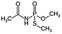 |
100 ppm | 160 kHz and 0–50 W | Ozone treatment, (O3 flow rate 2 L min−1) | Temperature 25 °C; pH 7; sonication time 60 min | Primary products (CH3O(CH3S)P(O)NH2 and CH3COOH), intermediate products (CH3O(CH3S)P(O)OH, CH3O(HO)P(O)OH, and CH3S(O)2SCH3), and final products (NH4+, NO3− QUOTE , SO42−, CO2, H2O, and H3PO4) | • 22.9% and 60.6% of the acephate was removed by ultrasonic irradiation and ozonation, respectively | 72 |
| • The degradation efficiency of acephate enhances to 87.6% by combined ultrasonic/ozonation process | |||||||||
| • The combined method led to thoroughly acephate degradation and most final products were innocuous to the environment | |||||||||
| 2 | Alachlor, M (g mol−1) = 269.8, pKa = 0.62, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.09 P: 3.09 |
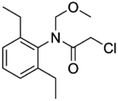 |
20 ppm | An ultrasonic horn: 20 kHz and 0–100 W | Fenton process, additives (hydrogen peroxide and carbon tetrachloride) | Temperature 28 °C; pH 2–11; sonication time 120 min | Major byproducts: 2 hydroxy-2′,6′-diethyl-N-methyl acetanilide and 2-chloro-2′,6′-diethyl-N-methyl acetanilide | • Only 3.5% degradation was obtained using ultrasonic bath after 60 min | 8 |
| An ultrasonic bath: 400 kHz and 80 W | • 86.4% degradation of alachlor was achieved by ultrasonic horn at initial pH 3 after 120 min | ||||||||
| • Nearly 98% degradation was obtained using US/H2O2 (0.07 g L−1) after 120 min | |||||||||
| • 98% degradation was obtained using US/CCl4 (1 g L−1) after 90 min | |||||||||
• Complete degradation was obtained after 50 min US/FeSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 (0.035 g L−1 H2O2 (0.035 g L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07 g L−1) 0.07 g L−1) |
|||||||||
| • At the 1 g L−1 loading of CCl4, 98% degradation was achieved after 90 min sonication | |||||||||
| 3 | Atrazine, M (g mol−1) = 215.7, pKa = 4.14, 10.7, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: −0.97 P: −0.97 |
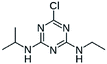 |
5 ppm | An ultrasonic generator: 20 kHz, 0–2000 W | • Ultraviolet irradiation (UV lamp 254 nm) | Temperature 20 °C; pH 12; treatment time 4 h | Not reported | • 97.68% degradation was achieved under the conditions of 142.5 W ultrasound power, 75 W UV power and 10.75 g h−1 O3 flow rate | 73 |
| • Ozonation (two 300 W O3 generators) | • The degradation of Atrazine followed the second-order polynomial model | ||||||||
| • The presence of other organic compounds in the matrix approximately avoided the degradation of atrazine by consuming radicals | |||||||||
| 4 | Carbofuran, M (g mol−1) = 221.2, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 1.8 P: 1.8 |
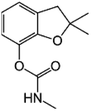 |
10–200 ppm | A probe system: 20 kHz and 300 W | • Fenton process (H2O2: 0–500 mg L−1, Fe2+: 0–0.306 mM) | Temperature 25 °C; pH 7.3; treatment time 60 min | Not reported | • Carbofuran degradation the enhanced from 22% to 44% with increasing H2O2 dosages of 0–200 mg L−1 within 120 min | 74 |
| • Almost 99% of the carbofuran was degraded by combined ultrasound/Fenton process after 30 min for the initial carbofuran concentration of 20 mg L−1 and Fe2+ and H2O2 dosages of 20 mg L−1 and 100 mg L−1, respectively, all at pH 3 | |||||||||
| • The degradation of carbofuran followed the first-order kinetics model | |||||||||
| 5 | Chlorpyrifos, M (g mol−1) = 350.57, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 4.96 P: 4.96 |
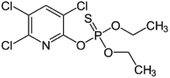 |
900 ppm | An ultrasonic generator: 40 kHz, 40–320 W | Electrooxidation process (voltage 5–30 V, Na2SO4 concentration 0.5–3 g L−1) | Temperature 15–35 °C; treatment time 60 min | Not reported | • The optimum conditions for degradation were: electrolyte concentration of 2 mg L−1, voltage of 20 V, ultrasonic power of 200 W and temperature 20 °C, which led to 93.3% and 72.8% of degradation in US-EC system and EC system | 71 |
| • The chlorpyrifos degradation followed pseudo-first-order kinetics | |||||||||
| • Ultrasound in the US-EC system gradually improve the amount of ˙OH production compared with the EC system | |||||||||
| 6 | Dichlorvos, M (g mol−1) = 221, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 1.43 P: 1.43 |
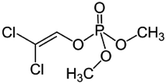 |
20 ppm | An ultrasonic horn: 36 kHz, 0–150 W | Photocatalysis, ozone and Fenton process | Temperature 20 °C; pH 3; sonication time 120 min | Not reported | • Only 6.4% and 20% degradation of dichlorvos was achieved after 120 min of US and US/H2O2 (0.07 g L−1) | 75 |
| • 3% and 78.4% degradation was obtained by US/TiO2 (0.1 g L−1) and US/TiO2/solar (0.1 g L−1) in 2 h treatment | |||||||||
• Combination of US/Fenton’s reagent (80 mg L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 mg L−1) increased the extent of degradation 81.2% 80 mg L−1) increased the extent of degradation 81.2% |
|||||||||
| • Complete degradation was obtained in 30 min of reaction time by using combination of ozone (1.95 g h−1) and ultrasound | |||||||||
| 7 | Diazinon, M (g mol−1) = 304.4, pKa = 2.6, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.69 P: 3.69 |
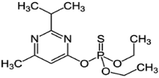 |
50 ppm | A probe system: 20 kHz and 100 W | Fenton process (H2O2: 0–4.41 mM, Fe2+: 0–0.306 mM) | Temperature 15–55 °C; pH 7.3; treatment time 60 min | Diethyl phosphonate, 2-isopropyl-6-methyl-4-pyrimidinol, diazoxon and hydroxydiazin | • The degradation efficiencies subjected to US, US/Fe2+ and US/H2O2 were 22, 25 and 26%, respectively | 76 |
| • 98% degradation occurred by sono-Fenton under optimal condition: 20 ppm Fe2+, 150 ppm, H2O2, 25 °C and pH 3 | |||||||||
| • OH˙ attack, hydroxylation and hydrolysis were the major degradation pathway of diazinon | |||||||||
| 8 | Diazinon | 20–80 ppm | An ultrasonic bath: 200–400 W L−1 | Catalyst (catalyzed persulfate (5–10 mmol L−1) with Fe3O4@MOF-2 nanocomposite: Fe3O4@MOF-2 (0.4–1 g L−1) | Temperature 20 °C; pH 3–12; treatment time 120 min | 2-Isopropyl-6-methylpyrimidin-4-ol, 2-(thiophos-phonooxy)acrylic acid, (1E,2E)-3-((dimethoxyphosphoryl)oxy)-N-methylbut-2-enimidic acid, 3,7-dimethyloct-6-enal, (methylamino)methyl dihydrogen phosphite, (E)-2,6-dimethylocta-2,6-diene and propionic acid | • Degradation of diazinon enhanced by increasing the Fe3O4@MOF-2 dosage and the US bath power, along with reducing the diazinon concentration | 15 | |
| • 100% degradation was achieved by Fe3O4@MOF-2/US/PS under follows condition: [diazinon]0 = 30 mg L−1, [PS] = 10 mmol L−1, Fe3O4@MOF-2 = 0.7 g L−1, pH = 3 | |||||||||
| 9 | Dimethoate, M (g mol−1) = 229.3, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 0.704 P: 0.704 |
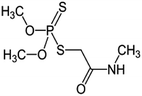 |
20 ppm | A probe system: 40 kHz and 0–250 W | Ozonation (0.1–0.55 0.41 m3 h−1) | Temperature 25 °C; pH 7.0; sonication time 5–30 min | 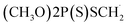 |
• Pseudo-first-order kinetics | 77 |
| • US, O3 and combined US/O3 process resulted in 14.5%, 20.1% and 90.8% dimethoate degradation, respectively, under the optimal conditions: treatment time 4 h, O3 flow rate of 0.41 m3h−1, ultrasonic power of 4.64 W cm−2, pH of 10.0, temperature of 25 °C, and initial dimethoate concentration of 20 mg L−1 | |||||||||
| 10 | Fenitrothion, M (g mol−1) = 277.2, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.32 P: 3.32 |
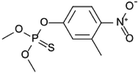 |
10 ppm | An ultrasonic generator: 20 kHz, 150 W | Photo-Fenton process: (light intensity: 1.0 mW cm−2 Fe3+: 0–1 × 10−3 oxalate: 0–5 × 10−3 M) | Temperature 25 °C; pH 6; treatment time 30 min | Nitrite and sulfate ions | • Almost 100% degradation was obtained by US/ferrioxalate/UV system under optimum conditions: pH 6, 5 × 10−4 M Fe(III), 5 × 10−3 M oxalate | 78 |
| 11 | Linuron, M (g mol−1) = 249.1, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3 P: 3 |
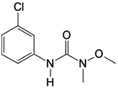 |
10 ppm | An ultrasonic generator: 200 kHz, 100 W | Photo-Fenton process (Fe(II): 0–5 × 10−4 mol L−1) | Temperature 25 °C; treatment time 60 min; pH 2.5–5.5 | Chloride, nitrite and nitrate ions | • Complete decomposition of linuron was achieved by US/Fe (II)/UV system after 20 min under follows conditions: Fe(II) concentration of 1.2 ×10−4 mol L−1 and pH 3.0; while only 79.3% decomposition of linuron was obtained by sonolysis | 79 |
| • First-order constant for degradation of linuron by ultrasound//Fe (II)/UV process (0.17 min−1) was about 2 times greater than that in individual ultrasonic process (0.08 min−1) | |||||||||
| 12 | Metazachlor, M (g mol−1) = 277.75, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.49 P: 2.49 |
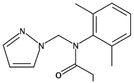 |
An ultrasonic generator: 20 kHz, 100 W | • Oxygen (oxygen flow rate of 2.0 L min−1) | Temperature 22 °C; treatment time 120 min; pH 3 | 2-Chloro-N-(2-methylphenyl)-N-[(1H-pyrazol-1-yl)methyl]acetamide; 2-chloro-N-[2-(hydroxymethyl)phenyl]-N-[(1H-pyrazol-1-yl)methyl]acetamide; 2-chloro-N-(2-formyl-6-methylphenyl)-N-methylacetamide; 2-chloro-N-(2,6-dimethylphenyl)acetamide; 2-chloro-N-(2-formyl-6-methylphenyl)acetamide; N-(2-chloroacetyl)-N-(2-methylphenyl)formamide | • The metazachlor decomposition by sonolysis fitted in pseudo-first order kinetics | 80 | |
| • Fenton-like oxidation process (initial concentration of ferric oxyhydroxide of 50 mg L−1) | • First-order constant for degradation of metazachlor enhanced from 1.11 × 10−2 min−1 for conventional sonolysis to 1.79 × 10−2 and 2.88 × 10−2 min−1 for O2-saturated and Fe2O3-added solutions, respectively | ||||||||
| • Almost 97% degradation was achieved by sonolysis in the presence of ferric oxyhydroxide | |||||||||
| 13 | Methomyl, M (g mol−1) = 162.2, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 1.24 P: 1.24 |
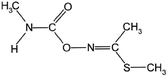 |
25 ppm | An ultrasonic generator: 20 kHz, 500 W | • H2O2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1 10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 1 20, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 1 30, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 1 40 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) 50) |
Temperature 28 °C; pH 2.5–7.5; treatment time 60 min | Not reported | • 28.57% degradation was achieved by sonolysis after 72 min at the optimal pH of 2.5 and power density of 0.155 W mL−1 | 59 |
• Fenton (Fe2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 1 H2O2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1 50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 1 40 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) 30) |
• Combination of ultrasound with H2O2, Fenton and photo-Fenton process led to complete degradation of methomyl after 27 min, 18 min and 9 min, respectively | ||||||||
| • Photo-Fenton process (two UV lamps of 8 W) | |||||||||
| 14 | Methyl parathion, M (g mol−1) = 263.2 | 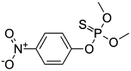 |
20 ppm | A probe system: 20 kHz, 0–270 W A ultrasonic bath | Additives (TiO2, CCl4 and H2O2); Fenton process | Temperature 30 °C; pH 2.5–9.3; sonication time 60 min | Not reported | • 10.2% degradation occurred by ultrasonic horn under acidic conditions at pH 2.5 | 52 |
| • Presence of solid particles TiO2, CCl4 and H2O2 during sonication led to a considerable increase in the extent of parathion degradation | |||||||||
• The extent of degradation in the presence of Fenton chemistry in cavitation condition was 96% for 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of FeSO4 1 ratio of FeSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 H2O2 |
|||||||||
| 15 | Monocrotophos, M (g mol−1) = 223.2, pKa = n.a, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: −0.2 P: −0.2 |
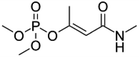 |
0.01–0.12 mM | An ultrasonic generator: 213 kHz, 16–55 m W mL−1 | Photocatalysis and sonophotocatalysis (1 g L−1 TiO2) | Temperature 25 °C; treatment time 60 min; pH 2.7 | Dimethyl phosphate, dimethylphosphonate, 3-hydroxy-2-buteneamide and N-methyl-3-oxobutanamide | • Sonodegradation followed first order dependence with respect to MCP while TiO2 photocatalytic degradation showed a zero order dependence | 81 |
| • The presence of TiO2 during the sonolysis inhibited the degradation of monocrotophos due to the interference of phosphate ions formed as an intermediate | |||||||||
| • About 15 fold enhancement was found for degradation rate in the presence of Fe3+ during photolysis | |||||||||
| 16 | Simazine, M (g mol−1) = 201.7, pKa = 1.62, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 2.3 P: 2.3 |
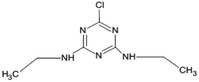 |
5 ppm | An ultrasonic generator: 42 kHz | Photocatalysis | pH 6; treatment time 7 h | 6-Chloro-N,N′-diethyl-1,3,5-triazine-2,4-diamine; 1,1′-[(6-chloro-1,3,5-triazine-2,4-diyl)diimino]diethanol; 4,6-bis(ethylamino)-1,3,5-triazin-2-ol; 6-chloro-1,3,5-triazine-2,4-diamine; N-(4-amino-6-hydroxy-1,3,5-triazin-2-yl)acetamide; 4,6-diamino-1,3,5-triazin-2-ol; 1,3,5-triazine-2,4,6-triol | • The first order kinetics was observed for the degradation of Simazine | 82 |
| • The extent of the TOC removal by sonolysis, sonocatalytic, photocatalytic and sonophotocatalytic were 11%, 31%, 26% and 43%, respectively | |||||||||
| 17 | Triazophos, M (g mol−1) = 313.31, pKa = −0.15, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: 3.55 P: 3.55 |
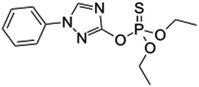 |
20 ppm | An ultrasonic generator: 40 kHz, 0–1500 W | H2O2 (triazophos: H2O2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1 1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); ozone (100–400 mg h−1) and Fenton's reagent (triazophos 5); ozone (100–400 mg h−1) and Fenton's reagent (triazophos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeSO4 FeSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 1 H2O2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1 1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Temperature 37 °C; treatment time 90 min; pH 2.3–7.3; flow rate 480 mL min | 3Z-Undecene-5,7,10-triynoic acid; 1-(2-hydroxyethyl)-2-hydroxymethyl-5-nitroimidazole; acetylisoniazid; zolpidem metabolite I; dihydrodeoxystreptomycin; swietenine; crocetin | • Combination of ultrasound with H2O2, ozone and Fenton's reagent result in 48.6%, 54.6% and 92.2% triazophos degradation, respectively | 61 |
• The best reaction parameters were: ultrasonic power 203.6 W, flow rate 480 mL min−1, pH of 3.2, ratio of triazophos to H2O2, ozone flow rate 400 mg h−1 and triazophos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeSO4 FeSO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2 1 H2O2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|||||||||
| • The triazophos degradation using ultrasound followed a first order reaction kinetics |
In another study, Shriwas and Gogate52 investigated degradation of methyl Parathion using ultrasonic horn and ultrasonic bath reactors with operating frequency of 20 kHz and maximum power rating of 270 W and 230 W, respectively. They reported that extents of degradation for only sonication were about 8% and 10.2% obtained on ultrasonic horn and ultrasonic bath, respectively, which can be attributed to lower operating power density in the case of the ultrasonic bath (72 W L−1 on the basis of rated power input) as compared to ultrasonic horn (2700 W L−1 on the basis of rated power input). In addition, they revealed that though the power dissipation levels are considerably higher in the case of horn reactor, similar level of enhancement in the extent of degradation was not observed. They explained their observations by the more uniform distribution of cavitational activity in the bath reactor owing to broader area of transducers as compared to horn reactor.
Most of the studies describing the degradation of pesticides used ultrasonic probe system due to its high intensity and optimum performance at different amplitudes. It should be noted here that though the treatment studies have been with horn or bath system, the scale up prospects of these ultrasonic reactors are quite poor. Indeed, in spite of extensive studies on laboratory scale and immense application potential for degradation of pesticides, not many researches are available in the open literature related to pesticide degradation on an industrial scale. The main drawback of horn-type ultrasonic system is high-energy consumption and the limited cavitation zone around the transducer, which is less efficient for the treatment of large volumes of liquid.49 Changing the configuration or geometry of the sonochemical reactor is one of the efficient ways for decreasing the energy consumption. It is recommended to ensure power dissipation over broader area using multiple transducers to get higher intensities of cavitation. Multiple transducer irradiations (with or without multiple frequencies) also lead to remarkably higher cavitational activity as compared to single transducer operation and thus these novel configurations of ultrasonic equipment show good prospects for scale up.
Agarwal et al.16 studied the effect of ultrasound frequency (35 and 130 kHz) on sonolysis of chlorpyrifos in aqueous solutions and they observed that pesticide removal increases with increasing of the ultrasound frequency.
Yao et al.48 also investigated the degradation of parathion in aqueous solutions at different frequencies (200, 400, 600, and 800 kHz). The results showed that parathion degradation reached a maximum at 600 kHz. They explained that the optimal frequency for degradation parathion, as a nonvolatile and hydrophobic compound, is determined by both the optimal hydroxyl radicals yield and the efficient mass transfer of the molecule from the liquid phase to the interfacial region.
Numerous studies have evaluated the effect of acoustic power on the extent of pesticide degradations. Schramm and Hua45 investigated the effect of acoustic power (86, 124 and 161 W) on the degradation of dichlorvos and also reported that increasing total acoustic power input from 86 to 161 W resulted in a change in the rate constant from 0.018 ± 0.001 min−1 to 0.037 ± 0.002 min−1. They also attributed these results to the increasing the number of collapsing bubbles and concentration of free-radicals into the bubble–bulk interface region and aqueous solution with increasing acoustic power. The similar results were reported by Agarwal et al.,16 Zhang et al.,41 Golash and Gogate,44 Schramm and Hua,45 Shayeghi et al.46 and Yao et al.48
Debabrata and Sivakumar43 investigated the degradation of dicofol in aqueous media under sonolysis process. They observed that the degradation increases with a rise in acoustic power up to 375 W, beyond which a reduction in the degradation rate. These authors explained their results by an excessive heat production during sonication which led to a less violent bubble collapse.
The initial pH of the solution controls the rate of formation of hydroxyl radicals during sonochemical degradation and hence affects the final extents of degradation. Acidic conditions inside the cavitation reactors lead the higher rate of formation as well as accumulation of hydroxyl radicals due to hampering of the recombination reaction to form H2O2. In other words, in higher pH solutions, a higher number of hydroxyl radicals recombine to form H2O2 that leads to decrease in the quantum of the hydroxyl radicals available for the desired degradation reaction.8,9 Furthermore, a high pH value may create more free radical scavengers and results in the diminish in the concentration of HO˙.44,59 Thus, higher reactivity of hydroxyl radicals in the acidic medium than that at neutral and basic pH enhances degradation kinetics under the ultrasonic irradiation.
Sonochemical degradation kinetics at different pH is dependent on the state of the pollutant molecule, i.e., whether the pollutant is present as ionic species or as a molecule. Hence, the physicochemical property, pKa value of ionizable organic pollutants plays a major role in determining the effect of pH on the rate of degradation. When the pH value is lower than pKa value, the molecular form of the pollutant is dominate and hence can accumulate at the bubble interface (gas–liquid film region) and more subjected to the highly reactive hydroxyl radicals.9,60,61 Also, a fraction of this molecular form may even vaporizes into the cavitation bubbles (gaseous) for volatile compounds. Thus, when pH is less than the pKa the overall decomposition of pollutants at is considered to take place in both the gaseous and interfacial film regions by pyrolysis and free radical attack. At lower pH, the electrostatic attractive force between the charged of bubble–water interface and oppositely charged hydrophobic compounds resulted in faster degradation kinetics under acidic conditions.62–64 If the pH is higher than the pKa value, the amount of ionic form predominates, which cannot vaporize into the cavitation bubbles. This ionic species is restricted only into the interfacial film region and react with the OH radicals.37,60 Therefore, solution pH must be kept lower than the pKa for higher degradation of pesticides during sonication. For pollutants without ionizable groups little degradation variance was observed in tested pH range.63
Zhang et al.40 monitored degradation of chlorpyrifos at different pH values and reported that the extent of chlorpyrifos degradation increased from 41% to 55% with an increase in its initial pH from 5 to 7; however, the degradation percentages declined, as the pH value increased from 7 to 8. They attributed the highest degradation efficiency at pH 7 to the occurrence of complex degradation pathway during sonolysis.
Similar findings of an enhancement in the extent of degradation of pesticides under acidic conditions during sonolysis are also reported in the literature. Golash and Gogate44 investigated the effect of initial pH on sonochemical degradation of dichlorvos and reported that maximum extent of degradation was obtained at operating pH of 2. They attributed this to the improved formation of free radicals under acidic conditions and also higher oxidation potential of hydroxyl radical under acidic conditions. Debabrata and Sivakumar43 have also reported that the maximum extent of degradation of dicofol is obtained at operating pH of 3. Similarly, Kida et al.47 have also reported that the best efficiency of degradation of pesticides was obtained at operating pH of 3.
Many studies have investigated the influences of temperature on sonochemical degradation of pesticides. For instance,43 studied the effect of solution temperature on the sonochemical degradation of dicofol. They found that temperature of 20 °C as an optimum temperature for the highest rate of dicofol degradation and the degradation rate constant declined to 0.009 min−1 and 0.008 min−1 with a change in the temperature to 10 °C and 30 °C, respectively. Golash et al.44 also reported that maximum extent of dichlorvos degradation using sonochemical reactors was obtained at an optimum temperature of 25 °C. They explained that higher operating temperatures resulting in a net diminish in the energy being released at the implosion and subsequently decreasing the extent of free radicals in the system. Zhang et al.40 observed similar results in their survey.
In literature there are many studies analyzing the influence of initial concentration of pesticide on the efficiency of sonochemical degradation. For instance, Zhang et al.41 observed a decrease in the degradation percentage from 51.3% to 10.8% when the initial concentration of diazinon was increased from 7.82 μM to 65.19 μM. Furthermore, similar results of a decrease in the degradation rate of pesticides with an increase in initial concentration during sonolysis have also been reported by Shayeghi et al.,46 Kida et al.47 and Yao et al.48
In another study, Debabrata and Sivakumar43 investigated sonochemical degradation of dicofol with initial concentrations of 5.4 to 54 μM. They reported 27 μM, as the optimum initial concentration for the highest degradation rate. Also, they attributed the higher degradation rates of dicofol at concentration of 27 μM to higher pyrolysis of dicofol along with radical attack at interfacial region. Matouq et al.42 observed an optimum initial concentration of 3.9 mM for diazinon degradation by high frequency of ultrasound.
Agarwal et al.16 studied the sonolysis of azinphos-methyl for 20, 40 and 60 min treatment time. They observed that the removal efficiency of azinphos-methyl enhanced very quickly during the first 20 min and then it proceeded more slowly until 60 min. At the end of sonolysis, the yield of pesticide removal remained constant.
4.2. Synergetic effect of ultrasound and other degradation technologies
Although sonochemical reactions were quite efficient for degradation of pesticides, complete degradation was not achieved in most of the cases. This might be due to higher polarity of the organic compound, low availability of hydroxyl radical or lack of dissipated power.71 Hydrophilic molecules with low vapor pressures tend to remain in the bulk phase; however, the extent of hydroxyl radicals is lesser than the interfacial region liquid during sonication.62 To overcome these limitations, the sonochemical process can be combined with advanced oxidation processes or other technologies. Some of the synergetic effects of ultrasound and other degradation processes are presented in Table 2.| H2O2 + Fe2+ → Fe3+ + OH− + HO˙ | (8) |
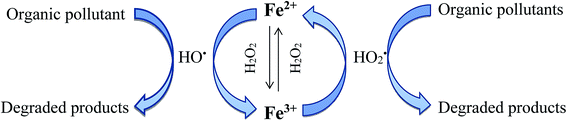
The generated ferric iron reacts again with excess hydrogen peroxide to form more radicals (reaction (9)).83 This reaction which is called Fenton-like reaction and slower about 6000 times than Fenton reaction, leads Fe2+ regeneration in an efficient cyclic mechanism.84 In Fenton like reaction, besides ferrous ion regeneration, hydroperoxyl radicals 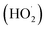 are produced which can also attack pesticides, but they are less sensitive than hydroxyl radicals. Reactions (9)–(12) represent the rate limiting steps in the Fenton chemistry since H2O2 is consumed and Fe2+ are regenerated from Fe3+ through these reactions. Formation of hydroxyl radicals includes a complex sequence of radical–radical reactions or hydrogen peroxide–radical reaction (reactions (13)–(16)).83 Highly reactive hydroxyl radicals can degrade the pesticides, oxidizing and transforming them into by-products; they can react with other radicals; or with other ions/compounds in water (inefficient equations).83,84,86 The oxidation mechanism for the Fenton process is shown in Fig. 4.
are produced which can also attack pesticides, but they are less sensitive than hydroxyl radicals. Reactions (9)–(12) represent the rate limiting steps in the Fenton chemistry since H2O2 is consumed and Fe2+ are regenerated from Fe3+ through these reactions. Formation of hydroxyl radicals includes a complex sequence of radical–radical reactions or hydrogen peroxide–radical reaction (reactions (13)–(16)).83 Highly reactive hydroxyl radicals can degrade the pesticides, oxidizing and transforming them into by-products; they can react with other radicals; or with other ions/compounds in water (inefficient equations).83,84,86 The oxidation mechanism for the Fenton process is shown in Fig. 4.
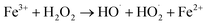 | (9) |
| Fe2+ + HO˙ → Fe3+ + OH− | (10) |
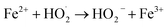 | (11) |
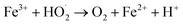 | (12) |
| HO˙ + HO˙ → H2O2 | (13) |
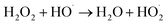 | (14) |
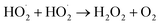 | (15) |
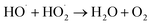 | (16) |
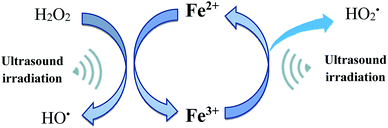
To enhance the hydroxyl radical concentration in the bulk phase, Fenton and ultrasonic cavitation can be combined together. These methods utilize the advantages of sonolysis and Fenton's reagent, allowing increased degradation of pollutants. The studies reported in the literature related to ultrasound/Fenton process on pesticides degradation are summarized in Table 2. The synergistic effect of ultrasonic cavitation and Fenton process leads to a higher formation of hydroxyl radicals. Ferric ions will react with hydrogen peroxide and produce a complex intermediate Fe–(HO2)2+ (reaction (17)). Although Fe–(HO2)2+ can be decomposed to Fe2+ and 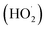 spontaneously, the decomposition rate is much smaller. However, combined with the ultrasound, the decomposition rate of Fe–(HO2)2+ can be greatly increased (reaction (18)).38 Once ferrous iron had formed, it reacted with hydrogen peroxide and produced hydroxyl radical again, and then a cycle mechanism was established.8,75 In other words, coupling US irradiation and Fenton oxidation (in the so-called sono-Fenton or US/Fenton process) can promote faster pesticide degradation due to (i) higher formation of HO˙, (ii) improved mixing and contact between HO˙ and pesticide, and (iii) enhanced regeneration of ferrous ions.87 Thus, using a combination of ultrasound and Fenton process leads to higher oxidation potential, improving degradation and mineralization rate of the pollutant. In addition, intense turbulence created by cavitation phenomena also results in promoting mass transfer rate and also enhancing utilization of hydroxyl radicals. Furthermore, in combined ultrasound/Fenton system, some part of hydrogen peroxide directly dissociates in the presence of ultrasonic cavitation generating additional hydroxyl radicals.31 The reaction mechanism for the sono-Fenton process is shown in Fig. 5.
spontaneously, the decomposition rate is much smaller. However, combined with the ultrasound, the decomposition rate of Fe–(HO2)2+ can be greatly increased (reaction (18)).38 Once ferrous iron had formed, it reacted with hydrogen peroxide and produced hydroxyl radical again, and then a cycle mechanism was established.8,75 In other words, coupling US irradiation and Fenton oxidation (in the so-called sono-Fenton or US/Fenton process) can promote faster pesticide degradation due to (i) higher formation of HO˙, (ii) improved mixing and contact between HO˙ and pesticide, and (iii) enhanced regeneration of ferrous ions.87 Thus, using a combination of ultrasound and Fenton process leads to higher oxidation potential, improving degradation and mineralization rate of the pollutant. In addition, intense turbulence created by cavitation phenomena also results in promoting mass transfer rate and also enhancing utilization of hydroxyl radicals. Furthermore, in combined ultrasound/Fenton system, some part of hydrogen peroxide directly dissociates in the presence of ultrasonic cavitation generating additional hydroxyl radicals.31 The reaction mechanism for the sono-Fenton process is shown in Fig. 5.
| Fe3+ + H2O2 → Fe–(HO2)2+ + H+ | (17) |
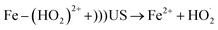 | (18) |
As shown in Table 2, many studies have investigated the degradation of pesticides using a combination of ultrasound and Fenton process. For example, Ma et al.88 investigated the degradation of carbofuran by a combined ultrasound/Fenton process. They observed that more than 99% of the carbofuran was degraded by the combination of ultrasonic irradiation and Fenton process within short reaction time periods. They reported an increase of 66% in the extent of carbofuran degradation when the Fenton process was combined with ultrasound.
Also, Wang et al.76 studied the degradation of diazinon using ultrasonic irradiation facilitated by Fenton process. They reported that 98% degradation of diazinon was achieves by sono-Fenton process which increased by 61% compared with Fenton process.
| H2O2 + hv → 2HO˙ | (19) |
| Fe(OH)2+ + hv → Fe2+ + HO˙ | (20) |
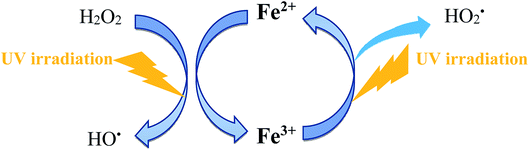
Additionally, the ferrous ions regenerated reacts with hydrogen peroxide. As a result, almost all hydrogen peroxide formed by sonication was consumed. Therefore, ultrasound irradiation combined with photo-Fenton reaction would exhibit significant enhancement in the degradation of pesticides. The sonication combined with photo-Fenton process has several unique advantages, including simple handling, rapid degradation, and wide applicable pH range. The additional advantage of using sono-photo-Fenton process would be that SPF process declines the extent of ferrous ions present in the treated water, and this is crucial in an industrial point of view.89
Katsumata et al.78 investigated the application of US/ferrioxalate/UV process for the degradation of fenitrothion. The initial pH was maintained at 6. After 30 min, almost complete degradation of fenitrothion was observed with sono-photo-Fenton process whereas 87% and 40% degradation was observed with ferrioxalate/UV and ultrasonic, respectively.
In another study,79 they conducted an investigation on degradation of linuron by using ultrasound/Fe(II)/UV process. The effect of Fe(II) concentration and initial pH, on the degradation of linuron was investigated. The optimum initial concentration of Fe(II) and pH were found to be 1.2 × 10−4 mol L−1 and 3, respectively. The results showed that linuron was completely degraded after 20 min using ultrasound/UV/Fe(II) process.
| H2O2 + )))US → 2HO˙ | (21) |
Thus, using a combination of ultrasound and hydrogen peroxide causing higher degradation as compared to both the treatment schemes operated individually, due to the enhancement in the quantum of free radicals generated by the decomposition of hydrogen peroxide. Loadings of hydrogen peroxide decides the rate of dissociation of hydrogen peroxide and hence the rate of generation of the enhanced hydroxyl radicals and also on the reactivity of the generated free radicals especially hydroxyl radicals with the pollutant. The use of hydrogen peroxide in conjunction with sonication is only beneficial to the point where optimum loading is achieved. Above the optimum loadings, additional hydrogen peroxide acts as the scavenger of hydroxyl radicals and hence it results in a marginal enhance in the extent of degradation (reaction (22)).64
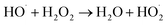 | (22) |
Thus, there exists an optimum loading of hydrogen peroxide where the extent of decomposition of hydrogen peroxide into hydroxyl radicals is appreciable and scavenging action of residual hydrogen peroxide is not dominating. The optimum concentration of hydrogen peroxide depends on the type of compound, reactor geometry, the operating conditions such as ultrasonic intensity.
Several studies have evaluated the effect of addition of hydrogen peroxide on sonochemical degradation of pesticides. For instance, Bagal et al.8 studied the degradation of alachlor (initial concentration of 20 mg L−1) sonochemical reactor irradiated with 20 kHz ultrasound at the power dissipation of 100 W and reported that the optimum concentration of hydrogen peroxide is 0.07 g L−1 giving a ratio of oxidant to pollutant as 3.5. Beyond this loading, a further increase in the hydrogen peroxide loading to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio resulted in a marginal increase in the extent of degradation.
1 ratio resulted in a marginal increase in the extent of degradation.
Raut-Jadhav et al.59 studied the degradation of methomyl by using the ultrasound cavitation in combination with H2O2. They found that complete degradation of methomyl was obtained after 27 min.
In other experiments, Shriwas et al.52 reported an increase in the extent of degradation of methyl Parathion with an addition of the hydrogen peroxide till an optimum loading of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of hydrogen peroxide to methyl Parathion. They found also that the extent of degradation decreases at addition ratio of 20
1 ratio of hydrogen peroxide to methyl Parathion. They found also that the extent of degradation decreases at addition ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
| CCl4 + )))US → ˙CCl3 + ˙Cl | (23) |
| CCl4 + )))US → :CCl2 + Cl2 | (24) |
| ˙CCl3 + )))US → :CCl2 + ˙Cl | (25) |
| ˙CCl3 + ˙CCl3 → CCl4 + :CCl2 | (26) |
| ˙CCl3 + ˙CCl3 → C2Cl6 | (27) |
| :CCl2 + :CCl2 → C2Cl4 | (28) |
| ˙Cl + ˙Cl → Cl2 | (29) |
| Cl2 + H2O → HClO + HCl | (30) |
Along with hydroxyl radicals attack due to the sonolysis of water vapor in the cavitation bubble, the additional oxidizing species attack the organic compound present in the bulk phase or at the gas–liquid interface. Thus, this combined attack in the presence of CCl4 remarkably enhances the rate of pollutant degradation. It is also important to note that adding CCl4 can affect final toxicity levels of the system, if the additive is not utilized completely during the treatment scheme. Furthermore, the excessive amount of CCl4 beyond an optimal value in the reaction system leads to formation of vaporous cavitation bubbles due to highly volatile nature of the additive and this reduces the net release of energy during the bubble implosion leading to decreased generation of oxidants. Hence, in order to prevent residual amount of CCl4, minimize the toxicity and achieve maximum intensification of degradation, selecting an optimum loading is a critical design consideration. The optimum loading of CCl4 is strongly dependent on the type of the pollutant.44
Bagal et al.8 have studied the ultrasonic degradation of alachlor in presence of CCl4 (0.1 to 3 g L−1). They found that the extent of degradation increased with an increase in the CCl4 loading till an optimum loading of 1 g L−1 beyond which marginal increase in the extent of degradation was observed. Similar trends have been observed in the literature for sonochemical degradation of methyl Parathion in the presence of CCl4.52
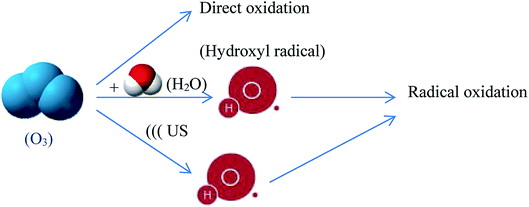
Ultrasound/ozone combination can be more effective and beneficial than US or O3 alone treatment for the degradation of pesticides. The use of ultrasound in combination with ozone can allow the decline of both ozone consumption and ultrasonic energy, with the consequent decline of operating costs.51 In a collapsing cavitation bubble, the pyrolytic decomposition of ozone and subsequent hydroxyl radical formation occurs as follows:
| O3 + )))US → O2 + O(3P) | (31) |
| O(3P) + H2O → 2HO˙ | (32) |
Ultrasonic irradiations produce better utilization of the oxidant through improving the decomposition of ozone and results in generation of more active species, such as hydroxyl radicals and nascent oxygen. Furthermore, in US/O3 process, cavitation effect of ultrasound leads to formation of myriad of tiny air bubbles which enable most O3 to enter the liquid medium or react on the bubble–liquid interface. In other words, ultrasound promotes the mass transfer of O3 to the solution by the turbulence produced from cavitational effects. Thus, ultrasound overcomes mass transfer resistance, as a major limiting factor for the application of ozone alone. Hence, the enhancement of the mass transfer and dissociation processes of ozone coupled with high-localized temperatures and pressures upon the collapsing of cavities increase the generation of highly reactive hydroxyl radicals which results in a higher reaction rate.70 The ultrasound/ozonation mechanism is proposed and illustrated in Fig. 7.
The extent of degradation increases with an increase in the ozone flow rate up to optimum value, which can attribute to the improvement of the mass transfer rate of O3 from air–ozone bubbles to the liquid medium. The enhancement in gas holdup enhances the bubble–liquid interfacial area with a consequent enhance in the mass transfer rate of O3 from the gas bubbles to the solution and the increase in the free radicals concentration. Beyond the optimum, the degradation rate diminishes with increasing ozone flow rate due to transfer of the bubbly regime to the heterogeneous regime where large bubbles size start to form as a result of ozone bubble collision and coalescence. The formation of the large bubbles decreases the bubble–liquid interfacial region considerably, with a consequent reduction in the O3 transfer rate from the gas phase to the liquid phase.70,91
Wang et al.72 reported that the degradation efficiency of acephate by combined ultrasonic/ozonation method was 27% greater than the degradation efficiency when individual ozonation method was used after 60 min.
Patil et al.75 found that combination of ozone and ultrasonic irradiations was the best approach for effective removal of dichlorvos and complete degradation of dichlorvos was obtained by using the combined method.
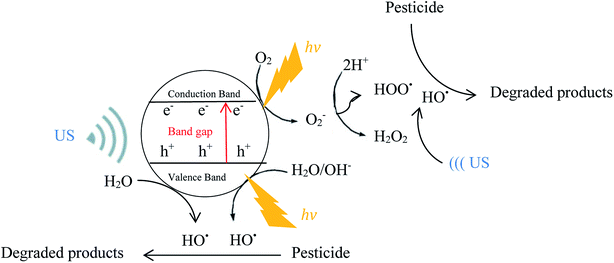
 free radicals. In addition, ultrasound irradiation increases the catalyst surface due to reducing particle size and continuously cleans the photocatalyst surface during the operation.81,94 Schematic of the pesticides degradation mechanism by photocatalytic and sono-photocatalytic processes are shown in Fig. 8 and 9, respectively.
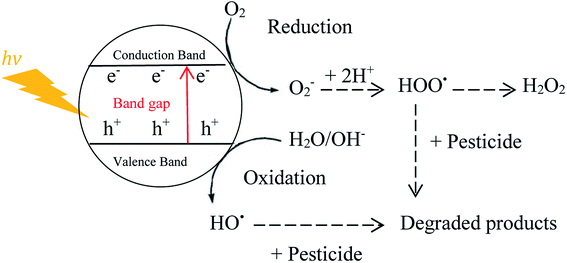
free radicals. In addition, ultrasound irradiation increases the catalyst surface due to reducing particle size and continuously cleans the photocatalyst surface during the operation.81,94 Schematic of the pesticides degradation mechanism by photocatalytic and sono-photocatalytic processes are shown in Fig. 8 and 9, respectively.| Photo-excitation: TiO2 + hv → TiO2 (e− + h+) | (33) |
| h+ + OHads− → HO˙ | (34) |
| e− + O2 → O2˙− → HO˙ | (35) |
Upon irradiation of catalyst with light energy higher or equal to the band gap energy, an electron from the valence band elevated to the conduction band with simultaneous production of a hole in the valence band.95 The valence band holes have the ability to oxidize the organic compounds, or they can react with OH− or H2O to form strong oxidizing hydroxyl radicals.96 On the other hand, the conduction band electrons can also react with dissolved oxygen to produce superoxide radical anion (O2˙−) which can also lead to generation of additional hydroxyl radicals as a major active species.75
As shown in Table 2, numerous studies evaluated the effectiveness of ultrasound application in combination with photocatalysis process for pesticide removal. Patil et al.75 examined sonophotocatalytic degradation of dichlorvos using TiO2 at different loadings of TiO2 (0.01 g L−1, 0.075 g L−1, 0.1 g L−1 and 0.2 g L−1). They reported that the maximum degradation efficiency after 2 h treatment was obtained at a TiO2 concentration of 0.1 g L−1 (78.5%) beyond which the extent of degradation is nearly constant. Similar results were found by Sathishkumar et al.82 for sonophotocatalytic (42 kHz) degradation of Simazine in the presence of Au–TiO2 nanocatalysts. They reported that the order of Simazine degradation was, sonophotocatalysis > sonocatalysis > photocatalysis.
Sajjadi et al.15 performed sonochemical degradation of diazinon using catalyzed persulfate with Fe3O4@MOF-2 nanocomposite. They reported that complete degradation was achieved by the Fe3O4@MOF-2/US/PS system and the combined system was a capable degradation process for pesticide treatment.
Madhavan et al.81 studied the sonophotocatalytic degradation of monocrotophos using TiO2. They reported that a slight improvement in the degradation rate was observed for sonophotocatalysis relative to sonolysis due to inhibition effect of phosphate ions.
5. Path forward and research needs
There are a variety of aspects in the application of ultrasound waves to removal pesticides that require to be addressed in the future. Several crucial issues where future studies should be directed are mentioned below. A detailed overview of the studies related to sonochemical degradation of pesticides has indicated that significant intensification in term of enhanced removal, can be obtained with the use of ultrasound either alone or in combination with other technologies. However, it has been observed that the majority of the studies have been based on the use of simple designs of batch sonochemical reactors such as ultrasonic bath and ultrasonic horn which may not be efficient at industrial scale operation. Further research may be directed in terms of improvement of novel sonoreactor designs based on the use of multiple transducers resulting into uniform and enhanced cavitational activity.Also, there is a need to develop continuous flow ultrasonic reactor with transducers attached to the reactor wall and the solution, hence, placing several units in parallel can omit scale up problems as the sonoreactor geometry remains constant and matches the penetration depth of the ultrasonic wave.
Another problem preventing the effective operation at industrial scale is possible erosion of transducer material with continuous utilization leading to a reduced transfer of energy and also require for frequent maintenance and/or replacement. Therefore, the further research studies need to be directed in terms of improvement of high power ultrasonic transducers, with higher power capacity, efficiency, radiating surface area and more sophisticated control system.
The analysis of scientific literature shows that most studies focus on low pesticide concentrations in synthetic samples; however, the pesticide wastewater has a very high strength wastewater that contained various toxic and detrimental contaminants. The natural water samples use is a crucial factor for the reliable assessment of removal process efficiency, as it permits the investigation of conditions close to the actual ones, therefore paving the way for process scale-up considerations.
Theoretical work is indeed required for efficient optimization of the large-scale design of the sonoreactor. Based on theoretical analysis, one can obtain the pressure field distribution in any new sonoreactor with various geometries and operating conditions, which can help in optimization for maximum/uniform distribution of cavitational activity. The modeling investigations can be extended to quantification of other operational parameters such as mass transfer coefficient, distribution of temperature, liquid streaming, etc., which can be controlling process parameters considering the specific application of pesticide removal.
In the perspective of large-scale applications of sonochemical degradation of pesticides, a fundamental aspect to be better clarified is the possible generation of hazardous intermediated or products: to this end, toxicological researches are needed.
From the economic value perspective, the feasibility of this approach full-scale needs to be assessed. Therefore, more research studies should be conducted in order to establish energy consumption levels, in order to evaluate both the technical and economic competitiveness of ultrasound towards conventional treatment methods. These items must be considered as future research directions.
6. Conclusions
In recent years, the majority of studies have been focused on the search for alternative and innovative techniques for removal of pesticides from aqueous solution. A detailed analysis into different aspects of pesticide degradation using ultrasonic technology alone or in combination with oxidation processes has been performed. This review clearly establishes that ultrasound as a promising technology has great potential in degradation of different types of pesticides. The pesticide degradation process under ultrasonic irradiation is controlled by pyrolysis and free radical reactions. The extent of degradation strongly depends on both sonochemical conditions, namely, frequency and power and operational parameters such as initial pesticide concentration, temperature, pH, dissolved gas, etc. Besides, the characteristics of the pesticides (vapor pressure, density, surface tension, etc.) influence sonochemical reactions. Ultrasound has been proved to be an effective technology with advantages of in terms of operational simplicity, safety, cleanliness, energy consumption, kinetic rate, and secondary pollutant formation. Nevertheless, sonochemical degradation is effective with compounds possessing a high vapor pressure and sometimes it needs much higher energy for complete decomposition which is economically not desirable. Literature data also pointed out that to overcome these limitations, the sonochemical process can be combined with advanced oxidation processes. Most of the studies on sonochemical degradation of pesticides are laboratory oriented which cannot be implemented directly in large-scale applications. On the basis of this literature review, major attention should be devoted in the future to the kinetics, reactor design, criteria for cost effectiveness and large industrial scale applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Research Council of Kermanshah University of Medical Sciences for the financial support.References
- P. Nicolopoulou-Stamati, S. Maipas, C. Kotampasi, P. Stamatis and L. Hens, Front. Public Health, 2016, 4, 1–8 CrossRef.
- R. A. Hamza, O. T. Iorhemen and J. H. Tay, Environ. Technol. Innov., 2016, 5, 161–175 CrossRef.
- M. Köck-Schulmeyer, M. Villagrasa, M. López de Alda, R. Céspedes-Sánchez, F. Ventura and D. Barceló, Sci. Total Environ., 2013, 458–460, 466–476 CrossRef.
- R. Kaur, G. K. Mavi, S. Raghav and I. Khan, Int. J. Curr. Microbiol. Appl. Sci., 2019, 8, 1889–1897 CrossRef CAS.
- M. Arias-Estévez, E. López-Periago, E. Martínez-Carballo, J. Simal-Gándara, J. C. Mejuto and L. García-Río, Agric., Ecosyst. Environ., 2008, 123, 247–260 CrossRef.
- A. Marican and E. F. Durán-Lara, Environ. Sci. Pollut. Res., 2018, 25, 2051–2064 CrossRef CAS PubMed.
- J. George and Y. Shukla, J. Proteomics, 2011, 74, 2713–2722 CrossRef CAS.
- M. V. Bagal and P. R. Gogate, Sep. Purif. Technol., 2012, 90, 92–100 CrossRef CAS.
- S. Raut-Jadhav, V. K. Saharan, D. V. Pinjari, D. R. Saini, S. H. Sonawane and A. B. Pandit, J. Environ. Chem. Eng., 2013, 1, 850–857 CrossRef CAS.
- R. Al-Shawabkah, Z. Al-Qodah and A. Al-Bsoul, Desalin. Water Treat., 2015, 53, 2555–2564 CrossRef CAS.
- S. Mostafalou and M. Abdollahi, Toxicol. Appl. Pharmacol., 2013, 268, 157–177 CrossRef CAS PubMed.
- H. Xie, L. Zhu, T. Ma, J. Wang, J. Wang, J. Su and B. Shao, J. Environ. Sci., 2010, 22, 1930–1935 CrossRef CAS.
- J. Rodriguez-Campos, L. Dendooven, D. Alvarez-Bernal and S. M. Contreras-Ramos, Appl. Soil Ecol., 2014, 79, 10–25 CrossRef.
- V. L. Gole, A. Fishgold, R. Sierra-Alvarez, P. Deymier and M. Keswani, Sep. Purif. Technol., 2018, 194, 104–110 CrossRef CAS.
- S. Sajjadi, A. Khataee, N. Bagheri, M. Kobya, A. Şenocak, E. Demirbas and A. G. Karaoğlu, J. Ind. Eng. Chem., 2019, 77, 280–290 CrossRef CAS.
- S. Agarwal, I. Tyagi, V. K. Gupta, M. H. Dehghani, A. Bagheri, K. Yetilmezsoy, A. Amrane, B. Heibati and S. Rodriguez-Couto, J. Mol. Liq., 2016, 221, 1237–1242 CrossRef CAS.
- I. Arslan-Alaton, G. Tureli and T. Olmez-Hanci, J. Photochem. Photobiol., A, 2009, 202, 142–153 CrossRef CAS.
- S. Wang, B. Huang, Y. Wang and L. Liao, Ultrason. Sonochem., 2006, 13, 506–510 CrossRef CAS PubMed.
- R. A. Torres, R. Mosteo, C. Pétrier and C. Pulgarin, Ultrason. Sonochem., 2009, 16, 425–430 CrossRef CAS PubMed.
- K. Thangavadivel, M. Megharaj, R. S. C. Smart, P. J. Lesniewski and R. Naidu, J. Hazard. Mater., 2009, 168, 1380–1386 CrossRef CAS PubMed.
- M. Ashokkumar, Handb. Ultrason. Sonochemistry, 2016, pp. 813–839 Search PubMed.
- N. C. J. David, Fundamentals and Applications of Ultrasonic Waves, CRC Publisher, USA, 2000, pp. 15–23 Search PubMed.
- K. S. Suslick, Sci. Am., 1989, 260, 80–86 CrossRef CAS.
- H. M. Santos, C. Lodeiro and J. L. Capelo-Martínez, Ultrasound in Chemistry, 2009, pp. 80–86 Search PubMed.
- X. Cheng, M. Zhang, B. Xu, B. Adhikari and J. Sun, Ultrason. Sonochem., 2015, 27, 576–585 CrossRef CAS.
- B. Gielen, J. Jordens, J. Janssen, H. Pfeiffer, M. Wevers, L. C. J. Thomassen, L. Braeken and T. Van Gerven, Ultrason. Sonochem., 2015, 25, 31–39 CrossRef CAS PubMed.
- M. Ashokkumar, J. Lee, Y. Iida, K. Yasui, T. Kozuka, T. Tuziuti and A. Towata, Phys. Chem. Chem. Phys., 2009, 11, 10118–10121 RSC.
- M. Ashokkumar, Ultrason. Sonochem., 2011, 18, 864–872 CrossRef CAS PubMed.
- H. Ghodbane and O. Hamdaoui, Ultrason. Sonochem., 2009, 16, 593–598 CrossRef CAS PubMed.
- L. K. Weavers, N. Malmstadt and M. R. Hoffmann, Environ. Sci. Technol., 2000, 34, 1280–1285 CrossRef CAS.
- S. Chitra, K. Paramasivan, P. K. Sinha and K. B. Lal, J. Cleaner Prod., 2004, 12, 429–435 CrossRef.
- P. Chowdhury and T. Viraraghavan, Sci. Total Environ., 2009, 407, 2474–2492 CrossRef CAS PubMed.
- T. G. Leighton, Prog. Biophys. Mol. Biol., 2007, 93, 3–83 CrossRef PubMed.
- R. S. Davidson, A. Safdar, J. D. Spencer and B. Robinson, Ultrasonics, 1987, 25, 35–39 CrossRef CAS.
- A. L. Marshall, J. Am. Chem. Soc., 1932, 54, 4460–4461 CrossRef CAS.
- M. A. Beckett and I. Hua, Water Res., 2003, 37, 2372–2376 CrossRef CAS PubMed.
- M. Sivakumar, P. A. Tatake and A. B. Pandit, Chem. Eng. J., 2002, 85, 327–338 CrossRef CAS.
- Y. L. Song, J. T. Li and H. Chen, J. Chem. Technol. Biotechnol., 2009, 84, 578–583 CrossRef CAS.
- K. Sekiguchi, C. Sasaki and K. Sakamoto, Ultrason. Sonochem., 2011, 18, 158–163 CrossRef CAS PubMed.
- Y. Zhang, Y. Hou, F. Chen, Z. Xiao, J. Zhang and X. Hu, Chemosphere, 2011, 82, 1109–1115 CrossRef CAS PubMed.
- Y. Zhang, W. Zhang, X. Liao, J. Zhang, Y. Hou, Z. Xiao, F. Chen and X. Hu, Ultrason. Sonochem., 2010, 17, 662–668 CrossRef CAS PubMed.
- M. A. Matouq, Z. A. Al-Anber, T. Tagawa, S. Aljbour and M. Al-Shannag, Ultrason. Sonochem., 2008, 15, 869–874 CrossRef CAS.
- P. Debabrata and M. Sivakumar, Chemosphere, 2018, 204, 101–108 CrossRef CAS.
- N. Golash and P. R. Gogate, Ultrason. Sonochem., 2012, 19, 1051–1060 CrossRef CAS PubMed.
- J. D. Schramm and I. Hua, Water Res., 2001, 35, 665–674 CrossRef CAS.
- M. Shayeghi, M. Dehghani and A. Fadaei, Iran. J. Public Health, 2014, 40, 122–128 Search PubMed.
- M. Kida, S. Ziembowicz and P. Koszelnik, Sep. Purif. Technol., 2018, 192, 457–464 CrossRef CAS.
- J. J. Yao, N. Y. Gao, C. Li, L. Li and B. Xu, J. Hazard. Mater., 2010, 175, 138–145 CrossRef CAS.
- M. D. Luque de Castro and F. Priego-Capote, Techniques and instrumentation in analytical chemistry - Analytical applications of ultrasound, 2007, vol. 26 Search PubMed.
- J. González-García, V. Sáez, I. Tudela, M. I. Díez-Garcia, M. D. Esclapez and O. Louisnard, Water, 2010, 2, 28–74 CrossRef.
- V. Naddeo, A. Cesaro, D. Mantzavinos, D. Fatta-Kassinos and V. Belgiorno, Global NEST J., 2014, 16, 561–577 CAS.
- A. K. Shriwas and P. R. Gogate, Sep. Purif. Technol., 2011, 79, 1–7 CrossRef CAS.
- L. Yang, J. Z. Sostaric, J. F. Rathman and L. K. Weavers, J. Phys. Chem. B, 2008, 112, 852–858 CrossRef CAS.
- H. M. Hung and M. R. Hoffmann, J. Phys. Chem. A, 1999, 103, 2734–2739 CrossRef CAS.
- M. A. Beckett and I. Hua, J. Phys. Chem. A, 2001, 105, 3796–3802 CrossRef CAS.
- M. Sivakumar and A. B. Pandit, Ultrason. Sonochem., 2001, 8, 233–240 CrossRef CAS.
- P. Kanthale, M. Ashokkumar and F. Grieser, Ultrason. Sonochem., 2008, 15, 143–150 CrossRef CAS.
- M. H. Entezari and P. Kruus, Ultrason. Sonochem., 1996, 3, 19–24 CrossRef CAS.
- S. Raut-Jadhav, D. V. Pinjari, D. R. Saini, S. H. Sonawane and A. B. Pandit, Ultrason. Sonochem., 2016, 31, 135–142 CrossRef CAS PubMed.
- L. Wang, Water Res., 2001, 35, 3927–3933 CrossRef.
- R. H. Jawale and P. R. Gogate, Ultrason. Sonochem., 2018, 40, 89–96 CrossRef CAS PubMed.
- Y. Jiang, C. Pétrier and T. D. Waite, Ultrason. Sonochem., 2002, 9, 163–168 CrossRef CAS.
- Z. Wei, R. Spinney, R. Ke, Z. Yang and R. Xiao, Environ. Chem. Lett., 2016, 14, 163–182 CrossRef CAS.
- S. Merouani, O. Hamdaoui, F. Saoudi and M. Chiha, Chem. Eng. J., 2010, 158, 550–557 CrossRef CAS.
- P. R. Gogate, A. Marie and A. B. Pandit, Ultrason. Sonochem., 2003, 10, 325–330 CrossRef CAS.
- K. S. Suslick and W. L. Nyborg, Sci. 80, 1989, 243, 1499 Search PubMed.
- M. H. Dehghani and A. Fadaei, Environ. Prot. Eng., 2013, 39, 5–14 CAS.
- E. Nikfar, M. H. Dehghani, A. H. Mahvi, N. Rastkari, M. Asif, I. Tyagi, S. Agarwal and V. K. Gupta, J. Mol. Liq., 2016, 213, 332–338 CrossRef CAS.
- M. Rahimi, N. Moradi, M. Faryadi and S. Safari, Desalin. Water Treat., 2016, 57, 1–9 CrossRef.
- X. D. Fan, W. L. Zhang, H. Y. Xiao, T. Q. Qiu and J. G. Jiang, RSC Adv., 2015, 5, 45622–45630 RSC.
- Q. Ren, C. Yin, Z. Chen, M. Cheng, Y. Ren, X. Xie, Y. Li, X. Zhao, L. Xu, H. Yang and W. Li, Microchem. J., 2019, 145, 146–153 CrossRef CAS.
- B. Wang, C. p. Zhu, R. h. Gong, J. Zhu, B. Huang, F. Xu, Q. g. Ren, Q. b. Han and Z. b. He, Water Sci. Eng., 2015, 8, 233–238 CrossRef.
- L. Jing, B. Chen, D. Wen, J. Zheng and B. Zhang, J. Environ. Manage., 2017, 203, 182–190 CrossRef CAS PubMed.
- Y. S. Ma and C. F. Sung, Sustainable Environ. Res., 2010, 20, 213–219 CAS.
- P. N. Patil and P. R. Gogate, J. Water Process Eng., 2015, 8, e58–e65 CrossRef.
- C. K. Wang and Y. H. Shih, Sustainable Environ. Res., 2016, 26, 110–116 CrossRef CAS.
- Y. N. Liu, D. Jin, X. P. Lu and P. F. Han, Ultrason. Sonochem., 2008, 15, 755–760 CrossRef CAS PubMed.
- H. Katsumata, T. Okada, S. Kaneco, T. Suzuki and K. Ohta, Ultrason. Sonochem., 2010, 17, 200–206 CrossRef CAS PubMed.
- H. Katsumata, T. Kobayashi, S. Kaneco, T. Suzuki and K. Ohta, Chem. Eng. J., 2011, 166, 468–473 CrossRef CAS.
- M. Kask, M. Krichevskaya and J. Bolobajev, J. Environ. Chem. Eng., 2019, 7, 103095 CrossRef.
- J. Madhavan, P. S. Sathish Kumar, S. Anandan, F. Grieser and M. Ashokkumar, J. Hazard. Mater., 2010, 177, 944–949 CrossRef CAS PubMed.
- P. Sathishkumar, R. V. Mangalaraja, H. D. Mansilla, M. A. Gracia-Pinilla and S. Anandan, Appl. Catal., B, 2014, 160–161, 692–700 CrossRef CAS.
- A. Babuponnusami and K. Muthukumar, J. Environ. Chem. Eng., 2014, 2, 557–572 CrossRef CAS.
- M. h. Zhang, H. Dong, L. Zhao, D. x. Wang and D. Meng, Sci. Total Environ., 2019, 670, 110–121 CrossRef CAS PubMed.
- S. Dogruel, T. Olmez-Hanci, Z. Kartal, I. Arslan-Alaton and D. Orhon, Water Res., 2009, 43, 3974–3983 CrossRef CAS PubMed.
- J. J. Pignatello, E. Oliveros and A. MacKay, Crit. Rev. Environ. Sci. Technol., 2006, 36, 1–84 CrossRef CAS.
- S. Adityosulindro, L. Barthe, K. González-labrada, J. J. Haza, H. Delmas, C. Julcour, S. Adityosulindro, L. Barthe, K. González-labrada, U. Javier and J. Haza, Ultrason. Sonochem., 2018, 39, 889–896 CrossRef PubMed.
- Y. S. Ma, C. F. Sung and J. G. Lin, J. Hazard. Mater., 2010, 178, 320–325 CrossRef CAS PubMed.
- P. Vaishnave, A. Kumar, R. Ameta, P. B. Punjabi and S. C. Ameta, Arabian J. Chem., 2014, 7, 981–985 CrossRef CAS.
- W. K. Lafi and Z. Al-Qodah, J. Hazard. Mater., 2006, 137, 489–497 CrossRef CAS PubMed.
- S. Song, Z. He and J. Chen, Ultrason. Sonochem., 2007, 14, 84–88 CrossRef CAS PubMed.
- S.-Y. Lee and S.-J. Park, J. Ind. Eng. Chem., 2013, 19, 1761–1769 CrossRef CAS.
- B. Shahmoradi, A. Maleki and K. Byrappa, Catal. Sci. Technol., 2011, 1, 1216–1223 RSC.
- A. Paleologou, H. Marakas, N. P. Xekoukoulotakis, A. Moya, Y. Vergara, N. Kalogerakis, P. Gikas and D. Mantzavinos, Catal. Today, 2007, 129, 136–142 CrossRef CAS.
- M. Moradi, F. Ghanbari, M. Manshouri and K. A. Angali, Korean J. Chem. Eng., 2016, 33, 539–546 CrossRef CAS.
- S. Tabasideh, A. Maleki, B. Shahmoradi, E. Ghahremani and G. McKay, Sep. Purif. Technol., 2017, 189, 186–192 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |