Open Access Article
Open Access ArticleEffects of deterioration and mildewing on the quality of wheat seeds with different moisture contents during storage
Ruolan Wang *a,
Lulu Liua,
Yapeng Guoa,
Xin Hea and
Qian Lu*ab
*a,
Lulu Liua,
Yapeng Guoa,
Xin Hea and
Qian Lu*ab
aSchool of Food Science and Technology, Henan University of Technology, Zhengzhou, 450001, China
bSchool of Resources, Environmental & Chemical Engineering, Key Laboratory of Poyang Lake Environment and Resource Utilization, Ministry of Education, Nanchang University, Nanchang 330031, China. E-mail: wangruol@163.com
First published on 16th April 2020
Abstract
Deterioration and mildewing caused by moisture of wheat seeds are serious problems that should be addressed for safe storage. In this work, simulated storage facilities were employed to preserve wheat seeds with different moisture contents (16%, 18%, 20% and 22%). The temperature changes in grain bulk, the mildewing situation, and the biochemical properties of wheat seeds were analyzed. Microstructures of endosperm and embryos were observed under electron microscopy. The results showed that with the increase of moisture content in wheat seeds, the deterioration and mildewing became serious. In the storage of wheat seeds with high moisture content, serious damage to the internal structure of endosperm and embryo was observed. Besides, the temperature increase and the fungal growth in grain bulk are attributed to the moisture of wheat seeds. Particularly, with the nutrient loss and cell damage, germination efficiency decreased with the extension of the storage period and the biochemical properties of wheat seeds are negatively impacted. Based on the results of this study, to prevent the deterioration and mildewing of wheat seeds and protect the biochemical properties in long-term storage, moisture should be controlled during the storage.
1 Introduction
Wheat seeds are a staple feedstock for both food and feed industry.1 To alleviate the conflict between continuous food/feed consumption and seasonal wheat production, a large number of facilities have been constructed for wheat seed storage.1 Large-scale grain storage could not only prevent a food shortage crisis, but also maintain the prices of grain products in the market, playing a key role in the development of modern society.2 However, a detrimental result of the large-scale grain storage is the grain deterioration and mildewing, which can be caused by temperature fluctuation, insect activity, air humidity, fungal growth, and many other factors.1,3 To reduce the economic loss of grain storage, the deterioration and mildewing under different storage conditions should be fully understood.According to previous studies, a variety of mechanisms are involved in the grain deterioration and mildewing during storage. Firstly, temperature increase, which can be caused by the grain cell respiration and solar radiation, widely occurs during the grain storage.4 The high temperature can further create a favorable environment for grain cell respiration, causing the nutrients loss and weight loss in grain seeds. Secondly, insects' activities can damage the structure of grain seeds and accelerate the deterioration process during storage.5 Although fumigation can limit the insects' activities or densities, it is not possible to reduce the number of insects to zero in the large-scale grain storage facilities. Hence, the damages caused by insects' activities are along with the grain storage. Thirdly, in the storage conditions with high humidity, fast deterioration of wheat seeds has been reported by previous studies.6,7 One of the main mechanisms is that the humid environment can favor the fast growth of fungi, which damage the surface of wheat seeds and consume the nutrients.
Moisture is an important concern during the storage of wheat seeds. On one hand, moisture content is important to the edible properties of wheat seeds. On the other hand, moisture may change the storage environment and further impact the properties of wheat seeds. Reed et al. (2007) reported that moisture can accelerate the respiration of wheat seeds during storage and promote the spoilage.8 Previous studies also reported the promotional effects of moisture on the respiration and spoilage of many other types of grains.9,10 However, effects of moisture gradients in wheat seeds on the temperature fluctuations of grain bulk have been rarely studied. In addition, previous studies mainly focused on the cell respiration while neglected the relation between moisture and fungal growth.1,11 To our knowledge, during the storage, moisture transfer always occurs in the grain bulk, resulting in the dramatic increase of moisture content in a portion of wheat seeds.12,13 Also, microbial activities, particularly fungal activities, in grain bulk might damage the external structure of wheat seeds and cause the spoilage.14 Therefore, it is necessary to fully understand the effects of high moisture content and fungal growth on the storage of wheat seeds.
The main aim of this work was providing answers to four important questions: (1) What are the temperature changes in the three-dimensional grain bulk during storage? (2) Is fungal activity contributing to the temperature increase of wheat seeds with high moisture contents? (3) What are the effects of storage on biochemical properties of wheat seeds with different moisture contents? (4) What are the microstructures of endosperm in wheat seeds before and after storage? With the solutions of aforementioned questions, effects of moisture content on the biochemical properties of wheat seeds can be fully understood. By the end of this work, strategies for wheat seeds storage and the evaluation of storage cost are provided.
2 Materials and methods
2.1 Wheat seeds and storage facility
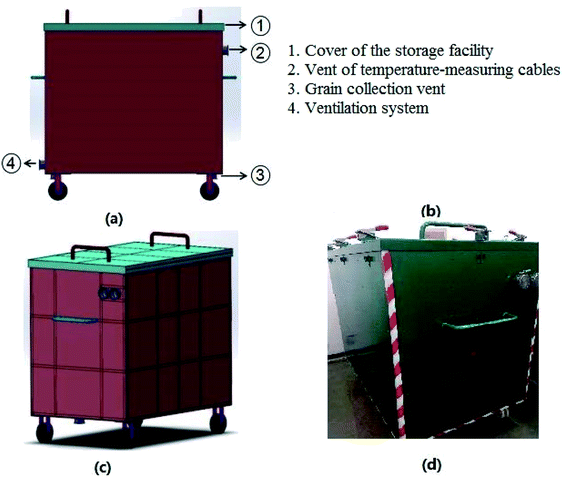
The wheat seeds used in this work were produced in Henan Province (China) and harvested in June, 2017. The storage facility used for the experiment was designed by our research team. The size of storage facility, of which the length, width, and height are 1.5 m, 0.95 m, and 1.2 m, respectively, is made of carbon steel. Specific structures of the storage facility are shown in Fig. 1. There are vents for temperature-measuring cables, which can monitor the actual temperature of the grain bulk, on the sides of storage facility. Ventilation system is equipped with the storage facility to prevent the temperature increase caused by the wheat seeds respiration. In this work, the wheat seeds were stored at room temperature (15–25 °C).To evaluate the effects of moisture contents on the biochemical properties of wheat seeds during storage, the moisture contents of wheat seeds were set as 16%, 18%, 20% and 22%. The storage periods of wheat seeds with 16%, 18%, 20% and 22% moisture contents were set as 103 days, 49 days, 28 days, and 24 days, respectively.
2.2 Experimental design
The experiments in this study were carried out in four steps. Firstly, the temperature changes of wheat seeds with different moisture contents were measured to confirm the effects of moisture content on the storage conditions. To improve the reliability of the research results, both vertical temperature and horizontal temperature of the grain bulk were measured. Secondly, effects of moisture content on the mildewing of wheat seeds during storage were studied by quantifying the number of fungi. Thirdly, microstructures of endosperm and embryo of wheat seeds before and after storage were demonstrated. The changes of large starch granules, small starch granules, protein matrices, broken starch, and broken protein in the endosperm were observed at different storage stages. In the embryo, changes of cell wall, mitochondria, nucleus, nucleolus, and nucleus membrane were observed. Fourthly, biochemical properties, including the germination efficiency, farinograph properties, and extensograph properties, of wheat seeds before storage and after storage were measured. By the end of this work, strategies to alleviate the negative effects of moisture on the quality of wheat seeds during storage were discussed.In this work, all the experiments and tests were performed in triplicate and the experimental results were expressed as mean or mean ± standard deviation.
2.3 Analysis methods and experimental procedures
The amount of fungi on wheat seeds was expressed as colony-forming unit (CFU) (104/g). The measurement of CFU was performed according to the national standard (GB 4789.15-2016) of China. Specific procedures are listed as follows: (1) 25 g samples are mixed with 225 mL sterile distilled water and then the solution is diluted by 10 times; (2) 1 mL diluted samples are sprayed on Petri dish with Bengal red agar medium; (3) the samples were preserved in incubator at 28 ± 1 °C for 5 days; (4) optical microscope is used to quantify the number of fungal colonies on the agar medium; (5) the CFU is calculated.
In this work, the mildewing degrees of wheat seeds were identified according to the CFU. When the values of CFU fell in the scopes of 4.4 × 104–1.2 × 105, 1.2 × 105–7.1 × 105, and >7.1 × 105, the wheat seeds would be considered as mild mildewing, moderate mildewing, and severe mildewing, respectively.15
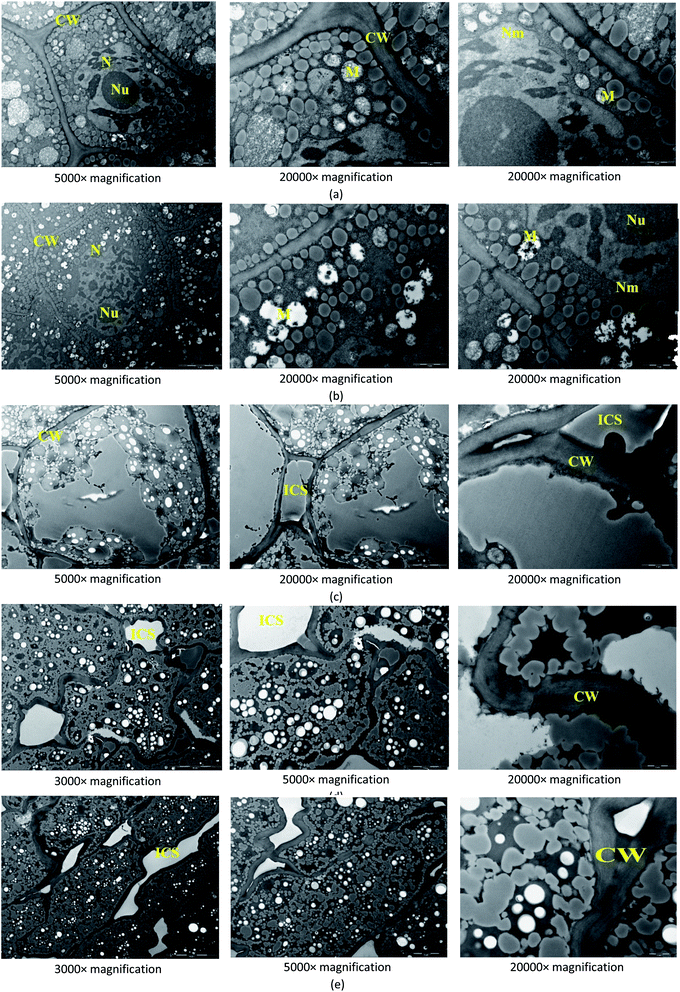
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnifications with focus on the cell wall, intercellular space, mitochondria, nucleus, nucleolus, and nucleus membrane.
000× magnifications with focus on the cell wall, intercellular space, mitochondria, nucleus, nucleolus, and nucleus membrane.3 Results
3.1 Effects of moisture contents on storage heating
 | ||
| Fig. 2 Temperature changes (vertical level) of wheat seeds during storage: (a) 16% moisture content; (b) 18% moisture content; (c) 20% moisture content; (d) 22% moisture content. | ||
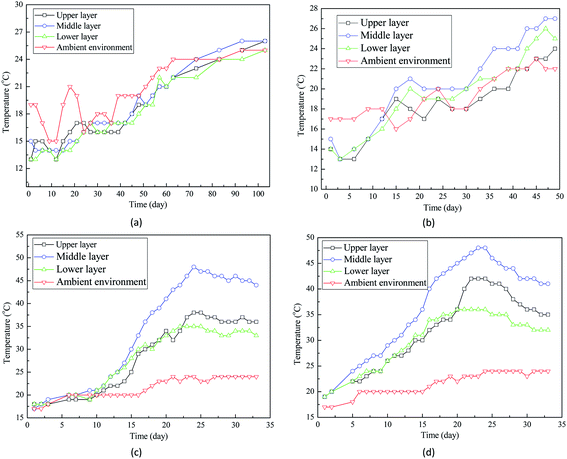
Fig. 2(c) and (d) demonstrated that the temperatures of grain seeds at three layers were much higher than the ambient temperature. For example, after 30 day storage, ambient temperature increased to around 22 °C while the final temperatures of grain bulk ranged between 30 °C and 44 °C (Fig. 2(c)). Such a difference suggests that in addition to the ambient temperature increase, some other factors contributed to the temperature increase of grain bulk. Interestingly, the difference between ambient temperature and grain bulk temperature is related with the moisture content of wheat seeds. Fig. 2 indicated that the higher the moisture content, the larger the temperature difference. Therefore, it is hypothesized that high moisture content accelerated the temperature increase of grain bulk.
Not only the temperature fluctuation, but also the temperature gradient was related with moisture content of wheat seeds. Fig. 3 indicated that the temperature gradient in grain bulk was only around 2 °C before storage while the temperature gradient of some grain bulks increased dramatically after storage. For example, the final temperature gradient of wheat seeds with 16% moisture content was still around 2 °C after 103 day storage. However, the temperature gradients of wheat seeds with 20% and 22% moisture contents were over 10 °C. Interestingly, temperature of the central part of grain bulk was much higher than that of surrounding part (Fig. 3(d) and (h)). The most possible reason for this phenomenon is that self-heating caused by the moisture of wheat seeds contributed to the temperature increase in grain bulk.
Two theories have been proposed to explain the moisture-related temperature increase in the storage of grain seeds. Firstly, semi-closed grain bulk creates a favorable environment for fungal activities, which can generate heat and further increase the temperature. Secondly, it has been fully documented that high moisture can accelerate the respiration of wheat seeds, releasing heat and increasing storage temperature. Ghazavi and Houshmand (2014) reported that the over intensive respiration of grain seeds during storage can consume the grain nutrients and even damage the cell structures.21 To verify if the endogenous activity (wheat seeds' respiration) or the exogenous activity (fungal growth) contributed to the temperature increase in grain bulk, more studies have been conducted in this work.
3.2 Effects of moisture contents on mildewing
 | ||
| Fig. 4 Measurement of CFU for the analysis of wheat seeds mildewing: (a) 16% moisture content; (b) 18% moisture content; (c) 20% moisture content; (d) 22% moisture content. | ||
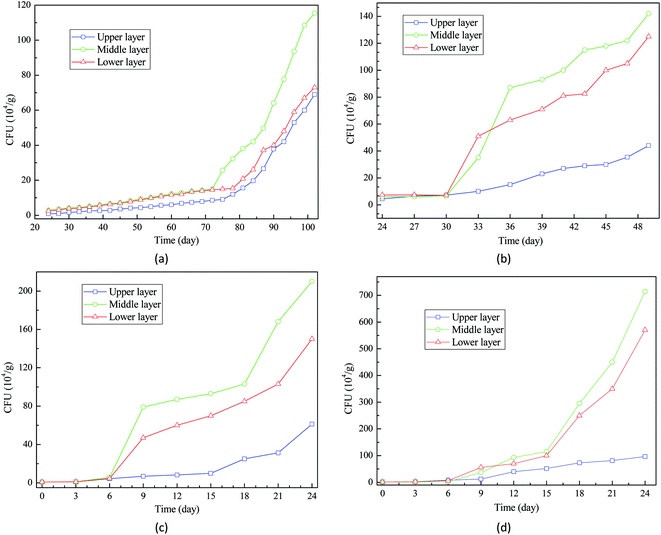
Interestingly, the CFU of wheat seeds at upper layer was much lower than those of wheat seeds at middle layer and lower layer. This result suggests that the wheat seeds at middle layer and lower layer are more likely to be impacted by the deterioration caused by fungal activities. This is in accordance with the study of White et al. (2010) that explored the relationship between fungal growth and wheat seeds deterioration in storage facilities.22 Therefore, during on the long-term storage, appropriate technologies should be applied to hinder the fast growth of fungi at the middle layer and lower layer of grain bulk.
As reported by Awasthi et al. (2014), the microbial activities can generate heat, further causing temperature increase in the closed grain storage facilities.23 Therefore, based on the observation of a large quantity of fungi on wheat seeds, the first hypothesis that fungal growth and activity contributed to the temperature increase in grain bulk is confirmed.
| Mildewing degree | Moisture contents of wheat seeds | |||
|---|---|---|---|---|
| 16% | 18% | 20% | 22% | |
| Mild | Day 33 | Day 24 | Day 6 | Day 6 |
| Moderate | Day 60 | Day 33 | Day 9 | Day 9 |
| Severe | Day 93 | Day 36 | Day 12 | Day 12 |
The mechanisms for the correlation of moisture content and mildewing are complex. Firstly, the moisture is favorable to the growth of fungi in grain bulk since most fungal metabolisms require moisture.24,25 The wheat seeds with high moisture content can continuously supply extra moisture to the fungi surviving in grain bulk. Secondly, wheat seeds with high moisture content are prone to having higher respiration rate, generating more heat and increasing the temperature in storage facility. The warm and humid environment would accelerate the growth of fungi, resulting in mildewing of wheat seeds during storage.26
If the wheat seeds with severe mildewing are considered unsuitable for further storage, the storage periods of wheat seeds with 16%, 18%, 20%, and 22% moisture contents should be no longer than 93, 36, 12, and 12 days, respectively (Table 1). This result confirmed that it is a practically feasible way to extend the storage period of wheat seeds by lowering the moisture content before storage. In most cases, to ensure the food security for the society, wheat seeds need to be preserved in the storage facilities for even over two years. Therefore, moisture content of wheat seeds should be lowered below 16% to avoid severe mildewing. Additionally, some other advanced technologies, such as fumigation, ventilation, and temperature control, should be adopted to maintain the quality of wheat seeds during the long-term storage.
3.3 Microstructure of endosperm and embryo during storage
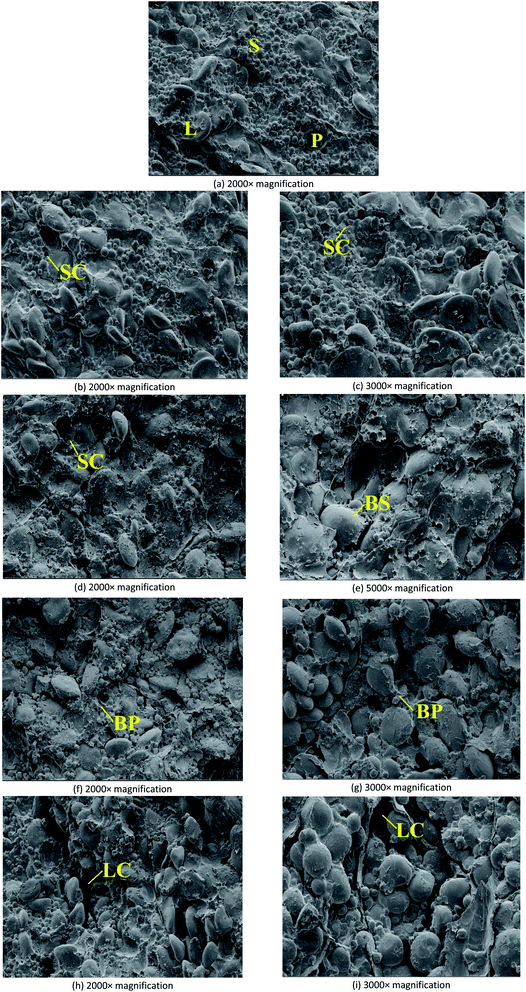
With the extension of storage period, the abscission of small starch granule was observed firstly (Fig. 5(b)). More holes caused by the abscission of small starch granules were observed in Fig. 5(d) and (f), suggesting that when the storage period was extended, the internal structure of endosperm can be seriously damaged. As shown in Fig. 5(f), on Day 12, the damages of protein matrices in endosperm were observed, resulting in some broken protein matrices. Fig. 5(h) demonstrated that large starch granules dropped from the endosperm when the storage period was extended 24 days. Therefore, in the storage of wheat seeds with high moisture content (20%), serious destruction of endosperm was observed during the 24 day storage. Fig. 5(c), (e), (g), and (i), which reflected the internal structures of endosperm at 2000× magnification, also confirmed the results concluded based on Fig. 5(b), (d), (f), and (h).
Generally, a variety of factors can contribute to the damage or deterioration of wheat seeds during storage.30,31 Firstly, the fungal activities can damage the cell structure of wheat seeds and further cause the nutrients loss in endosperm. Secondly, the internal deterioration caused by the intensive respiration of wheat seeds may cause the nutrients loss in endosperm. As a result, with the damage of endosperm and embryo, the biochemical properties of wheat seeds would be negatively impacted. To further explore the negative effects of storage on the biochemical properties of wheat seeds with different moisture contents, germination efficiency, water absorption of gluten, farinograph properties, and extensograph properties were measured.
3.4 Effects of moisture contents on the biochemical properties of wheat seeds
 | ||
| Fig. 7 Germination efficiency of wheat seeds at different time during storage: (a) 16% moisture content; (b) 18% moisture content; (c) 20% moisture content; (d) 22% moisture content. | ||
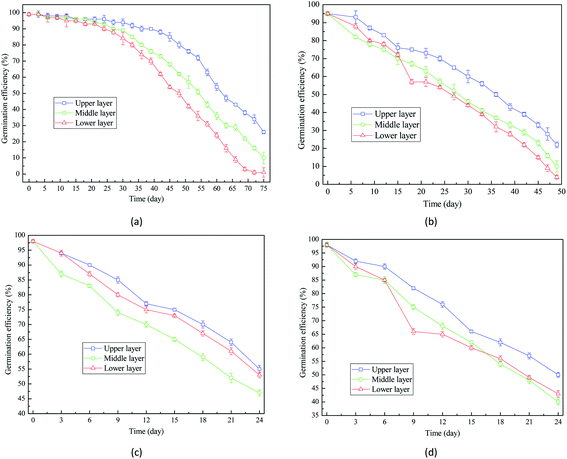
Comparison between Fig. 7(a) and (b) indicated that when the moisture content increased from 16% to 18%, the decrease rate of germination efficiency was improved. Fig. 7(d) showed that the germination efficiency of wheat seeds with 22% moisture content decreased to around 40% after 24 day storage. Therefore, the extension of storage period is detrimental to the germination capacity of wheat seeds and the high moisture content accelerates the decrease of germination efficiency.
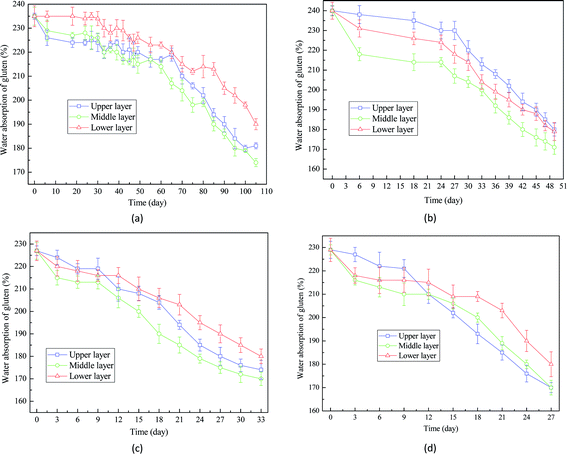
Similar trends were also observed in the analysis of water absorption of gluten (Fig. 8). It took around 103 days, 49 days, 28 days, and 24 days to reduce the water absorption of gluten of wheat seeds with 16%, 18%, 20% and 22% from 230% to 170%, suggesting that low moisture content has positive effects on the water absorption of gluten. This result is in accordance with the changes of microstructure of endosperm shown in Fig. 5. The main mechanism for the decrease of water absorption of gluten is that the broken protein observed in wheat seeds during storage could damage the properties of gluten.33,34
| Moisture content | Storage time (day) | Development time (min) | Maximum consistency (FU) | Water absorption (%) | Stability time (min) | Softening (FU) |
|---|---|---|---|---|---|---|
| 16% | 0 | 3.19 ± 0.25 | 493 ± 12 | 66.3 ± 3.5 | 2.40 ± 0.16 | 80 ± 2.9 |
| 24 | 3.34 ± 0.19 | 487 ± 24 | 60.1 ± 4.9 | 2.82 ± 0.08 | 84 ± 4.5 | |
| 48 | 3.71 ± 0.26 | 502 ± 21 | 66.0 ± 1.5 | 3.26 ± 0.10 | 87 ± 3.8 | |
| 72 | 4.03 ± 0.09 | 507 ± 16 | 66.3 ± 1.9 | 3.47 ± 0.05 | 93 ± 2.7 | |
| 96 | 3.85 ± 0.12 | 501 ± 19 | 66.1 ± 2.5 | 3.79 ± 0.09 | 102 ± 5.9 | |
| 18% | 0 | 2.86 ± 0.33 | 507 ± 30 | 63.8 ± 2.7 | 2.61 ± 0.11 | 80 ± 4.2 |
| 12 | 3.89 ± 0.31 | 483 ± 24 | 66.7 ± 2.6 | 4.05 ± 0.21 | 71 ± 1.5 | |
| 24 | 4.04 ± 0.28 | 492 ± 38 | 66.8 ± 3.3 | 5.57 ± 0.15 | 75 ± 1.9 | |
| 36 | 4.68 ± 0.15 | 513 ± 36 | 67.3 ± 3.8 | 5.71 ± 0.16 | 82 ± 2.8 | |
| 48 | 4.52 ± 0.17 | 495 ± 24 | 67.9 ± 1.5 | 5.13 ± 0.32 | 78 ± 2.6 | |
| 20% | 0 | 3.14 ± 0.12 | 467 ± 11 | 65.3 ± 2.4 | 2.73 ± 0.15 | 64 ± 3.5 |
| 6 | 3.23 ± 0.22 | 485 ± 18 | 65.8 ± 3.6 | 2.85 ± 0.22 | 68 ± 3.9 | |
| 12 | 3.60 ± 0.25 | 494 ± 26 | 70.5 ± 1.1 | 3.73 ± 0.27 | 75 ± 4.1 | |
| 18 | 6.26 ± 0.16 | 488 ± 42 | 67.5 ± 2.8 | 4.61 ± 0.16 | 118 ± 4.8 | |
| 24 | 5.09 ± 0.31 | 483 ± 31 | 66.6 ± 4.2 | 4.31 ± 0.24 | 124 ± 3.8 | |
| 22% | 0 | 3.21 ± 0.32 | 502 ± 25 | 66.9 ± 4.1 | 2.98 ± 0.26 | 62 ± 3.5 |
| 6 | 3.44 ± 0.22 | 506 ± 27 | 67.0 ± 3.8 | 3.02 ± 0.30 | 71 ± 2.9 | |
| 12 | 4.99 ± 0.28 | 493 ± 11 | 67.0 ± 3.2 | 4.96 ± 0.27 | 87 ± 3.1 | |
| 18 | 4.45 ± 0.26 | 508 ± 15 | 66.4 ± 1.5 | 5.37 ± 0.17 | 92 ± 1.5 | |
| 24 | 6.76 ± 0.40 | 482 ± 22 | 66.2 ± 2.8 | 4.82 ± 0.22 | 120 ± 4.9 |
No obvious changes were observed in the measurement of water absorption, suggesting that the water absorption capacity of wheat flour remained stable during the storage. The softening of wheat flour is a factor that reflects the softness of wheat dough. As shown in Table 3, softening values increased with the extension of storage periods and the increase of moisture content. For example, the softening value of wheat seeds with 22% moisture content increased from 62 FU to 120 FU when the storage period was extended to 24 days. In a real-world application, wheat flour with high softening values may not be suitable for the food production.
| Moisture content | Storage time (day) | 45/90/135 min | |||||
|---|---|---|---|---|---|---|---|
| Area (cm2) | Resistance (BU) | Extensibility (mm) | Maximum resistance (BU) | R/E | Max R/E | ||
| 16% | 0 | 25/34/40 | 138/182/232 | 117/122/114 | 139/183/235 | 1.2/1.5/2 | 1.2/1.5/2.1 |
| 24 | 29/33/39 | 141/181/186 | 113/117/119 | 142/181/188 | 1.2/1.5/1.6 | 1.3/1.5/1.6 | |
| 48 | 43/41/45 | 241/237/245 | 114/114/118 | 241/239/245 | 2.1/2.1/2.1 | 2.1/2.1/2.1 | |
| 72 | 44/46/51 | 267/267/279 | 109/116/120 | 263/269/281 | 2.4/2.3/2.3 | 2.4/2.3/2.3 | |
| 96 | 47/49/53 | 249/251/273 | 119/121/124 | 249/253/274 | 2.1/2.1/2.2 | 2.1/2.1/2.2 | |
| 18% | 0 | 24/31/37 | 134/176/213 | 114/117/114 | 141/176/213 | 1.2/1.5/1.9 | 1.2/1.5/1.9 |
| 12 | 29/37/45 | 182/231/279 | 104/107/109 | 182/231/280 | 1.7/2.2/2.5 | 1.7/2.2/2.6 | |
| 24 | 27/33/45 | 156/195/233 | 112/109/110 | 156/196/234 | 1.4/1.8/2.1 | 1.4/2.8/2.1 | |
| 36 | 37/42/44 | 206/228/257 | 116/121/111 | 206/230/258 | 1.8/1.9/2.3 | 1.8/1.9/2.3 | |
| 48 | 31/38/43 | 174/210/234 | 116/117/121 | 174/211/236 | 1.5/1.8/1.9 | 1.5/1.8/1.9 | |
| 20% | 0 | 21/27/31 | 120/161/179 | 103/114/117 | 121/164/183 | 1.2/1.4/1.5 | 1.2/1.4/1.6 |
| 6 | 21/33/39 | 122/183/236 | 108/120/108 | 129/184/237 | 1.1/1.5/2.2 | 1.2/1.5/2.2 | |
| 12 | 28/36/41 | 149/188/216 | 118/121/123 | 149/190/218 | 1.3/1.6/1.8 | 1.3/1.6/1.8 | |
| 18 | 43/46/48 | 222/241/264 | 126/126/124 | 226/247/268 | 1.8/1.9/2.1 | 1.8/2.0/2.2 | |
| 24 | 34/40/42 | 178/206/218 | 120/124/125 | 180/212/226 | 1.5/1.7/1.8 | 1.5/1.7/1.8 | |
| 22% | 0 | 27/31/39 | 162/207/221 | 107/109/111 | 162/208/223 | 1.5/1.9/2.0 | 1.5/1.9/2.0 |
| 6 | 29/37/42 | 167/218/260 | 113/112/109 | 169/218/261 | 1.5/2.0/2.4 | 1.5/2.0/2.4 | |
| 12 | 37/46/47 | 224/268/280 | 108/116/119 | 225/268/283 | 2.1/2.3/2.4 | 2.1/2.3/2.4 | |
| 18 | 35/46/47 | 202/264/282 | 114/114/112 | 202/264/282 | 1.8/2.3/2.5 | 1.8/2.3/2.5 | |
| 24 | 45/46/45 | 221/245/252 | 121/124/118 | 225/253/257 | 1.8/1.8/2.1 | 1.9/2.3/2.2 | |
4 Discussion
4.1 Problems caused by high moisture content
High moisture content poses a serious threat to the storage of wheat seeds. Firstly, moisture can accelerate the cell respiration of wheat seeds during storage, resulting in the temperature increase of grain bulk. As a consequence, the high temperature of the grain bulk will increase the deterioration rate of grain and negatively impact their biochemical properties.35 Secondly, with the continuous respiration of wheat seeds during storage, air humidity in storage facilities will be improved to a higher level. Both high moisture content in wheat seeds and high air humidity favor the growth of fungi in grain bulk. The metabolisms of some fungi can damage the complete structure of wheat seeds and obtain nutrients from wheat seeds. Also, the fungal activities can further promote the temperature increase in storage facility.36This study firmly confirmed aforementioned two explanations. According to the analysis of temperature changes, it was discovered that grain bulk with higher moisture content had higher temperature. The dramatic increase of temperature was partly attributed to the intensive cell respiration of wheat seeds during storage. In addition, the fast growth of fungi and the damage of cell structures were observed, confirming that the fungal activities are correlated with the deterioration of wheat seeds during storage.
The negative effects of moisture on the biochemical properties of wheat seeds indicate that the storage process should be strictly controlled. Otherwise, the deterioration and mildewing would jeopardize the utilization of wheat seeds. On one hand, the germination efficiency of wheat seeds with 20% moisture content decreased to about 50% after 24 day storage. In China, the storage period of wheat seeds can be as long as four years. As a result, after the long-term storage, the wheat seeds would not be used for seeding. On the other hand, due to the nutrients loss and microstructure damage, extensograph properties and farinograph properties of flour made of wheat seeds after storage are negatively impacted.37 Consequently, the wheat seeds after storage may not be suitable for further processing in food industry.
4.2 Strategies for the wheat seeds storage
To mitigate the negative effects of moisture content on the wheat seeds during storage, some strategies are available. In a real-world application, these strategies could be adapted according to the actual situations.Firstly, moisture content of wheat seeds can be reduced to a lower level before the storage by appropriate dehydration process. Wheat seeds with lower moisture content would be more suitable for storage since the fungal activities and the cell respirations could be hindered under the low-moisture condition. However, since the grain with low moisture content could not be directly used for food production, the wheat seeds after storage should be remoisten before further processing in food industry. This step may make the wheat seeds processing more complicated and cost-consuming.
Secondly, air conditioning can be applied to prevent the dramatic increase of temperature during the long-term storage. Under the low temperature, fungal growth and metabolism would be limited, lowering the threats to wheat seeds storage. According to previous studies, low-temperature maintenance has been proven to be a good way for long-term storage of wheat seeds or rice seeds.2,38 However, the cost and energy consumption of maintaining low temperature in the huge storage facilities are high. As a result, the large-scale grain storage may not be affordable.
Thirdly, ventilation is another feasible method to limit the fungal growth and reduce the risks caused by high moisture content.39 It was reported that ventilation can accelerate the air low in storage facilities and hinder the fast growth of fungi and insects.40 In most cases, the internal circulation ventilation is integrated with the utilization of fumigation as an effective way to kill fungi and insects in the grain bulk.41 The disadvantages of this technology include the potential contamination by residual fumigants and the moisture loss by ventilation.
5 Conclusions
It is concluded that (1) moisture plays a key role in the storage of wheat seeds by impacting the biochemical properties, fungal growth, and temperature fluctuation. (2) High moisture content promoted the temperature increase in grain bulk and shortened the storage period of wheat seeds. (3) During the storage, microstructures of endosperm and embryo were damaged in the wheat seeds with high moisture content. (4) Fungal growth in grain bulk was observed to be correlated with the moisture content of wheat seeds. (5) With the damage of internal structures and the nutrients loss, wheat seeds had low germination efficiency and poor extensograph properties and farinograph properties. Thus, the utilization of wheat seeds in downstream industry would be seriously hindered. In a real-world application, therefore, strategies, such as moisture content reduction, low-temperature maintenance, ventilation, and fumigation, should be adapted appropriately for the storage of wheat seeds with high moisture content.Conflicts of interest
There is no conflict to declare.Acknowledgements
This work was supported by the funding from National Key R&D Program of China (Grant No. 2016YFD0401005).References
- R. Wang, L. Zhang and Q. Lu, Exploration of mechanisms for internal deterioration of wheat seeds in postharvest storage and nitrogen atmosphere control for properties protection, Crop Sci., 2018, 58(2), 823–836 CrossRef CAS.
- R. Wang, L. Xiao, L. Yang and Q. Lu, Oxidative stress with the damage of scavenging system: a mechanism for the nutrients loss in rice seeds during post-harvest storage, CyTA–J. Food, 2019, 17(1), 260–271 CrossRef CAS.
- S. B. Zhang, Y. Y. Lv, Y. L. Wang, F. Jia, J. S. Wang and Y. S. Hu, Physiochemical changes in wheat of different hardnesses during storage, J. Stored Prod. Res., 2017, 72, 161–165 CrossRef.
- A. A. Sawant, S. C. Patil, S. B. Kalse and N. J. Thakor, Effect of temperature, relative humidity and moisture content on germination percentage of wheat stored in different storage structures, Agric Eng Int: CIGR Journal, 2012, 14(2), 110–118 Search PubMed.
- Z. L. Liu, S. S. Chu and G. H. Jiang, Toxicity of Schizonpeta multifida essential oil and its constituent compounds towards two grain storage insects, J. Sci. Food Agric., 2011, 91(5), 905–909 CrossRef CAS PubMed.
- A. A. Sawant, S. C. Patil, S. B. Kalse and N. J. Thakor, Effect of temperature, relative humidity and moisture content on germination percentage of wheat stored in different storage structures, Agric Eng Int: CIGR Journal, 2012, 14(2), 110–118 Search PubMed.
- K. Shehu and M. T. Bello, Effect of environmental factors on the growth of Aspergillus species associated with stored millet grains in Sokoto, Nig. J. Basic Appl. Sci., 2011, 19(2), 218–223 Search PubMed.
- C. Reed, S. Doyungan, B. Ioerger and A. Getchell, Response of storage molds to different initial moisture contents of maize (corn) stored at 25 C, and effect on respiration rate and nutrient composition, J. Stored Prod. Res., 2007, 43(4), 443–458 CrossRef.
- Z. G. Weinberg, Y. Yan, Y. Chen, S. Finkelman, G. Ashbell and S. Navarro, The effect of moisture level on high-moisture maize (Zea mays L.) under hermetic storage conditions—in vitro studies, J. Stored Prod. Res., 2008, 44(2), 136–144 CrossRef CAS.
- T. Genkawa, T. Uchino, A. Inoue, F. Tanaka and D. Hamanaka, Development of a low-moisture-content storage system for brown rice: storability at decreased moisture contents, Biosyst. Eng., 2008, 99(4), 515–522 CrossRef.
- B. Lu, Y. Ren, Y. Z. Du, Y. Fu and J. Gu, Effect of ozone on respiration of adult Sitophilus oryzae (L.), Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.), J. Insect Physiol., 2009, 55(10), 885–889 CrossRef CAS PubMed.
- K. S. O. Rocha, J. H. Martins, M. A. Martins, J. A. O. Saraz and A. F. L. Filho, Three-dimensional modeling and simulation of heat and mass transfer processes in porous media: an application for maize stored in a flat bin, Dry. Technol., 2013, 31(10), 1099–1106 CrossRef CAS.
- K. M. Lo, C. S. Chen, J. T. Clayton and D. D. Adrian, Simulation of temperature and moisture changes in wheat storage due to weather variability, J. Agric. Eng. Res., 1975, 20(1), 47–53 CrossRef.
- V. Chelladurai, D. S. Jayas and N. D. G. White, Thermal imaging for detecting fungal infection in stored wheat, J. Stored Prod. Res., 2010, 46(3), 174–179 CrossRef.
- R. Wang, Grain and Oil Storage Science, China Light Industry Press, Beijing, China, 2016 Search PubMed.
- H. Jackowiak, D. Packa, M. Wiwart and J. Perkowski, Scanning electron microscopy of Fusarium damaged kernels of spring wheat, Int. J. Food Microbiol., 2005, 98(2), 113–123 CrossRef CAS PubMed.
- P. P. Tian, Y. Y. Lv, W. J. Yuan, S. B. Zhang and Y. S. Hu, Effect of artificial aging on wheat quality deterioration during storage, J. Stored Prod. Res., 2019, 80, 50–56 CrossRef.
- C. Spanò, S. Bottega, R. Lorenzi and I. Grilli, Ageing in embryos from wheat grains stored at different temperatures: oxidative stress and antioxidant response, Funct. Plant Biol., 2011, 38(7), 624–631 CrossRef.
- P. Qin, Z. Kong, X. Liao and Y. Liu, Effects of accelerated aging on physiological and biochemical characteristics of waxy and non-waxy wheat seeds, J. NE Agric. Univ., 2011, 18(2), 7–12 CAS.
- Q. Lu, Y. He and X. Liu, Property assessment of steamed bread added with cellulase by using fuzzy mathematical model, J. Texture Stud., 2015, 46(6), 420–428 CrossRef.
- M. A. Ghazavi and S. Houshmand, Effects of mechanical damage and temperature on potato respiration rate and weight loss, World Appl. Sci. J., 2010, 8(5), 647–652 CAS.
- S. D. White, P. T. Murphy, C. J. Bern and J. H. van Leeuwen, Controlling deterioration of high-moisture maize with ozone treatment, J. Stored Prod. Res., 2010, 46(1), 7–12 CrossRef CAS.
- M. K. Awasthi, A. K. Pandey, J. Khan, P. S. Bundela, J. W. Wong and A. Selvam, Evaluation of thermophilic fungal consortium for organic municipal solid waste composting, Bioresour. Technol., 2014, 168, 214–221 CrossRef CAS PubMed.
- D. Abramson, R. Hulasare, R. K. York, N. D. G. White and D. S. Jayas, Mycotoxins, ergosterol, and odor volatiles in durum wheat during granary storage at 16% and 20% moisture content, J. Stored Prod. Res., 2005, 41(1), 67–76 CrossRef CAS.
- T. Filbakk, O. A. Høibø, J. Dibdiakova and J. Nurmi, Modelling moisture content and dry matter loss during storage of logging residues for energy, Scand. J. For. Res., 2011, 26(3), 267–277 CrossRef.
- C. Karunakaran, W. E. Muir, D. S. Jayas, N. D. G. White and D. Abramson, Safe storage time of high moisture wheat, J. Stored Prod. Res., 2001, 37(3), 303–312 CrossRef CAS PubMed.
- E. Chiotelli and M. Le Meste, Effect of small and large wheat starch granules on thermomechanical behavior of starch, Cereal Chem., 2002, 79(2), 286–293 CrossRef CAS.
- J. D. Wilson, D. B. Bechtel, T. C. Todd and P. A. Seib, Measurement of wheat starch granule size distribution using image analysis and laser diffraction technology, Cereal Chem., 2006, 83(3), 259–268 CrossRef CAS.
- H. S. Kim and K. C. Huber, Physicochemical properties and amylopectin fine structures of A-and B-type granules of waxy and normal soft wheat starch, J. Cereal. Sci., 2010, 51(3), 256–264 CrossRef CAS.
- A. A. Barreto, R. Abalone, A. Gastón and R. Bartosik, Analysis of storage conditions of a wheat silo-bag for different weather conditions by computer simulation, Biosyst. Eng., 2013, 116(4), 497–508 CrossRef.
- E. Raudienė, D. Rušinskas, G. Balčiūnas, G. Juodeikienė and D. Gailius, Carbon dioxide respiration rates in wheat at various temperatures and moisture contents, Mapan, 2017, 32(1), 51–58 CrossRef.
- P. V. Hung, T. Maeda, S. Yamamoto and N. Morita, Effects of germination on nutritional composition of waxy wheat, J. Sci. Food Agric., 2012, 92(3), 667–672 CrossRef CAS PubMed.
- U. Nithya, V. Chelladurai, D. S. Jayas and N. D. G. White, Safe storage guidelines for durum wheat, J. Stored Prod. Res., 2011, 47(4), 328–333 CrossRef.
- A. Miœ, Influence of the storage of wheat flour on the physical properties of gluten, Int. Agrophys., 2003, 17, 71–75 Search PubMed.
- R. Rajarammanna, D. S. Jayas and N. D. G. White, Comparison of deterioration of rye under two different storage regimes, J. Stored Prod. Res., 2010, 46(2), 87–92 CrossRef.
- M. E. J. Al-Defiery and A. F. Merjan, Mycoflora of mold contamination in wheatflour and storage wheat flour, Mesop. Environ. J., 2015, 1(2), 18–25 Search PubMed.
- A. F. Doblado-Maldonado, O. A. Pike, J. C. Sweley and D. J. Rose, Key issues and challenges in whole wheat flour milling and storage, J. Cereal. Sci., 2012, 56(2), 119–126 CrossRef.
- U. Nithya, V. Chelladurai, D. S. Jayas and N. D. G. White, Safe storage guidelines for durum wheat, J. Stored Prod. Res., 2011, 47(4), 328–333 CrossRef.
- Y. Wang, H. Duan, H. Zhang and Z. Fang, Modeling on heat and mass transfer in stored wheat during forced cooling ventilation, J. Therm. Sci., 2010, 19(2), 167–172 CrossRef.
- P. Fandohan, B. Gnonlonfin, K. Hell, W. F. O. Marasas and M. J. Wingfield, Impact of indigenous storage systems and insect infestation on the contamination of maize with fumonisins, Afr. J. Biotechnol., 2006, 5(7), 546–552 Search PubMed.
- D. Befikadu, Factors affecting quality of grain stored in Ethiopian traditional storage structures and opportunities for improvement, Int. J. Sci. Basic Appl. Res., 2014, 18(1), 235–257 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |