Open Access Article
Open Access ArticleWide visible-range fluorescence of Eu3+ located in the macroscopic bi-layer ceramic/glass composite
Haifeng Shia,
Jiaxin Yanga,
Zhimin Yua,
Yu Songa,
Edwin Yue Bun Punb,
Xin Zhao*a and
Hai Lin *ab
*ab
aSchool of Textile and Material Engineering, Dalian Polytechnic University, Dalian 116034, P. R. China. E-mail: zhaoxin@dlpu.edu.cn; lhai8686@yahoo.com
bDepartment of Electronical Engineering and State Key Laboratory of Terahertz and Millimeter Waves, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, P. R. China
First published on 21st May 2020
Abstract
The Eu3+ doped fluoride bi-layer ceramic/glass composite (GCZBL-Eu) was prepared by a one-step method and the effective wide visible-range fluorescence was recorded. The de-population rates of the 5D0, 5D1, 5D2, and 5D3 multi-levels in the glass layer (GZBL-Eu) were estimated to be 214, 746, 1163, and 680 s−1, respectively, and that in the ceramic layer (CZBL-Eu) were 211, 730, 1075, and 654 s−1, which implies multi-channel radiative transitions due to the non-radiative relaxation limitation of low OH content and low phonon energy. Simultaneously, the quantum efficiencies of the 5D0 levels in GZBL-Eu and CZBL-Eu were as high as 98.5% and 94.8%, respectively, thus demonstrating the effectiveness of the radiative transition emissions from Eu3+. Besides, GCZBL-Eu with the glass forming layer increases the emission intensity by 24% compared to CZBL-Eu, which is attributed to the multiple-cycle reflection in the composite structure of the glass–ceramic transition region, and the color coordinates of CZBL-Eu (0.483, 0.385) and GCZBL-Eu (0.469, 0.389) show that they can release yellowish-white light. The hetero-structured GCZBL-Eu provides a new approach for laser lighting, fluorescent display, and up-conversion applications.
Introduction
White light-emitting diodes (WLEDs) have recently attracted considerable attention as next generation light sources and as alternatives for conventional incandescent and fluorescent lamps.1–4 One of the most popular ways to obtain WLEDs is to combine near UV-LEDs with a glass or crystal phosphor emitting in the blue, green, and red spectral regions.5–7 For this purpose, multiple luminescence centers in the phosphors are widely adopted to achieve high brightness and high color rendering index of white light emission.8–12 However, rare earth (RE) co-doping leads to complex cross relaxation processes, which cannot release photons efficiently. In view of the high fluorescence quantum efficiency in visible white light illumination, the exploration focusing on the compound material with single RE3+ ions doping and multi-channel emission becomes urgent.The emission spectrum of Eu3+ located in the hosts, characterized by the existence of high energy phonons such as the oxides, is strong only for the case of the bands produced by the radiative relaxations from 5D0.13–19 The emissions from 5D0 are favored mostly because the energy difference between the 5D3, 5D2, 5D1, and 5D0 levels of the Eu3+ cation can be covered by non-radiative transitions from the higher levels to 5D0. The resulting emission profile, with intense bands mostly confined to a narrow range, is not suitable for the preparation of white-light phosphors if the intention is to use only a single RE3+ dopant. However, in hosts having weak phonons, such as fluorides, the non-radiative transitions from the higher electronic levels to 5D0 become difficult and the electronic transitions originating in those levels acquire higher intensity than in the oxide hosts. The result is a fluorescence spectrum exhibiting bands of significant intensity (albeit emission at wavelength higher than 500 nm remains strongest) at various positions of the visible range; such a spectral profile makes Eu3+, in fluorides, a possible candidate for the fabrication of white light phosphors based on a single RE3+. In this work, for the first time, it has been examined whether the emission of Eu3+, introduced in a Zr–Ba–La–F host (macroscopic ceramic/glass bi-layer), can be used to obtain such phosphors. In addition, the transitions 5D0 → 7F1 and 5D0 → 7F2 have different dependences on the crystal field environment belonging to the magnetic dipole and electric dipole transitions.20–25 Also, Eu3+ ions have similar ionic radius and crystalline properties as other RE3+ ions, which can be used as a probe of the local structure to explore the influence of the micro-environment of RE3+ ions on the luminescence of materials.
In this work, Eu3+-doped fluoride ceramics with a glass layer (GCZBL-Eu) have been prepared and intense yellowish-white fluorescence, attributed to multi-peak emission, was observed. With the formation of the thin glass layer on the ceramic surface, the GCZBL-Eu increases its emission intensity by 24% compared to CZBL-Eu, which is ascribed to multiple-cycle reflection in the complex structure of the glass–ceramic transition region. The micro-environment symmetry of GZBL-Eu and CZBL-Eu is judged according to the value of the J-O parameter Ω2 with different emission intensity ratios between 5D0 → 7F2 and 5D0 → 7F1. The differential fluorescence characteristics, such as the radiative lifetimes of the multi-levels and the quantum efficiency of the 5D0 levels from Eu3+ in GZBL-Eu and CZBL-Eu, were analyzed. In addition, the color coordinates of CZBL-Eu and GCZBL-Eu are located in the yellowish-white lighting region, which indicates that the hetero-structured materials can be well applied to optical devices.
Preparation and measurements of GCZBL-Eu
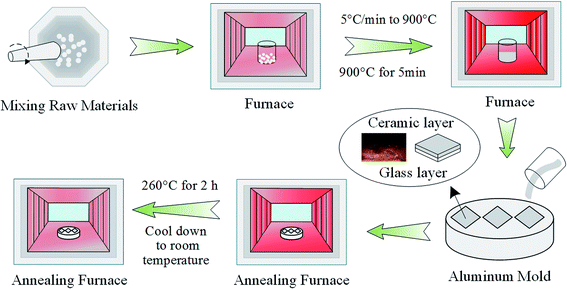
The fluoride bi-layer ceramic/glass composites were prepared according to the molar host composition of 60ZrF4–30BaF2–10LaF3 (ZBL) via the melt-quench method and 0.2 wt% EuF3 was introduced into the ZBL matrix as the dopant. The well-mixed high-purity fluoride raw materials were melted at 900 °C for 5 min in a platinum crucible using an electric furnace at the rate of 5 °C min−1 from room temperature to 900 °C. In addition, NH4F on the raw materials provides a reducing atmosphere and thus, the molten glass was quenched onto an aluminum plate. Herein, the bottom molten glass, when in contact with the aluminum plate, takes away a lot of heat rapidly and forms an ultrathin glass layer owing to the process of efficient heat conduction. Correspondingly, the upper liquid starts to crystallize at the interface with air because air cannot take away a lot of heat. Subsequently, the molded samples were annealed at 260 °C for 2 h and then cooled down slowly to room temperature. The schematic diagram of the systematic preparation procedure is exhibited in Fig. 1. Relevant tests were performed on the glass surface of the bi-layer ceramic/glass composite, the ceramic layer, and the glass layer, which were named as GCZBL-Eu, CZBL-Eu, and GZBL-Eu, respectively (CZBL-Eu and GZBL-Eu samples were ground to the glass or ceramic layer by the GCZBL-Eu composite).The differential thermal analysis (DTA) scan was carried out on a WCR-2D differential thermal analyzer at the rate of 10 °C min−1 from room temperature to 900 °C. X-ray diffraction (XRD) measurements for the powders of GZBL-Eu and CZBL-Eu were carried out on a Shimadzu XRD-7000 diffractometer (40 kV, 30 mA). The morphological behavior of the section of GCZBL-Eu was observed by a field-emission scanning electron microscope (SEM instrument, JEOL JSM-7800F). The glass layer thickness of GCZBL-Eu was measured by a fluorescence microscope (Imaging system CK-500). The refractive index of GZBL-Eu was measured by using the Metricon 2010 prism coupler. The visible fluorescence spectra and the fluorescence decay curves were obtained using a Hitachi F-7000 fluorescence spectrophotometer equipped with an R928 photomultiplier tube (PMT) as the detector and a commercial Xe-lamp as the excitation source.
Results and discussion
The transition temperature Tg = 305 °C, the onset crystallization temperature Tx = 364 °C, and the peak temperature Tc = 379 °C of the DTA curve are shown in Fig. 1(a). The parameters including the temperature difference value ΔT = Tx − Tg = 59 °C, the thermal stability parameter H = ΔT/Tg = 0.19, and the Saad–Poulain criterion S = ΔT(Tc − Tx)/Tg = 2.9 °C demonstrate that this sample is more effortless to crystallize than other oxide glasses or oxyfluoride glasses, and is suitable for the preparation of the fluoride ceramic-based composite glass. The inset of Fig. 2(a) shows the cross-sectional morphology of GCZBL-Eu under an optical microscope. The shiny part of this picture is the glass layer, whose thickness was identified to be ∼0.81 mm, the dark part is ceramic-based, and the intersection part of the two layers is called the GC transition region.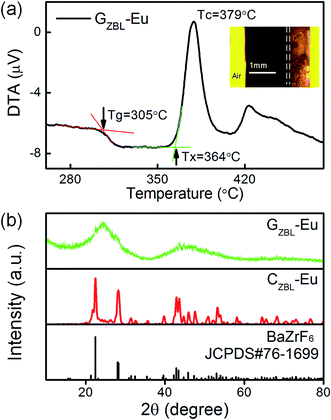 | ||
| Fig. 2 (a) The DTA curve of GZBL-Eu. Inset: the cross-section morphology of the GCZBL-Eu sample under an optical microscope. (b) The XRD patterns of GZBL-Eu and CZBL-Eu. | ||
The typical XRD pattern of GZBL-Eu in Fig. 2(b) exhibits a broad diffuse scattering at lower angles without the narrow diffraction peaks of the crystal phase and the amorphous state of the prepared glass layer is well-identified. At the same time, by comparing the sharp diffraction peaks in the XRD spectrum of CZBL-Eu, the crystal phase of CZBL-Eu is identified to be pure BaZrF6. The slight differences in the cell parameters between CZBL-Eu (a = 7.744 Å, b = 11.691 Å, c = 5.404 Å, α = β = γ = 90°) and the standard BaZrF6 phase (a = 7.681 Å, b = 11.357 Å, c = 5.511 Å, α = β = γ = 90°) indicate the possibility of lattice deformation caused by the introduction of Eu3+. The cell volumes of orthorhombic CZBL-Eu and BaZrF6 judged by the cell parameters were calculated to be 489.25 and 480.74 Å3 by the equation V = a × b × c, indicating that the doping of Eu3+ has little effect on the cell volume before and after substitution. In order to maintain electrical neutrality, Eu3+, whose radius of 0.95 Å is between that of Zr4+ (0.80 Å) and Ba2+ (1.35 Å), is more likely to replace Zr4+ in the lattice and forms F− vacancies.
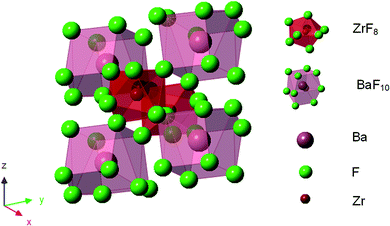
Molten glass appears to be disordered owing to increased interatomic amplitudes and irregular diffusion of the molecules, and the disordered state is fixed to form a glass on sudden cooling. When molten glass cools down slowly, each atom returns to the lowest energy state and forms crystals. In this matrix, BaZrF6 is a crystal with lower lattice energy and is easier to precipitate; Fig. 3 shows the schematic diagram of the crystal structure of BaZrF6.
Spectrum 1 and spectrum 2 with the same element type were chosen from the SEM images of the sample cross-section in Fig. 4(a) for elemental analysis, as shown in Fig. 4(c) and (d). In comparison, the element contents in GZBL-Eu and CZBL-Eu have little difference within the allowable error range, indicating that there is no obvious element migration during the crystallization process. In addition, the absence of oxygen and other elements in the EDS spectra indicates that there is no foreign matter in the prepared GZBL-Eu and CZBL-Eu and the surface of the samples is not oxidized. The SEM image of the ceramic region displays that the BaZrF6 crystalline grain covered by glass in CZBL-Eu grows in an ordered direction and arrangement, as shown in Fig. 4(b). The elemental mapping in the regions contained within the red frames of Fig. 4(e–i) exhibits that Zr and Ba are densely distributed whereas it is the opposite for La, which indicates that the red frame region represents the cross-section of the BaZrF6 grains coated with glass.
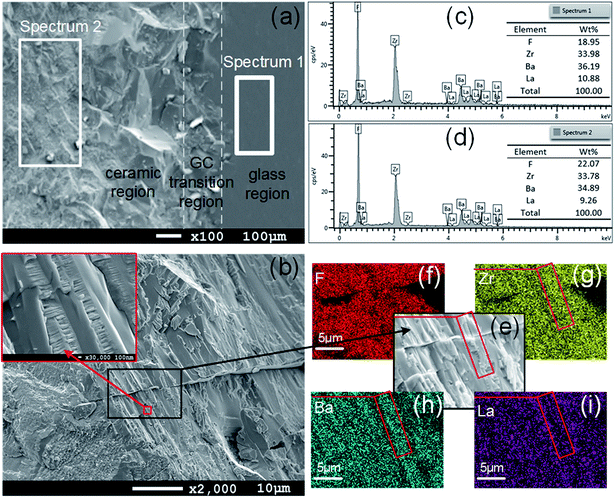 | ||
| Fig. 4 (a and b) SEM images of the cross-section of GCZBL-Eu. (c and d) EDS spectra and the elemental content of GZBL-Eu and CZBL-Eu. (e–i) Elemental mapping of CZBL-Eu. | ||
Based on the FT-IR spectrum of the glass layer in Fig. 5(a), the molar absorption coefficient αOH can be used to evaluate the residual OH content in the glass samples and was found to be 0.91 cm−1 in the 75TeO2–10ZnO–10Na2O–5GeO2 glasses,26 while the value in this work is as low as 0.57 cm−1. The low OH content of the samples is helpful in achieving the anticipated photon emission by reducing the fluorescence loss. In view of the high sensitivity of the multi-channel transition to the phonon energy, the maximum phonon energy (E) of the glass samples was estimated through the empirical formula E = 92.9 + 0.4257R, where R corresponds to the wavenumber at 10% transmittance of the infrared transmission sideband and the maximum phonon energy of GZBL-Eu is identified to be ∼483 cm−1.
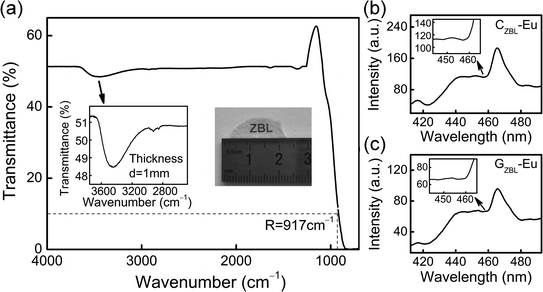 | ||
| Fig. 5 (a) The FT-IR spectrum of the glass layer. (b and c) The excitation spectra of GZBL-Eu and CZBL-Eu monitored at 615 nm emission. | ||
The energy difference between the phonon sideband spectrum and the zero-phonon line is the coupled phonon energy for the electron transition of the RE3+ ions. The phonon energies of GZBL-Eu and CZBL-Eu were estimated to ∼452 and ∼424 cm−1 according to the fitting of the two curves shown in Fig. 5(b) and (c), respectively. The local vibrational modes with the corresponding RE3+ ions are generally considered to be slightly less than the maximum phonon energy of the material.27 GZBL-Eu and CZBL-Eu with low phonon energies, which have been confirmed by the two methods mutually, can reduce the possibility of non-radiative transitions and facilitate fluorescence emission.
The emission spectra of GZBL-Eu and CZBL-Eu were recorded under 395 nm excitation as shown in Fig. 6(a). The emission intensity of CZBL-Eu is much higher than that of GZBL-Eu owing to the ordered arrangement of BaZrF6 particles and another factor is that Eu3+ maybe be retained in CZBL-Eu more than in GZBL-Eu. Eleven emission peaks of GZBL-Eu and CZBL-Eu are located at 429, 445, 464, 488, 509, 525, 535, 554, 590, 615, and 698 nm, which originate from the 5D3 → 7F2,3,4, 5D2 → 7F2,3, 5D1 → 7F0,1,2, and 5D0 → 7F1,2,4 transitions of Eu3+, respectively. The energy level diagram in Fig. 6(b) shows the radiation and relaxation processes of multiple channels and the values of energy gaps among the 5D0, 5D1, 5D2, and 5D3 levels. In the GZBL-Eu and CZBL-Eu systems, the multiphonon relaxation processes from the 5D3 and 5D2 levels are accomplished by six-phonon bridging and that from 5D1 level is completed by four-phonon bridging. All of them belong to high order processes (more than 3 phonons) and thus, the probability of energy loss by non-radiative transitions is lowered. Therefore, the blue and green light emission peaks from 5D3, 5D2, and 5D1 levels cooperating with the red-light emission peaks from the 5D0 level can be well applied to luminescent color tuning, thus expanding the application direction of single RE3+ ions.
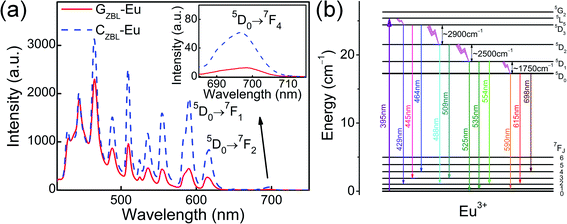 | ||
| Fig. 6 (a) Emission spectra of GZBL-Eu and CZBL-Eu under 395 nm excitation. (b) The schematic diagram of multimodal emission energy levels of Eu3+ under 395 nm excitation. | ||
By referring to the halogen lamp, the relative spectral power distribution is converted from the emission spectrum and is shown in Fig. 7(a). Depending on the relative spectral power distribution, photon distribution N(ν) can be deduced as 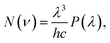 where ν, λ, h, c, and P(λ) represent the wavenumber, wavelength, Planck constant, vacuum light velocity, and spectral power distribution, respectively.28–30 Under 395 nm excitation, the emission photon number distribution curves of GZBL-Eu and CZBL-Eu are derived as shown in Fig. 7(b).
where ν, λ, h, c, and P(λ) represent the wavenumber, wavelength, Planck constant, vacuum light velocity, and spectral power distribution, respectively.28–30 Under 395 nm excitation, the emission photon number distribution curves of GZBL-Eu and CZBL-Eu are derived as shown in Fig. 7(b).
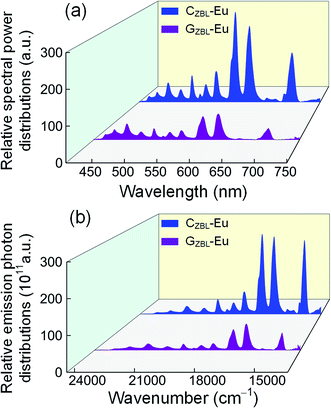 | ||
| Fig. 7 The relative spectral power distributions (a) and the relative emission photon distributions (b) of GZBL-Eu and CZBL-Eu under 395 nm excitation. | ||
Ω2, which is influenced by the local structure symmetry of Eu3+ in GZBL-Eu and CZBL-Eu, is usually used to describe the emission distribution of the 5D0 level and can be calculated based on the emission photon number distributions instead of the general absorption spectra. In the calculation, the ordinary refractive index n value of GZBL-Eu is measured to be 1.555 and the value of CZBL-Eu is estimated to be 1.590.31 The Ω2 value of Eu3+ in CZBL-Eu is 1.75 × 10−20 cm2, which is lower than 2.49 × 10−20 cm2 in GZBL-Eu. A smaller Ω2 value indicates a higher symmetry of the local structure of Eu3+ ions and weaker covalency around the metal–donor interaction. Also, compared with CZBL-Eu, GZBL-Eu, and other substrates,32–37 a smaller Ω2 value (higher symmetry) corresponds to lower red-light emission ratio of the 5D0 → 7F2 transition. In other words, at a higher symmetry site, the intensity ratio of the 5D0 → 7F2 emission is reduced and thus, it becomes more similar to that of the 5D0 → 7F1 transition. This means that CZBL-Eu can better limit the emission of red light and using the shorter-wavelength orange light generated in the 5D0 → 7F1 transition, it is easier to synthesize white light with blue and green light from the 5D3, 5D2, and 5D1 level transitions. Such a change towards more equal intensity emission at various wavelengths is more suitable for realizing an overall white emission of the phosphor and the excitation source system.
To better express the capacity of the potential radiative transition among the levels, the experimental average lifetimes and the de-population rate of Eu3+ in GZBL-Eu and CZBL-Eu can be derived from the fluorescence decay curves, as shown in Fig. 8, and the related results are calculated and listed in Table 1. In the fluoride system with low phonon energy, photons can be effectively released and the low de-population rate represents that the radiative transition occupies a major proportion. In order to evaluate the effective radiation of CZBL-Eu and GZBL-Eu, the quantum efficiencies of the 5D0 level was calculated. The data of theoretical radiative fluorescent lifetimes (including the relevant calculation data), experimental average lifetimes, and quantum efficiencies are listed in Table 2. The quantum efficiencies of Eu3+ from the 5D0 level in GZBL-Eu and CZBL-Eu were calculated to be 98.5% and 94.8%, which demonstrate the effectiveness of radiative transition emission from Eu3+ in GZBL-Eu and CZBL-Eu. The difference in the quantum efficiencies of GZBL-Eu and CZBL-Eu is consistent with the conclusion that the luminous efficiency of Eu3+ decreases in a more symmetrical crystal field environment. The high quantum efficiencies of GZBL-Eu and CZBL-Eu demonstrate the effectiveness of radiative transition emissions from Eu3+ and show the application prospects in energy conversion and energy saving.
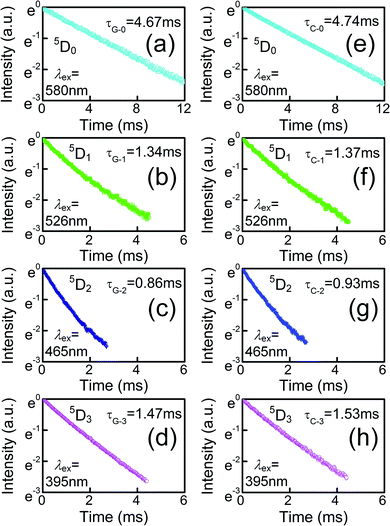 | ||
| Fig. 8 Fluorescence decay curves from the 5D0, 5D1, 5D2, and 5D3 level of Eu3+ in GZBL-Eu (a–d) and CZBL-Eu (e–h) monitored at 615, 535, 509, and 464 nm, respectively. | ||
| Energy level | GZBL-Eu | CZBL-Eu | ||
|---|---|---|---|---|
| Experimental lifetime (ms) | De-population rate (s−1) | Experimental lifetime (ms) | De-population rate (s−1) | |
| 5D0 | 4.67 | 214 | 4.74 | 211 |
| 5D1 | 1.34 | 746 | 1.37 | 730 |
| 5D2 | 0.86 | 1163 | 0.93 | 1075 |
| 5D3 | 1.47 | 680 | 1.53 | 654 |
| Sample | Transition | Energy (cm−1) | Photon number ratio | Aij (s−1) | βij (%) | τrad (ms) | τexp (ms) | Quantum efficiency (%) |
|---|---|---|---|---|---|---|---|---|
| GZBL-Eu | 5D0 → 7F1 (N1) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 949 949 |
— | 64.3 | 30.5 | 4.74 | 4.67 | 98.5 |
| 5D0 → 7F2 (N2) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271 |
N2/N1 = 1.30 | 83.8 | 39.7 | ||||
| 5D0 → 7F4 (N4) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 318 |
N4/N1 = 0.98 | 63.0 | 29.8 | ||||
| CZBL-Eu | 5D0 → 7F1 (N1) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938 938 |
— | 68.7 | 34.4 | 5.00 | 4.74 | 94.8 |
| 5D0 → 7F2 (N2) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207 207 |
N2/N1 = 0.98 | 63.3 | 31.7 | ||||
| 5D0 → 7F4 (N4) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 351 351 |
N4/N1 = 1.06 | 67.9 | 33.9 |
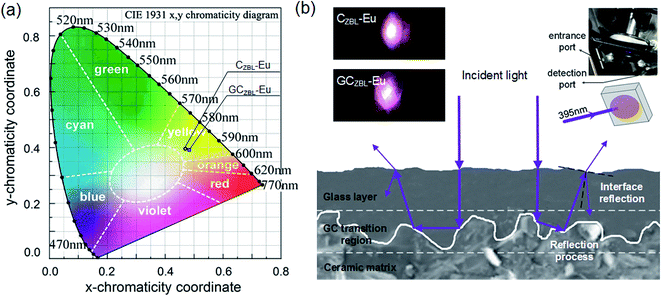
The emission spectra and the relative spectral power distributions of CZBL-Eu and GCZBL-Eu are shown in Fig. 9(a) and (b) under 395 nm excitation, and both them show multi-channel radiative transitions of Eu3+ in CZBL-Eu and GCZBL-Eu. Herein, the CIE-1931 chromaticity coordinates for the luminescence of CZBL-Eu and GCZBL-Eu were derived to be (0.483, 0.385) and (0.469, 0.389), which fall into the yellowish-white region, as shown in Fig. 10(a). The chromaticity coordinates prove that the emission light of CZBL-Eu and GCZBL-Eu is yellowish-white, suggesting that the Eu3+-activated GCZBL-Eu system has application prospect as a single phosphor.
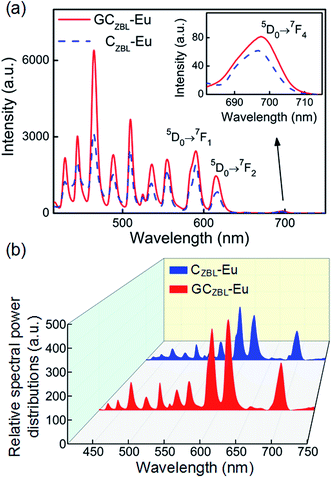 | ||
| Fig. 9 Comparison of emission intensities (a) and the relative spectral power distributions (b) between CZBL-Eu and GCZBL-Eu under 395 nm excitation. | ||
The emission intensity of GCZBL-Eu at 590 nm is 1.24 times higher than that for CZBL-Eu, where the significant improvement in the emission intensity can be attributed to the complex surface morphology of the ceramic matrix and the intense dispersion effect of the glass layer, and the schematic diagram of increasing the incident light absorption efficiency and enhancing the emission is shown in Fig. 10(b). When the incident light shines into GCZBL-Eu, the under-utilized light is re-reflected to the ceramic boundary, forming a multiple-cycle effect owing to the specular reflection of the glass layer. Then, the incident light will be absorbed by the glass phase in the reflection process of the glass–ceramic transition region and is converted into other wavelengths for emission.
As shown in the inset of Fig. 10(b), intense lighting is observed in CZBL-Eu and GCZBL-Eu, and the violet lighting in the picture is illuminated by a 395 nm Xe lamp. Simultaneously, the purple source overlaps with the emitted yellowish-white light to form the pink-white light. Effective fluorescence emission enhancement facilitates better application of GCZBL-Eu with warm-white light emission of laser lighting.
Conclusion
Hetero-structured GCZBL-Eu with glass region, ceramic region, and GC transition region obtained by the one-step method exhibits yellowish-white light and the crystal phase of CZBL-Eu is identified as BaZrF6. Multi-channel radiative transitions were discovered through multi-peak emission, long fluorescence lifetimes, and de-population rates of the 5D0, 5D1, 5D2, and 5D3 levels in GZBL-Eu and CZBL-Eu, which is because the low OH content and low phonon energy limit the non-radiative relaxation. The effective radiative transition emission of the 5D0 levels in GZBL-Eu and CZBL-Eu is demonstrated by the high quantum efficiency of 98.5% and 94.8%, respectively. The release of yellowish-white light is indicated by the color coordinates of CZBL-Eu (0.483, 0.385) and GCZBL-Eu (0.469, 0.389), and the emission intensity of GCZBL-Eu is 1.24 times higher than that of CZBL-Eu due to multiple-cycle reflection in the complex structure of the GC transition region. The effective yellowish-white lighting proves that the GCZBL-Eu system with fluorescence enhancement has potential in the application direction of laser illumination.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research work was supported by the Scientific Research Funding Project from the Educational Department of Liaoning Province, China (Grant No. J2019021) and the Research Grants Council of the Hong Kong Special Administrative Region, China (Grant No. CityU 11218018).References
- T. Mondal, S. Mondal, S. Bose, D. Sengupta, U. K. Ghorai and S. K. Saha, J. Mater. Chem. C, 2018, 6, 614–621 RSC.
- C. Y. Sun, X. L. Wang, X. Zhang, C. Qin, P. Li, Z. M. Su, D. X. Zhu, G. G. Shan, K. Z. Shao, H. Wu and J. Li, Nat. Commun., 2013, 4, 2717 CrossRef PubMed.
- Q. Wang and D. Ma, Chem. Soc. Rev., 2010, 39, 2387–2398 RSC.
- X. Q. Liao, X. Yang, R. Zhang, J. Cheng, J. Li, S. Y. Chen, J. Zhu and L. Li, J. Mater. Chem. C, 2017, 5, 10001–10006 RSC.
- H. Li, H. B. Liu, X. M. Tao, J. Su, P. F. Ning, X. F. Xu, Y. Zhou, W. Gu and X. Liu, J. Mater. Sci., 2014, 49, 4439–4444 CrossRef.
- Z. Gao, S. Liu, P. Sun, J. H. Jeong, R. Yu and B. Deng, J. Rare Earths, 2018, 36, 917–923 CrossRef CAS.
- M. Mazzeo, V. Vitale, F. Della Sala, M. Anni, G. Barbarella, L. Favaretto, G. Sotgiu, R. Cingolani and G. Gigli, Adv. Mater., 2005, 17, 34–39 CrossRef CAS.
- R. S. Deshpande, V. Bulović and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 888–890 CrossRef CAS.
- H. P. Barbosa, I. G. N. Silva, M. C. F. C. Felinto, E. E. S. Teotonio and H. F. Brito, J. Alloys Compd., 2017, 696, 820–827 CrossRef CAS.
- N. Guo, Y. Huang, H. You, M. Yang, Y. Song, K. Liu and Y. Zheng, Inorg. Chem., 2010, 49, 10907–10913 CrossRef CAS PubMed.
- P. Y. Poma, W. Q. Santos, T. O. Sales, A. S. Gouveia-Neto and C. Jacinto, J. Lumin., 2017, 188, 18–23 CrossRef CAS.
- S. A. Ying, S. Xiao, J. W. Yao, Y. F. Dai, D. Z. Yang, X. F. Qiao, J. S. Chen, T. F. Zhu and D. G. Ma, Adv. Opt. Mater., 2019, 7, 1901291 CrossRef CAS.
- X. Liu, Y. Li, T. Aidilibike, J. Guo, W. Di and W. Qin, J. Lumin., 2017, 185, 247–250 CrossRef CAS.
- L. Han, S. Xie, M. Wang, T. Sun, Q. Liu, G. Jiang, Y. Shi and Y. Tang, Mater. Lett., 2019, 234, 241–244 CrossRef CAS.
- Y. Yu, D. Chen, P. Huang, H. Lin and Y. Wang, Ceram. Int., 2010, 36, 1091–1094 CrossRef CAS.
- A. Balamurugan, M. L. Reddy and M. Jayakannan, J. Mater. Chem. A, 2013, 1, 2256–2266 RSC.
- J. N. Hao and B. Yan, J. Mater. Chem. A, 2014, 2, 18018–18025 RSC.
- X. Shen and B. Yan, J. Mater. Chem. C, 2015, 3, 7038–7044 RSC.
- J. J. Velázquez, J. Mosa, G. Gorni, R. Balda, J. Fernández, A. Durán and Y. Castro, J. Non-Cryst. Solids, 2019, 520, 119447 CrossRef.
- X. Liu, J. Zhou, S. Zhou, Y. Yue and J. Qiu, Prog. Mater. Sci., 2018, 97, 38–96 CrossRef CAS.
- L. Li, H. K. Yang, B. K. Moon, Z. Fu, C. Guo, J. H. Jeong, S. S. Yi, K. Jang and H. S. Lee, J. Phys. Chem. C, 2008, 113, 610–617 CrossRef.
- B. Rajamouli, P. Sood, S. Giri, V. Krishnan and V. Sivakumar, Eur. J. Inorg. Chem., 2016, 2016, 3900–3911 CrossRef CAS.
- S. K. Gupta, M. Mohapatra, V. Natarajan and S. V. Godbole, J. Mater. Sci., 2012, 47, 3504–3515 CrossRef CAS.
- N. Wantana, E. Kaewnuam, B. Damdee, S. Kaewjaeng, S. Kothan, H. J. Kim and J. Kaewkhao, J. Lumin., 2018, 194, 75–81 CrossRef CAS.
- Y. Y. Li and S. Q. Xu, RSC Adv., 2018, 8, 31905–31910 RSC.
- V. A. G. Rivera, M. El-Amraoui, Y. Ledemi, Y. Messaddeq and E. Marega Jr, J. Lumin., 2014, 145, 787–792 CrossRef CAS.
- S. Todoroki, K. Hirao and N. Soga, J. Non-Cryst. Solids, 1992, 143, 46–51 CrossRef CAS.
- R. K. Pushpendra Kunchala, S. N. Achary and B. S. Naidu, ACS Appl. Nano Mater., 2019, 2, 5527–5537 CrossRef.
- R. K. Pushpendra Kunchala, S. N. Achary, A. K. Tyagi and B. S. Naidu, Cryst. Growth Des., 2019, 19, 3379–3388 CrossRef.
- B. S. Naidu, B. Vishwanadh, V. Sudarsan and R. K. Vatsa, Dalton Trans., 2012, 41, 3194–3203 RSC.
- G. De Leede, R. Beerkens, E. Van Duin and H. De Waal, J. Mater. Sci., 1992, 27, 2309–2315 CrossRef CAS.
- N. Li, Y. Xue, D. Wang, B. Liu, C. Guo, Q. Song, X. Xu, J. Liu, D. Li, J. Xu, Z. Xu and J. Xu, J. Lumin., 2019, 208, 208–212 CrossRef CAS.
- M. Kaczkan, S. Turczyński and M. Malinowski, J. Lumin., 2018, 196, 111–115 CrossRef CAS.
- Y. Tian, B. Chen, R. Hua, N. Yu, B. Liu, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, B. Tian and H. Zhong, Crystengcomm, 2012, 14, 1760–1769 RSC.
- L. Liu and X. Chen, Nanotechnology, 2007, 18, 255704 CrossRef.
- P. A. Loiko, V. I. Dashkevich, S. N. Bagaev, V. A. Orlovich, X. Mateos, J. M. Serres, E. V. Vilejshikova, A. S. Yasukevich, K. V. Yumashev, N. V. Kuleshov, E. B. Dunina, A. A. Kornienko, S. M. Vatnik and A. A. Pavlyukf, J. Lumin., 2015, 168, 102–108 CrossRef CAS.
- P. Loiko, E. V. Vilejshikova, A. A. Volokitina, V. A. Trifonov, J. M. Serres, X. Mateos, N. V. Kuleshov, K. V. Yumashev, A. V. Baranov and A. A. Pavlyuk, J. Lumin., 2017, 188, 154–161 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |