Open Access Article
Open Access ArticleEffects of different potassium and nitrogen pretreatment strategies on anaerobic digestion performance of rice straw
Juan Luo a,
Juan Lib,
Liang Zhanga,
Nankun Liac,
Akiber Chufo Wachemoad,
Chunmei Liua,
Hairong Yuana and
Xiujin Li
a,
Juan Lib,
Liang Zhanga,
Nankun Liac,
Akiber Chufo Wachemoad,
Chunmei Liua,
Hairong Yuana and
Xiujin Li *a
*a
aDepartment of Environmental Science and Engineering, Beijing University of Chemical Technology, 15 Beisanhuan East Road, Chaoyang District, Beijing 100029, PR China. E-mail: xjlibuct@gmail.com; xjli@mail.buct.edu.cn
bBeijing Municipal Environmental Monitoring Center, 14 Chegongzhuang West Road, Haidian District, Beijing 100048, PR China
cAppraisal Center for Environment & Engineering Ministry of Environmental Protection, 8 Dayangfang, Anwai Beiyuan, Chaoyang District, Beijing 100012, PR China
dDepartment of Water Supply and Environmental Engineering, Arba Minch University, P.O. Box 21, Arba Minch, Ethiopia
First published on 6th July 2020
Abstract
The effects of different potassium and nitrogen pretreatment strategies on the anaerobic digestion (AD) performance of rice straw (RS) were investigated. KOH, NH3·H2O and KOH + NH3·H2O combined pretreatments were applied. The results showed that KOH + NH3·H2O combined pretreatment achieved the highest biomethane production and TS (TS: total solid) removal rate of 274 mL g VS−1 and 43.9%, which were 6.2–75.8% and 4.3–29.5% higher than that of single alkali pretreatments and untreated RS, respectively. The NH3·H2O groups improved the process stability, which maintained the NH3–N concentration in the range of 265–580 mg L−1. It was also found that Bacteroidetes and Firmicutes were the dominant bacterial at phyla level, and the populations of acetate methanogen (Methanosarcina and Methanosaeta) were enriched in the AD system by KOH + NH3·H2O pretreatment. Furthermore, the cost of pretreatment agents can be recovered by the increased digestate nutritional value due to the K and N remaining in the digestate after AD. The results indicated that the KOH + NH3·H2O combined pretreatment might be a promising method for efficient AD of straw in future industrial applications.
1. Introduction
China is one of the largest agricultural countries in the world. The annual production of rice straw (RS) averages in the range of 180–270 million tons.1 However, a considerable amount of RS has been burned in the open air, resulting in serious environmental pollution and the waste of a reusable resource.2 Anaerobic digestion (AD) is a highly recommended waste-to-energy conversion method and the most promising method for renewable energy production from agricultural residues.3 However, two shortcomings were observed in direct utilization of RS. One is the recalcitrant lignocellulosic structure, resisting hydrolysis of the substrate and further conversion by anaerobic microorganisms; the other is the excessive carbon/nitrogen ratio (C/N, 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–78
1–78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), while the suggested optimum C/N for AD is in the range from 20
1), while the suggested optimum C/N for AD is in the range from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 30
1 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4,5 Excessive C/N can inhibit the methane production due to the changes occurred in the microbial composition and metabolic pathway transfer.6,7 Therefore, overcoming the recalcitrance of lignocellulosic biomass and optimize the C/N in digesters are critical to further improve the AD performance of RS.
1.4,5 Excessive C/N can inhibit the methane production due to the changes occurred in the microbial composition and metabolic pathway transfer.6,7 Therefore, overcoming the recalcitrance of lignocellulosic biomass and optimize the C/N in digesters are critical to further improve the AD performance of RS.
Pretreatment of straw prior to AD can effectively destroy the inherent recalcitrant lignocellulosic structure and increase biodegradability of lignocellulosic biomass.8 Several different single alkali pretreatments were conducted. Shetty et al.9 conducted AD at room temperature with 1% NaOH pretreated RS, the biogas production increased by more than 34%. Mancini et al.10 using 1.6% (w/w) NaOH for the pretreatment of RS achieved an enhancement of 21.4% for the final biomethane production yield (318 mL g VS−1) compared to the untreated substrate. However, the high load of Na+ in the biogas slurry is harmful to environment because it is difficult to recover and might cause salinization.11 Potassium hydroxide (KOH) pretreatment is accepted due to potassium ability to be recycled and used as fertilizer after pretreatment and digestion. Muhammad et al.12 received a 41% higher biomethane yield of 258 mL g VS−1 with 6% KOH pretreatment than untreated wheat straw, and yielded a digestate with higher fertilizer values potassium (138%). Liu et al.13 studied ambient temperature KOH pretreatment at a loading of 20% and achieved a 52.5% higher methane yield compared to untreated wheat straw. Although these single alkali pretreatments can promote the AD performance of straw, the C/N in the digestion system cannot be regulated and extra nitrogen is needed during AD. Oji et al.14 indicated that ammonia monohydrate (NH3·H2O) pretreatment could show enhanced biodegradability of straw for better AD performance and increase the nitrogen content in the digestion system. Zhang et al.15 pretreated rice straw with 2% NH3·H2O, which showed a 17.5% biogas yield increase over the untreated one. Yuan et al.16 reported that 4% NH3·H2O pretreated wheat straw achieved 427.1 mL g VS−1 of biogas yield, which was 26.7% higher than that of untreated sample. However, there has been few studies paying attention to the combined alkali pretreatment of RS with KOH + NH3·H2O thus far. It might be possible to gain higher AD efficiency than single KOH pretreatment and single NH3·H2O pretreatment. Because KOH is a strong monobasic alkali, which can quickly weaken the ester bond connecting the hemicellulose and lignin in the substrate. This may increase the damaging effect of NH3·H2O on lignocelluloses. Moreover, KOH + NH3·H2O combined pretreatment can provide nitrogen for AD system and has potential potassium and nitrogen nutrient for digestate. This method can recover the cost of pretreatment agents and reduce the negative environmental impacts such as soil salinization and water pollution.
Different pretreatment methods could result in differences in the substrate bioconversion characteristics and further the microbial community structure, diversity and activity of specific populations.17 In the study of Zhou et al., the overall performance of waste activated sludge digestion was significantly dependent on the initial chemical pretreatments, which in turn influenced and was related to the microbial community structures.18 A few studies have been studied on the biogas performance for single KOH and single NH3·H2O pretreatment.19 However, little information is available on the effects of KOH + NH3·H2O combined pretreatment on AD performances and micro-organisms.
The objectives of this research were to: (1) investigate the biomethane production performance and conversion rate of main compositions for different potassium and nitrogen pretreatment strategies (KNPSs). (2) Compare the microbial community structures for different KNPSs. (3) Analyze the nutritional value of digestate for different KNPSs.
2. Methods and materials
2.1. Materials
The RS used in this study was collected from Tianjin District, China and then air-dried in open field. The collected RS was chopped by a knife mill (YSW-180, Zhengde, China) for size reduction and then grounded into a size of 20 meshes by a universal pulverizer (YSW-180, Yanshan Zhengde Co., Beijing, China) to increase the surface area for the next pretreatment and microbial attack. The inoculum for AD process was taken from a biogas continuously operated stable station in Shunyi District, Beijing, China. The characteristics of RS and inoculum are listed in Table 1.| Items | Value (%) | |
|---|---|---|
| Rice straw | Inoculum | |
| a Values are means ± SD (n = 3).b Content of fresh matter.c Content of dry matter. | ||
| TS (%)b | 93.70 ± 0.09 | 11.95 ± 0.06 |
| VS (%)b | 80.82 ± 0.48 | 8.31 ± 0.13 |
| MLSS (g L−1)b | — | 112.00 ± 3.50 |
| TC (%)c | 38.14 ± 0.13 | 35.15 ± 0.11 |
| TN (%)c | 0.51 ± 0.03 | 2.56 ± 0.28 |
| C/N (%)c | 74.78 ± 0.06 | 13.15 ± 0.45 |
| K (g kg−1)c | 10.42 ± 0.23 | 20.09 ± 0.42 |
| P (g kg−1)c | 2.86 ± 0.08 | 36.07 ± 0.67 |
| Cellulose (%)c | 36.44 ± 0.75 | — |
| Hemicellulose (%)c | 26.87 ± 2.35 | — |
| Lignin (%)c | 4.84 ± 0.18 | — |
2.2. Pretreatment
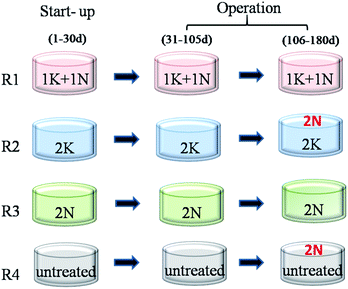
In order to compare the biogas production performance of RS with different pretreatments, NH3·H2O and KOH were used as pretreatment reagents in this study. The RS was pretreated by 1% KOH +1% NH3·H2O (1K + 1N), 2% KOH (2K) and 2% NH3·H2O (2N), which was based on dry weight of RS at 30 °C for 2 d, and the water amount addition of all pretreatment groups was 6 times that of the dry weight of RS. Then, the pretreated RS was directly used for AD tests.2.3. Start-up and operation of reactors
Four lab-scale continuously stirred tank reactors (CSTRs) were applied for AD experiment. The total volume of each CSTR was 10 L with the working volume of 8 L. The four reactors (R1, R2, R3 and R4) were respectively started up with 1K + 1N, 2K, 2N and untreated RS at 60 g VS L−1 and inoculum at mixed liquid suspended solids (MLSS) of 30 g L−1. Stable reactor temperature was maintained at 35 ± 1 °C by circulating water through the surrounding water jacket. Each reactor was agitated with a stainless-steel stirrer at a speed of 80 rpm for 10 min every 2 h.After 30 days of adaption period, the reactors were considered ready for semi-continuous feed. The feeding organic loading rate (OLR) of each CSTR was 1.7 g VS L−1 d−1 and hydraulic retention time (HRT) was 45 d.
The pretreatment method of feed in each reactor respectively corresponded to that at start-up period. Moreover, to supplement the N-source, 2% NH3·H2O was daily added to R2 (2K_2N) and R4 (untreated_2N) during AD process after being fed for 75 d. Different KNPSs in four reactors were shown in Fig. 1.
The biogas residue and biogas slurry samples were periodically collected from reactors at different time which was used for chemical composition analysis and system stability measurements. The steady state was defined to be the point when biogas production rates varied within 5% of their average values after an operating time to more than one HRT period.20
2.4. Analytic methods
The samples for VFAs, TAC and NH4+–N concentration analysis were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min and then the supernatants divided into two parts. A portion of the supernatants were filtered with a 0.22 μm membrane for VFAs determination, and the other supernatant was directly used for NH3–N concentration and TAC analysis. The VFAs containing ethanol, acetic, propionic, n-butyric, iso-butyric, n-valeric and iso-valeric acids concentrations were analyzed using a gas chromatograph (GC-2014, Shimadzu, Japan) equipped with a flame ionization detector. TAC was determined by pH titration method using 0.1 M HCl and expressed in g equivalent CaCO3 L−1 using the APHA standard methods.21 The concentration of NH4+–N was measured by a Kjeldahl analyzer (KT-260, Foss, Suzhou, China). The pH value of each digester was detected by a pH meter (Thermo Electron, USA).
000 rpm for 15 min and then the supernatants divided into two parts. A portion of the supernatants were filtered with a 0.22 μm membrane for VFAs determination, and the other supernatant was directly used for NH3–N concentration and TAC analysis. The VFAs containing ethanol, acetic, propionic, n-butyric, iso-butyric, n-valeric and iso-valeric acids concentrations were analyzed using a gas chromatograph (GC-2014, Shimadzu, Japan) equipped with a flame ionization detector. TAC was determined by pH titration method using 0.1 M HCl and expressed in g equivalent CaCO3 L−1 using the APHA standard methods.21 The concentration of NH4+–N was measured by a Kjeldahl analyzer (KT-260, Foss, Suzhou, China). The pH value of each digester was detected by a pH meter (Thermo Electron, USA).
Amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) according to the manufacturer's instructions and quantified using QuantiFluor™-ST (Promega, U.S.). The purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250) on an Illumina MiSeq platform according to the standard protocols. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database.
3. Results and discussion
3.1. Biogas production performance
Different KNPSs of anaerobic digestion were carried out. The DBP trend in each CSTR was shown in Fig. 2(a). After initial steady state start-up (1–30 d), reactors were operated at OLR of 90 g TS L−1 (31–180 d). The R1 and R3, in which respectively fed with 1K + 1N and 2N pretreated RS, showed the DBP kept at 7.17 L d−1 and 6.80 L d−1, relatively, indicating a stable DBP performance. The DBP of R2 (2K) decreased from 6.32 L d−1 (80 d) to 4.78 L d−1 (105 d). R4 (untreated) also showed the same trend in DBP, which declined from 5.50 L d−1 to 3.14 L d−1 during 65–105 d. When 2% NH3·H2O was respectively daily added with feeding from day 106–180 d, the DBP of R2 (2K_2N) and R4 (untreated_2N) was recovered and maintained at a steady state (Fig. 2(a)). This might be the recovery of microbial activity after NH3·H2O supplement.22 The average DBP of 1K + 1N pretreatment was higher than that of other KNPSs. However, the average daily methane content of different KNPSs had no significant difference (52.5–53.0%), but were a little higher than untreated RS (50.2%) (Fig. 2(b)).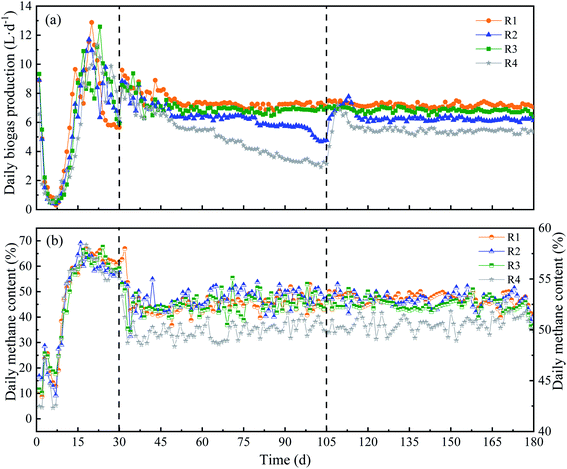 | ||
| Fig. 2 Daily biogas production (a) and daily methane content (b) of four reactors with different KNPSs. | ||
The average DMP-V and DMP-VS of CSTRs with different KNPSs were shown in Table 2. The 1K + 1N pretreatment resulting the highest average DMP-V and DMP-VS of 473 mL L−1 d−1 and 274 mL g VS−1 respectively, followed by 2N pretreatment (446 mL L−1 d−1, 258 mL g VS−1); the DMP-V and DMP-VS of 2K_2N and untreated_2N experiment groups were higher than that of 2K pretreated and untreated RS. Compared to the sample without NH3·H2O supplementation during AD, the methane production from the ammonia added groups increased by 6.6–25.6%. The DMP-VS of 1K + 1N pretreatment was 6.2%, 13.8% and 39.8% higher than that of 2N, 2K_2N and untreated_2N groups, respectively. This result was 32.2% higher than that of RS pretreated with 3% KOH, as reported by Muhammad et al.12 The DMP-VS of the 1K + 1N pretreated RS was also 63.7% higher than that for corn stalk pretreated with 2K reported by Liu et al.13 The results illustrated that KOH + NH3·H2O pretreatment showed good system stability and achieved a significant effect on the biodegradability of RS during AD in long-term CSTR operation. This was mainly because the addition of KOH and NH3·H2O enhanced the destruction of rigid lignocelluloses structures, which was benefit to the effects of the RS pretreatment.
| Reactors | Experiment groups | DMP-V (mL L−1 d−1) | DMP-VS (mL g VS−1) |
|---|---|---|---|
| R1 | 1K + 1N | 473 ± 11 | 274 ± 7 |
| R2 | 2K | 390 ± 34 | 226 ± 18 |
| R3 | 2N | 446 ± 11 | 258 ± 6 |
| R4 | Untreated | 269 ± 40 | 156 ± 23 |
| R2 | 2K_2N | 416 ± 15 | 241 ± 9 |
| R4 | Untreated_2N | 337 ± 10 | 196 ± 6 |
3.2. Conversion rate of main compositions
The main characteristics of RS digestate collected from reactors with different KNPSs are shown in Table 3. RS, like any other lignocellulosic biomass, is composed of cellulose, hemicellulose and lignin (LCH), which can be converted to biomethane through AD process. Additionally, the reduction of LCH components during the process of AD can be used to evaluate the performance of digestion process.23,24 The conversion rates of cellulose and hemicellulose in reactors with different KNPSs were analyzed (Table 3). Lignin is a complex molecule composed of phenylpropane units linked in a three dimensional structure which is particularly difficult to biodegrade by anaerobic bacteria.25 In this study, the lignin content from the feedstock was low (below 5%) and its conversion was very insignificant, as a result, the data of lignin was not shown in tables.| Reactors | Experiment groups | Content (%) | Conversion rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | TS | VS | Cellulose | Hemi-cellulose | TS | VS | ||
| R1 | 1K + 1N | 16.2 ± 1.2 | 14.4 ± 0.9 | 5.1 ± 0.2 | 4.5 ± 0.1 | 55.4 ± 3.5 | 46.3 ± 2.6 | 43.9 ± 1.2 | 57.2 ± 1.0 |
| R2 | 2K | 18.4 ± 2.2 | 16.3 ± 2.2 | 5.5 ± 0.5 | 5.1 ± 0.5 | 49.6 ± 6.4 | 39.4 ± 4.7 | 38.6 ± 2.1 | 51.2 ± 2.2 |
| R3 | 2N | 16.9 ± 1.3 | 15.1 ± 0.8 | 5.2 ± 0.2 | 4.6 ± 0.2 | 53.6 ± 3.1 | 43.8 ± 2.4 | 42.1 ± 1.3 | 55.7 ± 1.2 |
| R4 | Untreated | 21.2 ± 3.6 | 17.9 ± 3.7 | 6.0 ± 0.6 | 5.6 ± 0.5 | 41.8 ± 7.8 | 33.4 ± 5.3 | 33.9 ± 4.5 | 46.0 ± 4.7 |
| R2 | 2K_2N | 17.9 ± 0.6 | 15.9 ± 0.8 | 5.5 ± 0.2 | 4.8 ± 0.1 | 50.8 ± 3.1 | 40.8 ± 2.0 | 39.4 ± 1.4 | 53.8 ± 1.5 |
| R4 | Untreated_2N | 19.3 ± 0.8 | 16.5 ± 0.8 | 5.6 ± 0.1 | 5.2 ± 0.1 | 46.9 ± 2.7 | 38.2 ± 2.1 | 37.7 ± 1.4 | 49.8 ± 1.7 |
1K + 1N pretreatment achieved 55.4% and 46.3% removal efficiencies for cellulose and hemicellulose, respectively, which was 3.4–18.1% and 5.7–21.2% higher than that of single alkali pretreatment and untreated groups (46.9–53.6% and 38.2–43.8%) (Table 3). The TS and VS removal rate of all groups was 33.9–43.9% and 46.0–57.2%, respectively, and 1K + 1N group received the highest TS and VS removal rate of 43.9% and 57.2%, which were 4.3–29.5% and 2.7–24.3% higher than other groups. The 2K and untreated groups showed low TS conversion rate (33.9–38.6%). This indicated that ammonia directly added to the digesters during AD can regulate nutritional balance and also had better effect on DBP as well as the substrate conversion rate. The substrate conversion rate is arranged in descending order as follows: 1K + 1N > 2N > 2K_2N > 2K > untreated_2N > untreated, indicating that high main compositions conversion rates correspond to high biogas production. Ammonia added during feeding period promoted the conversion of RS showing that lack of nitrogen leading to lower TS conversion and biogas production. Whereas, KOH + NH3·H2O combined pretreatment promoted further conversion of RS into biogas production.
3.3. System stability
Stability is a critical factor during the operation of anaerobic digesters whereas instability is a problem that has plagued digesters for many years which can be resulted from any material or related effects that interfere with methane formation.26 The stability of AD system mainly reflected in the indicators of NH3–N, pH, VFAs, and alkalinity.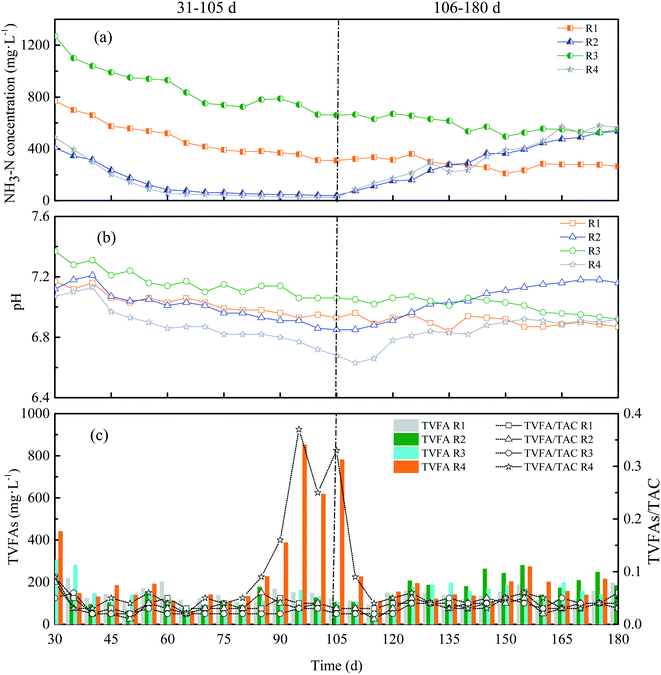 | ||
| Fig. 3 NH3–N concentration (a), pH (b), TVFAs and TVFAs/TAC (c) of four reactors with different KNPSs. | ||
As shown in Fig. 3(a), R1 and R3 had a relatively high NH3–N concentration of 770 mg L−1 and 1270 mg L−1 at the beginning due to the 1% and 2% NH3·H2O addition during pretreatment, respectively. Then, the NH3–N concentration in R1 and R3 gradually decreased when the feeding continued, and then kept at a relatively stable level of 265–580 mg L−1. The NH3–N concentration in R2 and R4 was 410 mg L−1 and 490 mg L−1 on the first feeding day, and continuously decreased with the feeding proceeding. On the 105th day, The NH3–N concentration in R2 and R4 dropped to 39 mg L−1 and 24.5 mg L−1 respectively. When ammonia supplemented with feeding, the NH3–N concentration of R2 and R4 gradually recovered and reached up to 280–565 mg L−1. This is consistent with the reports of Rajagopal et al.28 who regulated the NH3–N concentration by adjusting the carbon and nitrogen ratio of the system.
As can be seen in Fig. 2(a) and 3(a), the biogas production of each reactor maintained stable when the NH3–N concentration is in between 55-1270 mg L−1, and then gradually declined while the NH3–N concentration is less than 55 mg L−1. This result is not consistent with other researchers. For instance, Procházka et al.27 indicated that low NH3–N concentration (500 mg L−1) caused low methane yield, loss of biomass and loss of the acetoclastic methanogenic activity. Additionally, Benjamin et al.29 reported that the optimum growth conditions for Methanosaeta concilii, which is the most ammonia-sensitive methanogen, were in the range of 250–1100 mg L−1 NH3–N concentration. Wang and Yuan30,31 achieved the highest biogas yield with 0.7% and 4% ammonia concentration, respectively. When 1% and 2% NH3·H2O were used in our study, the ammonia concentration in the digesters maintained 265–580 mg L−1, which were lower than the levels mentioned above. Therefore, no ammonium toxicity existed in our study.
As shown in Fig. 3(c), the change in trend of TVFAs/TAC was consistent with TVFAs. After feeding for five days, TVFAs values of R1, R2 and R3 were less than 300 mg L−1 and the TVFAs/TAC ratio fluctuated in a small range (0.01–0.08) during the whole operation period. The TVFAs of R4 rose to more than 600 mg L−1 and the TVFAs/TAC had a sudden fluctuation that increased from 0.05 to 0.33 (80–105 d), which restored stability after ammonia addition with feeding (Fig. 3(c)). TVFAs concentration was kept at a low level, indicating that no inhibition occurred during the fermentation of RS in CSTRs. Under the same conditions, the lower VFAs value was, the better biogas production performance showed. The sudden increase in TVFAs concentration in R4 was attributed to the nitrogen deficiency. This resulted in low methanogenic activity and less VFAs that converted to methane in R4. Moreover, Hun et al.33 pointed out that the TVFAs/TAC ratio must be observed in a dynamic way during AD period and a sudden increment of the ratio reflects a potential instability of the process. The reason for the instability of R4 was mainly due to low buffering capacity and VFAs accumulation for insufficient nitrogen and low methanogenic activity. However, R2 was in low nitrogen content but no fluctuation of TVFAs/TAC and instability was observed due to the enhanced buffer capacity for KOH pretreatment.13 Compared to pretreatment without ammonia, the 1K + 1N and 2N pretreatments were more suitable for long-term stable AD operation. Similarly, N-source added during AD also could promote the stability of anaerobic digester.
3.4. Microbial community structure of different KNPSs
| Items | Experiment groups | Samples | OTUs | Ace | Chao | Coverage | Shannon | Simpson |
|---|---|---|---|---|---|---|---|---|
| Bacteria | 1K + 1N | R1 | 552 | 603 | 618 | 0.9986 | 4.34 | 0.0359 |
| 2K | R2 | 415 | 510 | 516 | 0.9972 | 3.52 | 0.0914 | |
| 2N | R3 | 531 | 575 | 578 | 0.9984 | 4.10 | 0.0392 | |
| Untreated | R4 | 551 | 584 | 586 | 0.9983 | 3.90 | 0.0758 | |
| 2K_2N | R2_2N | 489 | 564 | 568 | 0.9976 | 3.98 | 0.0431 | |
| untreated_2N | R4_2N | 516 | 592 | 591 | 0.9976 | 3.65 | 0.0759 | |
| Archaea | 1K + 1N | R1 | 47 | 47 | 47 | 1.0000 | 1.44 | 0.3406 |
| 2K | R2 | 19 | 19 | 19 | 1.0000 | 1.57 | 0.2795 | |
| 2N | R3 | 27 | 30 | 29 | 0.9999 | 1.38 | 0.3430 | |
| Untreated | R4 | 32 | 32 | 32 | 1.0000 | 1.63 | 0.2990 | |
| 2K_2N | R2_2N | 21 | 23 | 21 | 0.9999 | 1.38 | 0.3439 | |
| untreated_2N | R4_2N | 19 | 19 | 19 | 1.0000 | 0.95 | 0.5150 |
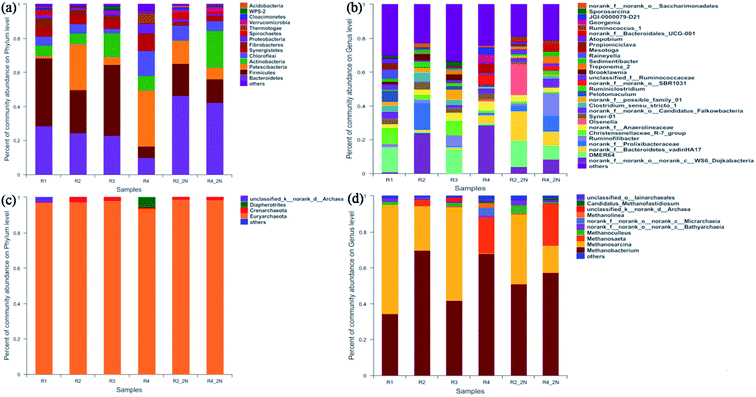 | ||
| Fig. 4 Bacterial and archaeal sequence distributions at phylum (a and c) and genus (b and d) level of different KNPSs. | ||
The genus level in the six samples was observed. The result showed that the dynamic profiles of bacterial genus differed significantly in different KNPSs (Fig. 4(b)). Twenty-nine major genera (represented by greater than 2.5% of total bacterial sequences in at least one sample) were found in all samples, and the 10 top species genus were DMER64, norank_c_WS6_Dojkabacteria, norank_f_Prolixibacteria, norank_c_Bacteroidetes_-vadinHA17, Rumininofilibacter, Christensenellaceae_R-7_group, Syner-01, norank_f_Anaerolineaceae, Olsenella, Clostridium_sensu_stricto_1. DMER64 and Christensenellaceae_R-7_group can establish magnetite-mediated direct electron transfer with methanogens during methanogenic degradation of VFAs.39 In addition, Christensenellaceae_R-7_group also promotes hydrolysis acidification, especially for cellulose that is difficult to degrade.40 The total abundance of DMER64 and Christensenellaceae_R-7_group in R1, R3, R2_2N and R4_2N was 24.2%, 23.0%, 15.6% and 8.2%, respectively. In R2 and R4, there were no DMER64 and Christensenellaceae_R-7_group observed. This indicated that the NH3·H2O addition increases the diversity of acid hydrolytic microorganisms. In addition, the bacterial diversity in R1 was higher than the other KNPSs groups, which demonstrating that the KOH + NH3·H2O combined pretreatment improved the ability of bacteria to hydrolyse lignocellulosic matter, thereby resulting in the remarkable increment of the hemicellulose and cellulose reduction efficiency. As a result, the diversity of bacteria increased in the final stage of AD.
The dynamic profiles of the archaeal community at the genus level were significantly different in the six samples (Fig. 4(d)). Methanosarcina and Methanosaeta are the methanogens have been found to decomposing acetic acid for methane production, and metabolic transformation could occur under certain conditions from Methanosaeta to Methanosarcina.42 The genus Methanobacterium is a typical hydrogen-nutrient methanogen existing in anaerobic system and has strong viability in producing methane from H2 and CO2.43 As can be seen in Fig. 4(d), archaeal communities demonstrated a clear dominance of the genus Methanosarcina and Methanosaeta (20.3–55.2%) and Methanobacterium (40.1–69.7%). The relative abundance of Methanosarcina and Methanosaeta in R1 (55.2%) and R3 (52.5%) was higher than those in R2 (28.4%) and R4 (20.3%). After NH3·H2O addition during AD, the Methanosarcina and Methanosaeta accounted for 39.2% and 38.6% in samples of R2_2N and R4_2N, respectively. This demonstrated that the KOH and/or NH3·H2O added in the digesters make more genera to use acetate as a substrate for methanogenesis. During the AD process, more than 70% of methane in biogas comes from the cleavage of acetate.44 Therefore, the addition of KOH and/or NH3·H2O can enrich the abundance of acetate-nutrient methanogen and the higher biomethane production. It was observed that the proportion of Methanosarcina and Methanosaeta species in the 1K + 1N group was higher than that of 2K, 2N and untreated groups, which was contributed to the increment concentration of VFAs after KOH + NH3·H2O combined pretreatment. The data presented in this study indicated that both KOH and NH3·H2O pretreatment had significant impacts on microbial community structures and the metabolic pathway for biomethane production in an AD digester.
3.5. Fertilizer value analyses of the digestate
Digestate, as a multi-component quick-acting compound organic fertilizer, plays an important role in improving soil fertility and improving the quality of agricultural products, and is getting more and more attention. In this study, a simple fertilizer value analysis of digestate was provided (Table 5). The content of nitrogen ranged from 24.0 g kg−1 TS−1 to 31.9 g kg−1 TS−1. The content of the ammonia addition samples were 2.0–7.9 g kg−1 TS−1 more than that of none ammonia addition samples. This indicated that most of the nitrogen, whether added in pretreatment or during AD process, remains in the digestate. A small portion of nitrogen may be volatilized in the form of NH3 during pretreatment or AD. As shown in Table 5, it was found that there was no much difference in the content of phosphorus (4.1–4.2 g kg−1 TS−1) in each sample for there was no extra phosphorus addition in each digester. The potassium contents in digastate were 15.2–29.9 g kg−1 TS−1 in all samples. Compared to groups without potassium addition, the content of the potassium addition groups increased 6.8–14.7 g kg−1 TS−1, which was consistent with the theoretical addition of potassium (7.0–14.0 g kg−1 TS−1). This indicated that the potassium added in pretreatment was not consumed as the AD proceeding, and retained in the digestate. The more potassium in digestate, the better fertilizer effect because sufficient potassium in the soil plays an active role in activation of enzymes for healthy plant growth.45| Groups | N | P | K |
|---|---|---|---|
| (g kg−1 TS−1) | (g kg−1 TS−1) | (g kg−1 TS−1) | |
| 1K + 1N | 28.2 ± 0.9 | 4.2 ± 0.1 | 23.1 ± 1.0 |
| 2K | 26.2 ± 1.2 | 4.1 ± 0.2 | 29.9 ± 1.4 |
| 2N | 30.3 ± 0.6 | 4.1 ± 0.1 | 15.2 ± 0.7 |
| Untreated | 24.0 ± 1.1 | 4.1 ± 0.2 | 16.3 ± 0.8 |
| 2K_2N | 31.9 ± 0.9 | 4.2 ± 0.0 | 28.5 ± 0.9 |
| untreated_2N | 31.3 ± 1.2 | 4.1 ± 0.1 | 15.2 ± 0.5 |
Therefore, the potassium and nitrogen added in the digesters can significantly increase fertilizer value of the digestate. Compared with untreated RS, although different KNPSs require a certain amount of reagents cost, these costs can be recovered by the increasing fertilizer value in the digestate. Moreover, three different KNPSs used in this study could all increase methane production efficiency. It was found that 1K + 1N pretreatment achieved the highest biomethane production and provided an environmental solution that had no potential environment pollution. Therefore, 1K + 1N pretreatment provides a meaningful insight for exploring efficient potassium and nitrogen pretreatment strategy to enhance AD performance for practical application in an affordable and environmentally friendly way.
4. Conclusions
KOH + NH3·H2O combined pretreatment was proved to be efficient for bioconversion of rice straw to biomethane. The rice straw pretreated by KOH + NH3·H2O achieved the highest methane production of 274 mL g VS−1, which was 6.2–75.8% higher than that of single alkali pretreatments and untreated RS. KOH + NH3·H2O pretreatment also showed higher abundance of the dominant bacterial phyla (Bacteroidetes and Firmicutes) and the acetate methanogen (Methanosarcina and Methanosaeta). Moreover, digestate nutritional value was improved due to the K and N remaining in digestate after AD. KOH + NH3·H2O combined pretreatment could be one of alternative methods for high-efficient biomethane production and environmentally friendly from RS.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the fund supports from National “Thirteenth Five-Year” Plan for Science & Technology Support (2018YFC1900901).References
- M. Elsayed, A. E. Abomohra, P. Ai, D. Wang, H. M. El-Mashad and Y. Zhang, Bioresour. Technol., 2018, 268, 183–189 CrossRef CAS PubMed.
- X. Qian, G. Shen, Z. Wang, X. Zhang, X. Chen, Z. Tang, Z. Lei and Z. Zhang, Bioresource Technology Reports, 2019, 7, 100208 CrossRef.
- J. Li, A. C. Wachemo, H. Yuan, X. Zuo and X. Li, Bioresour. Technol., 2019, 288, 121518 CrossRef CAS PubMed.
- R. Guan, X. Li, A. C. Wachemo, H. Yuan, Y. Liu, D. Zou, X. Zuo and J. Gu, Sci. Total Environ., 2018, 637–638, 9–17 CrossRef CAS PubMed.
- J. Xu, X. Wang, S. Sun, Y. Zhao and C. Hu, Sci. Rep., 2017, 7, 10897 CrossRef PubMed.
- G. Tian, W. Zhang, M. Dong, B. Yang, R. Zhu, F. Yin, X. Zhao, Y. Wang, W. Xiao, Q. Wang and X. Cui, Energy, 2017, 139, 571–579 CrossRef CAS.
- M. J. Fernández-Rodríguez, D. de la Lama-Calvente, A. Jiménez-Rodríguez, R. Borja and B. Rincón-Llorente, Process Saf. Environ. Prot., 2019, 128, 167–175 CrossRef.
- J. Du, Y. Qian, Y. Xi and X. Lü, Renew. Energy, 2019, 139, 261–267 CrossRef CAS.
- D. J. Shetty, P. Kshirsagar, S. Tapadiamaheshwari, V. Lanjekar, S. K. Singh and P. K. Dhakephalkar, Bioresour. Technol., 2016, 226, 80–88 CrossRef PubMed.
- G. Mancini, S. Papirio, G. Riccardelli, P. N. L. Lens and G. Esposito, Bioresour. Technol., 2018, 247, 897–903 CrossRef CAS PubMed.
- L. Li, C. Chen, R. Zhang, Y. He, W. Wang and G. Liu, Energy Fuels, 2015, 29, 5841–5846 CrossRef CAS.
- M. Jaffar, Y. Pang, H. Yuan, D. Zou, Y. Liu, B. Zhu and R. M. Korai, Chin. J. Chem. Eng., 2016, 24(3), 404–409 CrossRef.
- X. Liu, S. M. Zicari, G. Liu, Y. Li and R. Zhang, Bioresour. Technol., 2015, 185, 150–157 CrossRef CAS PubMed.
- U. I. Oji, H. E. Etim and F. C. Okoye, Small Rumin. Res., 2007, 69(1), 232–236 CrossRef.
- R. Zhang and Z. Zhang, Bioresour. Technol., 1999, 68(3), 235–245 CrossRef CAS.
- H. Yuan, R. Li, Y. Zhang, X. Li, C. Liu, M. Ying, M. Lin and Z. Yang, Biosyst. Eng., 2015, 129, 142–148 CrossRef.
- J. Li, A. C. Wachemo, H. Yuan, X. Zuo and X. Li, Waste Manag., 2019, 97, 52–62 CrossRef CAS PubMed.
- A. Zhou, J. Zhang, C. Varrone, K. Wen, G. Wang, W. Liu, A. Wang and X. Yue, Energy, 2017, 137, 457–467 CrossRef CAS.
- Q. Yu, R. Liu, K. Li and R. Ma, Renewable Sustainable Energy Rev., 2019, 107, 51–58 CrossRef CAS.
- D. G. Mulat, H. F. Jacobi, A. Feilberg, A. P. S. Adamsen, H. H. Richnow and M. Nikolausz, Appl. Environ. Microbiol., 2015, 82(2), 438–449 CrossRef PubMed.
- APHA, A. W., Standard Methods for the Examination of Water and Wastewater, American Water Works Association, 2005 Search PubMed.
- J.-H. Park, J.-J. Yoon, G. Kumar, Y.-S. Jin and S.-H. Kim, Waste Manag., 2018, 80, 218–223 CrossRef CAS PubMed.
- S. D. Pore, A. Engineer, S. S. Dagar and P. K. Dhakephalkar, Bioresour. Technol., 2019, 279, 25–33 CrossRef CAS PubMed.
- Z. Weizhang, Z. Zhongzhi, L. Yijing, S. Shanshan, Q. Wei and X. Meng, Bioresour. Technol., 2011, 102(24), 11177–11182 CrossRef PubMed.
- M. T. Jin, S. G. Sommer, H. B. M Ller, M. R. Weisbjerg and Y. J. Xin, Bioresour. Technol., 2011, 102(20), 9395–9402 CrossRef PubMed.
- J. Keenan, Environ. Lett., 1976, 11(8–9), 525–548 CAS.
- J. Procházka, J. Máca and M. Dohányos, Appl. Microbiol. Biotechnol., 2012, 93(1), 439–447 CrossRef PubMed.
- R. Rajagopal, D. I. Massé and G. Singh, Bioresour. Technol., 2013, 143(17), 632–641 CrossRef CAS PubMed.
- S. Benjamin, M. L. Garcia, A. Q. Shen and L. T. Angenent, Appl. Environ. Microbiol., 2007, 73(5), 1653 CrossRef PubMed.
- D. Wang, Y. Xin, H. Shi, P. Ai, L. Yu, X. Li and S. Chen, Closing ammonia loop in efficient biogas production: recycling ammonia pretreatment of wheat straw, Biosyst. Eng., 2019, 180, 182–190 CrossRef.
- H. Yuan, R. Guan, A. Wachemo, Y. Zhang, X. Zuo and X. Li, Chin. J. Chem. Eng., 2020, 28(2), 541–547 CrossRef.
- Y. Q. Li, C. M. Liu, A. C. Wachemo, H. R. Yuan, D. X. Zou, Y. P. Liu and X. J. Li, Bioresour. Technol., 2017, 235, 380–388 CrossRef CAS PubMed.
- K. S. Hun and K. G. Krishna, Biosystems Engineering, 2010, 35(1), 53–62 CrossRef.
- B. Si, Z. Liu, Y. Zhang, J. Li, R. Shen, Z. Zhu and X. Xing, Int. J. Hydrog. Energy, 2016, 41(7), 4429–4438 CrossRef CAS.
- Y. Liu, A. C. Wachemo, H. Yuan and X. Li, Bioresour. Technol., 2019, 287, 121339 CrossRef CAS PubMed.
- I. Ramirez, E. I. P. Volcke, R. Rajinikanth and J. Steyer, Water Res., 2009, 43(11), 2787–2800 CrossRef CAS PubMed.
- M. Kim, M. Abdulazeez, B. M. Haroun, G. Nakhla and M. Keleman, Microbial communities in co-digestion of food wastes and wastewater biosolids, Bioresour. Technol., 2019, 289, 121580 CrossRef CAS PubMed.
- L. Sun, T. Liu, B. Müller and A. Schnürer, Biotechnol. Biofuels, 2016, 9(1), 128 CrossRef PubMed.
- J. Lee, T. Koo, A. Yulisa and S. Hwang, J. Environ. Manage., 2019, 241, 418–426 CrossRef CAS PubMed.
- L. Dong, G. Cao, J. Wu, S. Yang and N. Ren, Bioresour. Technol., 2019, 284, 248–255 CrossRef CAS PubMed.
- J. H. Ko, N. Wang, T. Yuan, F. Lü, P. He and Q. Xu, Bioresour. Technol., 2018, 266, 516–523 CrossRef CAS PubMed.
- M. B. Kurade, S. Saha, E. Salama, S. M. Patil, S. P. Govindwar and B. Jeon, Bioresour. Technol., 2019, 272, 351–359 CrossRef CAS PubMed.
- S. Feng, X. Hong, T. Wang, X. Huang, Y. Tong and H. Yang, Bioresour. Technol., 2019, 288, 121509 CrossRef CAS PubMed.
- D. G. Mulat, H. F. Jacobi, A. Feilberg, A. P. Adamsen, H. H. Richnow and M. Nikolausz, Appl. Environ. Microbiol., 2016, 82, 438–449 CrossRef CAS PubMed.
- D. Holthusen, S. Peth and R. Horn, Soil Tillage Res., 2010, 111(1), 75–85 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |