Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells†
Melanie Reißa,
Andreas Brietzkeb,
Thomas Eicknerb,
Florian Steina,
Alexander Villinger a,
Christian Vogela,
Udo Kragl
a,
Christian Vogela,
Udo Kragl a and
Stefan Jopp
a and
Stefan Jopp *a
*a
aInstitute of Chemistry, University of Rostock, Albert-Einstein-Straße 3a, 18059 Rostock, Germany. E-mail: stefan.jopp@uni-rostock.de
bInstitute of Biomedical Engineering, University of Rostock, Friedrich-Barnewitz-Straße 4, 18119 Rostock, Germany
First published on 7th April 2020
Abstract
The large pool of naturally occurring carbohydrates with their diversity in chirality and structure led to the idea of a systematic investigation of carbohydrate based ILs. To this end, we investigated the influence of different ether groups, mainly methyl or ethyl ether, on the secondary OH groups as well as different configurations on physical properties such as melting point, thermostability and especially the influence on cell toxicity. For this investigation we chose α- and β-methyl-, β-allyl- and β-phenyl D-glucopyranose as well as four 1-deoxy-pentoses. In order to be able to classify the results, more ionic liquids with different structural motives were examined for cytotoxicity. Here, we present data that confirm the biocompatibility of such ILs consisting of naturally occurring molecules or their derivatives. The synthesized carbohydrate based ILs were tested for their suitability as additives in coatings for medical applications such as drug-eluting balloons.
Introduction
In recent years, ionic liquids have aroused the interest of scientists in the medical research field due to their wide variety of structures and potential applications.1 Current areas of investigation include IL based drugs (Active Pharmaceutical Ingredients-Ionic Liquids)2 as well as other applications of ILs in the pharmacological field such as drug delivery systems.1,3 In such systems, the IL serves as a solvent or solvent promoter for sparingly water-soluble drugs and can thus enhance pharmacokinetic and pharmacodynamic properties considerably.Another interesting medical application are drug-eluting balloons, which are typically used in re-stenotic lesions.4 Previous works of our group have shown that the ionic liquid cetylpyridinium salicate [CetPyr][Sal] is a potential candidate for such an application.5 This IL however, while showing good properties in drug release and homogenous coating ability, is not suited for an actual application due to cytotoxicity.
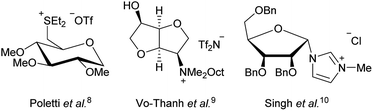
Thus we decided to further look into ILs based on natural products like amino acids6 and carbohydrates7 Especially carbohydrate based ionic liquids have piqued our interest as potential ILs with low cytotoxicity. While there have been a few previous studies on carbohydrate based ILs,7–10 most of them focused on the synthesis. Examples of previous ILs based on carbohydrates are shown in Fig. 1.
While the use of some of these carbohydrate based ILs as solvents or for asymmetric induction9 has been studied, a study for potential medical application has yet to be done.
Results and discussion
Synthesis
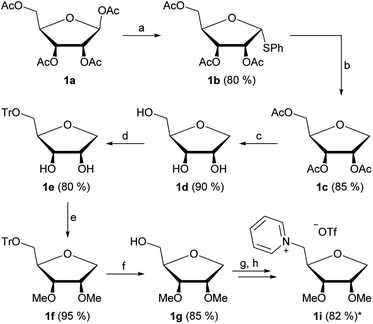
The first part of our strategy was the synthesis of four new ionic liquids derived from pentoses. We chose the peracetylated species of D-ribose 1a, D-lyxose 2a, D-xylose 3a and L-arabinose 4a as starting materials, which were prepared by procedures known from literature.11 Fig. 2 shows the general synthetic pathway exemplary for D-ribose.Starting from the peracetylated D-ribose 1a we investigated a direct reduction of the anomeric center using TMSOTf and Et3SiH.12 While this strategy successfully led to the 1-deoxy-ribose derivative 1c in a high yield of 92%, it could not be applied for the other three starting materials 2–4a. The yields were low and the products impure. Thus we switched to a two-step strategy of first introducing a thiophenyl group at the anomeric center followed by reduction with tributyltin hydride (Fig. 2a and b). This strategy could be applied for all four products in yields from 50 to 85%.
The next four steps were the deprotection of the acetyl groups, 5-O-tritylation, introduction of the methyl ether groups on the secondary OH groups and lastly deprotection of the 5-O-trityl group, leading to the products 1–4g (Fig. 2c–f). These steps were generally performed with high yields ranging from 80 to 95%.
Our strategy was finalized by converting the unprotected 5-OH group into a triflate, directly followed by the quarternization reaction with pyridine, yielding the products 1–4i in overall high yields after an 8-step synthesis (Fig. 2g and h).
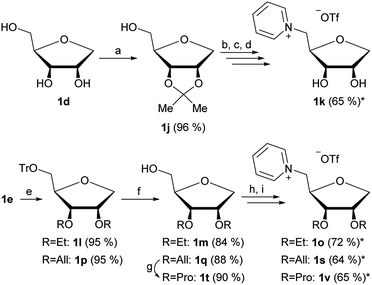
By using 1d and 1e as starting materials in a similar pathway as shown in Fig. 1 we were furthermore able to produce four additional 1-deoxy-ribose based ILs with varying groups in positions 2 and 3.
The isopropylidene protection of 1d (Fig. 3a) allows the production of ionic liquid 1k, which has free OH groups in the positions 2 and 3. Due to the instability of the isopropylidene group under acidic conditions, the synthesis of an isopropylidene protected 1-deoxy-ribose based IL was not possible at first. Said group was partly cleaved during the introduction of the 5-O-triflate group, thus a full cleavage of the isopropylidene group was performed after quarternization (Fig. 3b–d). We were however able to produce the isopropylidene protected 1-deoxy-ribose based IL 1x by using mesylate instead of triflate (Fig. 4).
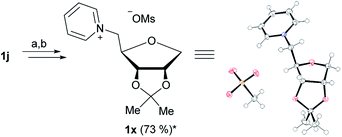 | ||
| Fig. 4 Synthesis and ORTEP13 of 1x. (a) Mesyl chloride, pyridine, r.t., 12 h; (b) pyridine, 125 °C, 5 h; *yield over two steps. | ||
By changing the reagent used for the introduction of the ether groups starting from 1e, ethyl and allyl ethers 1l and 1p have also been synthesized successfully (Fig. 3e). By further reducing the allyl group to a propyl group and carrying on with the already established strategy of introduction of the triflate followed by quarternization with pyridine, products 1o, 1s and 1v were achieved.
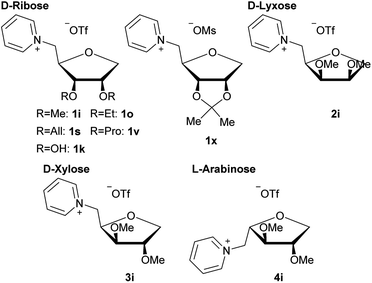
All nine new pyridine salts derived from pentoses are shown in Fig. 5. These ionic structures all classify as ionic liquids, as further shown under “Thermal analysis”.
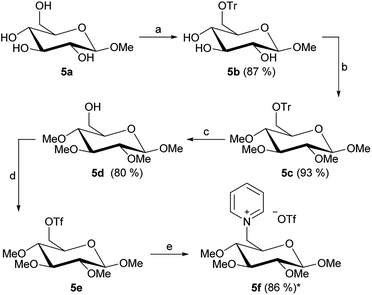
Our second synthetic goal was to apply the established strategy for 1-deoxy-pentoses on different glucosides. Our starting materials were β-D-methyl, allyl and phenyl glucosides 5–7a as well as α-D-methyl glucoside 8a. Fig. 6 shows the general synthetic pathway exemplary for the β-D-methyl glucoside 5a.
The 5-step reaction starts with the introduction of the 6-O-trityl group (Fig. 6a). This step was performed with yields from 66% when using the β-D-allyl glucoside up to 87% for the β-D-methyl glucoside. The follow-up reactions are, similar to the synthesis shown in Fig. 2, the introduction of the methyl ether groups and afterwards the 6-O-trityl deprotection. These two steps were generally performed in high yields up to 94% (Fig. 6b and c). The resulting free 6-OH group of the products 5–8d was then converted into a triflate group followed by quarternization with pyridine, leading to the carbohydrate-based pyridinium triflate salts 5–8f (Fig. 6d and e).
By following the same idea as applied before for the 1-deoxy-pentoses, the methyl ether groups were also changed to ethyl ether groups on the β-D-methyl glucoside structure. This leads to product 5j (Fig. 7). Lastly, two further salts were derived from 5d by changing the leaving group to mesylate or tosylate, leading to 5l and 5n, respectively (Fig. 7).
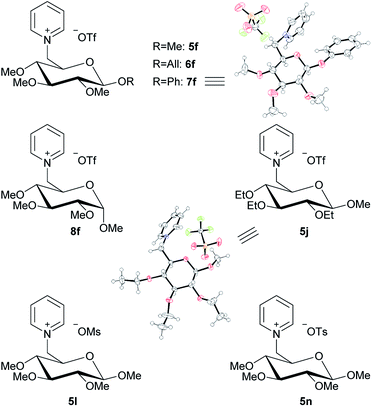 | ||
| Fig. 7 Overview of glucoside-based pyridinium salts and ORTEPs13 of 7f and 5j. | ||
All seven new pyridine salts derived from glucosides are shown in Fig. 7. Most of these new products classify as ionic liquids, as further shown under “Thermal analysis”.
Biocompatibility
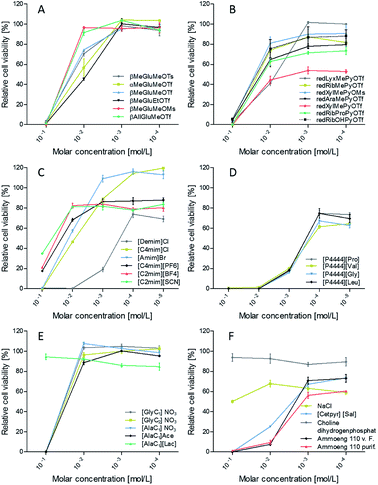
For biocompatibility measurements, several commonly known ILs, whose cytotoxicity has already been thoroughly explored,14 have been studied in comparison to the new carbohydrate based ILs we synthesized. A few examples of these additionally tested ILs are shown in Fig. 8.Cell viability assay revealed a strong impact on cell viability at 0.1 mol L−1 molar concentration for the predominant share of the evaluated ionic liquids (Fig. 9A–F). According to that only [AlaC1][Lac], choline dihydrogenphosphate and sodium chloride, which was tested for comparison, exhibit no zytotoxic effects. Due to the natural occurrence of these substances in cells, this meets our expectations.
Besides that, further discriminations between the ionic liquids presented herein can be made. Comparison between the different glucose derivatives (Fig. 9A) shows almost no viability for any substance at c = 0.1 mol L−1, whereas major differences can be found at a concentration of 10−2 mol L−1. A change of the anomeric form from β-glucose to α-glucose results in a decreased viability.
Moreover, results indicate a major impact of the sort of anion on biocompatibility. For βMeGluMePyr+ viability rises for following anions in the order: −OTs < −OTf < −OMs. This effect is supported by redXylMePyr+ with the counterions −OTf < −OMs (Fig. 9B). Surprisingly, the substitution of the methyl group by an allylic group at the anomeric center diminishes the cytotoxicity of the triflate anion.
For 1-deoxy-pentoses different viabilities could be observed in the following order: lyxose ≤ xylose < arabinose < ribose. Ribose derivatives are naturally found in all nucleotides (DNA, RNA) and hence in all cells, while lyxose can be found in the cell walls of few bacteria and xylose and arabinose in the polysaccharides of plants. Hence, ribose derivatives should be tolerated better by cells with mammalian origin.
Thus, the use of substances with biological origin such as carbohydrates and amino acids is a promising approach for improving the biocompatibility of ionic liquids. To test this, a common tetrabutylphosphonium cation [P4444] was countered with four different anions derived from proteinogenic hydrophobic amino acids: proline, valine, glycine and leucine (Fig. 9D). The resulting ionic liquids exhibit cytotoxic effects over the whole concentration range. There are no significant differences between the different anions.
In contrast amino acid derivatives from alanine and glycine as cations exhibit no cytotoxic effects at a concentration of 10−2 mol L−1 (Fig. 9E). There is also no difference between methoxy- or ethoxy esters in the glycine derivatives. According to the counter ion no significant differences between nitrate and acetate can be found.
Hence, using naturally occurring substances and their derivatives to generate the cation opens up new fields of application, such as biomedical applications. It is worth noting that the IL [AlaC1][Lac] shows good viability already at concentrations of 0.1 mol L−1, but compared to the other [AlaC1] derivatives a slightly worse impact on the biocompatibility at lower concentrations. Further studies have to be performed to shed light on this phenomenon (Tables 1 and 2).
| No. | Cation | Anion | EC50 [mmol L−1] |
|---|---|---|---|
| a 7f was not measured due to its very poor solubility in water. | |||
| 1i | redRibMePyr | OTf | 46.40 |
| 2i | redLyxMePyr | OTf | 25.19 |
| 3i | redXylMePyr | OTf | 38.65 |
| 4i | redAraMePyr | OTf | 40.37 |
| 1k | redRibOHPyr | OTf | 54.80 |
| 1v | redRibPrPyr | OTf | 136.62 |
| 5f | βMeGluMePyr | OTf | 41.73 |
| 6f | βAllGluMePyr | OTf | 36.40 |
| 7f | βPhGluMePyr | OTf | n.m.a |
| 8f | αMeGluMePyr | OTf | 49.88 |
| 5l | βMeGluMePyr | OMs | 57.60 |
| 5n | βMeGluMePyr | OTs | 51.49 |
| Cation | Anion | EC50 [mmol L−1] |
|---|---|---|
| P4444 | Pro | 0.575 |
| P4444 | Val | 0.775 |
| P4444 | Gly | 0.875 |
| P4444 | Leu | 0.725 |
| GlyC1 | NO3 | 35 |
| GlyC2 | NO3 | 20 |
| AlaC1 | NO3 | 20 |
| AlaC1 | Lac | 25 |
| Choline | H2PO4 | 30 |
| Amim | Br | 15 |
| C2mim | BF4 | 4 |
| C2mim | SCN | 8 |
| C4mim | Cl | 10 |
| C4mim | PF6 | 7 |
| C10mim (demim) | Cl | 0.035 |
| Ammoeng 110® | — | 1.07 |
| Ammoeng 110® purif. | — | 3.08 |
| Cetpyr | Sal | 0.038 |
| Na | Cl | 51.85 |
Thermal analysis
The analysis of the melting points of our 16 new carbohydrate based pyridinium salts allows the categorization into the class of ionic liquids, which are per definition salts with a melting point under 100 °C.As such, only βPhGluMePyrOTf 7f, βMeGluEtPyrOTf 5j and βMeGluMePyrOTs 5n do not qualify as ionic liquids, while all other glucoside- and 1-deoxy-pentose products exhibit melting points under 100 °C, with many of them even being room temperature ionic liquids (Tables 3 and 4).
| No. | Cation | Anion | mp [°C] | dp [°C] |
|---|---|---|---|---|
| 5f | βMeGluMePyr | OTf | Liquid at r.t. | 225 |
| 6f | βAllGluMePyr | OTf | 66–70 | 205 |
| 7f | βPhGluMePyr | OTf | 164–168 | 225 |
| 8f | αMeGluMePyr | OTf | 95–100 | 242 |
| 5j | βMeGluEtPyr | OTf | 118–120 | 215 |
| 5l | βMeGluMePyr | OMs | 60–63 | 250 |
| 5n | βMeGluMePyr | OTs | 135–138 | 242 |
| No. | Cation | Anion | mp [°C] | dp [°C] | Tg [°C] |
|---|---|---|---|---|---|
| 1i | redRibMePyr | OTf | 48–51 | 345 | −18 |
| 2i | redLyxMePyr | OTf | Liquid at r.t. | 325 | −41 |
| 3i | redXylMePyr | OTf | 32–36 | 345 | −28 |
| 4i | redAraMePyr | OTf | Liquid at r.t. | 340 | −38 |
| 1o | redRibEtPyr | OTf | Liquid at r.t. | 316 | −26 |
| 1s | redRibAllPyr | OTf | Liquid at r.t. | 301 | n.m. |
| 1v | redRibPrPyr | OTf | Liquid at r.t. | 235 | −27 |
| 1k | redRibOHPyr | OTf | Liquid at r.t. | 297 | −30 |
| 1x | redRibIsoPyr | OMs | 92–94 | 296 | n.m. |
While most of the 1-deoxy-pentose based ILs are liquid at room temperature (Table 4) and such don't allow a direct comparison of the impact of configuration and varying groups on the melting point, such comparisons can be made for the glucoside based products (Table 3).
The type and configuration of the group at the anomeric center has a high influence on the melting point. A trend of βOMe < βOAll ≤ βOPh was found for the glucosides 5–7f. The double bonds of allyl and phenyl groups allow more interactions between the cations than the methyl group, leading to this trend. Furthermore, βMeGluMePyrOTf 5f is liquid at room temperature, while αMeGluMePyrOTf 8f has a melting point close to 100 °C, showing that the configuration of the anomeric center alone has a high impact on the melting point. The change of methyl ether groups in 5f to ethyl ether groups in 5j also heightens the melting point significantly. Lastly the corresponding anion also has a high influence, showing a clear trend of OTf < OMs ≤ OTs.
The decomposition points of the glucoside products vary from 205 to 250 °C, while the 1-deoxy-pentose products have higher values with 297 to 345 °C. In general, these new products have a good thermal stability.
Glass transition temperatures we measured for the 1-deoxy-pentose based IL's to acquire further information of the influences of configuration and varying groups on physical properties. Here we see a trend of lyxose 2i < arabinose 4i < xylose 3i < ribose 1i. The impact of the varying groups in positions 2 and 3 on the glass transition temperature is small, with the methyl ether product 1i having the highest and the free OH group product 1k the lowest Tg in direct comparison.
Methods
Cell viability assay
In order to evaluate the biocompatibility of the carbohydrate-based ILs cytotoxicity was tested with Cell Viability Assay Kit (BioAssay systems, Hayward, CA, USA).L929 mouse fibroblasts (CCL-1, ATCC) were cultured in DMEM (PAN BIOTECH, Aidenbach, Germany) with 4.5 mg glucose and 10% fetal calf serum (FCS), 1% Penicillin/Streptomycin and 3.7 g L−1 NaHCO3.
For screening tests 2 × 104 L929 mouse fibroblasts were seeded in a 96-well microtiter plate with 200 μL culture medium per well and incubated under cell culture conditions (37 °C, 5% CO2) for 24 hours.
To proof cell viability CellQuanti-Blue Cell Viability Assay Kit (BioAssay systems, Hayward, CA, USA) was implemented. 10% CellQuanti-Blue supplement was added to the wells followed by an incubation of another 2 hours under same conditions. The reductive activity of the cells conducts the metabolic turnover from resazurin to the fluorescent resorufin (absorption 544 nm, emission 590 nm) which was detected with the Fluostar optima (BMG LABTECH, Ortenberg, Germany).
Thermal analysis
Melting points were determined with a micro heating stage (Mikroheiztisch BOETIUS, Dresden, Germany).Decomposition points have been measured via thermogravimetric analysis using a Labsys 1600 TGA-DSC (SETARAM Instrumentation, Caluire, France).
The differential scanning calorimetry using a Pyris 1 DSC (PerkinElmer, Waltham, USA) allowed the analysis of glass transition temperatures.
Conclusion
In this work we presented a reproducible strategy to synthesize new carbohydrate based pyridinium salts. Said synthetic pathway was successfully applied on 4 different 1-deoxy-pentoses as well as 4 different glucosides, leading to overall 16 new products with varying configurations and groups.Thermal analysis shows that 13 of these new carbohydrate based pyridinium salts qualify as ionic liquids per definition, with most of them even being room temperature ionic liquids. Further biocompatibility tests have proven that these ionic liquids are suitable for biomedical applications, as they exhibit a much higher viability than common imidazolium or phosphonium based ILs, which have been tested in comparison. A potential application currently under testing is the usage of these new carbohydrate based ionic liquids as additives in coatings for drug-eluting balloons.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof. H. Ohno for providing us the four tetrabutylphosphonium ILs paired with amino acid anions, which we used in the biocompability tests. We also thank Prof. Schick of the University of Rostock for conducting DSC with our samples.This work was supported by the European Union (ESF/14-BM-A55-0048/16) as well as by the Federal Ministry of Education and Research (BMBF) project REMEDIS (FKZ:03IS2081).Notes and references
- (a) K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132 CrossRef CAS PubMed; (b) P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, Wiley-VCH, Weinberg, 2002 CrossRef.
- Selected examples: (a) J. Stoimenovski, D. R. Mac Farlane, K. Bica and R. D. Rogers, Pharm. Res., 2010, 27, 521 CrossRef CAS PubMed; (b) K. Bica, H. Rodriguez, G. Gurau, O. A. Cojocaru, A. Riisager, R. Fehrmann and R. D. Rogers, Chem. Commun., 2012, 48, 5422 RSC; (c) J. L. Shamshina, S. P. Kelley, G. Gurau and R. D. Rogers, Nature, 2015, 528, 188 CrossRef CAS; (d) A. Cojocaru, J. L. Shamshina and R. D. Rogers, Chim. Oggi, 2013, 31, 24 Search PubMed; (e) R. Ferraz, L. C. Branco, C. Prudêncio, J. P. Noronha and Ž. Petrovski, ChemMedChem, 2011, 6, 975 CrossRef CAS PubMed.
- N. Adawiyah, M. Moniruzzaman, S. Hawatulaila and M. Goto, MedChemComm, 2016, 7, 1881 RSC.
- D. Jackson, D. Tong and J. Layland, Int. J. Cardiol., 2017, 226, 77 CrossRef PubMed.
- (a) S. Kaule, I. Minrath, F. Stein, U. Kragl, W. Schmidt, K.-P. Schmitz, K. Sternberg and S. Peterson, PLoS One, 2015, 10, e0116080 CrossRef PubMed; (b) S. Peterson, S. Kaule, F. Stein, I. Minrath, K.-P. Schmitz, U. Kragl and K. Sternberg, Mater. Sci. Eng. C, 2013, 33, 4244 CrossRef PubMed.
- H. Ohno and K. Fukumoto, Acc. Chem. Res., 2007, 40, 1122 CrossRef CAS PubMed.
- For review articles see 7a and 7b, for papers of carbohydrate based ILs see 7c–l and 8–10. (a) A. Marra, C. Chiappe and A. Mele, Chimia, 2011, 65, 76 CrossRef CAS PubMed; (b) N. Kaur, A. Singh and H. K. Chopra, Mini-Rev. Org. Chem., 2018, 15, 208 CrossRef CAS; (c) V. Kumar, C. E. Olsen, S. J. C. Schäffer, V. S. Parmar and S. V. Malhotra, Org. Lett., 2007, 9, 3905 CrossRef CAS PubMed; (d) V. Kumar, C. Pei, C. E. Olsen, S. J. C. Schäffer, V. S. Parmar and S. V. Malhotra, Tetrahedron: Asymmetry, 2008, 19, 664 CrossRef CAS; (e) O. N. Van Buu, A. Aupoix, N. D. T. Hong and G. Vo-Thanh, New J. Chem., 2009, 33, 2060 RSC; (f) M. D. R. Gomes da Silva, M. Manuela and A. Pereira, Carbohydr. Res., 2011, 346, 197 CrossRef CAS; (g) N. Ferlin, S. Gatard, A. N. Van Nhien, M. Courty and S. Bouquillon, Molecules, 2013, 18, 11512 CrossRef CAS PubMed; (h) R. Jayachandra and S. R. Reddy, Trends Carbohydr. Res., 2015, 7, 60 CAS; (i) R. Jayachandra, R. Lakshmipathy and S. R. Reddy, J. Mol. Liq., 2016, 219, 1172 CrossRef CAS; (j) R. Jayachandra and S. R. Reddy, RSC Adv., 2016, 6, 39758 RSC; (k) R. Jayachandra, S. R. Reddy and Balakrishna, ChemistrySelect, 2016, 1, 2341 CrossRef CAS; (l) R. Yuan, Y.-j. Wang, Y. Fang, W.-h. Ge, W. Lin, M.-q. Li, J.-b. Xu, Y. Wan, Y. Liu and H. Wu, Chem. Eng. J., 2017, 315, 1026 CrossRef.
- L. Poletti, C. Chiappe, L. Lay, D. Pieraccini, L. Polito and G. Russo, Green Chem., 2007, 9, 337 RSC.
- O. N. Van Buu and G. Vo-Thanh, Lett. Org. Chem., 2007, 4, 158 CrossRef CAS.
- P. G. J. Plaza, B. A. Bhongade and G. Singh, Synlett, 2008, 2973 CAS.
- For peracetylation of ribose, lyxose, xylose and arabinose see, in this order: (a) L. Li, B. Lin, Z. Yang, L. Zhang and L. Zhang, Tetrahedron Lett., 2008, 49, 4491 CrossRef CAS; (b) B. L. Kam, J.-L. Barascut and J.-L. Imbach, Carbohydr. Res., 1979, 69, 135 CrossRef CAS; (c) S. G. Patching, S. A. Baldwin, A. D. Baldwin, J. D. Young, M. P. Gallagher, P. J. F. Henderson and R. B. Herbert, Org. Biomol. Chem., 2005, 3, 462 RSC; (d) Y. Su, J. Xie, Y. Wang, X. Hu and X. Lin, Eur. J. Med. Chem., 2010, 45, 2713 CrossRef CAS PubMed.
- A. Jeffery and V. Nair, Tetrahedron Lett., 1995, 36, 3627 CrossRef CAS.
- CCDCs 1914143, 1916778 and 1916780 contains ESI crystallographic data for this paper.†.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352 CrossRef CAS.
- Most of the ionic liquids used in biocompability tests were purchased from commercial sources. All ILs using [P4444] cations were a generous gift of Prof. H. Ohno (Tokyo, Japan).
- Synthesis of [CetPyr][Sal]: K. Bica, C. Rijksen, M. Nieuwenhuyzen and R. D. Rogers, Phys. Chem. Chem. Phys., 2010, 12, 2011 RSC.
- Synthesis of [Amim][Br]: C. Chardin, J. Rouden, S. Livi and J. Baudoux, Green Chem., 2017, 19, 5054 RSC.
- Synthesis of ILs with amino acid ester cation: G.-H. Tao, L. He, N. Sun and Y. Kou, Chem. Commun., 2005, 3562 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Including experimental data for all products as well as 1H, 13C and 19F NMR spectra for key intermediates and all final ionic products. CCDC 1914143 1916778 1916780. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra01370f |
| This journal is © The Royal Society of Chemistry 2020 |