Open Access Article
Open Access ArticleNovel synthesis of divergent aryl imidazoles from ketones involving copper-catalyzed α-amination and oxidative C–C bond cleavage†
Jiangkun Huanga,
Lan Luoa,
Naiguo Xinga,
Linghui Gua,
Chen Lia,
Qiao Hana,
Shilong Zheng *b and
Ling He
*b and
Ling He *a
*a
aKey Laboratory of Drug-Targeting and Drug Delivery System of the Education Ministry, Sichuan Engineering Laboratory for Plant-Sourced Drug and Sichuan Research Center for Drug Precision Industrial Technology, Department of Medicinal Chemistry, West China School of Pharmacy, Sichuan University, Chengdu, Sichuan 610041, P. R. China. E-mail: heling2012@scu.edu.cn
bDepartment of Chemistry and RCMI Cancer Research Center, Xavier University of Louisiana, New Orleans, LA 70125, USA
First published on 6th April 2020
Abstract
A one-pot synthesis, initiated by a copper salt with inorganic (NH4)2CO3 as the nitrogen source, forms divergent aryl imidazole derivatives from ketones via α-amination and oxidative C–C bond cleavage reactions. The approach provides a simple and rapid synthesis of imidazole derivatives and has certain versatility.
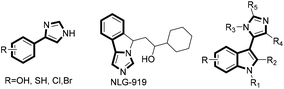
The aryl imidazole is a core skeleton for chiral ligands widely used in asymmetric synthesis,1 as well as many drug molecules and natural products that possess anti-cancer, anti-infection, anti-histamine, anti-ulcer, antihypertensive, and anti-malarial activities (Fig. 1).2 Therefore, how to construct an aryl imidazole core is of great significance and has been extensively studied for the development of different synthetic methods.
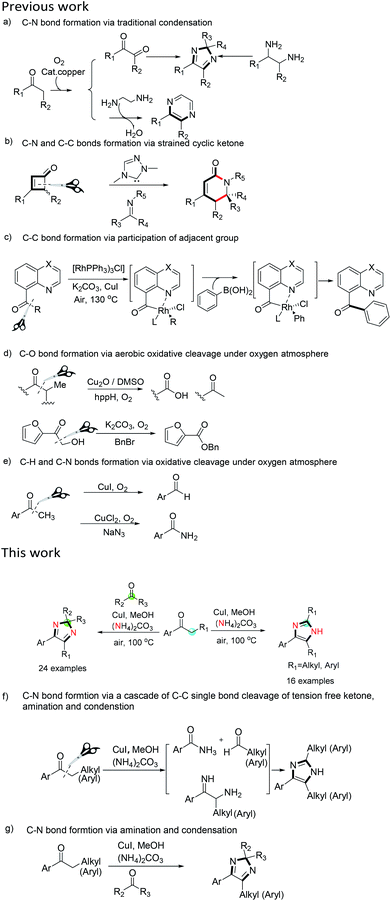
As early as 1858, imidazoles were first synthesized by Heinrich Debus' research group,3 and many other synthetic methods have been reported since then. These include the Bredereck synthetic method,4 the Leusen reaction,5 the Debus-Radziszewski reaction,3 the Claisen rearrangement reaction,6 the Phillips method,7 the isonitrile synthesis,8 and the ketones method (Scheme 1a).9 These methods mostly involved the traditional amination process which typically employs indirect synthesis from halogenated hydrocarbons, unsaturated bonds, and/or acyl chlorides. This leads to an increase in the number of reaction steps and limits the diversity of the substrates.
In the past decade, metal-catalyzed C–H bond activation and amination and the activation of C–C bond cleavage have been favored.10–13 The activation energy of C–H and C–C bonds cleavage can be reduced by directly metal-catalyzed activation of the C–H and C–C bonds, which makes the reaction easier and simplifies the synthetic process. This resulted in the development of synthetic methods for imidazole skeletons involving metal-catalyzed amination.14 However, a common problem with these syntheses is that the starting substrates are not readily available so that these solutions are economically unreliable. Recently, a few of the tandem new bond formations after C–C bond cleavage draw our attention. These examples offer the reutilization of the molecule fragments, which can be utilized to synthesize various nitrogen-containing heterocyclic rings with the atomic economy. However, these methods also have some drawbacks and limitations that do not have universal application, such as need of ring tension for C–C bond breaking (Scheme 1b),11 the participation of adjacent groups for C–C bond breaking (Scheme 1c), and C–C bond breaking in α-hydroxyketones and ketones (Scheme 1d and e).15
Following our interest in tandem metal-catalyzed transformation for the heterocyclic synthesis,16 especially the α-amination catalyzed by transition metal complexes,17 which is an attractive reaction for the synthesis of amine derivatives and amino-functionalized heterocycles, we explored the metal-catalyzed α-amination reaction of ketones using ammonia released from ammonium carbonate. In transition metal-catalyzed amination, the simple inorganic ammonium salts as the nitrogen source is a change from the conventional nitrogen sources including iodoimines, chloramines T, organic azides, hydroxylamines, NFSI, aromatic nitroso derivatives, and other organic nitrogen-containing compounds. Furthermore, it is surprising that the catalytic α-amination of the ketone results in the tandem reactions for the synthesis of imidazole while producing a small amount of amide. Herein, we report a novel approach of imidazole formation involving α-amination and C–C bond cleavage reaction with an excellent substrate scope.
Firstly, using propiophenone as the substrate, we investigated the effect of different reaction conditions on the reaction. As shown in ESI Table 1.† The use of inexpensive ammonium carbonate as a nitrogen source and copper iodide as a catalyst and readily available starting materials makes this protocol economically visible.
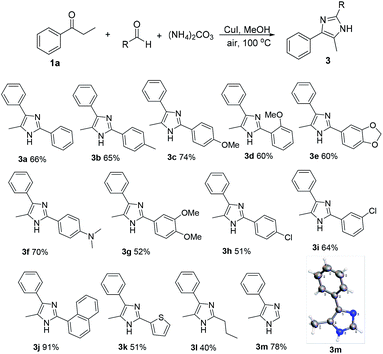
Under the optimal reaction conditions, we explored the substrate scope of the reaction (Scheme 2). In this investigation, the corresponding imidazole derivatives (2a–2p) were obtained from the reaction of a series of aromatic ketones. The results show that the substrates with electron-withdrawing groups on the aromatic ring have higher reactivity than the substrates with electron-donating substituents on the aromatic ring with the exception of 2k. The substrates containing electron-withdrawing groups on the aromatic ring afforded moderate to high yield of the imidazole products (2b–2g). In addition, the alkyl chain length of the aryl alkyl ketones also affected the yields of the reaction, and the longer the chain length, the lower the reactivity (2n, 2o). The α-aryl substituted aromatic ketones also can afford the corresponding imidazole product (2p).
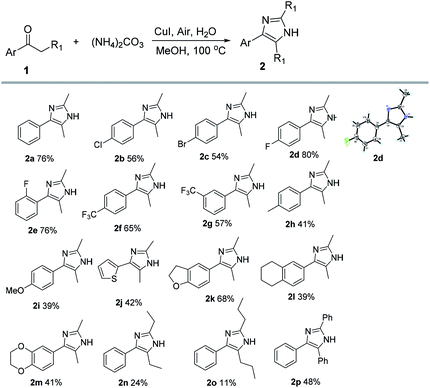 | ||
| Scheme 2 Reactivity of the aryl alkyl ketones[a]. [a] Reactions conditions: CuI (10 mol%), 1 (0.37 mmol), (NH4)2CO3 (7.4 mmol), air, H2O (0.37 mmol), MeOH (2 mL), and 100 °C for 6 h in a sealed tube. | ||
To understand the reaction for novel imidazole synthesis well, we designed a series of experiments to observe the special effects of some conditions on the imidazole synthesis (see ESI†). The effect of the nitrogen source on the reaction was first investigated. No reaction occurred in the absence of (NH4)2CO3. This result indicates that (NH4)2CO3 is really a nitrogen source for the reaction and it may decompose into ammonia to form a complex with copper and participates in the catalytic cycle. To confirm this point, ammonia-saturated methanol was used as the reaction solvent. The reaction occurred, but the yield of the corresponding product was only 46%. This lower yield may be due to the fact that only a limited amount of ammonia dissolved in methanol. Subsequently, the copper–ammonia complex (Cu[NH3]4SO4) was prepared by the reaction of the copper salt with excess ammonia water and was used in the reaction. The result shows that the product was obtained in 72% yield, which confirmed that the copper–ammonia complex was not only the catalyst of the reaction, but also the nitrogen source. In addition, the effects of air and water on the reaction were also investigated. In the absence of water (anhydrous methanol) or air, no target product was obtained, indicating that water and air play an important role in the cleavage of C–C bond of propiophenone. Further, to confirm the α-C–H activation and α-amination in the imidazole formation, α-perdeuterated propiophenone was used as the substrate using deuterated methanol as a solvent in the presence of dry air and D2O, the product was obtained in only 11% yield. The results show that C–H activation is a prerequisite for the reaction process.
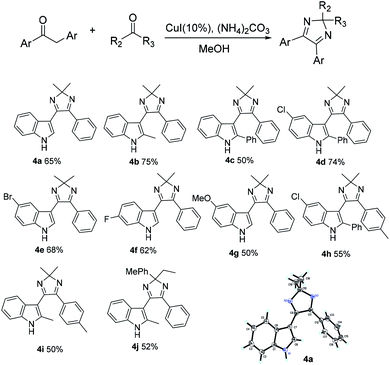
In addition, referring to the C–C bond cleavage reaction,11,15 during the formation of imidazoles, we speculated that a portion of propiophenone may undergo cleavage of the C–C bond to produce an aldehyde. In order to confirm this, benzaldehyde was added to the reaction system, and only trace amount of original product 2a was found. In contrast, the corresponding aldehyde condensation products was obtained in yields of 66%, respectively. If 4 equivalents of benzaldehyde are added, the formation of 2a can also be completely inhibited. The results indicate that a competitive reaction occurs when an excess of other aldehyde is present, and the root cause is related to more rapidly α-amination than the cleavage of the C–C bond of propiophenone during the reaction. The experimental results make us to use other aldehydes and ketones in the synthesis of divergent aryl imidazoles. As shown in Scheme 3, divergent imidazoles were obtained in excellent yield by adding four equivalents of the aldehyde to the reaction system. Both aliphatic and aromatic aldehydes yield the ideal results, but ketones failed to afford pure corresponding products, the reaction is messy and complicated. It is interesting that when 1,2-diarylethan-1-one replaces propiophenone 1a, the addition of ketones resulted in the formation of corresponding 2H-imidazoles (Scheme 4). Meanwhile, the formation of 4j further indicates that the reaction rate of α-amination is faster than that of C–C bond cleavage. The structures of 2d and 3m, 4a were further confirmed by the X-ray single crystal analysis (the detailed crystal data are provided in the ESI†).
At the same time, ethylamine was used as a nitrogen source and phenylacetone as a substrate to react under the same reaction conditions. The intermediates α-ethylpheny-lacetone, (E)-ethylacetone-1-(ethylimino)-1-phenyl-propan-2-amine and N-ethylbenzamide and their products 1,3-diethyl-4-methyl-ene-5-phenyl-2,3-dihydro-1-himidazole were found by LC-MS (see ESI†).
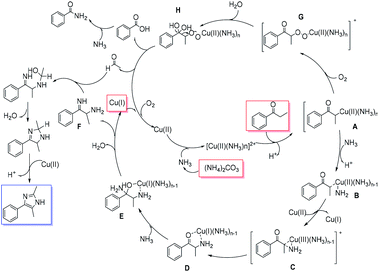
On the basis of experimental results and the previously proposed mechanisms,18 a reaction pathway for aryl imidazole synthesis was proposed (Scheme 5). First, Cu(I) is oxidized to Cu(II) under an oxygen atmosphere. The Cu(II) species then forms a complex with ammonia (released from ammonium carbonate). Subsequently, the copper–ammonia complex reacts with the substrate to give intermediate A. A is converted into intermediate B after loss of a proton. B then undergoes a disproportionation reaction with NH2 insertion to afford intermediate C. Intermediate D occurs via cleavage of C–Cu bond. Intermediate E forms via ammonia 1, 2-addition to species D. The intermediate E is then dehydrated to form compound F. Simultaneously, O2 insertion into the C–Cu bond of intermediate A results in intermediate G. Addition of water then forms intermediate H. An aldehyde is then formed by electron transfer and cleavage of the C–C bond. Finally, the nucleophilic addition reaction of the aldehyde with intermediate F occurs to obtain the imidazole product by further dehydration and loss of a proton.
In conclusion, under copper catalysis, the aryl alkyl ketones were reacted with an inorganic salt (NH4)2CO3 in one-pot to form the imidazole derivatives by C–H activated amination and C–C oxidative cleavage. We speculate that in the imidazole synthesis, a portion of aryl alkyl ketones underwent the C–C bond cleavage, and the cleavage fragment is further subjected to a multicomponent cascade reaction with other reaction intermediates to form imidazole derivatives. The approach provides a simple and rapid synthesis for imidazole derivatives and has certain versatility. When the applicability of the reaction was further explored, it was found that the addition of other aldehydes in the reaction of the arylalkyl ketone inhibited the formation of the original product, but produced a new imidazole derivative formed by condensation with the aldehydes. In the aryl alkyl ketone reaction, the addition of other aldehydes and ketones can produce more diverse imidazole derivatives.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21072131), 111 project (B18035) and Sichuan University – Lu Zhou Strategic Cooperation Projects (No. 2017 CDLZ-S34).Notes and references
- (a) V. Cesar, S. Bellemin-Laponnaz and L. H. Gade, Chem. Soc. Rev., 2004, 33, 619–636 RSC; (b) K. Yoshida and R. Yasue, Chem.–Eur. J., 2018, 24, 18575–18586 CrossRef CAS PubMed; (c) Q. Xu, P. Gu, H. C. Jiang, Y. Wei and M. Shi, Chem. Rec., 2016, 16, 2740–2753 CrossRef PubMed; (d) P. Gu, J. Zhang, Q. Xu and M. Shi, Dalton Trans., 2013, 42, 13599–13606 RSC; (e) A. Schumacher, M. Bernasconi and A. Pfaltz, Angew. Chem., Int. Ed., 2013, 52, 7422–7425 CrossRef CAS PubMed; (f) W. Zawartka, A. Gniewek and A. M. Trzeciak, Inorg. Chim. Acta, 2017, 455, 595–599 CrossRef CAS; (g) T. Soeta, Y. Hatanaka, T. Ishizaka and Y. Ukaji, Tetrahedron, 2018, 74, 4601–4605 CrossRef CAS; (h) V. Gauchot, J. Gravel, M. Vidal, M. Charbonneau, V. Kairouz and A. R. Schmitzer, Synlett, 2015, 26, 2763–2779 CrossRef CAS.
- (a) M. Sono and S. G. Cady, Biochemistry, 1989, 28, 5392–5399 CrossRef CAS PubMed; (b) Q. Huang, M. F. Zheng, S. S. Yang, C. X. Kuang, C. J. Yu and Q. Yang, Eur. J. Med. Chem., 2011, 46, 5680–5687 CrossRef CAS PubMed; (c) S. Kumar, D. Jaller, B. Patel, J. M. LaLonde, J. B. DuHadaway, W. P. Malachowski, G. C. Prendergast and A. J. Muller, J. Med. Chem., 2008, 51, 4968–4977 CrossRef CAS PubMed; (d) A. Nayak, Z. Hao, R. Sadek, R. Dobbins, L. Marshall, N. Vahanian, J. Ramsey, E. Kennedy, M. Mautino, C. Link, R. Lin, S. Royer-Joo, K. Morrissey, S. Mahrus, B. McCall, A. Pirzkall, D. Munn, J. Janik and S. Khleif, Eur. J. Cancer, 2015, 51, S69 CrossRef; (e) J. Wiesner, R. Ortmann, H. Jomaa and M. Schlitzer, Angew. Chem., Int. Ed., 2003, 42, 5274–5293 CrossRef CAS PubMed; (f) J. Wu, J. L. Mi and C. H. Zhou, Chin. Pharm. J., 2007, 42, 404–409 CAS.
- H. Debus, Justus Liebigs Ann. Chem., 1858, 107, 199–208 CrossRef.
- (a) T. N. Sorrell and W. E. Allen, J. Org. Chem., 1994, 59, 1589–1590 CrossRef CAS; (b) W. C. Wong and C. Gluchowski, Synthesis, 1995, 139–140 CrossRef CAS; (c) H. Bredereck and G. Theilig, Chem. Ber., 1953, 86, 88–96 CrossRef CAS; (d) A. Novelli and A. Desantis, Tetrahedron Lett., 1967, 265–269 CrossRef CAS.
- A. M. Vanleusen, J. Wildeman and O. H. Oldenziel, J. Org. Chem., 1977, 42, 1153–1159 CrossRef CAS.
- (a) N. D. Heindel and M. C. Chun, Tetrahedron Lett., 1971, 1439–1440 CrossRef CAS; (b) I. Lantos, W. Y. Zhang, X. Q. Shui and D. S. Eggleston, J. Org. Chem., 1993, 58, 7092–7095 CrossRef CAS.
- (a) M. A. Phillips, J. Chem. Soc., 1928, 172–177 RSC; (b) M. A. Phillips, J. Chem. Soc., 1928, 2393–2399 RSC.
- K. Nunami, M. Yamada, T. Fukui and K. Matsumoto, J. Org. Chem., 1994, 59, 7635–7642 CrossRef CAS.
- (a) B. S. Schmidt and S. Hauke, J. Org. Chem., 2013, 78, 5427–5435 CrossRef CAS PubMed; (b) J. Jayram and V. Jeena, Green Chem., 2017, 19, 5841–5845 RSC; (c) K. Wu, Z. L. Huang and X. T. Qi, Sci. Adv., 2015, 1, 1–7 Search PubMed.
- (a) R. H. Crabtree, Chem. Rev., 1985, 85, 245–269 CrossRef CAS; (b) M. Murakami and Y. Ito, Top. Organomet. Chem., 1999, 3, 97–129 CrossRef CAS; (c) M. Tobisu and N. Chatani, Chem. Soc. Rev., 2008, 37, 300–307 RSC; (d) A. Masarwa and I. Marek, Chem.–Eur. J., 2010, 16, 9712–9721 CrossRef CAS PubMed; (e) M. Murakami and T. Matsuda, Chem. Commun., 2011, 47, 1100–1105 RSC; (f) T. Shen, Y. Q. Zhang, Y. F. Liang and N. Jiao, J. Am. Chem. Soc., 2016, 138, 13147–13150 CrossRef CAS PubMed; (g) P. H. Chen, B. A. Billett, T. Tsukamoto and G. B. Dong, ACS Catal., 2017, 7, 1340–1360 CrossRef CAS PubMed.
- (a) O. G. Kulinkovich, Chem. Rev., 2003, 103, 2597–2632 CrossRef CAS PubMed; (b) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117–3179 CrossRef CAS PubMed; (c) T. Seiser and N. Cramer, Org. Biomol. Chem., 2009, 7, 2835–2840 RSC; (d) C. A. Carson and M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051–3060 RSC; (e) T. Seiser, T. Saget, D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 7740–7752 CrossRef CAS PubMed; (f) T. Seiser and N. Cramer, J. Am. Chem. Soc., 2010, 132, 5340–5341 CrossRef CAS PubMed; (g) B. S. Li, Y. H. Wang, Z. C. Jin, P. C. Zheng, R. Ganguly and Y. R. Chi, Nat. Commun., 2015, 6, 6207–6210 CrossRef PubMed.
- (a) A. M. Dreis and C. J. Douglas, J. Am. Chem. Soc., 2009, 131, 412–413 CrossRef CAS PubMed; (b) N. Chatani, Y. Ie, F. Kakiuchi and S. Murai, J. Am. Chem. Soc., 1999, 121, 8645–8646 CrossRef CAS; (c) J. J. Wang, W. Q. Chen, S. J. Zuo, L. Liu, X. R. Zhang and J. H. Wang, Angew. Chem., Int. Ed., 2012, 51, 12334–12338 CrossRef CAS PubMed; (d) F. D. Lewis and J. G. Magyar, J. Org. Chem., 1972, 37, 2102–2107 CrossRef CAS; (e) N. Zhang and J. Vozzolo, J. Org. Chem., 2002, 67, 1703–1704 CrossRef CAS PubMed; (f) Z. Q. Lei, H. Li, Y. Li, X. S. Zhang, K. Chen, X. Wang, J. Sun and Z. J. Shi, Angew. Chem., Int. Ed., 2012, 51, 2690–2694 CrossRef CAS PubMed; (g) T. Morioka, A. Nishizawa, T. Furukawa, M. Tobisu and N. Chatani, J. Am. Chem. Soc., 2017, 139, 1416–1419 CrossRef CAS PubMed.
- Y. Park, Y. Kim and S. Chang, Chem. Rev., 2017, 117, 9247–9301 CrossRef CAS PubMed.
- (a) J. H. Li and L. Neuville, Org. Lett., 2013, 15, 1752–1755 CrossRef CAS PubMed; (b) H. C. Ma, X. Y. Zhang, L. L. Chen and W. Yu, J. Org. Chem., 2017, 82, 11841–11847 CrossRef CAS PubMed; (c) S. D. Pardeshi, P. A. Sathe, K. S. Vadagaonkar, L. Melone and A. C. Chaskar, Synthesis, 2018, 50, 361–370 CrossRef CAS.
- (a) H. Liu, C. Dong, Z. G. Zhang, P. Y. Wu and X. F. Jiang, Angew. Chem., Int. Ed., 2012, 51, 12570–12574 CrossRef CAS PubMed; (b) L. Zhang, X. H. Bi, X. X. Guan, X. Q. Li, Q. Liu, B. D. Barry and P. Q. Liao, Angew. Chem., Int. Ed., 2013, 52, 11303–11307 CrossRef CAS PubMed; (c) C. H. Tang and N. Jiao, Angew. Chem., Int. Ed., 2014, 53, 6528–6532 CrossRef CAS PubMed; (d) Q. Xing, H. Lv, C. G. Xia and F. W. Li, Chem. Commun., 2016, 52, 489–492 RSC; (e) A. S.-K. . Tsang, A. Kapat and F. Schoenebeck, J. Am. Chem. Soc., 2016, 138, 518–526 CrossRef CAS PubMed.
- (a) C. L. Zhong, B. Y. Tang, P. Yin, Y. Chen and L. He, J. Org. Chem., 2012, 77, 4271–4277 CrossRef CAS PubMed; (b) C. Li, N. Liu, Q. R. Qi, Y. Chen and L. He, Eur. J. Org. Chem., 2015, 2585–2589 CrossRef CAS; (c) L. H. Gu, P. Wang, Q. Zhong, Y. X. Deng, J. P. Xie, F. Liu, F. Xiao, S. L. Zheng, Y. Chen, G. D. Wang and L. He, RSC Adv., 2017, 7, 9412–9416 RSC.
- (a) L. He, J. Yu, J. Zhang and X. Q. Yu, Org. Lett., 2007, 9, 2277–2280 CrossRef CAS PubMed; (b) N. Liu, B. Y. Tang, Y. Chen and L. He, Eur. J. Org. Chem., 2009, 2059–2062 CrossRef; (c) L. H. Gu, D. D. Zhang, Q. R. Qi, P. Yin and L. He, Tetrahedron, 2014, 70, 8155–8160 CrossRef CAS.
- (a) X. D. Tang, J. D. Yang, Z. Z. Zhu, M. F. Zheng, W. Q. Wu and H. F. Jiang, J. Org. Chem., 2016, 81, 11461–11466 CrossRef CAS PubMed; (b) S. Itoh, Acc. Chem. Res., 2015, 48, 2066–2074 CrossRef CAS PubMed; (c) X. D. Tang, H. L. Gao, J. D. Yang, W. Q. Wu and H. F. Jiang, Org. Chem. Front., 2014, 1, 1295–1298 RSC; (d) X. D. Tang, L. B. Huang, Y. L. Xu, J. D. Yang, W. Q. Wu and H. F. Jiang, Angew. Chem., Int. Ed., 2014, 53, 4205–4208 CrossRef CAS PubMed; (e) X. D. Tang, L. B. Huang, J. D. Yang, Y. L. Xu, W. Q. Wu and H. F. Jiang, Chem. Commun., 2014, 50, 14793–14796 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1853653, 1882199 and 1882198. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra01408g |
| This journal is © The Royal Society of Chemistry 2020 |