Open Access Article
Open Access ArticleA unique polymersome covered by loop-cluster polyamine corona†
Wen-Li Wang and
Ren-Hua Jin *
*
Department of Material and Life Chemistry, Kanagawa University, 3-27-1 Rokkakubashi, Yokohama 221-8686, Japan. E-mail: rhjin@kanagawa-u.ac.jp
First published on 1st April 2020
Abstract
In this work, we synthesized a new comb-like copolymer (c-iMP) possessing amphiphilic diblock side chains consisting of poly(methyloxazoline) (PMOZ) and poly(phenyloxazoline) (PPOZ) via reversible addition-fragmentation chain-transfer (RAFT) polymerization of p-choloromethylstyene (CMS) and cationic ring-opening polymerization (CROP) of 2-methyl-2-oxazoline (MOZ) and 2-phenyl-2-oxazoline (POZ). Then, by performing selective hydrolysis of the hydrophilic PMOZ block, the PMOZ block was transformed into PEI (polyethylenimine) from which comb-like copolymers (c-iEP) possessing two blocks of PEI (E) and PPOZ (P) were produced. The comb-copolymers c-iEP showed unique self-assembling behavior in water to give an unusual polymersome covered by a loop-cluster polyamine corona. The polymersome showed extreme toughness and stability without collapse and fusion even at dry conditions and could be transformed into capsules. This is the first example of polymesome without free-ends on the vesicle wall which could include Ag ions inside and could deposit silica around the wall to form hybrid hollow spheres.
Introduction
Polymersomes (polymeric vesicles) possessing inside and outside corona and a sandwiched membrane wall between the two-side coronas are easily generated via self-assembly of amphiphilic block or alternative copolymers. Polymersomes are very important functional materials which could be used as vehicles in medicine,1 as reactors and templates in chemical reactions,2 as artificial cells in life-like adaptivity3 and as precursors in the preparation of soft/hard hollow objects.4 An important point in the preparation and application of polymersomes is to design polymers with specially structured components and dimensions. There are vast reports of polymers with linear or dendritic components which can self-assemble into polymersomes. However, almost all of the designed polymers give polymersomes with the same vesicular structure. That is, the polymersomes have a lot of hydrophilic free-ends on the polymer chains in the inside and the outside brush-like corona which sandwich the hydrophobic membrane wall. It is well known that the properties and function of polymersomes not only depend upon the chemical components of the corona chain but are also affected by the corona structure (chain length and chain conformation). Therefore, control of corona-dimension surrounding the membrane wall would be very meaningful for understanding more properly the property and function of polymersomes and for expecting the potentials of polymersomes.Construction of polymeric loop without free-end is a useful strategy to design polymeric materials. Previously, we synthesized porphyrin-centred star polymers possessing four arms of amphiphilic diblock with different order of the two blocks.5 One is location of hydrophilic block near the porphyrin-core and other one is location of hydrophilic block far away the porphyrin-core. The self-assembly of the latter resulted in usual polymer micelle with free-ends of corona. In contrast, the former offered unusual polymer flower micelle with loop-covered corona. The interest in the loop-covered micelle is that the hydrophobic porphyrin residues could be located on the corona which communicated freely with the substrates dissolved in the water phase.
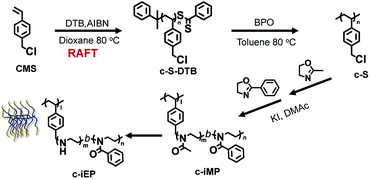
This experience promoted us to try the preparation of polymersomes with loop-covered corona. For this purpose, we synthesized specially structured comb polymer (c-iEP) consisted of polystyrenic main chain and di-blocked side chain (teeth) possessing hydrophilic linear polyethyleneimine (PEI) block (inner block near to the main chain) and hydrophobic poly(phenyloxazoline) (PPOZ) block (outer block far away the main-chain) (see Scheme 1). In this polymer, the hydrophilic PEI block has not free-end because it is sandwiched between the polystyrenic main chain and the block of PPOZ on teeth. We found that the comb could self-assemble into a polymersome with unusual corona dimension without free-ends of polymeric chain in aqueous medium, i.e., the hydrophilic PEI without free-ends endowed corona with loop-cluster structure which covered the hydrophobic PPOZ wall to form extraordinarily stable polymersome not only keeping globular shape without collapsing but remaining also in isolation without fusion each other even in air-dried and ultra-high vacuum conditions. Furthermore, after cross-linking the PEI loop by bis-epoxide, a capsule with ca. 2 nm thickness wall became more stable and played well as catalytic template to give silica/polymersome hybrid hollow.
Experimental
Materials
4-Chloromethylstyene (CMS, 90%, TCI) was purified via basic alumina column chromatography before use. 2-Methyl-2-oxazoline (MOZ, 98%, Aldrich) and 2-phenyl-2-oxazoline (POZ, 99%, Aldirich) were distilled from sodium hydroxide and then stored under a dry nitrogen atmosphere prior to use. Azobis(isobutyronitrile) (AIBN, 98%, TCI) was recrystallized from ethanol and dried. Cumyl dithiobenzoate (DTB, 98%, Aldirich), benzoyl peroxide (BPO, 75%, TCI), postassium iodide (KI, 99.5%, TCI), toluene (super dehydrated, wako), dimethyl acetamide (super dehydrated, wako), hydrochloric acid (5 M, wako), ammonia solution (28 vol%, wako), tetramethoxysilane (TMOS, 98%, TCI) and other chemicals were used as received.Synthesis of c-S macroinitiators
The c-S macroinitiators were synthesized via reversible addition-fragmentation chain-transfer (RAFT) polymerization of CMS and then removal of dithiobenzoate end groups procedure.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of BPO to the dithiobenzoate group. After purging the solution with nitrogen gas at room temperature for 1 hour, the temperature was raised to 80 °C until colorless under a nitrogen atmosphere. Then, the solution was added to n-hexane for precipitation of the polymer and this precipitation was repeated four times. The white powders (c-S72) were finally obtained after vacuum drying at 40 °C for 1 day. GPC analysis indicated the apparent Mn and Mw/Mn (PDI) values to be 9.9 kg mol−1 and 1.24, respectively. The polymer was also subjected to 1H NMR and UV-Vis spectroscopes (see Fig. S2 and S3†).
1 molar ratio of BPO to the dithiobenzoate group. After purging the solution with nitrogen gas at room temperature for 1 hour, the temperature was raised to 80 °C until colorless under a nitrogen atmosphere. Then, the solution was added to n-hexane for precipitation of the polymer and this precipitation was repeated four times. The white powders (c-S72) were finally obtained after vacuum drying at 40 °C for 1 day. GPC analysis indicated the apparent Mn and Mw/Mn (PDI) values to be 9.9 kg mol−1 and 1.24, respectively. The polymer was also subjected to 1H NMR and UV-Vis spectroscopes (see Fig. S2 and S3†).Synthesis of comb-like 2-oxzaoline block copolymers (c-iMP)
A typical procedure for preparing the comb polymer with poly(methyloxazoline) (PMOZ)-b-poly(2-phenyloxazoline) (PPOZ) side chains was as follows. The macroinitiator (c-S72) (0.10 g, –C7H6Cl: 6.5 × 10−4 mol, 1 equiv.) and potassium iodide (0.216 g, 1.3 × 10−3 mol, two-fold excess to benzyl chloride unit) were added into a 50 mL brown round-bottom two-necked flask. Then 20 mL of N,N-dimethylacetoamide and 3.25 mL of MOZ (38.4 mmol, 30 equiv.) was added to the flask under a dry nitrogen atmosphere. The mixture was heated at 85 °C for 24 hours. At this condition, the conversion of MOZ estimated by 1H-NMR was more than 99%, from which the DP of PMOZ was calculated to be 30 (Fig. S4†). Continuously, 8.5 mL of POZ (62.7 mmol, 50 equiv.) was added to the mixture and heated at 120 °C for 48 hours. Then the mixture was dissolved in CHCl3 and precipitated into a large amount of diethyl ether twice. The white powders of comb-like block polymer c-iMP were obtained. It should be noted here that the GPC data could not be obtained for these two types of comb-like polymers since the injection of the solutions of comb polymers did not give any fraction peaks although linear PMOZ or PMOZ-b-PPOZ with different polymerization degrees are easily characterized by the same GPC system. Taking the DP of the first block of PMOZ as reference, we calculated the DP of the second block of PPOZ to be 37, based on the integration ratio of the PMOZ and PPOZ in the 1H NMR spectrum of c-iMP (Fig. S5†).Synthesis of c-iEP by selective hydrolysis of PMOZ block
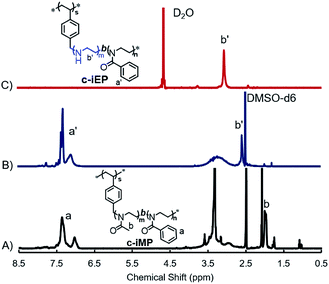
Comb-like block copolymers c-iEP were prepared by selective hydrolysis of PMOZ block on c-iMP in 3 M HCl as follows: 1 g of c-iMP was mixed in 15 mL of 3 M HCl aqueous solution. The mixture was reflexed at 100 °C for 2.5 hours. After cooled the reaction solution by ice-water, a certain amount of ammonia solution (28 vol%) was added until pH > 10 and stirred for 24 hours. Finally, the precipitates of c-iEP from the reaction solution were recovered by centrifugation (rpm = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 10 min) and washed with deionized water until pH 8. Finally, white powders were obtained by lyophilisation and characterized by 1H NMR spectroscope (see Fig. 1).
000, 10 min) and washed with deionized water until pH 8. Finally, white powders were obtained by lyophilisation and characterized by 1H NMR spectroscope (see Fig. 1).
Self-assembly of comb-like block copolymer c-iEP
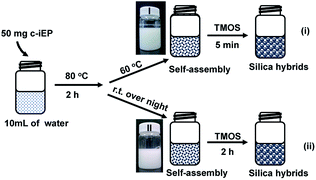
The self-organization process of comb-like block copolymer c-iEP in water was as follows: 50 mg of c-iEP was added to 10 mL of aqueous solution, followed by heating at 80 °C for 2 hours, then naturally cooled and allowed to stand at room temperature for 24 hours (or held at 60 °C for 1 h). One drop of the solution was dropped on a silicon wafer, after drying, it was subjected to SEM observation.Silica deposition on self-assemblies of c-iEPs
A small amount of TMOS (0.3 mL) was added to 10 mL of aqueous dispersion of c-iEP prepared as above, and the mixture was stirred at room temperature for 2 hours (or at 60 °C for 5 min). Then the white precipitates were recovered by centrifugation and washed with water and acetone. The silica obtained was characterized by FT-IR, TGA and SEM.Characterizations
The measurement of proton nuclear magnetic resonance (1H NMR) of the polymers were conducted on a JEOL ECA-500 spectrometer operating at 500 MHz. Fourier transform infrared (FT-IR) spectra were obtained using a JASCO FT-IR-4600 spectrometer by diffusing the samples in KBr pellets. GPC analyses of c-S-DTB and c-S were conducted by TOSOH HLC-8220 apparatus, using DMF solution containing 10.0 mM of LiBr and 10.0 mM of H3PO4 as eluent. The polymersome and its silica hybrids were observed using a HITACHI SU8010 scanning electron microscope (SEM) at 5 kV after samples were sputtered with Pt particles. The TEM observation was performed on a JEOL JEM-2010 instrument with an acceleration voltage of 200 kV. The samples for the TEM were prepared by dropping a suspension sample self-assembled onto a commercial copper micro grid coated with a polymer film. The samples on grid were thoroughly dried in vacuum at room temperature for 24 h prior to observation. The content of silica hybrids (c-iEP@SiO2) were investigated using a Seiko Instruments EXSTER 6000 thermogravimetric analysis (TGA) at 30–900 °C and a heating rate of 10 °C min−1 under air atmosphere.Results and discussion
Scheme 1 showed our strategy of accessing comb-like block copolymer c-iEP, where the reversible addition-fragmentation chain-transfer (RAFT) polymerization of CMS, cationic ring-opening co-polymerizations (CROP) of 2-methyl-2-oxazoline and 2-phenyl-2-oxazoline by using macroinitiator (c-S), and selective hydrolysis reaction of the block of poly(2-methyl-2-oxazoline) were combined. Well-defined poly(4-chloro-methylstyrene) (c-S72-DTB) homopolymer with degree of polymerization (DP) of 72 were synthesized by RAFT polymerization of chloromethylstyrene (CMS) with cumyl dithiobenzoate (DTB) as RAFT agent and azobis(isobutyronitrile) (AIBN) as initiator (see Fig. S1†).6,7 Then the end group of dithiobenzoate was removed via a radical reaction in the presence of excess of radical reagent (BPO) to obtain end-inactive macroinitiator of c-S (see Fig. S2 and S3†). Afterward, using c-S (DP = 72) as macroinitiatior and potassium iodide as activator, we performed the block copolymerization of MOZ and POZ from which the precursor of c-iMP (here, M shows inner PMOZ30 block while P shows outer PPOZ37 block) was resulted (see Experimental section, Table 1, Fig. S4 and S5†). Finally, the c-iMP was used in hydrolyzation by refluxing it in 3 M HCl at 100 °C for 2.5 hours. It should be noted here that PMOZ is hydrophilic block while the PPOZ is hydrophobic. Therefore, the precursor can form micelle in the reaction condition with hydrophobic core of PPOZ and hydrophilic shell of PMOZ. The part of hydrophilic shell could be selectively hydrolyzed under the above reaction conditions (see Table 1).8 Fig. 1A and B showed 1H-NMR spectra of the precursor c-iMP and its hydrolyzed product of c-iEP in DMSO-d6, respectively. Before hydrolyzation, c-iMP showed two typical peaks assigned to the Ph (6.9–7.5 ppm) on PPOZ and CH3 (ca. 2.0 ppm) on PMOZ (Fig. 1A). After hydrolysis, as shown in Fig. 1B, the characteristic peak at 2 ppm due to CH3 (ca. 2.0 ppm) on PMOZ was completely disappeared while the peaks due to the Ph (6.9–7.5 ppm) on PPOZ was retained. This indicates that the PMOZ block was hydrolyzed selectively. Because the total degrees of polymerization in the teeth remained unchanged after hydrolysis and the hydrolyzation of hydrophilic block was nearly complete, we estimated that the degree of polymerization of PEI and PPOZ is 30 and 37 for the c-iEP. The details of the c-iMP and c-iEP were summarized in Table 1.To understand the physical performance of the comb of c-iEP, we compared 1H NMR spectrum of c-iEP in DMSO-d6 and in hot D2O (see Fig. 1B and C). In DMSO-d6, the comb of c-iEP showed characteristic peaks due to the two different components of PPOZ (Ph: 6.9–7.5 ppm; [O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCH2CH2–]: broaden, 2.75–3.60) and PEI (–NHCH2CH2–, at 2.55 ppm). This means that the two components of PPOZ and PEI are soluble in DMSO-d6. However, the same polymer dissolved in hot D2O (70 °C) showed only the peak due to PEI component with relatively sharp peak at 3.0 ppm, while the peaks due to PPOZ block completely disappeared. This indicated that PPOZ block aggregated each other in hot D2O. From this comparison, we are sure that c-iEP is an amphiphilic polymer with soluble PEI and insoluble PPOZ in water.
NCH2CH2–]: broaden, 2.75–3.60) and PEI (–NHCH2CH2–, at 2.55 ppm). This means that the two components of PPOZ and PEI are soluble in DMSO-d6. However, the same polymer dissolved in hot D2O (70 °C) showed only the peak due to PEI component with relatively sharp peak at 3.0 ppm, while the peaks due to PPOZ block completely disappeared. This indicated that PPOZ block aggregated each other in hot D2O. From this comparison, we are sure that c-iEP is an amphiphilic polymer with soluble PEI and insoluble PPOZ in water.
It is well known that amphiphilic diblock copolymers with linear dimensions can easily form various self-assembled entities such as micelles, vesicles and lamellar structure because of the aggregation between the hydrophobic blocks in water. We particularly interested here what could be expectable for the comb of c-iEP in the self-assembly and catalytic function in silica deposition. It should be noted here that the silica deposition on PEI aggregates could be used as a tool to investigate the morphological structures of PEI aggregates because the morphologies of PEI aggregates can be effectively transferred to the resulting hard silica which are easily handleable in SEM observation.7,9–11 PEI homopolymer is easily soluble in hot water but assembles at room temperature due to crystalline property of PEI. Although the comb of c-iEP has not crystalline feature (see XRD pattern Fig. S6†), for promoting enough solvation of PEI block, we performed the self-organization of c-iEP by stirring the copolymers (50 mg) in 10 mL of hot water (80 °C) for 2 hours and then placed them in the cooling (room temperature (RT)) or warming (60 °C) conditions as shown in Fig. 2. Then, to the aqueous dispersions, silica source of tetramethoxysilane (TMOS) was added and stirred for definite times (5 min in the warmed condition or 2 h in the RT condition). The inset photographs in Fig. 2 showed the state of the dispersion self-assembled from c-iEP at warmed (60 °C) and RT conditions. The aggregates from c-iEP seem completely opaque but such state remained without precipitation even after placed for one month. After reacted with TMOS, the dispersions resulted in precipitation due to the formation of hybrid structures with deposition of silica onto the aggregates.
The two samples from the warmed and RT dispersions and their corresponding silica hybrids generated from the two conditions were dropped on silicon wafer (or TEM grid) and subjected to SEM (or TEM) observation. As shown in Fig. 3, the aggregates images in Fig. 3a, b and e, f appeared as spheres with the larger diameters 200–700 nm. We found that the hybrids prepared from the reaction of the aggregates of the warmed dispersions with TMOS only by 5 min remained spherical with unchanged and appeared as isolated state with relatively larger diameters in the range of 200–550 nm. Interestingly, from some individual broken hybrids spheres (obtained from the different temperatures), it can be clearly seen that the spheres are hollow. The formation of the hybrids spheres with large diameter and structural hollow should be attributed to the globular vesicular structures of the aggregates of c-iEP. From the TG analysis, it was found that the silica content for the two spherical hybrids was very low (<20%, see Fig. S7†), indicating also hollow spherical features of the hybrids.
 | ||
| Fig. 3 SEM images of self-assembled entities from c-iEP and c-iPE@silica hybrids. ((a, b) and (e, f)) c-iEP, ((c, d) and (g, h)) c-iEP@SiO2; (a–d) formed at room temperature; (e–h) formed at 60 °C. | ||
In order to more clearly clarify the spherical aggregates from c-iEP formed under the warmed conditions, we also visualized them by TEM (Fig. 4). As can be seen in Fig. 4a, the self-assembled entities from c-iEP were large spherical aggregates (maximum of nearly 600 nm in diameter) with many hollows inside the spheres and there is no any collapse or rupture. Interestingly, from the relatively smaller spheres in diameter about 200 nm (Fig. 4b), we can see clearly that they include inside hollows which have diameters about 100 nm, with nearly 3 nm thickness wall. These images are just due to non-unilamellar polymeric vesicles (polymersomes) of hollow in hollow.12–14 Generally, polymeric globular vesicles are not robust in the ambient drying conditions and easily collapse into circular form, i.e., their globular hollow shape could not remain under dried conditions being likely an air-leaked balloon.15 Therefore, the spherical TEM image of vesicles samples is often conducted to the special technical protocols such as freeze-fracture and/or cryogenic electron microscope.16,17 In our case, the self-assembled globular vesicular samples are available easily in the ambient-drying conditions. As shown in the SEM images from top and side view (Fig. S8†), the globular spheres without collapse were clearly observed. Herein, it should be noted that the comb of c-iMP (before hydrolysis) could not form vesicular entity but give micelles about 50 nm in diameters. It seems that the polyamine block (but not polyamide) block is indispensable for the formation of loop-cluster covered vesicle.
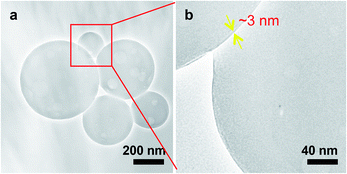 | ||
| Fig. 4 TEM images of self-assemblies of c-iEP. (a) Low magnification. (b) Magnified image of the red box area in (a). | ||
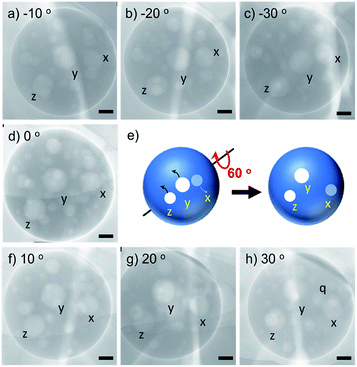
To further image the globular polymersome obtained via ambient drying, we focused on a large polymersome and looked the changes of the inside hollow positions by tilting the polymersome-loaded grid from −30° to +30°. As shown in Fig. 5, three inside hollows labelled with x, y and z moved their position, and the distance between the three hollows changed in 2-dimentional images when the angle was tilted by every 10 degrees. Very interestingly, after tilting 60 degrees from −30 to +30°, a new hollow (q) appeared near the hollow x, indicating that before tilting, the hollow q is located below the vertical of hollow x. These changes observed on 2-dimentional images of the inside hollows exhibit that the small hollows are attached around the inner wall of large polymersome without fusion and collapse even in super vacuum (<10−6 Pa, TEM condition) at ambient temperature. This indicates the robustness of the membrane wall of the polymersome self-assembled from the comb of c-iEP.
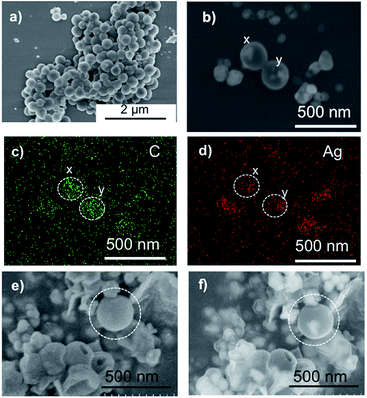
Furthermore, as a preliminary experiment, we examined the encapsulation of silver nanoparticles in the polymersome. For example, 50 mg of c-iEP and L(+)-ascorbic acid (52 mg, 2.95 × 10−4 mol) was mixed in 4 mL of methanol. Then 1 mL of silver acetate aqueous solution (25 mg mL−1, 1.50 × 10−4 mol) and 5 mL of water was added into the mixture. After 5 min, the brown precipitates were recovered by centrifugation and washed with water twice. Finally, the Ag@c-iEP obtained were dispersed in water and used in characterization by SEM and EDX. As shown in Fig. 6, we can see clearly the globular polymersome which are smaller than 300 nm (under 3 kV). To confirm whether Ag nanoparticles exist inside the spheres, we performed EDX focusing individual spheres with increasing the acceleration voltage to 10 kV (see Fig. 6b–d). We can see from Fig. 6b that the spheres (denoted by x and y) have internal nanoparticles appeared in bright spots which just are attributed to the Ag nanoparticles (see EDX image in Fig. 5d). Using the polymersome of Ag@c-iEP, we also performed silicification by addition of TMOS and the obtained hybrids SiO2@Ag@c-iEP were subjected to SEM observation by changing acceleration voltage (see Fig. 6e and f). Under the lower 3 kV acceleration voltage condition, the spheres did not show internal information. However, under the higher 10 kV acceleration voltage condition, the spheres of different sizes showed internal bright spots with different contrast to the peripheral rings indicating that the Ag nanoparticles were enclosed in the very thin hollow silica wall.
In order to examine the potentials of the polymersomes self-assembled from c-iEP, we further tried the transformation of the polyemrsome to a hollow capsule via cross-linking PEI on the loop-cluster by using diepoxide (1,2-diglycidyloxyethane, DGE, see Fig. 7A and S9†). From the comparison of 1H NMR before and after cross-linking (Fig. S9†), we confirmed that the degree of cross-linking is about 40%. After cross-linking, the particle size distributions in DLS (Fig. S10†) became slightly smaller indicating that the crosslinking occurred only intra-polymersome. To understand the property of the capsule of CL-c-iEP, we also performed the silica deposition on the capsules. The related SEM and TEM images were shown in Fig. S11† and 7B, respectively. It is clear from the SEM images (Fig. S11a and b†) that the capsules remained globular with size of smaller than 600 nm indicating the shape stability of the capsule without collapse. On the other hand, the hybrids silica formed on the capsules template showed spheres with size smaller than 400 nm (see Fig. S11c and d†). From the contrast image of the edge on the sphere of CL-c-iEP (see Fig. 7B(a)), we estimated that the wall thickness is about 2 nm. In comparison, the hollow silica hybrids showed shell thickness nearly 6 nm (see Fig. 7B(b)). This means that the capsule is not only very stable keeping their shape in silica deposition but also could give very thin silica hollow.
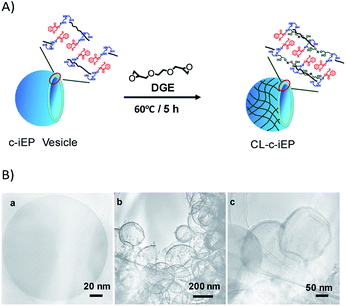 | ||
| Fig. 7 (A) Schematic representation of the cross-linked polymersome. (B) TEM images of (a) capsule CL-iEP, (b and c) SiO2@CL-iEP. | ||
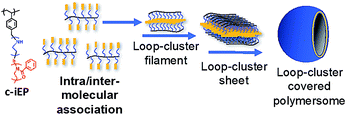
Based on the above results, we could propose the mechanism of the formation of polymersome as shown in Scheme 2. In case of c-iEP, the PEI block (near to the backbone of polystyrene) has no crystalline feature and thus is solvated easily in water while the hydrophobic association between the block of PPOZ (far from the backbone of polystyrene) becomes loose in hot water (80 °C) after stirring for 2 h to form opaque dispersion. When the hot water dispersion is placed in the warmed or ambient water, PPOZ blocks on the comb interact each other by intra- and inter-molecular hydrophobic association forces. These forces promote the formation of special ladder-like entities with hydrophobic centre-line in which the blocks of PPOZ prefer to be lay down each other to form very thin central hydrophobic filament. Continuously, the ladder-like entities further associate with driving hydrophobic forces between the centre-filaments to form bi-layered two-dimensional sheet where the hydrophobic domain is sandwiched by loop-cluster-like hydrophilic PEI layers (see Scheme 2). These sheets finally curved to form hollow vesicular structure with large diameters (300–800 nm) accompanying the inclusion of small hollows on the inside. The importance here is that the two-sides of the loop-cluster-like hydrophilic layers on the hollow surface have not free-ends of polymeric chains, which are completely different to the usual polymeric vesicles of possessing a lot of free-ends in their surface wall formed by linear amphiphilic block copolymers.
Conclusion
Comb-like polymers c-iEP containing block-copolymeric side chains of PEI-b-PPOZ on teeth were firstly synthesized via a combination of CROP (cationic ring-opening polymerization) of 2-methyl-2-oxazoline and 2-phenyl-2-oxazoline and acidic hydrolysis of the resulting poly(methyloxazoline) block. Especially, different to the usual knowledge on amphiphilic block copolymers, the comb containing the teeth of PEI block without its free-ends could self-organize into unusual polymersome without brush-like free-ends shell but with loop-cluster-like corona on hydrophobic vesicular membrane, and surprisingly, the polymersome with very thin wall bellow 3 nm showed high stability and toughness to keep spherical structure even at ambient-drying conditions.Furthermore, the polymersome with PEI loop could be transformed into very stable capsule via cross-linking the PEI corona and the capsule could play as catalytic template to deposit spontaneously silica on the wall of the capsule to produce very thin hollow silica. We expect that the comb designed as like c-iEP without free-ends of PEI is potentially applicable in drug delivery, templating and catalysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research was supported in part by the JSPS KAKEHI Grant-in-Aid for Scientific Research (B), 19H02767.Notes and references
- (a) M. Mohammadi, S. Taghavi, K. Abnous, S. M. Taghdisi, M. Ramezani and M. Alibolandi, Adv. Funct. Mater., 2018, 28, 1802136 CrossRef; (b) F. Wang, J. Xiao, S. Chen, H. Sun, B. Yang, J. Jiang, X. Zhou and J. Du, Adv. Mater., 2018, 30, 1705674 CrossRef PubMed.
- (a) T. Nishimura and K. Akiyoshi, Adv. Sci., 2018, 5, 1800801 CrossRef PubMed; (b) Y. Mai and A. Eisenberg, Acc. Chem. Res., 2012, 45, 1657 CrossRef CAS PubMed; (c) X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983 CrossRef CAS PubMed; (d) P. Tanner, P. Baumann, R. Enea, O. Onaca, C. Palivan and W. Meier, Acc. Chem. Res., 2011, 44, 1039 CrossRef CAS PubMed.
- B. C. Buddingh and J. C. M. van Hest, Acc. Chem. Res., 2017, 50, 769 CrossRef CAS PubMed.
- R. Dong, W. Liu and J. Hao, Acc. Chem. Res., 2012, 45, 504 CrossRef CAS PubMed.
- R.-H. Jin, Adv. Mater., 2002, 14, 889 CrossRef CAS.
- D.-D. Yao and R.-H. Jin, Polym. Chem., 2015, 6, 2255 RSC.
- D.-D. Yao, H. Kurosawa, D. Souma and R.-H. Jin, Polymer, 2016, 86, 120 CrossRef CAS.
- R.-H. Jin, ChemPhysChem, 2003, 4, 1118 CrossRef CAS PubMed.
- R.-H. Jin and J.-J. Yuan, Macromol. Chem. Phys., 2005, 206, 2160 CrossRef CAS.
- J.-J. Yuan and R.-H. Jin, Langmuir, 2010, 26, 4212 CrossRef CAS PubMed.
- J.-J. Yuan and R.-H. Jin, Langmuir, 2005, 21, 3136 CrossRef CAS PubMed.
- A. Napoli, M. Valentini, N. Tirelli, M. Müller and J. A. Hubbell, Nat. Mater., 2004, 3, 183 CrossRef CAS PubMed.
- S. Chiruvolu, S. Walker, J. Israelachvili, F. J. Schmitt, D. Leckband and J. A. Zasadzinski, Science, 1994, 264, 1753 CrossRef CAS PubMed.
- B. M. Discher, Y. Y. Won, D. S. Ege, J. C. Lee, F. S. Bates, D. E. Discher and D. A. Hammer, Science, 1999, 284, 1143 CrossRef CAS PubMed.
- Y. Xiao, H. Sun and J. Du, J. Am. Chem. Soc., 2017, 139, 7640 CrossRef CAS PubMed.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967 CrossRef CAS PubMed.
- L. E. Franken, E. J. Boekema and M. C. A. Stuart, Adv. Sci., 2017, 4, 1600476 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterizations of the comb polymer, SEM, TEM, DLS, UV-Vis. See DOI: 10.1039/c9ra10704e |
| This journal is © The Royal Society of Chemistry 2020 |