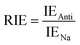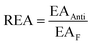Open Access Article
Open Access ArticleFunctionalization and antioxidant activity of polyaniline–fullerene hybrid nanomaterials: a theoretical investigation†
Nguyen Minh Thong *a,
Quan V. Vo
*a,
Quan V. Vo *b,
Trinh Le Huyen
*b,
Trinh Le Huyen cd,
Mai Van Baye,
Nguyen Nho Dungf,
Pham Thi Thu Thaodg and
Pham Cam Nam
cd,
Mai Van Baye,
Nguyen Nho Dungf,
Pham Thi Thu Thaodg and
Pham Cam Nam *d
*d
aThe University of Danang, Campus in Kon Tum, 704 Phan Dinh Phung, Kon Tum 580000, Vietnam. E-mail: nmthong@kontum.udn.vn
bThe University of Danang, University of Technology and Education, 48 Cao Thang, Danang 550000, Vietnam. E-mail: vvquan@ute.udn.vn
cDepartment of Applied Chemistry, National Chiao Tung University, Hsinchu 30010, Taiwan
dDepartment of Chemical Engineering, The University of Danang, University of Science and Technology, 54 Nguyen Luong Bang, Danang 550000, Vietnam. E-mail: pcnam@dut.udn.vn
eThe University of Danang, University of Science and Education, Danang 550000, Vietnam
fDanang University of Physical Education and Sports, Danang 550000, Vietnam
gDepartment of Chemistry, University of Sciences, Hue University, 77 Nguyen Hue, Hue 530000, Vietnam
First published on 9th April 2020
Abstract
Functionalized fullerene is one of the most advantageous nanotechnologies to develop novel materials for potential biomedical applications. In this study, we applied the ONIOM-GD3 approach to explore the nucleophilic addition reaction mechanism between polyaniline (emeraldine and leucoemeraldine forms) and fullerene. Potential energy surfaces were also analyzed to predict the predominantly formed products of the functionalized reaction. The themoparameters, such as bond dissociation enthalpy (BDE), ionization energy (IE), and electron affinity (EA), characterized by two mechanisms HAT and SET, were used to evaluate the antioxidant activities of the selected compounds. Moreover, the calculated HOMO, LUMO, and DOS results indicate that the electronic structures of polyaniline–fullerene were significantly affected by the presence of fullerene. The computational results show that C60-L1 seems to be the best antioxidant following the SET mechanism.
1. Introduction
Nowadays, hybrid nanomaterials formed between conjugated polymers and carbon nanomaterials have been attracting considerable attention from experimental and theoretical researchers because of their important role in developing science and technologies.1–4Polyaniline belong to the family of π-conjugated polymers and its low cost as well as high application potential has attracted significant attention. Recently, the antioxidant activity of polyaniline has attracted substantial interest in biomedical applications. Polyaniline can be combined with carbon nanomaterials such as fullerenes,5,6 carbon nanotubes,7 and graphene.8,9 Among them, [60]fullerene has raised much interest in the field of biomedical applications and materials science10–14 due to their attractive chemical–physical characteristics. In fact, fullerene has been regarded as ‘radical sponges’ to trap multiple radicals per molecule.15,16 Functionalization of fullerene is well known as a worthy way to develop the new fullerene based nanomaterials. In fact, the nucleophilic addition reactions between aromatic amines and fullerene by using solvent-free gas phase have been one of the most widely used functionalization approaches because this technique helps to prevent the contamination by impurities and organic solvents, which is important for biomedical applications.17
For the large molecular systems such as fullerene derivatives, in order to determine accurately the reaction mechanism of fullerene functionalization, the ONIOM method developed by Morokuma and coworkers is one of the suitable choices.18 In fact, numerous previous studies applied the two-layered ONIOM approach for the investigation of chemical reactions involving fullerene.19–22 To get an accurate description of the energy profile for the study of the reaction of fullerene functionalization,21 the GD3 empirical dispersion correction, according to Grimme,23 was also taken into account in the ONIOM approach (ONIOM-GD3).
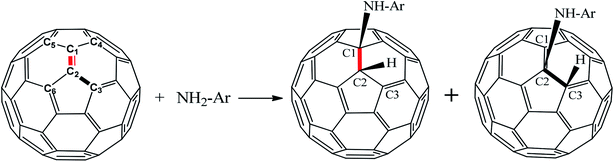
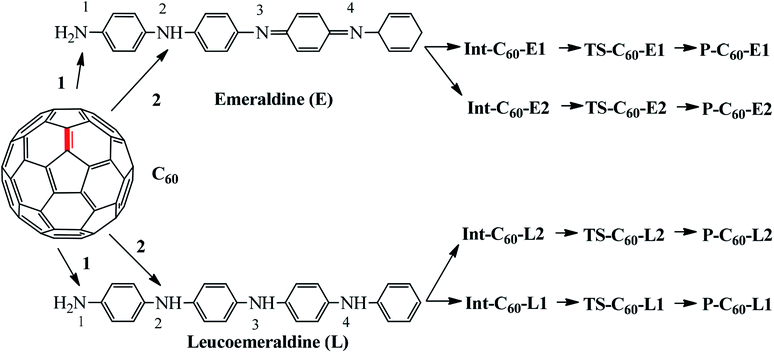
Based on the experimental reaction,17 the reaction mechanism between aniline and fullerene (Fig. 1) was reproduced and studied in detail via the ONIOM-GD3 method. Then, we systematically explored the possible reaction paths for fullerene functionalization based on the reactions of polyaniline in the form of emeraldine (E) and leucoemeraldine (L) using the same ONIOM-GD3 method (Fig. 2). A question was raised about what kind of these products is preferably formed. To solve this problem, the potential energy surface (PES) was investigated to clarify the mechanism of these reactions.
To date, numerous studies on antioxidant activity of polyaniline24,25 and fullerene26 have been reported. For aromatic amine antioxidants, the reaction mechanism and rate depend on the hydrogen atom's donating ability to peroxyl radicals. Moreover, the characteristics of fullerenes have an extended π-bond system, and their electron affinity is higher than that of primary antioxidants. Thus, the question is whether the derivatization of fullerenes with polyaniline is expected to increase their antioxidant capacity. However, theoretical approaches on the radical scavenging activity of new polyaniline–fullerene have not been realized in the literature yet. Therefore, we aim to predict the antioxidant activities of polyaniline–fullerene via single electron transfer (SET) and hydrogen atom transfer (HAT) mechanisms along with the important thermal-parameters such as ionization energy (IE), electron affinity (EA), and bond dissociation enthalpy (BDE) parameters.
In this study, we focus on studying the mechanism for the reaction of fullerene with the two forms of polyaniline as well as to evaluate the antioxidant ability of polyaniline–fullerene hybrid nanomaterials using the ONIOM-GD3 (B3LYP/6-31G(d):PM6) and hybrid density functional theory (DFT) methods.
2. Computational methods
All the electronic structures of species involved in the title reaction as reactants, intermediates, transition states, and products were optimized using the two-layered ONIOM-GD3 (B3LYP/6-31G(d):PM6) method including Grimme's dispersion corrections.23 The transition states were first obtained and then confirmed by the presence of one imaginary frequency. Furthermore, the intrinsic reaction coordinate (IRC) was also performed for analyzing the connection between the transition states (TS) and two related local minimum structures.The geometry and the vibrational frequency of the composited polyaniline–fullerene and the related neutral, cationic, and anionic radicals were carried out at the B3LYP/6-31G(d) method.27,28 From these optimized structures, the single-point energy calculations were employed at the level of B3LYP/6-311++G(d,p).
Moreover, the full-electron donor–acceptor map (FEDAM) was established to analyze the radical scavenging activity of the studied compounds via the electron acceptance (REA) and electron donation (RIE) indices, which were defined according to the equations:
where Anti, Na, and F represent the antioxidant molecule, sodium, and fluorum, respectively.
The calculations for this study were performed using the Gaussian 09 software.29
3. Results and discussion
3.1. Functionalized fullerene using the ONIOM method
First, the reliability of the model of fullerene and aniline using the ONIOM-GD3 (B3LYP/6-31G(d):PM6) method in this study was tested by comparing the optimized structures and thermodynamic parameters of reactants, transition states, and products with the computed values using B3LYP-GD3/6-31+G(d) for all atoms in this system. The results are shown in Table 1. For convenience, the intermediates, transition states, and products are marked as “Int”, “TS”, and “P” acronyms, respectively.| Species | ONIOM-GD3 (B3LYP/6-31G(d):PM6) | B3LYP-GD3/6-31+G(d) | Deviationc | |
|---|---|---|---|---|
| a Distance is in angstroms Å.b Relative energy in kcal mol−1 (zero-point energies were included).c Values (B3LYP-GD3/6-31+G(d)) – values (ONIOM-GD3 (B3LYP/6-31G(d):PM6)).d Ref. 30. | ||||
| Optimized structures (bond distance)a | ||||
| Fullerene | C1–C2 | 1.401 | 1.396 (1.401)d | −0.005 (0.000) |
| C2–C3 | 1.458 | 1.454 (1.458)d | −0.004 (0.000) | |
| TS(5,6) | C1–C2 | 1.486 | 1.483 | −0.003 |
| C2–C3 | 1.610 | 1.582 | −0.028 | |
| C2–C6 | 1.529 | 1.525 | −0.004 | |
| C2–N | 1.580 | 1.608 | 0.028 | |
| TS(6,6) | C1–C2 | 1.574 | 1.555 | −0.019 |
| C1–C4 | 1.523 | 1.521 | −0.002 | |
| C1–C5 | 1.525 | 1.523 | −0.002 | |
| C1–N | 1.582 | 1.590 | 0.008 | |
| P(5,6) | C1–C2 | 1.537 | 1.527 | −0.010 |
| C2–C3 | 1.640 | 1.624 | −0.016 | |
| C2–C6 | 1.547 | 1.543 | −0.004 | |
| C2–N | 1.467 | 1.473 | 0.006 | |
| P(6,6) | C1–C2 | 1.627 | 1.601 | −0.026 |
| C1–C5 | 1.544 | 1.544 | 0.000 | |
| C1–C4 | 1.556 | 1.553 | −0.003 | |
| C1–N | 1.465 | 1.472 | 0.007 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Relative energiesb | ||||
| Reactants | 0.0 | 0.0 | 0.0 | |
| Int | −4.0 | −3.1 | 0.9 | |
| TS(5,6) | 44.1 | 48.4 | 4.3 | |
| TS(6,6) | 32.8 | 36.4 | 3.6 | |
| P(5,6) | 9.9 | 15.2 | 5.3 | |
| P(6,6) | −4.9 | −2.4 | 2.5 | |
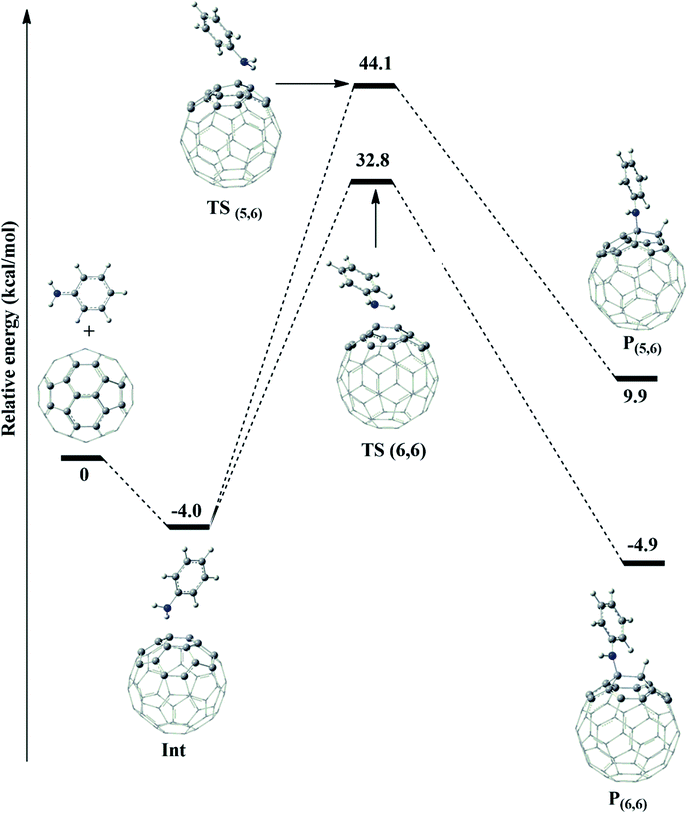
As can be seen in Table 1, the optimized bond distances of fullerene using the ONIOM-GD3 (B3LYP/6-31G(d):PM6) method agree well with the experimental data. Indeed, the calculated/experimental [5-6] (C2–C3) and [6-6] (C1–C2) bonds were 1.458/1.458 Å and 1.401/1.401 Å, respectively.30 The calculated data at the ONIOM-GD3 (B3LYP/6-31G(d):PM6) and B3LYP-GD3/6-31+G(d) levels of theory in Table 1 show that the geometrical parameters of the transition states and products have a very small deviation in the range of −0.028 to 0.028 Å. For the thermodynamic parameters, the deviation between the two methods is in the range of 0.9 to 5.3 kcal mol−1. It can be observed that the calculated values obtained in the proposed ONIOM-GD3 model were in very good agreement with the corresponding data obtained using the B3LYP-GD3/6-31+G(d) method for the whole system. Thus, this ONIOM-GD3 partition is recommended as a reliable and computationally affordable approach to be exploited in this study.
In the initial step, the association of fullerene and aniline formed an intermediate (Int) with a binding energy of −4.0 kcal mol−1 lower than the reactants (see Fig. 3). This intermediate can proceed through two channels for the abstraction process of fullerene. The first one was a process at the (6,6)–bond to yield TS(6,6), whose energy barrier was 36.8 kcal mol−1. The second one occurs to form P(5,6) at the (5,6)−bond via the transition state TS(5,6) during the reaction, as confirmed by one imaginary vibrational frequency and IRC analysis presented in Table S1 and Fig. S1 of ESI.† As shown in Fig. 3, the energy barrier of TS(5,6) was higher than that of TS(6,6) by 11.3 kcal mol−1. Therefore, a conclusion can be drawn that the process for the P(6,6) product was energetically favored than that for P(5,6) product. It is clear that the theoretical results obtained from this section are also similar to the experimental procedures in order to synthesize aniline–fullerene.17
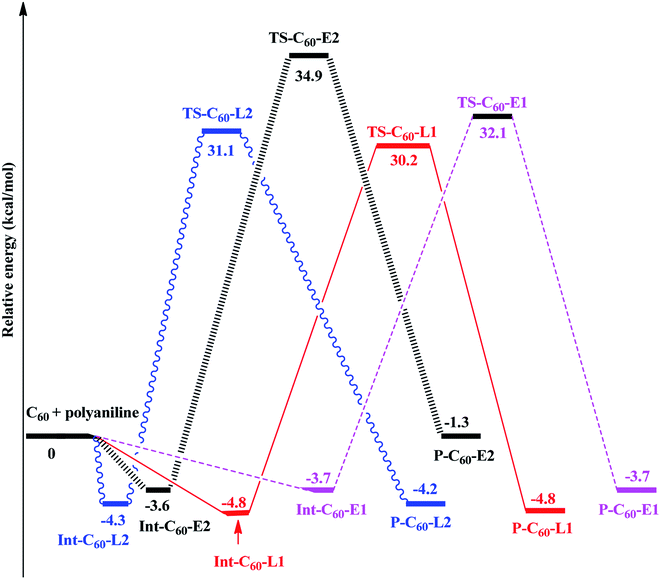
Thus, the PES of the reaction between fullerene and polyaniline (PANI) focus only on the (6-6)–bond, which is illustrated in Fig. 3. Depending on the environment, the structure of PANI consists of three forms, including leucoemeraldine (PANI-L), pernigraniline (PANI-P), and emeraldine (PANI-E). However, in this context, we chose two forms, namely PANI-L and PANI-E, to investigate the functionalized reaction of fullerene with polyaniline because the structure of pernigraniline does not possess the –NH group to perform the amination reaction.
Fig. 4 shows that all the obtained reactions of two forms of polyaniline with fullerene have tended to be quite similar. Energies of all the species and energy barriers for the reaction between fullerene and two forms of polyaniline using the ONIOM-GD3 (B3LYP/6-31G(d):PM6) method are summarized in Tables S1 and S2 (ESI†). The intermediates being a local minimum on the potential energy surface were formed from the reactants, without transition states. The relative energies to the corresponding intermediates Int-C60-E1, Int-C60-E2, Int-C60-L1, and Int-C60-L2 were −3.7, −3.6,−4.8, and −4.3 kcal mol−1, respectively. The reaction pathway diagram shows that the energy barriers of TS-C60-E1, TS-C60-E2, TS-C60-L1, and TS-C60-L2 were 35.8, 38.5, 35.0, and 35.4 kcal mol−1, respectively. The relative energies of products, namely P-C60-E1, P-C60-E2, P-C60-L1, and P-C60-L2, were predicted to be −3.7, −1.3, −4.8, and −4.2 kcal mol−1, respectively, in comparison to the reactants. Thus, this obtained result suggests that P-C60-E1, P-C60-L1, and P-C60-L2 products were predominantly formed from a thermodynamic point of view.
3.2. Antioxidant mechanisms
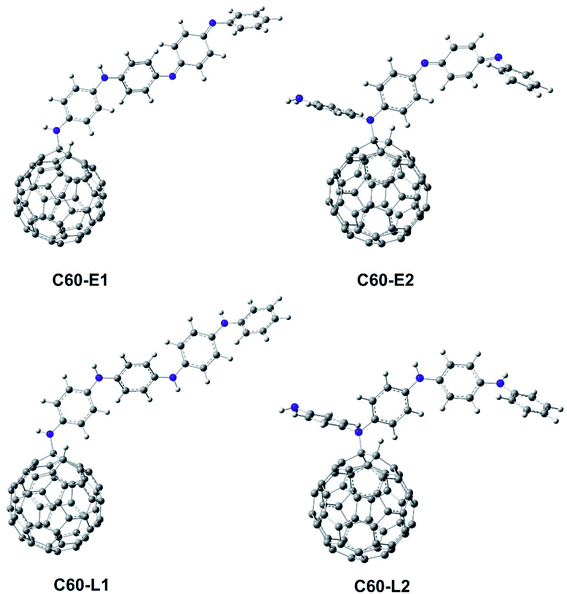
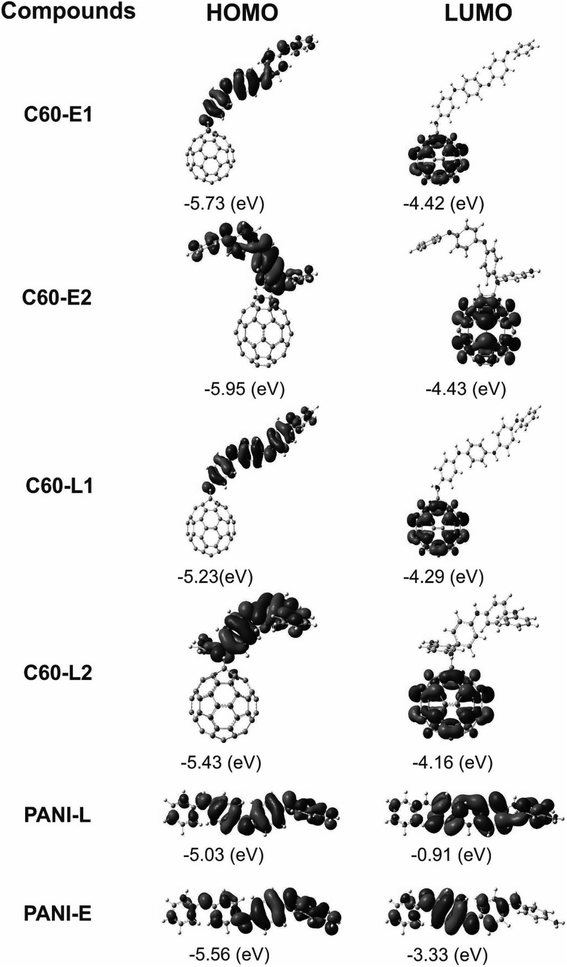
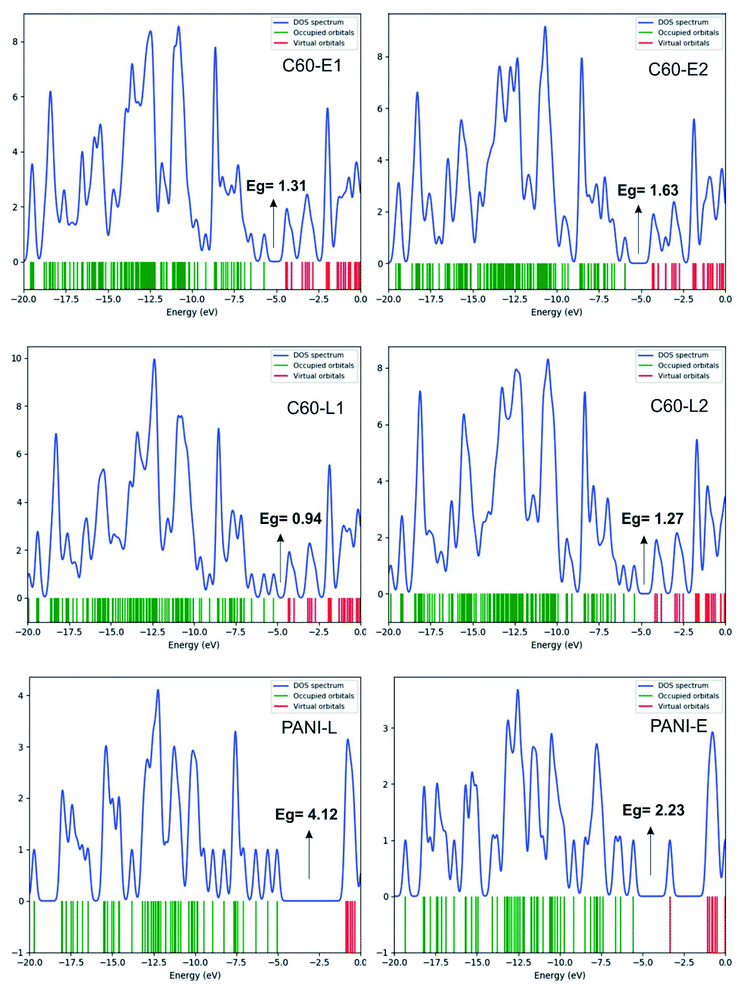
The structure of polyaniline–fullerene consists of two typical segments: polyaniline known as chain-breaking antioxidants25,31 and fullerene known as an extended π-bond system and a high electron affinity.32 For this reason, both HAT and SET mechanisms were used to analyze the radical quenching capacity of the products of reactions between fullerene and the two forms of polyaniline. The structures of C60-L1, C60-L2, C60-E1, and C60-E2 are shown in Fig. 5. The thermochemical parameters characterized by antioxidant activity (BDE, IE, and EA) were calculated using the B3LYP/6-311++G(d,p)//B3LYP/6-31G(d) model. Moreover, the Grimme's dispersion corrections23 were included to evaluate the effects of dispersion interactions on the thermochemical parameters. As can be seen in Tables S3 and S4 (ESI†), the deviation between BDE, IE, and EA calculated at the B3LYP-GD3 and B3LYP levels were very small (<1.0 kcal mol−1). Therefore, the effects of dispersion interactions on the thermochemical parameters are insignificant.| Compounds | BDE(N–H) (kcal mol−1) | Compounds | BDE(N–H) (kcal mol−1) |
|---|---|---|---|
| PANI-L | C60-L2 | ||
| N1–H | 85.09 | N1–H | 89.78 |
| N2–H | 78.04 | N3–H | 79.75 |
| N3–H | 78.10 | N4–H | 81.14 |
| N4–H | 79.92 | ||
| PANI-E | C60-E1 | ||
| N1–H | 86.83 | N1–H | 83.86 |
| N2–H | 78.66 | N2–H | 78.93 |
| C60-L1 | C60-E2 | ||
| N1–H | 81.85 | N1–H | 90.88 |
| N2–H | 78.14 | ||
| N3–H | 78.31 | ||
| N4–H | 80.21 |
| Compounds | Adiabatic IE (kcal mol−1) | Adiabatic EA (kcal mol−1) |
|---|---|---|
| PANI-L | 130.36 | 0.58 |
| PANI-E | 140.74 | 45.62 |
| C60-L1 | 130.98 | 65.10 |
| C60-L2 | 133.97 | 61.74 |
| C60-E1 | 140.56 | 66.46 |
| C60-E2 | 143.84 | 65.18 |
It was found that the IE values of the materials (130.98–143.84 kcal mol−1) were larger than those of the polyanilines (130.36–140.74 kcal mol−1). This suggests that the polyaniline–fullerene hybrid nanomaterials hardly transferred an electron compared with the initial polyanilines. However, it is worth noticing that the EAs of the materials were significantly higher than those of the polyanilines (averaged about 29.83 kcal mol−1). Thus, the accepting-electron process seems to be the main mechanism responsible for the antioxidant activity of the polyaniline–fullerenes.
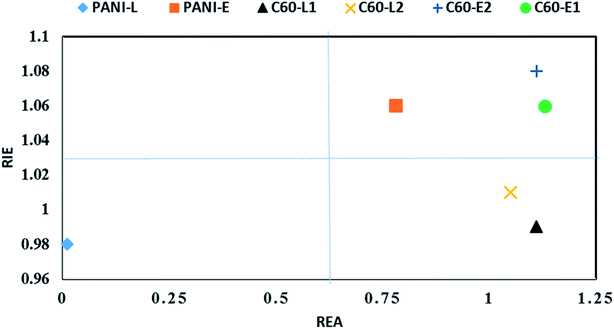
Based on FEDAM shown in Fig. 6, it can be seen that C60-L1 belongs to the best antioxidant sector. It demonstrates that C60-L1 simultaneously plays the two roles as a good electron donator and a good electron acceptor.
3.3. Reaction between CH3OO˙, O2˙−and polyaniline–fullerene
The interaction between polyaniline–fullerene and CH3OO˙ and O2˙− was evaluated through an electron transfer mechanism in the gas phase to provide more insight into their free radical quenching capacity following the reaction equations:| Anti + R˙ → Anti+˙+ R− | (1) |
| Anti + R˙ → Anti−˙ + R+ | (2) |
As can be seen in Table 4, the reactions Anti + CH3OO˙ following (1) and (2) are unspontaneous with the high positive thermal barriers of ΔH and ΔG (ΔH = 111.2 ÷ 266.6 kcal mol−1, ΔG = 114.7 ÷ 265.6 kcal mol−1). The trend was similar in the electron-donating process of the Anti + O2˙− reaction. However, it is worth noticing that the electron-accepting process of the Anti + O2˙− reaction following (2) was spontaneous (ΔH = −15.2 ÷ −20.8 kcal mol−1, ΔG = −16.7 ÷ −20.7 kcal mol−1), whereas that for the initial polymers was unspontaneous (ΔG > 0). This affirms that the polyaniline–fullerene materials are good in O2˙− scavenging following the electron-accepting mechanism. This is a good agreement with the previous studies.35
| Compounds | Anti + CH3OO˙ | Anti + O2˙− | ||||||
|---|---|---|---|---|---|---|---|---|
| Reaction (1) | Reaction (2) | Reaction (1) | Reaction (2) | |||||
| ΔH | ΔG | ΔH | ΔG | ΔH | ΔG | ΔH | ΔG | |
| C60-L1 | 113.4 | 114.7 | 190.1 | 190.3 | 339.9 | 340.6 | −19.0 | −18.9 |
| C60-L2 | 115.7 | 117.3 | 193.9 | 192.5 | 342.2 | 343.2 | −15.2 | −16.7 |
| C60-E1 | 123.6 | 124.6 | 188.3 | 188.5 | 350.0 | 350.5 | −20.8 | −20.7 |
| C60-E2 | 126.4 | 127.8 | 190.3 | 190.4 | 352.8 | 353.7 | −18.8 | −18.8 |
| PANI-L | 111.2 | 113.0 | 266.6 | 265.6 | 337.6 | 338.9 | 57.5 | 56.4 |
| PANI-E | 122.1 | 123.2 | 213.8 | 212.9 | 348.6 | 349.1 | 4.7 | 3.7 |
4. Conclusion
In this study, some conclusions in investigating the characteristics of new polyaniline–fullerene materials can be drawn as:- Potential energy surfaces of the reaction between fullerene and polyaniline (PANI) were analyzed via the ONIOM method. The obtained result suggests that P-C60-E1and P-C60-L1 products were predominantly formed.
- Evaluating the antioxidant activity of polyaniline–fullerene materials using these themoparameters (BDE, IE, and EA) established the HAT and SET mechanism and found that the favored mechanism is the electron-accepting one. In addition to the calculated HOMO, LUMO, DOS, and FEDAM results show that C60-L has the strongest antioxidant activity among the new polyaniline–fullerene materials.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.06-2018.42.References
- S. Xiong, F. Yang, H. Jiang, J. Ma and X. Lu, Covalently bonded polyaniline/fullerene hybrids with coral-like morphology for high-performance supercapacitor, Electrochim. Acta, 2012, 85, 235–242, DOI:10.1016/j.electacta.2012.08.056.
- S. Xiong, et al., Covalent bonding of polyaniline on fullerene: Enhanced electrical, ionic conductivities and electrochromic performances, Electrochim. Acta, 2012, 67, 194–200, DOI:10.1016/j.electacta.2012.02.026.
- H. Wang, X. Yan and G. Piao, A high-performance supercapacitor based on fullerene C 60 whisker and polyaniline emeraldine base composite, Electrochim. Acta, 2017, 231, 264–271, DOI:10.1016/j.electacta.2017.02.057.
- N. Politakos, I. Zalakain, B. Fernandez d'Arlas, A. Eceiza and G. Kortaberria, Optical, structural and electrical properties of polyaniline systems doped with C60 and small gap C60 fullerenes, Mater. Chem. Phys., 2013, 142, 387–394, DOI:10.1016/j.matchemphys.2013.07.033.
- M. Gizdavic-Nikolaidis, J. Vella, G. A. Bowmaker and Z. D. Zujovic, Rapid microwave synthesis of polyaniline–C 60 nanocomposites, Synth. Met., 2016, 217, 14–18, DOI:10.1016/j.synthmet.2016.03.009.
- I. Y. Sapurina, et al., Polyaniline composites with fullerene C60, Phys. Solid State, 2002, 44, 574–575, DOI:10.1134/1.1462712.
- M. Baibarac, I. Baltog, S. Lefrant, J. Y. Mevellec and O. Chauvet, Polyaniline and Carbon Nanotubes Based Composites Containing Whole Units and Fragments of Nanotubes, Chem. Mater., 2003, 15, 4149–4156, DOI:10.1021/cm021287x.
- L. Feng, et al., Structure stability of polyaniline/graphene nanocomposites in gamma-ray environment, J. Radioanal. Nucl. Chem., 2018, 315, 627–638, DOI:10.1007/s10967-018-5710-y.
- G. Wang, W. Xing and S. Zhuo, The production of polyaniline/graphene hybrids for use as a counter electrode in dye-sensitized solar cells, Electrochim. Acta, 2012, 66, 151–157, DOI:10.1016/j.electacta.2012.01.088.
- X. Zhang, H. Cong, B. Yu and Q. Chen, Recent Advances of Water-Soluble Fullerene Derivatives in Biomedical Applications, Mini-Rev. Org. Chem., 2018, 16, 92–99, DOI:10.2174/1570193x15666180712114405.
- M. Mohajeri, B. Behnam and A. Sahebkar, Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials, J. Cell. Physiol., 2018, 234, 298–319, DOI:10.1002/jcp.26899.
- F. Moussa, [60]Fullerene and derivatives for biomedical applications, 113–136, 2018, DOI:10.1016/b978-0-08-100716-7.00005-2.
- S. Goodarzi, T. Da Ros, J. Conde, F. Sefat and M. Mozafari, Fullerene: biomedical engineers get to revisit an old friend, Mater. Today, 2017, 20, 460–480, DOI:10.1016/j.mattod.2017.03.017.
- X. Zhu, M. Sollogoub and Y. Zhang, Biological applications of hydrophilic C60 derivatives (hC60s)- a structural perspective, Eur. J. Med. Chem., 2016, 115, 438–452, DOI:10.1016/j.ejmech.2016.03.024.
- P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Radical reactions of c60, Science, 1991, 254, 1183–1185, DOI:10.1126/science.254.5035.1183.
- J. R. Morton, F. Negri and K. F. Preston, Addition of Free Radicals to C60, Acc. Chem. Res., 1998, 31, 63–69, DOI:10.1021/ar950120p.
- I. J. Ramírez-Calera, et al., Solvent-free functionalization of fullerene C60 and pristine multi-walled carbon nanotubes with aromatic amines, Appl. Surf. Sci., 2015, 328, 45–62, DOI:10.1016/j.apsusc.2014.11.188.
- L. W. Chung, et al., The ONIOM Method and Its Applications, Chem. Rev., 2015, 115, 5678–5796, DOI:10.1021/cr5004419.
- S. Osuna, J. Morera, M. Cases, K. Morokuma and M. Sola, Diels-Alder reaction between cyclopentadiene and C60: an analysis of the performance of the ONIOM method for the study of chemical reactivity in fullerenes and nanotubes, J. Phys. Chem. A, 2009, 113, 9721–9726, DOI:10.1021/jp904294y.
- S. Filippone, et al., On the mechanism of the thermal retrocycloaddition of pyrrolidinofullerenes (retro-Prato reaction), Chem.–Eur. J., 2008, 14, 5198–5206, DOI:10.1002/chem.200800096.
- S. Osuna, M. Swart and M. Sola, Dispersion corrections essential for the study of chemical reactivity in fullerenes, J. Phys. Chem. A, 2011, 115, 3491–3496, DOI:10.1021/jp1091575.
- N. M. Thong, et al., Functionalization of fullerene via the Bingel reaction with alpha-chlorocarbanions: an ONIOM approach, J. Mol. Model., 2016, 22, 113, DOI:10.1007/s00894-016-2981-5.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- C. F. Hsu, H. Peng, C. Basle, J. Travas-Sejdic and P. A. Kilmartin, ABTS˙+ scavenging activity of polypyrrole, polyaniline and poly(3,4-ethylenedioxythiophene), Polym. Int., 2011, 60, 69–77, DOI:10.1002/pi.2912.
- M. Gizdavic-Nikolaidis, J. Travas-Sejdic, P. A. Kilmartin, G. A. Bowmaker and R. P. Cooney, Evaluation of antioxidant activity of aniline and polyaniline, Curr. Appl. Phys., 2004, 4, 343–346, DOI:10.1016/j.cap.2003.11.044.
- M. D. Tzirakis and M. Orfanopoulos, Radical reactions of fullerenes: from synthetic organic chemistry to materials science and biology, Chem. Rev., 2013, 113, 5262–5321, DOI:10.1021/cr300475r.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652, DOI:10.1063/1.464913.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789, DOI:10.1103/PhysRevB.37.785.
- Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- K. Hedberg, et al., Bond lengths in free molecules of buckminsterfullerene, c60, from gas-phase electron diffraction, Science, 1991, 254, 410–412, DOI:10.1126/science.254.5030.410.
- K. U. Ingold and D. A. Pratt, Advances in radical-trapping antioxidant chemistry in the 21st century: a kinetics and mechanisms perspective, Chem. Rev., 2014, 114, 9022–9046, DOI:10.1021/cr500226n.
- L. Echegoyen and L. E. Echegoyen, Electrochemistry of Fullerenes and Their Derivatives, Acc. Chem. Res., 1998, 31, 593–601, DOI:10.1021/ar970138v.
- A. Martinez, R. Vargas and A. Galano, What is important to prevent oxidative stress? A theoretical study on electron-transfer reactions between carotenoids and free radicals, J. Phys. Chem. B, 2009, 113, 12113–12120, DOI:10.1021/jp903958h.
- W. Cai, Y. Chen, L. Xie, H. Zhang and C. Hou, Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues, Eur. Food Res. Technol., 2013, 238, 121–128, DOI:10.1007/s00217-013-2091-x.
- J. J. Yin, et al., The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials, Biomaterials, 2009, 30, 611–621, DOI:10.1016/j.biomaterials.2008.09.061.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00903b |
| This journal is © The Royal Society of Chemistry 2020 |