Open Access Article
Open Access ArticleDesilylation of copolymer membranes composed of poly[1-(p-trimethylsilyl)phenyl-2-(p-trimethylsilyl)phenylacetylene] for improved gas permeability
Yi Lin,
Toshikazu Sakaguchi * and
Tamotsu Hashimoto
* and
Tamotsu Hashimoto
Department of Materials Science and Engineering, Graduatde School of Engineering, University of Fukui, Bunkyo 3-9-1, Fukui 910-8507, Japan. E-mail: sakaguchi@matse.u-fukui.ac.jp
First published on 13th April 2020
Abstract
Efficient gas-separation systems comprising gas-permeable membranes are important for energy conservation in various industrial applications. Herein, high-molecular-weight copolymers (2ab and 2ac) were synthesized in good yields by the copolymerization of 1-(p-trimethylsilyl)phenyl-2-(p-trimethylsilyl)phenylacetylene (1a) with 1-phenyl-2-(p-tert-butyl)phenylacetylene (1b) and 1-phenyl-2-(p-trimethylsilyl)phenylacetylene (1c) in various monomer feed ratios using TaCl5–n-Bu4Sn. Tough membranes were obtained by solution casting. The copolymers exhibited very high gas permeabilities (PO2: 1700–3400 barrers). Desilylation of 2ac membranes decreased the gas permeability, but desilylation of 2ab membranes resulted in a significant increase in the gas permeability. The highest oxygen permeability coefficient obtained was 9300 barrers, which was comparable to that of poly(1-trimethylsilyl-1-propyne), a polymer known to have the highest gas permeability.
Introduction
Efficient gas-separation systems have been extensively investigated for various industrial applications. Gas-permeable polymer membranes have attracted significant attention as they can simplify the systems and allow energy conservation.1,2 In recent decades, substituted polyacetylenes have been intensively examined for their practical application as gas-permeable materials.3–6 Polyacetylenes with bulky spherical substituents show large excess free volume and high gas permeabilities because of the presence of rigid main chains comprising alternating double bonds and the steric repulsion of the bulky substituents that inhibits an effective polymer chain packing.7,8 For instance, poly(1-trimethylsilyl-1-propyne) (PTMSP) exhibits the highest gas permeability, where the oxygen permeability coefficient (PO2) is greater than 9000 barrers.3 However, aliphatic polyacetylenes such as PTMSP are degraded by heat and oxidation.In contrast, diarylacetylene polymers are tolerant toward pyrolysis and oxidation. Poly[1-phenyl-2-(p-trimethylsilyl)phenylacetylene] (PTMSDPA) and poly[1-phenyl-2-(p-tert-butyl)phenylacetylene] (PTBDPA) are the examples of diarylacetylene polymers, and show high PO2 values of up to 1000 barrers.9–12 Therefore, these polymers are promising materials for gas separation membranes. Hu et al. synthesized polymethylated indan-containing poly(diphenylacetylene), which is the most gas-permeable polymer ever recorded to date.13,14 Fukui et al. incorporated fluorenyl groups into poly(diarylacetylene) to improve gas permeability,15 and the highest PO2 value among fluorenyl-containing poly(diarylacetylene)s is 9800 barrers.16 The incorporation of naphthyl,17 SiMe3,5,6,18 tert-butyl,18,19 halogen20–22 into diarylacetylene is also often considered as a way to obtain high gas-permeable materials, but their gas permeability still lower than PTMSP.
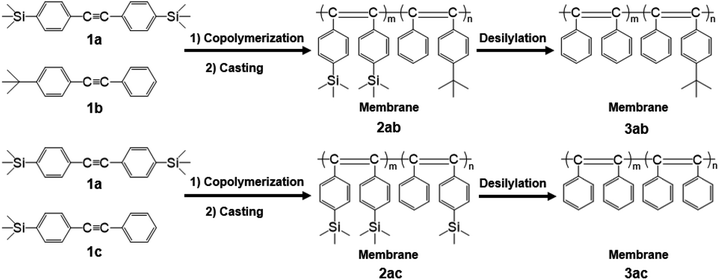
In this study, the copolymerization of 1a with a tert-butyl-containing monomer (1b) and trimethylsilyl-containing monomer (1c) in various feed ratios was accomplished (Scheme 1). Free-standing membranes were fabricated from the resultant copolymers, and the desilylation of the membranes was performed using a mixture of trifluoroacetic acid/hexane. Gas permeabilities of the copolymer membranes were studied.
Our research group previously reported that the copolymers of TMSDPA and TBDPA showed higher gas permeabilities than those of the homopolymers, PTMSDPA and PTBDPA.18 Furthermore, the desilylation of the copolymer membranes enhanced the gas permeability. This suggested that some micro-scale voids were generated by the elimination of silyl groups. Therefore, the desilylation of the copolymers of 1-(p-trimethylsilyl)phenyl-2-(p-trimethylsilyl)phenylacetylene (1a), with two trimethylsilyl groups, is expected to further improve the gas permeability.
Experimental
Materials
The polymerization solvent, toluene (Fujifilm Wako Pure Chemical Co., Ltd., Osaka, Japan), was purified twice by distillation in the presence of CaH2 (Fujifilm Wako Pure Chemical Co., Ltd.). The main catalyst, TaCl5 (99.999%, Sigma-Aldrich, St. Louis, Missouri, USA), was used without further purification, while the cocatalyst, n-Bu4Sn (Fujifilm Wako Pure Chemical Co., Ltd.), was used after distillation in the presence of CaH2. Phenylacetylene (Sigma-Aldrich), 1,4-dibromobenzene, 1-bromo-4-tert-butylbenzene, triethylamine, triphenylphosphine, copper(I) iodide, dichlorobis(triphenylphosphine)palladium(II), n-butyllithium, trifluoroacetic acid (TFA), chlorotrimethylsilane, 2-methyl-3-butyn-2-ol, sodium hydride, aqueous sodium nitrate, and other common solvents (Fujifilm Wako Pure Chemical Co., Ltd.) were used without further purification. 1-(p-Trimethylsilyl)phenyl-2-(p-trimethylsilyl)phenylacetylene (1a),23 1-phenyl-2-(p-tert-butyl)phenylacetylene (1b),11 and 1-phenyl-2-(p-trimethylsilyl)phenylacetylene (1c)10 were synthesized according to the methods reported in literature.Measurements
The molecular weight distribution (MWD) of the copolymers was measured by gel permeation chromatography (GPC) in CHCl3 (at a 1.0 mL min−1 flow rate) at 40 °C on a Shimadzu LC-10AD chromatography system (Shimadzu Corp., Kyoto, Japan) equipped with three polystyrene gel columns (Shodex K-804L, K-805L, and K-807L; Shodex, Tokyo, Japan) and a Shimadzu RID-6A refractive index detector (Shimadzu Corp.). The weight-average molecular weight (Mw) and polydispersity ratio were calculated from the chromatograms based on the polystyrene calibration. 1H (500 MHz) and 13C (125 MHz) NMR spectra were recorded on the Jeol ECX-500 instrument (JEOL Ltd., Tokyo, Japan) in CDCl3 at room temperature. IR spectra were recorded on a Nicolet IS 5 spectrometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The thickness of membrane was measured using a micrometer, and an average of ten points on each membrane was considered.Gas permeability was measured using a Rikaseiki K-315-N gas permeability apparatus equipped with an MKS Baratron detector (MKS Instruments, Inc., Andover, Massachusetts, USA) at 25 °C. The downstream side of the membrane was evacuated at 0.2–1.0 Pa, while the upstream side was filled with a gas at approximately 1 atm (105 Pa). The increase in pressure in a downstream receiving vessel was measured. The permeability coefficient P expressed in barrers [1 barrer = 1 × 10−10 cm3 (STP) × cm/(cm2 × sec × cmHg)] was calculated from the slopes of the time–pressure curves in the steady state where Fick's law was obeyed. The diffusion coefficient (D) was determined by the time lag method using the following equation:
| D = l2/6θ |
The density of membrane was determined by hydrostatic weighing using a Mettler Toledo balance (Mettler Toledo, Columbus, Ohio, USA) and a density determination kit. In this method, a liquid with a known density (ρ0) is required, and the membrane density (ρ) is obtained by the following equation:
| ρ = ρ0 × MA/(MA − ML) |
Copolymerization
Copolymerization was carried out in a Schlenk tube equipped with a three-way stopcock at 80 °C for 24 h under dry nitrogen with the following reagent concentrations: [1a] + [1b or 1c] = 0.20 M, [TaCl5] = 20 mM, and [n-Bu4Sn] = 80 mM. A detailed procedure for copolymerization is described as follows. The monomer solution was prepared in a Schlenk tube by mixing the monomers and dry toluene. An additional Schlenk tube was charged with TaCl5, n-Bu4Sn, and dry toluene; this catalyst solution was aged at 80 °C for 10 min. Then, the monomer solution was added to the catalyst solution. After 24 h, copolymerization was quenched with a small amount of methanol. The resulting copolymer was isolated by precipitation into excess methanol. The polymer yield was determined gravimetrically.Membrane fabrication and desilylation
The membranes (thickness: ca. 30–130 μm) of the copolymers were prepared by casting from toluene solution of the copolymers (0.15–0.50 wt%) into the Petri dishes. The dish was covered with a glass vessel to decrease the evaporation rate of the solvent (3–5 d). After the formation of the membrane, it was immersed in methanol for 24 h, followed by drying for 24 h at 25 °C. The desilylation of membranes was performed using the method described in literature.24 The detailed procedure is described as follows. The membrane was immersed in a mixture of trifluoroacetic acid and hexane (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature for 24 h. To remove the residual impurities in the polymer matrix, the membrane was immersed in acetone followed by methanol at room temperature for 24 h. Thereafter, it was dried under atmospheric pressure at room temperature for 24 h. The completion of the reaction was confirmed by comparing the IR spectra of the copolymers before and after desilylation.
1) at room temperature for 24 h. To remove the residual impurities in the polymer matrix, the membrane was immersed in acetone followed by methanol at room temperature for 24 h. Thereafter, it was dried under atmospheric pressure at room temperature for 24 h. The completion of the reaction was confirmed by comparing the IR spectra of the copolymers before and after desilylation.
Results and discussion
Copolymerization
It is well known that TaCl5/n-Bu4Sn is the best catalyst for the metathesis polymerization of diphenylacetylene derivatives to afford high-molecular-weight polymers.25–27 Therefore, in this study, the copolymerization of 1a with 1b and 1c was performed using TaCl5/n-Bu4Sn catalyst at 80 °C in toluene for 24 h under nitrogen. The results of copolymerization were summarized in Table 1.| Feed ratio | Copolymerb | |||
|---|---|---|---|---|
(1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1b or 1c) 1b or 1c) |
Yield (%) | Mwc (g mol−1) | Mw/Mnc | |
| a In toluene at 80 °C for 24 h; [1a] + [1b or 1c] = 0.20 M, [TaCl5] = 20 mM, [n-Bu4Sn] = 40 mM.b Methanol-insoluble product.c Measured by GPC (CHCl3). | ||||
| 1b | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
91 | 902![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.40 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
84 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.05 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81 | 546![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.55 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73 | 820![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.03 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
71 | Insoluble | ||
| 1c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
87 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.57 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
81 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.13 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.11 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90 | 872![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.62 | |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | Insoluble | ||
The copolymerization of 1a with 1b in the feed ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 afforded the copolymer, 2ab(1:4), whose Mw was up to 902
4 afforded the copolymer, 2ab(1:4), whose Mw was up to 902![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The copolymerizations of 1a with 1b in the feed ratios of 1
000. The copolymerizations of 1a with 1b in the feed ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4
1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in the production of copolymers, 2ab(1:2), 2ab(1:1), 2ab(2:1), and 2ab(4:1), respectively, in good yields. The molecular weights of 2ab(1:2), 2ab(1:1), and 2ab(2:1) were very high, but that of 2ab(4:1) could not be measured by GPC because 2ab(4:1) was insoluble in all solvents. The copolymerizations of 1a with 1c in the feed ratios of 1
1 resulted in the production of copolymers, 2ab(1:2), 2ab(1:1), 2ab(2:1), and 2ab(4:1), respectively, in good yields. The molecular weights of 2ab(1:2), 2ab(1:1), and 2ab(2:1) were very high, but that of 2ab(4:1) could not be measured by GPC because 2ab(4:1) was insoluble in all solvents. The copolymerizations of 1a with 1c in the feed ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 2
1, and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under the same conditions afforded the copolymers [2ac(1:4), 2ac(1:2), 2ac(1:1), and 2ac(2:1), respectively] in high yields with high molecular weights (Mw = 872
1 under the same conditions afforded the copolymers [2ac(1:4), 2ac(1:2), 2ac(1:1), and 2ac(2:1), respectively] in high yields with high molecular weights (Mw = 872![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2
000–2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540
540![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). Similar to 2ab(4:1), the copolymer 2ac(4:1) was obtained in good yield, but the solubility was poor.
000). Similar to 2ab(4:1), the copolymer 2ac(4:1) was obtained in good yield, but the solubility was poor.
Fabrication of copolymer membranes and their solubility
Tough free-standing membranes of 2ab and 2ac were fabricated by casting the copolymers from their toluene solutions, except 2ab(4:1) and 2ac(4:1), because of their insolubility. The desilylation was carried out using trifluoroacetic acid and hexane (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desilylated membranes (3ab and 3ac).
1) to afford the desilylated membranes (3ab and 3ac).
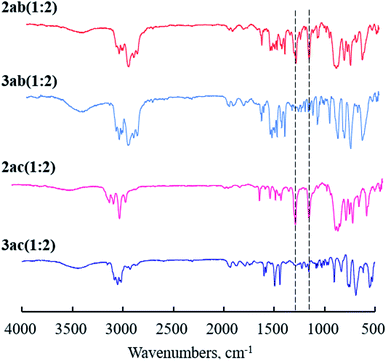
To verify the completion of the desilylation of copolymer membranes, IR measurements were carried out. The IR spectra of the membranes of 2ab(1:2), 2ac(1:2), and the desilylated analogues, 3ab(1:2) and 3ac(1:2), were shown in Fig. 1. The spectra of 2ab(1:2) and 2ac(1:2) showed the peaks at 1250 cm−1, assigned to the stretching of SiC–H bond, and 1120 cm−1 corresponding to the vibration of Si–C bond. No absorptions at 1250 cm−1 and 1120 cm−1 were observed in the spectra after desilylation (Fig. 1, 3ab and 3ac). The results suggest the completion of the desilylation reaction.
The solubilities of the copolymers were summarized in Table 2. Copolymers 2ab and 2ac showed the same solubility, and completely dissolved in relatively low polarity solvents such as toluene, chloroform, and tetrahydrofuran. However, 2ab(4:1) and 2ac(4:1) were insoluble in all solvents. Homopolymer of 1a is known to be insoluble in all solvents.23 Copolymers 2ab(4:1) and 2ac(4:1) mainly consist of the 1a component and therefore showed a similar solubility behavior as that of the homopolymer of 1a. Similar to 2ab, the desilylated polymer 3ab was soluble in low polarity solvents. However, 3ac was not soluble in any solvent. This variation in solubility before and after desilylation should come from the substituent of tert-butyl groups. Table 2 showed the substitution ratios for the phenyl groups. When the ratio was zero or more than 90%, copolymers were insoluble. Therefore, an appropriate ratio of substituents is necessary for the solubility of poly(diphenylacetylene).
| Toluene | CHCl3 | THF | DMF | DMSO | Methanol | Substitution ratiob [%] | |
|---|---|---|---|---|---|---|---|
| a Symbols: (+) soluble, (±) partly soluble, (−) insoluble.b Ratio of the substituent (tert-butyl or SiMe3) for the phenyl groups on polymer side chain. | |||||||
| 2ab(1:4) | + | + | + | − | − | − | 60 |
| 2ab(1:2) | + | + | + | − | − | − | 67 |
| 2ab(1:1) | + | + | + | − | − | − | 75 |
| 2ab(2:1) | + | + | + | − | − | − | 83 |
| 2ab(4:1) | − | − | − | − | − | − | 90 |
| 2ac(1:4) | + | + | + | − | − | − | 60 |
| 2ac(1:2) | + | + | + | − | − | − | 67 |
| 2ac(1:1) | + | + | + | − | − | − | 75 |
| 2ac(2:1) | + | + | + | − | − | − | 83 |
| 2ac(4:1) | − | − | − | − | − | − | 90 |
| 3ab(1:4) | + | + | + | − | − | − | 40 |
| 3ab(1:2) | + | + | + | − | − | − | 33 |
| 3ab(1:1) | ± | + | + | − | − | − | 25 |
| 3ab(2:1) | − | ± | ± | − | − | − | 17 |
| 3ac(1:4) | − | − | − | − | − | − | 0 |
| 3ac(1:2) | − | − | − | − | − | − | 0 |
| 3ac(1:1) | − | − | − | − | − | − | 0 |
| 3ac(2:1) | − | − | − | − | − | − | 0 |
Gas permeability and density of copolymer membranes
The permeability coefficients (P) of the membranes of 2ab, 2ac, 3ab, and 3ac toward various gases were measured at 25 °C after conditioning the membranes by immersing in methanol for 24 h and drying to constant weight at room temperature for an additional 24 h (Table 3). The density of the copolymer membranes are shown in Table 3.| Membrane | Ratio | PO2a | PCO2a | PN2a | PO2/PN2 | PCO2/PN2 | Densityb |
|---|---|---|---|---|---|---|---|
| a Units: 1 × 10−10 cm3 (STP) × cm/(cm2 × sec × cmHg) (=1 barrer).b Determined by hydrostatic weighing; units: g cm−3. | |||||||
| 2ab | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
2100 | 7830 | 1100 | 1.92 | 7.18 | 0.891 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2900 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1550 | 1.87 | 6.44 | 0.854 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2200 | 8080 | 1130 | 1.94 | 7.13 | 0.884 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1700 | 6900 | 740 | 2.30 | 9.31 | 0.902 | |
| 2ac | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1800 | 6940 | 870 | 2.07 | 7.98 | 0.900 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
2700 | 9280 | 1470 | 1.83 | 6.31 | 0.866 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1610 | 1.86 | 6.35 | 0.858 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2060 | 1.65 | 5.00 | 0.787 | |
| 3ab | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
6200 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4000 | 1.55 | 3.25 | 0.837 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9300 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
7500 | 1.24 | 2.49 | 0.757 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6600 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
4800 | 1.38 | 2.88 | 0.812 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4400 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
2990 | 1.47 | 3.66 | 0.848 | |
| 3ac | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
980 | 4000 | 380 | 2.54 | 10.39 | 0.984 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
990 | 4100 | 390 | 2.54 | 10.51 | 0.972 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1070 | 4300 | 480 | 2.24 | 8.98 | 0.957 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1080 | 4500 | 490 | 2.21 | 9.20 | 0.951 | |
| Poly(TMSDPA-co-TBDPA)18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2300 | 8300 | 1300 | 1.8 | 6.4 | |
| DSpoly(TMSDPA-co-TBDPA)18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2700 | 8600 | 1600 | 1.7 | 5.4 | |
| PTMSDPA12 | 1200 | 4900 | 560 | 2.1 | 8.7 | 0.91 | |
| PTBDPA11 | 1100 | 4800 | 500 | 2.2 | 9.6 | ||
| PTMSP12 | 9700 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6300 | 1.5 | 5.4 | 0.75 | |
| Indan-polyacetylenes13,14 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.13 | 2.66 | ||
| Naphthyl-polyacetylenes17 | 4300 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2700 | 1.6 | 4.8 | ||
| Flurenyl-polyacetylenes15,16 | 9800 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8100 | 1.2 | 3.0 | ||
| Halogen-polyacetylenes20–22 | 5400 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3700 | 1.5 | 5.1 | 1.01 | |
| PIM-1 (ref. 28) | 2270 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
823 | 2.8 | 16.5 | 1.09 | |
High gas permeabilities were observed for the membranes of 2ab and 2ac. The PO2 values of 2ab were in the range of 1700–2900 barrers, which were higher than that of PTBDPA (PO2: 1100 barrers). Copolymer 2ab(1:2) exhibited the highest gas permeability and its PO2 value was 2900 barrers, which was 2.6 times as that of PTBDPA. Similarly, 2ac showed a higher gas permeability than that of PTMSDPA. The PO2 values of 2ac were in the range of 1800–3400 barrers. The higher gas permeability of copolymers may be due to their asymmetrical structure of the repeating unit that generates micro-scale voids. It is obvious that incorporation of trimethylsilyl group or tert-butyl group into poly(diphenylacetylene)s in the way of copolymerization is effective to enhance the gas permeability.
The CO2 and N2 permeabilities of 2ab and 2ac were higher than those of PTBDPA and PTMSDPA, as well as the corresponding oxygen permeabilities. This suggests that the membranes of 2ab and 2ac have large free volumes. Accordingly, the membranes of 2ab and 2ac showed low densities of 0.787–0.902 g cm−3.
The desilylated membranes of 3ab exhibited significantly higher oxygen permeabilities than those of 2ab. The membrane density reduced upon desilylation. This indicates that the spaces occupied by the silyl groups were retained as micro-scale voids after desilylation due to the steric hindrance of tert-butyl groups. It is suggesting that the incorporation of tert-butyl groups prevents the chain packing effectively to enhance the gas permeability. Notably, 3ab(1:2) exhibited extremely high permeability and its PO2 value reached up to 9300 barrers, which was the highest value observed among all synthetic polymers. Contrary to 3ab, the desilylated membranes of 3ac showed lower gas permeabilities than those of the membranes of 2ac. The decrement of gas permeability through desilylation was due to the elimination of spherical bulky trimethylsilyl groups that caused the membrane to become dense. The PO2 values of 3ac were in the range of 980–1080 barrers. These were similar to the PO2 value of 900 barrers for poly(1-phenyl-2-phenylacetylene), which was formed by the desilylation of PTMSDPA.18 The densities of the membranes increased after desilylation. These results indicate that tert-butyl groups play an important role in the generation of micro-voids through membrane desilylation.
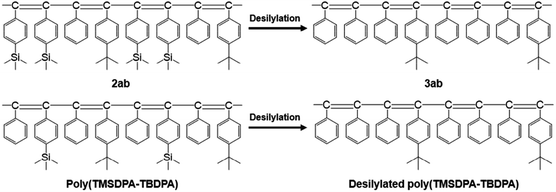
Interestingly, the PO2 values of 3ab were significantly higher than those of desilylated poly(TMSDPA–TBDPA) reported in our previous work,18 irrespective of the similarity in chemical structures (Fig. 2). For example, the PO2 of 3ab(1:1) was 6600 barrers, but the PO2 of desilylated poly(TMSDPA–TBDPA) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was 2700 barrers. The gas permeability increased as the amount of eliminated silyl group increased, when the desilylated polymer had tert-butyl groups.
1) was 2700 barrers. The gas permeability increased as the amount of eliminated silyl group increased, when the desilylated polymer had tert-butyl groups.
The oxygen/nitrogen separation factors (PO2/PN2) for all the membranes before and after desilylation were in the range of 1.2–2.5, and the values tended to increase with a decrease in the PO2 value. These findings are consistent with the general observation that the highly gas-permeable polymers typically exhibit low gas separation abilities.
Table 3 showed the data of several gas-permeable polyacetylenes and PIM-1 [a typical polymer in polymers of intrinsic microporosity (PIMs)].28 PTMSP3 has been known as the most gas-permeable polymer, whose PO2 value is as high as 9700 barrers. Indan-based polyacetylenes13,14 show the highest gas permeability among all the existing polymers. The other polyacetylenes containing various aromatic rings also show high gas permeability. PIM-1 (ref. 28) is known to show high gas permeability (PO2 = 2270 barrers). Compared to these highly gas-permeable polymers, the desilylated membranes 3ab(1:2) comparably showed ultrahigh permeability.
Diffusivity and solubility coefficients
To investigate the changes in gas permeability through desilylation in more detail, gas diffusion coefficients (D) were measured by the time lag method and the gas solubility coefficients (S) were calculated using the P and D values. Table 4 included the D and S values of 2ab, 2ac, 3ab, and 3ac.| Membrane | Ratio | DO2 × 107 | DCO2 × 107 | DN2 × 107 | SO2 × 103 | SCO2 × 103 | SN2 × 103 |
|---|---|---|---|---|---|---|---|
| a Determined by the “time lag” method at 25 °C; units: cm2 s−1.b Calculated using equation, S = P/D; units: cm3 (STP)/(cm3 × cmHg). | |||||||
| 2ab | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
130 | 160 | 110 | 16.3 | 48 | 9.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
160 | 163 | 135 | 18.1 | 59 | 11.6 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
135 | 160 | 118 | 16.1 | 48 | 9.6 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
105 | 120 | 70 | 15.7 | 54 | 10.0 | |
| 2ac | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
83 | 90 | 68 | 20.9 | 75 | 12.9 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
155 | 130 | 105 | 17.1 | 67 | 14.3 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
165 | 140 | 115 | 17.9 | 73 | 14.3 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
180 | 160 | 130 | 19.5 | 65 | 16.0 | |
| 3ab | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
135 | 180 | 120 | 43.9 | 80 | 33.2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
340 | 355 | 295 | 27.3 | 60 | 25.2 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
170 | 180 | 140 | 35.3 | 81 | 33.2 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
110 | 150 | 91 | 39.5 | 77 | 33.0 | |
| 3ac | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
78 | 68 | 63 | 12.5 | 59 | 7.0 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 68 | 64 | 12.0 | 58 | 7.1 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
87 | 70 | 65 | 12.3 | 60 | 7.3 | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
99 | 76 | 70 | 10.9 | 58 | 7.0 | |
The membranes of 3ab showed higher gas diffusivity and gas solubility values than those of 2ab. The increase in diffusivity and solubility was due to the increase in the free volume of the membrane. Contrary to 3ab, the membranes of 3ac exhibited lower gas diffusivities and solubilities than those of 2ac. These behaviors were observed for all gases. If the membrane has an affinity to a specific gas, the variation in diffusivity and solubility for a specific gas is different from those for the other gases. Therefore, the changes in gas permeability through desilylation are probably due to the change in free volume rather than the affinity to a specific gas.
Conclusions
Two types of diphenylacetylene copolymers (2ab and 2ac) were synthesized in various monomer feed ratios by metathesis copolymerization. The copolymers, 2ab and 2ac, exhibited high gas permeabilities and the PO2 values were in the range of 1700–3400 barrers. The desilylation of 2ab led to an extremely high gas permeability. Particularly, 3ab(1:2) showed the highest gas permeability in comparison to other copolymers, and a PO2 value of 9300 barrers was obtained. In contrast, the desilylation of 2ac resulted in a decrease in gas permeability. The desilylation generated free volume in the membranes with poly(diphenylacetylene) possessing tert-butyl groups.Conflicts of interest
There are no conflicts to declare.Notes and references
- Y. Tsujita, Prog. Polym. Sci., 2003, 28, 1377–1401 CrossRef CAS.
- P. Pandey and R. S. Chauhan, Prog. Polym. Sci., 2001, 26, 853–893 CrossRef CAS.
- K. Nagai, T. Masuda, T. Nakagawa, B. D. Freeman and I. Pinnau, Prog. Polym. Sci., 2001, 26, 721–798 CrossRef CAS.
- M. Ulbricht, Polymer, 2006, 47, 2217–2262 CrossRef CAS.
- K. Nagai, Y. M. Lee and T. Masuda, in Macromolecular Engineering, ed. K. Matyjaszewsky, Y. Gnanou and L. Leibler, Wiley-VCH, Weinheim, Germany, 2007, vol. 4, p. 2451 Search PubMed.
- T. Masuda and K. Nagai, in Materials Science of Membranes, ed. Y. Yampolskii, I. Pinnau and B. D. Freeman, Wiley, Chichester, UK, 2006, vol. 8, p. 231 Search PubMed.
- Y. Yampolskii, Macromolecules, 2012, 45, 3298–3311 CrossRef CAS.
- T. Aoki, T. Kaneko and M. Teraguchi, Polymer, 2006, 47, 4867–4892 CrossRef CAS.
- K. Tsuchihara, T. Masuda and T. Higashimura, Macromolecules, 1992, 25, 5816–5820 CrossRef CAS.
- T. Sakaguchi, K. Yumoto, M. Shiotsuki, F. Sanda, M. Yoshikawa and T. Masuda, Macromolecules, 2005, 38, 2704–2709 CrossRef CAS.
- H. Kouzai, T. Masuda and T. Higashimura, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 2523–2530 CrossRef CAS.
- L. G. Toy, K. Nagai, B. D. Freeman, I. Pinnau, Z. He, T. Masuda, M. Teraguchi and Y. P. Yampolskii, Macromolecules, 2000, 33, 2516–2524 CrossRef CAS.
- Y. Hu, M. Shiotsuki, F. Sanda, B. D. Freeman and T. Masuda, Macromolecules, 2008, 47, 8525–8532 CrossRef.
- Y. Hu, M. Shiotsuki, F. Sanda and T. Masuda, Chem. Commun., 2007, 4269–4270 RSC.
- A. Fukui, K. Hattori, Y. Hu, M. Shiotsuki, F. Sanda and T. Masuda, Polymer, 2009, 50, 4159–4165 CrossRef CAS.
- Y. Hu, K. Hattori, A. Fukui, M. Shiotsuki, F. Sanda and T. Masuda, Polymer, 2010, 51, 1548–1554 CrossRef CAS.
- T. Sakaguchi, G. Kwak and T. Masuda, Polymer, 2002, 43, 3937–3942 CrossRef CAS.
- T. Sakaguchi, Y. Lin and T. Hashimoto, RSC Adv., 2017, 7, 30949–30955 RSC.
- Y. Hu, T. Shimizu, K. Hattori, M. Shiotsuki, F. Sanda and T. Masuda, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 861–868 CrossRef CAS.
- M. Shiotsuki, F. Sanda and T. Masuda, Polym. Chem., 2011, 2, 1044–1058 RSC.
- T. Sakaguchi, M. Shiotsuki, F. Sanda, B. D. Freeman and T. Masuda, Macromolecules, 2005, 38, 8327–8332 CrossRef CAS.
- T. Sakaguchi, M. Shiotsuki, F. Sanda and T. Masuda, J. Membr. Sci., 2006, 280, 720–726 CrossRef CAS.
- G. Kwak, T. Aoki and T. Kaneko, Polymer, 2002, 43, 1705–1709 CrossRef CAS.
- M. Teraguchi and T. Masuda, Macromolecules, 2002, 35, 1149–1151 CrossRef CAS.
- T. Masuda, F. Sanda and M. Shiotsuki, in Comprehensive Organometallic Chemistry, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier, Oxford, 3rd edn, 2007, vol. 11, p. 557 Search PubMed.
- R. Nomura and T. Masuda, in Encyclopedia of Polymer Science and Technology, ed. H. F. Mark and J. I. Kroshwitz, Wiley-Interscience, New York, 3rd edn, 2003, vol. IA, p. 1 Search PubMed.
- T. Masuda and F. Sanda, in Handbook of Metathesis, ed. R. H. Grubbs, Wiley-VCH, Weinheim, Germany, 2003, vol. 3, p. 375 Search PubMed.
- C. G. Bezzu, M. Carta, A. Tonkins, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Adv. Mater., 2012, 24, 5930–5933 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |