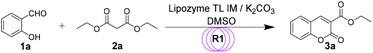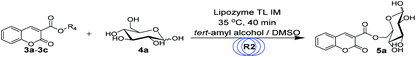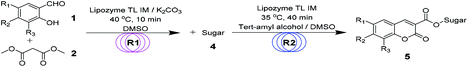Open Access Article
Open Access ArticleA sustainable innovation for the tandem synthesis of sugar-containing coumarin derivatives catalyzed by lipozyme TL IM from Thermomyces lanuginosus in continuous-flow microreactors†
Li-Hua Du *a,
Ping-Feng Chena,
Rui-Jie Longa,
Miao Xuea and
Xi-Ping Luo*b
*a,
Ping-Feng Chena,
Rui-Jie Longa,
Miao Xuea and
Xi-Ping Luo*b
aCollege of Pharmaceutical Science, ZheJiang University of Technology, Hangzhou, 310014, China. E-mail: orgdlh@zjut.edu.cn; orgdlh@gmail.com; Fax: +86 18969069399
bZhejiang Provincial Key Laboratory of Chemical Utilization of Forestry Biomass, Zhejiang A&F University, Hangzhou, 311300, China
First published on 2nd April 2020
Abstract
We developed an efficient and environmentally friendly two-step tandem methodology for the synthesis of sugar-containing coumarin derivatives catalyzed by lipozyme TL IM from Thermomyces lanuginosus in continuous-flow microreactors. Compared to those observed for other methods, the salient features of this work including green reaction conditions, short residence time (50 min), and catalysts are more readily available and the biocatalysis reaction process is efficient and easy to control. This two-step tandem synthesis of coumarin derivatives using the continuous-flow technology is a proof of concept that opens the use of enzymatic microreactors in coumarin derivative biotransformations.
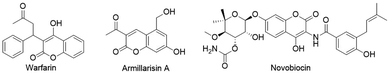
Coumarins and their derivatives have attracted considerable attention due to their extensive biological activities such as anti-bacterial, antiviral, anticancer, and antioxidant properties.1 Numerous studies including the separation and purification of naturally occurring coumarins from a variety of plants as well as the chemical synthesis of coumarin compounds with novel structures and properties have been conducted for the research and development of coumarins as potential drugs.2 So far, some coumarins, for example, warfarin,3 an anticoagulant that acts as a vitamin K antagonist, have been widely used in the treatment of thrombosis. Armillarisin A and novobiocin are commonly used as antibiotics (Fig. 1).4
As important coumarin derivatives, sugar-containing coumarin compounds have attracted special interest in organic synthesis and medicinal research due to their excellent physicochemical and pharmacokinetic characteristics.5 Thorson et al.6 reported that the glycosylation of the classical pharmacophore warfarin fundamentally alters the drug's mechanism of action, leading to a dramatic reduction in the anticoagulant function and a concomitant marked increase in anticancer cytotoxicity. In the past few years, several works on the synthesis of sugar-containing coumarins have been reported. Supuran et al.7 synthesized a series of glycosyl coumarin carbonic anhydrase IX and XII inhibitors, which strongly attenuated the growth of primary breast tumors. In 2016, Nilsson et al.8 reported a selective galactose-coumarin-derived galectin-3 inhibitor, which displayed efficacy similar to that of a known nonselective galectin-1/galectin-3 inhibitor.
The construction of sugar-containing coumarin derivatives can be achieved by basic synthetic approaches, and the most common synthesis strategy is the chemical method,9 which always needs several “protection–deprotection” steps. Recently, visible light has been used in the glycosylation reaction; however, most of the protocols for photoinduced glycosylation require transition metal catalysts in combination with expensive additives for the reaction to proceed.10
Enzymes are the most efficient catalysts, offering much more competitive processes compared to chemical catalysts.11 The use of enzymes as catalysts for the preparation of novel compounds has received increasing attention over the past few years. Some enzymes, such as the engineered C-glycosyltransferase MiCGTb-GAGM, were applied for the synthesis of coumarin C-glycosides, and two of them exhibited potent SGLT2 inhibitory activities.12 Nidetzky synthesized a new sugar-containing coumarin catalyzed by O-glycosyltransferase.13 Some other enzymes such as BLAP (alkaline protease from Bacillus licheniformis) have also been used to synthesize coumarin.14 The reactions catalyzed by enzymes are relatively mild; however, they always require a longer reaction time (24 h or more) to achieve the desired yields, and the enzymes needed for the reaction are difficult to obtain.15
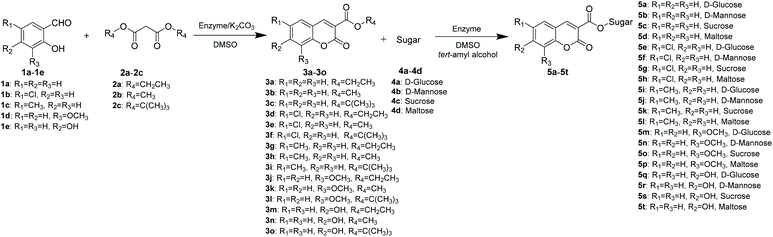
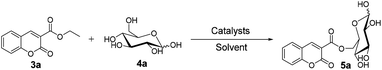
Continuous-flow microreactors coupled with enzymes have become an efficient way to increase the reaction efficiency and improve the yield.16 Modern synthetic chemistry faces the challenge of providing the society with high-performing, valuable products that are environmentally benign, cost-effective, safe, and atom-efficient. Due to a high surface-to-volume ratio, better heat exchange, and efficient mixing, the continuous-flow microreactor technology (MRT) has become increasingly popular as an alternative to conventional batch chemistry synthesis.17 In particular, with respect to the 12 principles of green chemistry, MRT can play a major role in improving chemical processes.18 To explore novel, eco-friendly and highly efficient protocols for sugar-containing coumarins and also as part of our ongoing study on the tandem microreaction technology, herein, we have reported a two-step tandem synthesis process of sugar-containing coumarin derivatives catalysed by lipozyme TL IM from Thermomyces lanuginosus in continuous-flow microreactors. The aim of this paper is to investigate using a two-step tandem continuous-flow microreactor the effect of various reaction parameters on the reaction yield. Furthermore, we hope to quickly build the related compound library through a new synthesis method for future drug screening (Scheme 1).
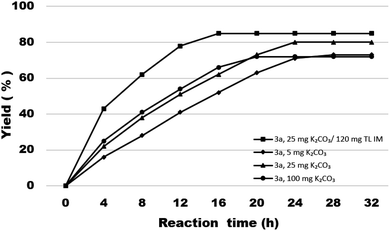
First, in order to determine whether lipozyme TL IM can be used to catalyze coumarin intermediates or sugar-containing coumarin synthesis reactions, we chose salicylaldehyde (1a) and diethyl malonate (2a) to react at 50 °C for 24 h in a shaker reactor (Table 1). The reaction catalyzed by lipozyme TL IM in DMSO gave the product 3a in 25% yield (entry 4, Table 1). We performed two groups of blank control trials and found that no product was detected without the enzyme (entry 1, Table 1). At the same time, this reaction did not occur with the denatured lipozyme TL IM (entry 2, Table 1) (lipozyme TL IM was heated in a water bath at 100 °C for 1 hour). When the reaction was catalyzed by a mixture of 25 mg K2CO3/120 mg lipozyme TL IM (K2CO3 accounts for 17% of the total mass of the catalysts), 85% yield of the target product was obtained in DMSO (entry 13, Table 1). To demonstrate the specific catalytic effect of lipozyme TL IM on the reaction, we monitored the reaction process; the time courses are shown in Fig. 2. It was found that the rate of the reaction catalyzed by the mixed catalyst was faster than that of the reaction catalyzed by K2CO3, especially in the range of 0–8 h. After 16 h, the product 3a catalyzed by the mixed catalyst reached equilibrium. Although the yield of 3a after catalysis by 25 mg K2CO3 peaked after 24 hours, it was not as high as the yield obtained after catalysis by the mixed catalyst.
| Entry | Solvent | Catalysts | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: salicylaldehyde (1a) (50 mM), diethyl malonate (2a) (100 mM), catalysts in 5 mL solvent, at 50 °C for 24 h.b Isolated yields. | |||
| 1 | DMSO | None | n.d. |
| 2 | DMSO | 100 mg lipozyme TL IM (denatured) | n.d. |
| 3 | Acetone | 100 mg lipozyme TL IM | 23 |
| 4 | DMSO | 100 mg lipozyme TL IM | 25 |
| 5 | tert-Amyl alcohols | 100 mg K2CO3 | <5 |
| 6 | Ethanol | 100 mg K2CO3 | 35 |
| 7 | Acetone | 100 mg K2CO3 | 61 |
| 8 | DMSO | 100 mg K2CO3 | 72 |
| 9 | DMSO | 50 mg K2CO3 | 78 |
| 10 | DMSO | 25 mg K2CO3 | 80 |
| 11 | DMSO | 5 mg K2CO3 | 71 |
| 12 | DMSO | 25 mg K2CO3/80 mg lipozyme TL IM | 84 |
| 13 | DMSO | 25 mg K2CO3/120 mg lipozyme TL IM | 85 |
| 14 | DMSO | 25 mg K2CO3/160 mg lipozyme TL IM | 83 |
For the synthesis of sugar-containing coumarins, considering that the enzyme and reaction media play a vital role in the reaction, we studied the effects of solvents and enzymes on the reaction (Table 2). As shown in Table 2, no reaction is observed in the presence of DMSO and THF (entry 2 and 6, Table 2). Also, no reaction occurs without the enzyme in tert-amyl alcohol (entry 1, Table 2). The best reaction yield of 29% can be achieved when the reaction is catalyzed by lipozyme TL IM in tert-amyl alcohol (entry 3, Table 2). Therefore, we chose tert-amyl alcohol as the solvent and lipozyme TL IM as the catalyst for the synthesis of sugar-containing coumarins.
| Entry | Solvent | Catalysts | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: ethyl coumarin-3-carboxylate (3a) (50 mM), D-glucose (4a) (25 mM), catalysts (200 mg) in 5 mL solvent, at 50 °C for 24 h.b Isolated yields. | |||
| 1 | tert-Amyl alcohol | None | n.d. |
| 2 | DMSO | Lipozyme TL IM | n.d. |
| 3 | tert-Amyl alcohol | Lipozyme TL IM | 29 |
| 4 | Acetone | Lipozyme TL IM | <5 |
| 5 | Isopropyl alcohol | Lipozyme TL IM | 13 |
| 6 | THF | Lipozyme TL IM | n.d. |
| 7 | Acetonitrile | Lipozyme TL IM | 17 |
| 8 | tert-Amyl alcohol | Subtilisin | n.d. |
| 9 | Acetonitrile | Subtilisin | n.d. |
| 10 | tert-Amyl alcohol | Nov 435 | 22 |
| 11 | Acetonitrile | Nov 435 | 9 |
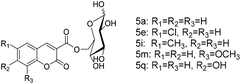
D-Glucose (4a) contains multiple hydroxyl groups, including 6-OH, 1-OH and secondary OHs. The acylation position of D-glucose was verified by 13C NMR according to the general strategy described by Yoshimoto et al.19 The 13C NMR data are shown in Table 3. The chemical signals for C-6α and C-6β of product 5a shifted downfield from 61.20 to 65.22 ppm and those for C-5α and C-5β shifted upfield from 71.80 to 69.20 ppm and from 76.70 to 73.49 ppm, respectively, while that for other C species did not change significantly. According to the general strategy described by Yoshimoto, the acylation of a hydroxyl group of the substrate results in a downfield shift of the O-acylated carbon and an upfield shift of the neighboring carbon. These results revealed that the acylation of D-glucose regioselectively occurred at the C-6 hydroxy position. A similar change was found in the 13C NMR data of the products 5e, 5i, 5m and 5q. We also determined the acylation positions of mannose (4b), sucrose (4c) and maltose (4d) in the same way. In the synthesis of sugar-containing coumarins catalyzed by lipozyme TL IM in tert-amyl alcohol, no other regioisomer was detected, indicating good regioselectivity.
| Carbon atom | D-Glucose | 5a | 5e | 5i | 5m | 5q |
|---|---|---|---|---|---|---|
| C-6α | 61.20 | 65.22 | 65.32 | 65.19 | 65.26 | 64.82 |
| C-6β | 61.20 | 65.22 | 65.36 | 65.19 | 65.26 | 64.82 |
| C-5α | 71.80 | 69.20 | 69.18 | 69.22 | 69.21 | 69.24 |
| C-5β | 76.70 | 73.49 | 73.48 | 73.51 | 73.50 | 73.55 |
| C-4α | 70.58 | 70.57 | 70.50 | 70.58 | 70.59 | 70.61 |
| C-4β | 70.30 | 70.17 | 70.10 | 70.18 | 70.19 | 70.22 |
| C-3α | 73.04 | 72.91 | 72.90 | 72.95 | 72.91 | 72.91 |
| C-3β | 76.79 | 76.46 | 76.45 | 76.49 | 76.46 | 76.47 |
| C-2α | 72.29 | 72.16 | 72.14 | 72.21 | 72.18 | 72.17 |
| C-2β | 74.78 | 74.71 | 74.68 | 74.75 | 74.72 | 74.71 |
| C-1α | 92.12 | 92.38 | 92.39 | 92.44 | 92.41 | 92.36 |
| C-1β | 96.79 | 97.00 | 97.01 | 97.06 | 97.02 | 96.98 |
Encouraged by these results, we wanted to design a two-step tandem continuous-flow protocol for the synthesis of sugar-containing coumarin derivatives. The experimental setup consisted of two flow microreactor systems: two syringe pumps (Harvard apparatus PHD 2000), two microtube reactors (R1 and R2) and two Y-shaped mixers (Y1 and Y2, φ = 1.8 mm) (Fig. 3). The microtube reactor R1 was filled with K2CO3/lipozyme TL IM (catalyst reactivity: 250 IUN g−1) and the microtube reactor R2 was filled with lipozyme TL IM. The whole flow microreactor system was dipped in a water bath to control the reaction temperature. A solution of the salicylaldehyde derivative (8.0 M) in DMSO and diester malonate derivative (16.0 M) in DMSO was passed through R1 (internal diameter 2.0 mm, length 100 cm) by a syringe pump with a flow rate of 62.4 μL min−1. The outlet mixture was mixed with a solution of sugar (50 mM in DMSO/tert-amyl alcohol) in Y2 and passed through R2 (flow rate: 15.6 μL min−1). After the reaction, the discharge was collected in a glass vessel. A final yield of 43–75% was obtained by column chromatography after the solvent was removed by vacuum distillation. The pure product was characterized by 1H NMR, 13C NMR and ESI-MS.
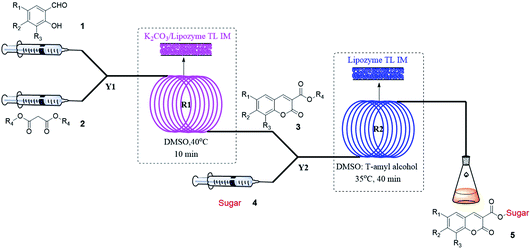 | ||
| Fig. 3 The experimental setup of two-step tandem sugar-containing coumarin derivative synthesis in microreactors. | ||
We studied the reaction parameters of each step before the two-step tandem synthesis of sugar-containing coumarin derivatives in continuous-flow microreactors. For the coumarin intermediate synthesis reaction, we selected the reaction of salicylaldehyde (1a) and diethyl malonate (2a) as the model reaction and studied the effect of the substrate molar ratio, catalysts, reaction temperature and residence time on the reaction. The results are summarized in Table 4. From entry 1–4, we can find that excess diethyl malonate (2a) can facilitate the reaction. Considering atom economy, we chose salicylaldehyde (1a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) diethyl malonate (2a) = 1
diethyl malonate (2a) = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as the optimum substrate molar ratio. From entry 3, 5, 6 and 7, we can find that the best reaction occurs when K2CO3 is 12% (mass percent) in the mixed catalyst K2CO3/lipozyme TL IM (entry 5, Table 4). We then explored the effect of the reaction temperature (entry 5, 8–10, Table 4) and found that the optimal temperature was 40 °C (entry 9, Table 4). The residence time of the reaction was a key factor in this process. We investigated the influence of residence time from 5 min to 30 min (entry 9, 11–13, Table 4); the results showed that the optimum yield (92%) was achieved after 10 minutes of reaction (entry 12, Table 4). Therefore, after studying the reaction parameters of the first step, we found that the optimal reaction could be achieved after a continuous reaction for 10 minutes at 40 °C under the catalysis of 12% K2CO3 (K2CO3 mass percent of the mixed catalyst K2CO3/lipozyme TL IM).
2 as the optimum substrate molar ratio. From entry 3, 5, 6 and 7, we can find that the best reaction occurs when K2CO3 is 12% (mass percent) in the mixed catalyst K2CO3/lipozyme TL IM (entry 5, Table 4). We then explored the effect of the reaction temperature (entry 5, 8–10, Table 4) and found that the optimal temperature was 40 °C (entry 9, Table 4). The residence time of the reaction was a key factor in this process. We investigated the influence of residence time from 5 min to 30 min (entry 9, 11–13, Table 4); the results showed that the optimum yield (92%) was achieved after 10 minutes of reaction (entry 12, Table 4). Therefore, after studying the reaction parameters of the first step, we found that the optimal reaction could be achieved after a continuous reaction for 10 minutes at 40 °C under the catalysis of 12% K2CO3 (K2CO3 mass percent of the mixed catalyst K2CO3/lipozyme TL IM).
| Entry | Substrate molar ratio (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) 2a) |
Catalystsb (%) | Temperature (°C) | Time (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: feed 1, salicylaldehyde (1a) was dissolved in 10 mL DMSO; feed 2, diethyl malonate (2a) was dissolved in 10 mL DMSO, reacted in continuous-flow microreactors catalyzed by mixed catalyst K2CO3/lipozyme TL IM.b Mass ratio of K2CO3 to mixed catalyst K2CO3/lipozyme TL IM.c Isolated yields. | |||||
| 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9 | 50 | 30 | 54 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9 | 50 | 30 | 55 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9 | 50 | 30 | 68 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
9 | 50 | 30 | 67 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 50 | 30 | 72 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
15 | 50 | 30 | 63 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 50 | 30 | 35 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 45 | 30 | 75 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 40 | 30 | 79 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 35 | 30 | 76 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 40 | 15 | 84 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 40 | 10 | 92 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
12 | 40 | 5 | 71 |
After we obtained the optimal synthesis conditions for the coumarin intermediates in the first step, we continued to study the optimal reaction conditions for the sugar-containing coumarins in the second step, including reaction solvents, substrate ratio, reaction temperature and residence time. We chose the reaction of ethyl coumarin-3-carboxylate (3a) and D-glucose (4a) as the template reaction. tert-Amyl alcohol was selected as the reaction solvent according to our results obtained for the batch method (entry 3, Table 2). However, considering that the solubility of D-glucose (4a) in tert-amyl alcohol is not very satisfactory, we added an appropriate amount of DMSO to tert-amyl alcohol to improve the solubility of D-glucose (4a), but the addition of DMSO inevitably affected the activity of the catalyst and the yield of the reaction; thus, we studied the effect of the volume ratio of DMSO to tert-amyl alcohol on the synthesis of sugar-containing coumarins. We studied the mixed solvent DMSO/tert-amyl alcohol (v/v) from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 to 1
8 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and found that the yield was higher in the DMSO/tert-amyl alcohol mixed solvent compared to that in tert-amyl alcohol (Fig. 4). Excess DMSO can cause enzyme inactivation; thus, we chose DMSO
20 and found that the yield was higher in the DMSO/tert-amyl alcohol mixed solvent compared to that in tert-amyl alcohol (Fig. 4). Excess DMSO can cause enzyme inactivation; thus, we chose DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tert-amyl alcohol = 1
tert-amyl alcohol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 as the optimal reaction solvent for the enzymatic synthesis of sugar-containing coumarin derivatives in continuous-flow microreactors.
18 as the optimal reaction solvent for the enzymatic synthesis of sugar-containing coumarin derivatives in continuous-flow microreactors.
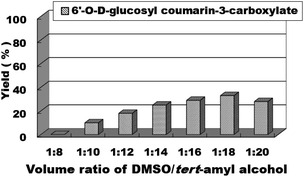 | ||
| Fig. 4 The effect of volume ratio of DMSO to tert-amyl alcohol on the sugar-containing coumarin synthesis reaction in continuous-flow microreactors. | ||
We then studied the effects of the molar ratio of ethyl coumarin-3-carboxylate (3a) to D-glucose (4a) on the synthesis reaction of sugar-containing coumarins in continuous-flow microreactors. We studied the substrate ratio of ethyl coumarin-3-carboxylate (3a) to D-glucose (4a) from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 5
3 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; we found that with the increase in ethyl coumarin-3-carboxylate (3a), the yield of the reaction increased correspondingly (Fig. 5). When the molecular ratio of ethyl coumarin-3-carboxylate (3a) to D-glucose (4a) reached 4
1; we found that with the increase in ethyl coumarin-3-carboxylate (3a), the yield of the reaction increased correspondingly (Fig. 5). When the molecular ratio of ethyl coumarin-3-carboxylate (3a) to D-glucose (4a) reached 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the best reaction yield of 51% was obtained. Therefore, the molar ratio of ethyl coumarin-3-carboxylate (3a)
1, the best reaction yield of 51% was obtained. Therefore, the molar ratio of ethyl coumarin-3-carboxylate (3a)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D-glucose (4a) = 4
D-glucose (4a) = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen as the optimal substrate ratio for the next step of reaction exploration.
1 was chosen as the optimal substrate ratio for the next step of reaction exploration.
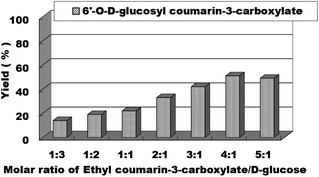 | ||
| Fig. 5 The effect of the molar ratio of ethyl coumarin-3-carboxylate to sugar on the sugar-containing coumarin synthesis reaction in microreactors. | ||
For enzymatic reactions, the reaction temperature is a very important reaction parameter; thus, we continued to study the effect of reaction temperature on the reaction yield. We studied the reaction temperature from 30 °C to 60 °C and found that the reaction could occur at 30 °C and reached the optimal level at 35 °C. After this, the reaction temperature continued to increase, but the yield rate of the reaction did not improve (Fig. 6). Therefore, the optimal reaction temperature for the synthesis of sugar-containing coumarins in the second step is 35 °C.
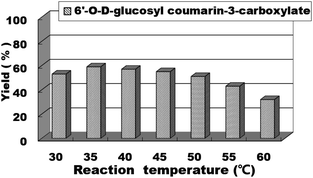 | ||
| Fig. 6 The effect of reaction temperature on the sugar-containing coumarin synthesis in microreactors. | ||
In continuous-flow microreactors, the residence time/flow rate has a great influence on the reaction yield. Therefore, we studied the effect of the residence time/flow rate on the reaction yield of sugar-containing coumarins in microreactors. We investigated the reaction from 10 min to 60 min and found that when the residence time was 10 min and the reaction flow rate was 62.4 μL min−1, 38% yield could be obtained. As the residence time was extended, the reaction yield also gradually increased. The best yield, i.e., 65% of sugar-containing coumarins was observed for a residence time of 40 min at a flow rate of 15.6 μL min−1. Thereafter, the residence time was extended, and the yield of the reaction did not increase correspondingly (Fig. 7). Therefore, the residence time/flow rate of 40 min/15.6 μL min−1 was chosen as the optimal residence time/flow rate for the enzymatic synthesis of sugar-containing coumarins in microreactors.
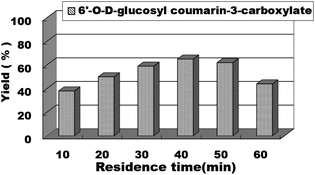 | ||
| Fig. 7 The effect of residence time on the sugar-containing coumarin synthesis reaction in microreactors. | ||
After obtaining the optimal reaction conditions for the two-step reaction, it was considered that the R4 group in compound 3 had a great influence on the synthesis of sugar-containing coumarin derivatives. Therefore, we chose compounds 3a, 3b and 3c to react with D-glucose (4a) (Table 5). It could be seen that after reacting with D-glucose (4a), tert-butyl (R4) (entry 3, Table 5) gave a much lower yield than ethyl (R4) (entry 1, Table 5) and methyl (R4) (entry 2, Table 5) due to steric hindrance. Hence, when R4 is a methyl group, the reaction result is the best. Therefore, dimethyl malonate (2b) was used for the next substrate expansion study.
| Entry | R4 | Yieldb (%) |
|---|---|---|
| a Reaction conditions: feed 1, coumarin-3-carboxylate derivative (2.0 mmol) was dissolved in tert-amyl alcohol/DMSO; feed 2, D-glucose (4a) (0.5 mmol) was dissolved in tert-amyl alcohol/DMSO, reacted in continuous-flow microreactors catalyzed by lipozyme TL IM (870 mg) at 35 °C for 40 min.b Isolated yields. | ||
| 1 | CH2CH3 | 65 |
| 2 | CH3 | 77 |
| 3 | C(CH3)3 | <5 |
Finally, we explored the scope and limitations of this two-step tandem methodology for the synthesis of sugar-containing coumarin derivatives catalyzed by lipozyme TL IM from Thermomyces lanuginosus in continuous-flow microreactors (Table 6). Five salicylaldehyde derivatives, namely, salicylaldehyde (1a), 5-chlorosalicylaldehyde (1b), 5-methylsalicylaldehyde (1c), 3-methoxysalicylaldehyde (1d), 2,4-dihydroxybenzaldehyde (1e), and dimethyl malonate (2b) and four sugar compounds, i.e., D-glucose (4a), D-mannose (4b), sucrose (4c), and maltose (4d) were subjected to the general reaction conditions using both shaker reactors and two-step tandem continuous-flow microreactors. It could be seen that a wide range of substrates were accepted by the enzyme (Table 6). For the shaker experiments, the reaction time needed to be about 40 h or more to obtain ideal conversion. Employing the enzymatic regioselective synthesis of sugar-containing coumarin derivatives under the two-step tandem continuous-flow microreactors, 18 compounds were synthesized in parallel in a single experiment under the same reaction conditions. From Table 6, we can find that the yield with monosaccharides is higher than that with disaccharides. It is worth mentioning that the two-step tandem continuous-flow synthesis protocol avoids the separation of intermediate products and simplifies the experimental steps. Furthermore, a final yield of 43–75% could be obtained; this result was better using two-step tandem continuous-flow microreactors than that obtained using shaker reactors. This two-step tandem process allows us to reduce the reaction time and simplify the purification of products, greatly improving the reaction efficiency.
| Entry | R1 | R2 | R3 | Sugar | Product | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Feed 1, salicylaldehyde derivative (20 mmol) was dissolved in 2.5 mL DMSO; feed 2, dimethyl malonate (2b) (40 mmol) was dissolved in 2.5 mL DMSO, reacted in continuous-flow microreactors catalyzed by mixed catalyst K2CO3/lipozyme TL IM (104.4 mg K2CO3 and 765.6 mg lipozyme TL IM) at 40 °C for 10 min; feed 3, 0.52 mL above reaction solution mixed with 9.48 mL tert-amyl alcohol; feed 4, sugar (0.5 mmol) was dissolved in 0.52 mL DMSO and 9.48 mL tert-amyl alcohol, reacted in continuous-flow microreactors catalyzed by lipozyme TL IM (870 mg) at 35 °C for 40 min.b Isolated yields. | ||||||
| 1 | H | H | H | D-Glucose | 5a | 75 |
| 2 | H | H | H | D-Mannose | 5b | 69 |
| 3 | H | H | H | Sucrose | 5c | 58 |
| 4 | H | H | H | Maltose | 5d | 59 |
| 5 | Cl | H | H | D-Glucose | 5e | 66 |
| 6 | Cl | H | H | D-Mannose | 5f | 73 |
| 7 | Cl | H | H | Sucrose | 5g | 47 |
| 8 | Cl | H | H | Maltose | 5h | 52 |
| 9 | CH3 | H | H | D-Glucose | 5i | 66 |
| 10 | CH3 | H | H | D-Mannose | 5j | 66 |
| 11 | CH3 | H | H | Sucrose | 5k | 45 |
| 12 | CH3 | H | H | Maltose | 5l | 52 |
| 13 | H | H | OCH3 | D-Glucose | 5m | 63 |
| 14 | H | H | OCH3 | D-Mannose | 5n | 69 |
| 15 | H | H | OCH3 | Sucrose | 5o | 43 |
| 16 | H | H | OCH3 | Maltose | 5p | 44 |
| 17 | H | OH | H | D-Glucose | 5q | 53 |
| 18 | H | OH | H | D-Mannose | 5r | 55 |
| 19 | H | OH | H | Sucrose | 5s | <5 |
| 20 | H | OH | H | Maltose | 5t | <5 |
In conclusion, we have developed an effective and environmentally friendly two-step tandem methodology for the synthesis of sugar-containing coumarin derivatives catalyzed by lipozyme TL IM from Thermomyces lanuginosus in continuous-flow microreactors. Lipozyme TL IM was first used to catalyze coumarin intermediates and sugar-containing coumarin synthesis reactions. For the first time, we designed two-step continuous flow reactors and used them in the synthesis of sugar-containing coumarins. We studied the effects of various reaction parameters including the reaction medium, substrate molar ratio, reaction temperature, residence time/flow rate and the effect of the substrate structure on the reaction. Using this technique, 18 sugar-containing coumarin derivatives were rapidly synthesized. Compared to traditional methods, the salient features of this method include mild reaction conditions (40 °C for the first step and 35 °C for the second step), short residence time (10 min for the first step and 40 min for the second step), high yields, and the use of an enzyme as a catalyst, resulting in high regioselectivity without the need for protection-deprotection steps. Furthermore, the two-step tandem continuous-flow synthesis protocol avoids the separation of intermediates and simplifies the experimental steps, which make our methodology a valuable contribution to the field of the synthesis of sugar-containing coumarin derivatives. The obtained sugar-containing coumarin derivatives may be potentially bioactive and can be used as pharmacological alternatives. We will continue our research to quickly build a library of related compounds for subsequent drug screening.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Natural Science Foundation of Zhejiang Province and Key Research & Development Projects of Zhejiang Province (LGN20C200020 and 2020C03090), the International Cooperation Project 948 (2014-4-29), the National Science and Technology Support Project (2015BAD14B0305), the National Natural Science Foundation of China (21306172), the Science and Technology Research Program of Zhejiang Province (2014C32094) as well as the Natural Science Foundation of Zhejiang University of Technology (116004029) for financial support.Notes and references
- (a) S. Z. Ferreira, H. C. Carneiro, H. A. Lara, R. B. Alves, J. M. Resende, H. M. Oliveira, L. M. Silva, D. A. Santos and R. P. Freitas, ACS Med. Chem. Lett., 2015, 6, 271–275 CrossRef PubMed; (b) M. Z. Hassan, H. Osman, M. A. Ali and M. J. Ahsan, Eur. J. Med. Chem., 2016, 123, 236–255 CrossRef PubMed; (c) Y. Q. Hu, Z. Xu, S. Zhang, X. Wu, J. W. Ding, Z. S. Lv and L. S. Feng, Eur. J. Med. Chem., 2017, 136, 122–130 CrossRef PubMed; (d) A. Fonseca, J. Reis, T. Silva, M. J. Matos, D. Bagetta, F. Ortuso, S. Alcaro, E. Uriarte and F. Borges, J. Med. Chem., 2017, 60, 7206–7212 CrossRef PubMed; (e) S. Zhang, X. Hu, D. Mang, T. Sasaki and Y. Zhang, Chem. Commun., 2019, 55, 7474–7477 RSC; (f) T. K. Mohamed, R. Z. Batran, S. A. Elseginy, M. M. Ali and A. E. Mahmoud, Bioorg. Chem., 2019, 85, 253–273 CrossRef PubMed.
- (a) D. Tian, F. Wang, M. Duan, L. Cao, Y. Zhang, X. Yao and J. Tang, J. Agric. Food Chem., 2019, 67, 1937–1947 CrossRef PubMed; (b) Z. Sen, W. Weida, M. Jie, S. Li, Z. Dongming and C. Xiaoguang, Phytomedicine, 2019, 57, 385–395 CrossRef PubMed; (c) H. Singh, J. V. Singh, K. Bhagat, H. K. Gulati, M. Sanduja, N. Kumar, N. Kinarivala and S. Sharma, Bioorg. Med. Chem., 2019, 27, 3477–3510 CrossRef CAS PubMed.
- M. J. Fasco, E. F. Hidebrandt and J. W. Suttie, J. Biol. Chem., 1982, 257, 11210–11212 CAS.
- (a) R. H. Flatman, A. Eustaquio, S. M. Li, L. Heide and A. Maxwell, Antimicrob. Agents Chemother., 2006, 50, 1136–1142 CrossRef CAS PubMed; (b) P. Wu, Y. Guo, F. Jia and X. Wang, Cell Biochem. Biophys., 2015, 72, 103–106 CrossRef PubMed.
- (a) J. R. Hwu, S. Y. Lin, S. C. Tsay, E. De Clercq, P. Leyssen and J. Neyts, J. Med. Chem., 2011, 54, 2114–2126 CrossRef PubMed; (b) Y. Otsuka, A. Sasaki, T. Teshima, K. Yamada and T. Yamamoto, Org. Lett., 2016, 18, 1338–1341 CrossRef PubMed; (c) M. Ghiasi and M. Seifi, Comput. Theor. Chem., 2017, 1118, 16–25 CrossRef; (d) F. De Moliner, K. Knox, A. Reinders, J. M. Ward, P. J. McLaughlin, K. Oparka and M. Vendrell, J. Exp. Bot., 2018, 69, 2473–2482 CrossRef PubMed.
- P. Peltier-Pain, S. C. Timmons, A. Grandemange, E. Benoit and J. S. Thorson, ChemMedChem, 2011, 6, 1347–1350 CrossRef PubMed.
- N. Touisni, A. Maresca, P. C. McDonald, Y. Lou, A. Scozzafava, S. Dedhar, J. Y. Winum and C. T. Supuran, J. Med. Chem., 2011, 54, 8271–8277 CrossRef PubMed.
- V. K. Rajput, A. MacKinnon, S. Mandal, P. Collins, H. Blanchard, H. Leffler, T. Sethi, H. Schambye, B. Mukhopadhyay and U. J. Nilsson, J. Med. Chem., 2016, 59, 8141–8147 CrossRef PubMed.
- (a) Y. Dai, B. Tian, H. Chen and Q. Zhang, ACS Catal., 2019, 9, 2909–2915 CrossRef; (b) J. Janssens, A. Bitra, J. Wang, T. Decruy, K. Venken, J. van der Eycken, D. Elewaut, D. M. Zajonc and S. van Calenbergh, ChemMedChem, 2019, 14, 147–168 CrossRef PubMed; (c) V. K. Rajput, H. Leffler, U. J. Nilsson and B. Mukhopadhyay, Bioorg. Med. Chem. Lett., 2014, 24, 3516–3520 CrossRef PubMed.
- (a) P. Wen and D. Crich, Org. Lett., 2017, 19, 2402–2405 CrossRef PubMed; (b) M. Krumb, T. Lucas and T. Opatz, Eur. J. Org. Chem., 2019, 2019, 4517–4521 CrossRef.
- (a) S. Sun and L. Tian, RSC Adv., 2018, 8, 37184–37192 RSC; (b) H. Li, Y. Qiu, C. Guo, M. Han, Y. Zhou, Y. Tong, G. Zheng and S. Zhu, Chem. Commun., 2019, 55, 8390–8393 RSC.
- D. Chen, R. Chen, K. Xie, T. Yue, X. Zhang, F. Ye and J. Dai, Org. Lett., 2018, 20, 1634–1637 CrossRef CAS.
- A. Gutmann, M. Schiller, M. Gruber-Khadjawi and B. Nidetzky, Org. Biomol. Chem., 2017, 15, 7917–7924 RSC.
- C. H. Wang, Z. Guan and Y. H. He, Green Chem., 2011, 13, 2048–2054 RSC.
- (a) A. Brodzka, D. Koszelewski, M. Zysk and R. Ostaszewski, Catal. Commun., 2018, 106, 82–86 CrossRef CAS; (b) Q. Pavic, S. Tranchimand, L. Lemiegre and L. Legentil, Chem. Commun., 2018, 54, 5550–5553 RSC; (c) S. G. Zhu, J. X. Huang, G. M. Zhang, S. X. Chen and F. L. Zhang, Org. Process Res. Dev., 2018, 22, 1548–1552 CrossRef CAS.
- (a) S. Kundu, A. S. Bhangale, W. E. Wallace, K. M. Flynn, C. M. Guttman, R. A. Gross and K. L. Beers, J. Am. Chem. Soc., 2011, 133, 6006–6011 CrossRef CAS; (b) E. Peris, O. Okafor, E. Kulcinskaja, R. Goodridge, S. V. Luis, E. Garcia-Verdugo, E. O'Reilly and V. Sans, Green Chem., 2017, 19, 5345–5349 RSC; (c) Y. Zhu, Q. Chen, L. Shao, Y. Jia and X. Zhang, React. Chem. Eng., 2020, 5, 9–32 RSC; (d) L. H. Du, J. H. Shen, Z. Dong, N. N. Zhou, B. Z. Cheng, Z. M. Ou and X. P. Luo, RSC Adv., 2018, 8, 12614–12618 RSC; (e) Y. Bi, H. Zhou, H. Jia and P. Wei, RSC Adv., 2017, 7, 12283–12291 RSC; (f) L. H. Du, Z. P. Jiang, L. L. Xu, N. N. Zhou, J. H. Shen, Z. Dong, L. Shen, H. Wang and X. P. Luo, Carbohydr. Res., 2018, 455, 32–38 CrossRef CAS PubMed.
- (a) P. D. Morse and T. F. Jamison, Angew. Chem., 2017, 129, 14187–14190 CrossRef; (b) J. Britton and C. L. Raston, Chem. Soc. Rev., 2017, 46, 1250–1271 RSC; (c) P. Zamani and A. R. Khosropour, Green Chem., 2016, 18, 6450–6455 RSC; (d) M. Rubens, J. H. Vrijsen, J. Laun and T. Junkers, Angew. Chem., Int. Ed., 2019, 58, 3183–3187 CrossRef CAS; (e) J. Fang, M. Ke, G. Huang, Y. Tao, D. Cheng and F.-E. Chen, RSC Adv., 2019, 9, 9270–9280 RSC; (f) R. Galaverna, T. McBride, J. C. Pastre and D. L. Browne, React. Chem. Eng., 2019, 4, 1559–1564 RSC.
- C. Wiles and P. Watts, Green Chem., 2012, 14, 38–54 RSC.
- K. Yoshimoto, Y. Itatani and Y. Tsuda, Chem. Pharm. Bull., 1980, 28, 2065–2076 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00879f |
| This journal is © The Royal Society of Chemistry 2020 |