Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pulsed laser deposited CoFe2O4 thin films as supercapacitor electrodes
S. M. Nikama,
A. Sharma b,
M. Rahaman
b,
M. Rahaman b,
A. M. Teli
b,
A. M. Teli c,
S. H. Mujaward,
D. R. T. Zahn
c,
S. H. Mujaward,
D. R. T. Zahn b,
P. S. Patilac,
S. C. Sahoo
b,
P. S. Patilac,
S. C. Sahoo e,
G. Salvan
e,
G. Salvan *b and
P. B. Patil
*b and
P. B. Patil *f
*f
aSchool of Nanoscience and Technology, Shivaji University, Kolhapur, Maharashtra – 416004, India
bSemiconductor Physics, Chemnitz University of Technology, 09107, Chemnitz, Germany. E-mail: salvan@physik.tu-chemnitz.de
cDepartment of Physics, Shivaji University, Kolhapur, Maharashtra – 416004, India
dDepartment of Physics, Yashavantrao Chavan Institute of Science, Satara, Maharashtra – 415001, India
eDepartment of Physics, Central University of Kerala, Kasaragod, Kerala – 671320, India
fDepartment of Physics, The New College, Shivaji University, Kolhapur, Maharashtra – 416012, India. E-mail: prashantphy@gmail.com
First published on 20th May 2020
Abstract
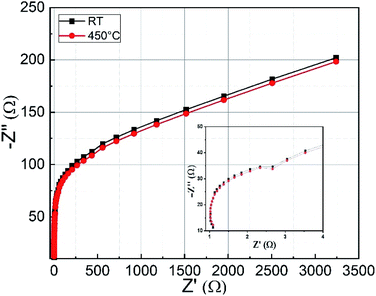
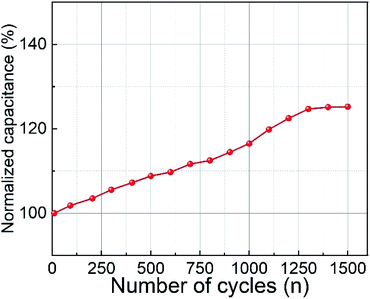
The influence of the substrate temperature on pulsed laser deposited (PLD) CoFe2O4 thin films for supercapacitor electrodes was thoroughly investigated. X-ray diffractometry and Raman spectroscopic analyses confirmed the formation of CoFe2O4 phase for films deposited at a substrate temperature of 450 °C. Topography and surface smoothness was measured using atomic force microscopy. We observed that the films deposited at room temperature showed improved electrochemical performance and supercapacitive properties compared to those of films deposited at 450 °C. Specific capacitances of about 777.4 F g−1 and 258.5 F g−1 were obtained for electrodes deposited at RT and 450 °C, respectively, at 0.5 mA cm−2 current density. The CoFe2O4 films deposited at room temperature exhibited an excellent power density (3277 W kg−1) and energy density (17 W h kg−1). Using electrochemical impedance spectroscopy, the series resistance and charge transfer resistance were found to be 1.1 Ω and 1.5 Ω, respectively. The cyclic stability was increased up to 125% after 1500 cycles due to the increasing electroactive surface of CoFe2O4 along with the fast electron and ion transport at the surface.
1. Introduction
With the endless desire for electricity and drift from the conventional power grid to renewable energy sources, the demand for efficient energy storage devices is rising exponentially. For this, the supercapacitor (SC) is an excellent solution due to its fast charging/discharging rates, high power density, long cycle life, and eco-friendly nature.1–4 In general, SCs can be classified into two types based on the operational charge–discharge storage mechanism: electric double-layer capacitors (EDLCs) and pseudocapacitors. EDLCs work on the principle of electrostatics, where the energy is stored by trapping ions at the electrode/electrolyte interface.2 A pseudocapacitor is based on the faradaic charge transfer process that utilizes reversible redox reactions and intercalation at electrodes to store charge. In particular, pseudocapacitive materials can achieve excellent specific capacitances and energy densities, and they can thus be an interesting source for constructing novel energy storage devices.5 However, the pseudo capacitor has a comparatively shorter cycle life than an EDLC, due to the not completely reversible redox reactions and the loss of electrical contact resulting from the disintegration of the crystal structure, which reduces electrochemical performance. This can be overcome by combining a pseudo-active material a with conductive support, also known as hybrid supercapacitors.6 Transition metal oxides, such as RuO2, Co3O4, and Fe2O3 have been considered as active materials for supercapacitors on account of their high theoretical capacitance, variable oxidation states, environmentally friendly nature, and low cost.7,8 Further, several researches have been focused to improve performance, storage, and energy densities for SCs by either tailoring the material properties9,10 and/or altering the surface11 of the electrode materials. Some of the prominent approaches include the electrode made of porous carbon,12 carbon nanotube,13 and metal–organic frameworks.14–16Recently, mixed metal oxides with spinel ferrite structure, MFe2O4 (M = Co, Mn, Zn, Mg, or Ni), were reported to have distinct hard or soft magnetic properties or even superparamagnetism, a large range of oxidation states, and chemical stability.17 In spinel ferrites, the divalent metal ion (M2+) occupies the tetrahedral site, and the trivalent metal ion (M3+ or Fe3+) occupies the octahedral position of the cubic close-packed oxygen lattice, see Fig. 1.18,19 D. Ravinder et al. reported that the ferrites have remarkably high permeability and can attain multiple redox states.20 These properties of ferrites were explored by several researchers who showed that the ferrite electrode has superior pseudo-capacitance.21–23 It is anticipated that mixed ferrites, with varying ratios of the M and Fe ions, improve the electrode performance, due to their higher number of cations for coordination sites.24
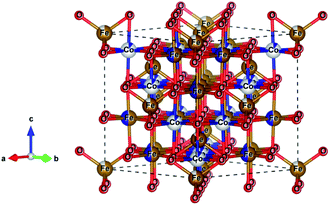 | ||
Fig. 1 Schematic crystal structure of spinel CoFe2O4 ferrite (Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group) showing Co and Fe cations as grey and gold spheres and O anions as red spheres, respectively. m space group) showing Co and Fe cations as grey and gold spheres and O anions as red spheres, respectively. | ||
Among the known mixed spinel ferrites, CoFe2O4 has a variety of merits, including excellent chemical stability, efficient electrocatalytic behaviour, high specific capacitance that makes it a suitable candidate for electrodes in supercapacitors.25–27 To harness these advantages of CoFe2O4 for supercapacitor applications, numerous chemical methods of preparation were reported, such as solvothermal, hydrothermal, electrodeposition, and spin coating.17,28–30 Even though all these chemical methods are cheap, they all have issues with the reproducibility of the stochiometric composition of the deposited material. On the other hand, the potential of the pulsed laser deposition (PLD) technique, which has an excellent stochiometric reproducibility of the target material,31,32 has not yet been explored for the deposition of cobalt ferrite electrodes for supercapacitor application.
The present study reports the efficacy of PLD deposited CoFe2O4 thin films for supercapacitive electrode applications. The films with different crystallinity were prepared by using different substrate temperatures. Mesoporous films prepared at RT can provide a higher surface area for an electrolyte to diffuse with low transfer limitation. The low crystallinity of the electrode material has improves the exposure to active sites accessible for the electrolyte on the surface.
2. Experimental
Cobalt ferrite thin films were deposited on a fused quartz substrate in a PLD vacuum chamber with a base pressure of 2 × 10−5 mbar. A sintered cobalt ferrite target comprised of α-Fe2O3 and Co3O4 in the stoichiometric ratio was repetitively (10 Hz) irradiated with the third harmonic (λ = 355 nm) of an Nd:YAG laser for 6 ns pulse duration delivering a typical fluence of 2.5 J cm−2 on the target during deposition. The fused quartz substrate was kept at a distance of 45 mm from the target. In this way, we fabricated CoFe2O4 thin films at room temperature (RT), 350 °C, and 450 °C substrate temperature. A clear evidence of crystallisation in the X-ray diffraction studies was observed only at 450 °C substrate temperature and the electrochemical performance of the 350 °C films (not shown here) was similar to that of the RT films. Thus, for the ease of discussion, only the RT and 450 °C samples are considered for the further investigations. Thicknesses of films prepared at RT and 450 °C substrate temperature were found to be around 110 nm and 200 nm, respectively.The crystallographic investigation of the films was performed by recording X-ray diffraction (XRD) 2θ–ω scans using a Bruker D2 PHASER X-ray diffractometer. Raman measurements were carried out using XploRA PLUS Raman spectrometer from Horiba scientific under 532 nm diode-pumped solid-state (DPSS) laser excitation using a 100×/0.9 NA objective with a laser power density of 1.33 mW μm−2 in a back-scattering geometry. The collected Raman signals were dispersed onto an electron multiplier charge-coupled device (EMCCD) using a 1200 l mm−1 grating. The sample surface morphology was examined using an Agilent 5420 atomic force microscope operated in intermittent contact mode. The electrochemical measurements were carried out in the three-electrode system using Autolab's potentiostat PGSTAT302.
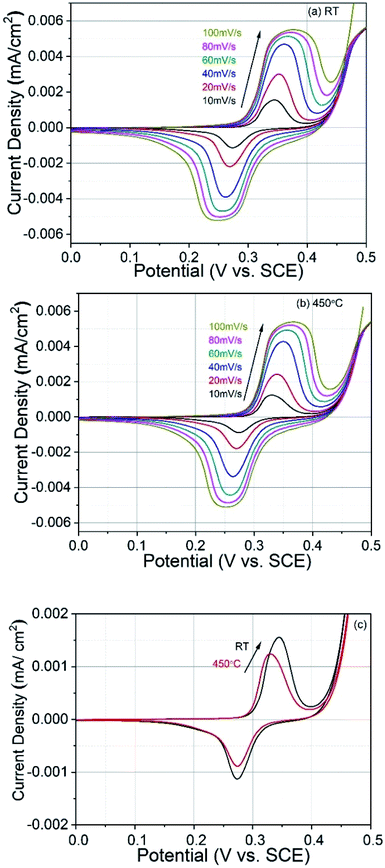
For electrochemical measurements, cobalt ferrite films were used as both electrode material and current collectors. For a seamless electrical contact to the film, a small piece of silver was clipped onto the conductive cobalt ferrite film by a toothless alligator clip to connect the battery analyser. Then the assembly was dipped in the 1 M KOH aqueous electrolyte solution for electrochemical measurements. Cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) were carried out with conventional three-electrode arrangement comprising platinum as a counter electrode, a saturated calomel electrode (SCE) as a reference electrode, and CoFe2O4 as a working electrode. Cyclic voltammetry measurements were carried out at various scan rates of 10–100 mV s−1 between the potential window of 0 V and 0.5 V. Galvanostatic charge–discharge measurements were carried out at a charging current density (0.5 mA cm−2) between 0 V and 0.5 V vs. SCE. The specific capacitance of the supercapacitor was calculated from GCD curves according to the equation19
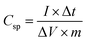 | (1) |
3. Results and discussion
3.1. Structural analysis
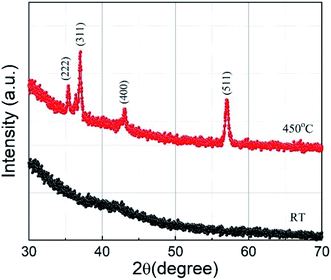
XRD 2θ–ω scans of the sample deposited at substrate temperatures of RT and 450 °C are shown in Fig. 2. The film deposited at RT does not show any XRD peak, implying that the film is amorphous. However, the film grown at a substrate temperature of 450 °C shows sharp diffraction peaks and the peaks observed can be indexed to the cubic Co-ferrite phase with the help of JCPDS data (ICDD, PDF 221086 for CoFe2O4) and assigned to the lattice planes (222), (311), (400), and (511) corresponding to the cubic spinel structure of CoFe2O4. The peaks with smaller intensity appearing around 36.40° and 42.52° are due to the presence of CoO (ICDD, PDF 431004 for CoO) in the sample. However, the relative intensity of the CoO peaks with respect to the CoFe2O4 peaks is minimal and the Co is thus expected not to influence the electrochemical performance of the CoFe2O4 electrode. We can also see in Fig. 2 that a lower number of peaks compared to the bulk were observed in the film. The films generally showed a preferred orientation in the (311) direction, as it is the case for many other reports of CoFe2O4 thin films.7,33 The lattice constant for the film deposited at 450 °C calculated using the (511) peak was found to be 8.4 Å which is comparable to the bulk crystallite value (ICDD, PDF 221086). For films deposited at 450 °C, the average crystallite size calculated by the Debye Scherrer formula using the (511) peak was found to be (19 ± 1) nm. The film deposited at RT may have grains with very small size, which could not be detected by XRD. In our previous work, reporting CoFe2O4 film preparation at different temperatures by PLD, we observed similar XRD behaviour for films prepared at RT.34 The selected area electron diffraction (SAED) pattern of these films prepared at RT showed diffraction rings which were identified as the Co-ferrite phase. This indicates that our films deposited at RT consist of low crystalline CoFe2O4 phase.3.2. Raman spectroscopy
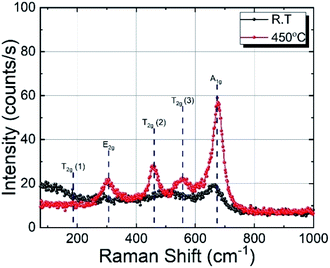
The Raman spectra of cobalt ferrite thin films prepared at RT and 450 °C are shown in Fig. 3. Raman spectroscopy is a powerful technique in understanding crystal structures of a material down to nano-size domains. CoFe2O4 belongs to the space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, predicting 39 phonon modes for this spinel structure. Among them, the five Raman active modes are A1g, Eg, and 3T2g.35,36 The modes below (above) 600 cm−1 are attributed to the oxygen motion around the octahedral (tetrahedral) lattice sites.37 The broadening of the Raman modes indicating its amorphous nature is in agreement with the XRD results. The film deposited at 450 °C substrate temperature shows four prominent Raman active modes at 302 cm−1, 458 cm−1, 556 cm−1, and 678 cm−1, which correspond to Eg, 2T2g, and A1g phonon vibrations, respectively. The Raman active T2g mode around 200 cm−1 is absent in the spectra, probably due to the weak Raman cross-section of this particular mode. The A1g Raman modes of the film prepared at 450 °C show a slightly asymmetric shape with a shoulder at the low-frequency end. This can be explained by the cationic radii at the octahedral and tetrahedral site (either Co or Fe) and the Co/Fe–O bond distance. Since Raman spectra are sensitive to the local structural change, the distribution of the local bond length can produce this double peak-like shape in the Raman spectra.38 In the case of other modes, this asymmetry is not visible, which may be due to the relatively low Raman intensity. The presence of four well-resolved Raman modes in the Raman spectrum of the sample prepared at 450 °C confirms the better crystalline quality compared to films prepared at RT. The Raman results are in good agreement with the XRD results presented in the previous section.
m, predicting 39 phonon modes for this spinel structure. Among them, the five Raman active modes are A1g, Eg, and 3T2g.35,36 The modes below (above) 600 cm−1 are attributed to the oxygen motion around the octahedral (tetrahedral) lattice sites.37 The broadening of the Raman modes indicating its amorphous nature is in agreement with the XRD results. The film deposited at 450 °C substrate temperature shows four prominent Raman active modes at 302 cm−1, 458 cm−1, 556 cm−1, and 678 cm−1, which correspond to Eg, 2T2g, and A1g phonon vibrations, respectively. The Raman active T2g mode around 200 cm−1 is absent in the spectra, probably due to the weak Raman cross-section of this particular mode. The A1g Raman modes of the film prepared at 450 °C show a slightly asymmetric shape with a shoulder at the low-frequency end. This can be explained by the cationic radii at the octahedral and tetrahedral site (either Co or Fe) and the Co/Fe–O bond distance. Since Raman spectra are sensitive to the local structural change, the distribution of the local bond length can produce this double peak-like shape in the Raman spectra.38 In the case of other modes, this asymmetry is not visible, which may be due to the relatively low Raman intensity. The presence of four well-resolved Raman modes in the Raman spectrum of the sample prepared at 450 °C confirms the better crystalline quality compared to films prepared at RT. The Raman results are in good agreement with the XRD results presented in the previous section.
3.3. Topographic analysis
For the topographic characterisation, atomic force microscopy (AFM) was performed at various regions on the CoFe2O4 thin films prepared at RT and 450 °C. Three-dimensional (3D) micrographs of representative AFM images of both samples are shown in Fig. 4. The AFM micrograph analysis reveals that the sample prepared at RT has higher roughness and porosity compared to films deposited at 450 °C. Thus, the sample prepared at RT has a higher surface-to-volume ratio, which provides an easy path for ion movement at the electrode–electrolyte interface, making it suitable for supercapacitor applications. The surfaces of samples prepared at RT and 450 °C have root mean square (RMS) roughness values of (20 ± 1) nm and (8.5 ± 0.5) nm, respectively. | ||
| Fig. 4 Pseudo-3D AFM images of CoFe2O4 thin films deposited at substrate temperatures of RT (a) and 450 °C (b). | ||
3.4. Electrochemical studies
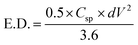 | (2) |
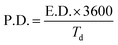 | (3) |
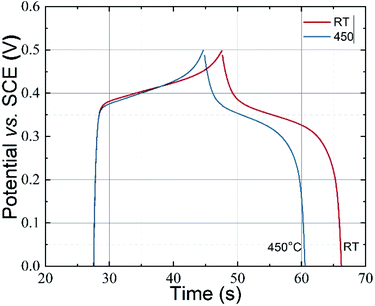 | ||
| Fig. 6 Galvanostatic charge–discharge curves for the CoFe2O4 electrodes prepared at RT and 450 °C in 1 M KOH electrolyte at 0.5 mA cm−2 current density. | ||
4. Conclusions
Cobalt ferrite thin films prepared by the pulsed laser deposition technique were used as electrodes for supercapacitor applications. The material formation was confirmed by XRD and Raman spectroscopy results. A specific capacitance of 777.4 F g−1 at 0.5 mA cm−2 current density was observed for the films prepared at RT, which is three times higher than that for the films prepared at 450 °C. This is attributed to the rough and porous surface of the films, which was confirmed by AFM. The films prepared at room temperature exhibited high power density (3277 W kg−1) and energy density (17 W h kg−1) values. The electrode material showed improved capacitance with increasing cycles due to the increase in the electroactive area. Keeping in mind the reproducibility and adherence of films prepared by PLD, the reported electrode materials could serve as a promising candidate for supercapacitor applications.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
We thank Prof. Shiva Prasad and Prof. N. Venkataramani, Indian Institute of Technology Bombay, India for the deposition of Co-ferrite thin films. The authors Apoorva Sharma and Georgeta Salvan would like to acknowledge the financial support by the Deutsche Forschungsgemeinschaft (DFG) under the project “Interfacial perpendicular magnetic anisotropy for next-generation monolithic 3D TMR sensors” (Project Number 282193534). The publication of this article was funded by Chemnitz University of Technology.References
- J. Kang, S. Zhang and Z. Zhang, Adv. Mater., 2017, 29, 1700515 CrossRef PubMed.
- H. Zhao, L. Liu, R. Vellacheri and Y. Lei, Adv. Sci., 2017, 4, 1700188 CrossRef PubMed.
- F. Wang, X. Wu, X. Yuan, Z. Liu, Y. Zhang, L. Fu, Y. Zhu, Q. Zhou, Y. Wu and W. Huang, Chem. Soc. Rev., 2017, 46, 6816–6854 RSC.
- M. Chen, Y. Zhang, L. Xing, Y. Liao, Y. Qiu, S. Yang and W. Li, Adv. Mater., 2017, 29, 69–76 Search PubMed.
- H. Gao, J. Xiang and Y. Cao, Appl. Surf. Sci., 2017, 413, 351–359 CrossRef CAS.
- A. E. Reddy, T. Anitha, C. V. V. Muralee Gopi, S. S. Rao, B. Naresh and H. J. Kim, Anal. Methods, 2018, 10, 223–229 RSC.
- J. S. Sagu, K. G. U. Wijayantha and A. A. Tahir, Electrochim. Acta, 2017, 246, 870–878 CrossRef CAS.
- S. Aloqayli, C. K. Ranaweera, Z. Wang, K. Siam, P. K. Kahol, P. Tripathi, O. N. Srivastava, B. K. Gupta, S. R. Mishra, F. Perez, X. Shen and R. K. Gupta, Energy Storage Materials, 2017, 8, 68–76 CrossRef.
- D. Wang, Y. Xiao, X. Luo, Z. Wu, Y.-J. Wang and B. Fang, ACS Sustainable Chem. Eng., 2017, 5, 2509–2515 CrossRef CAS.
- P. C. Metz, A. C. Ladonis, P. Gao, T. Hey and S. T. Misture, RSC Adv., 2020, 10, 1484–1497 RSC.
- C. Guan, J. Liu, Y. Wang, L. Mao, Z. Fan, Z. Shen, H. Zhang and J. Wang, ACS Nano, 2015, 9, 5198–5207 CrossRef CAS PubMed.
- J. Li, W. Zhang, X. Zhang, L. Huo, J. Liang, L. Wu, Y. Liu, J. Gao, H. Pang and H. Xue, J. Mater. Chem. A, 2020, 8, 2463–2471 RSC.
- J. Liu, L. Zhang, H. Bin Wu, J. Lin, Z. Shen and X. W. Lou, Energy Environ. Sci., 2014, 7, 3709–3719 RSC.
- S. Zheng, Q. Li, H. Xue, H. Pang and Q. Xu, Natl. Sci. Rev., 2020, 7, 305–314 CrossRef.
- Y. S. Wei, M. Zhang, M. Kitta, Z. Liu, S. Horike and Q. Xu, J. Am. Chem. Soc., 2019, 141, 7906–7916 CrossRef CAS PubMed.
- Y. Li, Y. Xu, Y. Liu and H. Pang, Small, 2019, 15, 1–8 Search PubMed.
- P. Richter, P. N. Plassmeyer, J. Harzdorf, T. Rüffer, H. Lang, J. Kalbacova, N. Jöhrmann, S. Schulze, M. Hietschold, S. S. P. K. Arekapudi, M. Albrecht, D. R. T. Zahn, C. J. Page and G. Salvan, Chem. Mater., 2016, 28, 4917–4927 CrossRef CAS.
- C. Himcinschi, I. Vrejoiu, G. Salvan, M. Fronk, A. Talkenberger, D. R. T. Zahn, D. Rafaja and J. Kortus, J. Appl. Phys., 2013, 113, 084101 CrossRef.
- A. E. Elkholy, F. El-Taib Heakal and N. K. Allam, RSC Adv., 2017, 7, 51888–51895 RSC.
- D. Ravinder and A. Ramana Reddy, Mater. Lett., 1999, 38, 265–269 CrossRef CAS.
- S.-L. Kuo, J.-F. Lee and N.-L. Wu, J. Electrochem. Soc., 2007, 154, A34 CrossRef CAS.
- P. Sen and A. De, Electrochim. Acta, 2010, 55, 4677–4684 CrossRef CAS.
- D. P. Lapham and A. C. C. Tseung, J. Mater. Sci., 2004, 39, 251–264 CrossRef CAS.
- B. Bhujun, M. T. T. Tan and A. S. Shanmugam, Results Phys., 2017, 7, 345–353 CrossRef.
- V. S. Kumbhar, A. D. Jagadale, N. M. Shinde and C. D. Lokhande, Appl. Surf. Sci., 2012, 259, 39–43 CrossRef CAS.
- L. Lv, Q. Xu, R. Ding, L. Qi and H. Wang, Mater. Lett., 2013, 111, 35–38 CrossRef CAS.
- J. S. Sagu, D. Mehta and K. G. U. Wijayantha, Electrochem. Commun., 2018, 87, 1–4 CrossRef CAS.
- H. Kennaz, A. Harat, O. Guellati, D. Y. Momodu, F. Barzegar, J. K. Dangbegnon, N. Manyala and M. Guerioune, J. Solid State Electrochem., 2018, 22, 835–847 CrossRef CAS.
- M. Sorescu, A. Grabias, D. Tarabasanu-Mihaila and L. Diamandescu, J. Mater. Synth. Process., 2001, 9, 119–123 CrossRef CAS.
- S. D. Sathaye, K. R. Patil, S. D. Kulkarni, P. P. Bakre, S. D. Pradhan, B. D. Sarwade and S. N. Shintre, J. Mater. Sci., 2003, 38, 29–33 CrossRef CAS.
- H. Fujioka, in Handbook of Crystal Growth, Elsevier, 2nd edn, 2015, vol. 3, pp. 365–397 Search PubMed.
- J. A. Greer and M. D. Tabat, J. Vac. Sci. Technol., A, 1995, 13, 1175–1181 CrossRef CAS.
- J. Hao, W. Yang, Z. Zhang, B. Lu, B. Zhang and J. Tang, Electrochim. Acta, 2015, 152, 13–18 CrossRef CAS.
- S. C. Sahoo, N. Venkataramani, S. Prasad, M. Bohra and R. Krishnan, J. Nanosci. Nanotechnol., 2010, 10, 3112–3117 CrossRef CAS PubMed.
- P. Chandramohan, M. P. Srinivasan, S. Velmurugan and S. V. Narasimhan, J. Solid State Chem., 2011, 184, 89–96 CrossRef CAS.
- S. C. Sahoo, N. Venkataramani, S. Prasad, M. Bohra and R. Krishnan, Appl. Phys. A: Mater. Sci. Process., 2012, 106, 931–935 CrossRef CAS.
- K. Gandha, K. Elkins, N. Poudyal and J. Ping Liu, J. Appl. Phys., 2015, 117, 17A736 CrossRef.
- N. D. Phu, N. Van Hung, L. H. Hoang, N. Van Minh, L. M. Oanh and D. B. Do, IEEE Trans. Magn., 2014, 50, 1–4 Search PubMed.
- J. N. Broughton and M. J. Brett, Electrochem. Solid-State Lett., 2002, 5, A279 CrossRef CAS.
- E. Laouini, Y. Berghoute, J. Douch, M. H. Mendonça, M. Hamdani and M. I. S. Pereira, J. Appl. Electrochem., 2009, 39, 2469–2479 CrossRef CAS.
- H. Gao, S. Cao and Y. Cao, Electrochim. Acta, 2017, 240, 31–42 CrossRef CAS.
- R.-N. Singh, M. Hamdani, J.-F. Koenig, G. Poillerat, J. L. Gautier and P. Chartier, J. Appl. Electrochem., 1990, 20, 442–446 CrossRef CAS.
- Y. Jiang and J. Liu, Energy Environ. Mater., 2019, 2, 30–37 CrossRef.
- F. Yu, T. Huang, P. Zhang, Y. Tao, F. Z. Cui, Q. Xie, S. Yao and F. Wang, Energy Storage Materials, 2019, 22, 235–255 CrossRef.
- L. Liu, H. Zhang, Y. Mu, Y. Bai and Y. Wang, J. Power Sources, 2016, 327, 599–609 CrossRef CAS.
- Y. Li, K. Huang, D. Zeng, S. Liu and Z. Yao, J. Solid State Electrochem., 2010, 14, 1205–1211 CrossRef CAS.
- H. Li, Y. Gao, C. Wang and G. Yang, Adv. Energy Mater., 2015, 5, 1401767 CrossRef.
- B. Bhujun, M. T. T. Tan and A. S. Shanmugam, Ceram. Int., 2016, 42, 6457–6466 CrossRef CAS.
- S. S. Raut and B. R. Sankapal, Electrochim. Acta, 2016, 198, 203–211 CrossRef CAS.
- V. Vignesh, K. Subramani, M. Sathish and R. Navamathavan, Colloids Surf., A, 2018, 538, 668–677 CrossRef CAS.
- W. Zhang, B. Quan, C. Lee, S. K. Park, X. Li, E. Choi, G. Diao and Y. Piao, ACS Appl. Mater. Interfaces, 2015, 7, 2404–2414 CrossRef CAS PubMed.
- P. Liu, H. Chen, X. Chang, Y. Xue, J. Zhou, Z. Zhao, H. Lin and S. Han, Electrochim. Acta, 2017, 231, 565–574 CrossRef CAS.
- J. Candler, T. Elmore, B. K. Gupta, L. Dong, S. Palchoudhury and R. K. Gupta, New J. Chem., 2015, 39, 6108–6116 RSC.
- M.-C. Liu, L.-B. Kong, X.-J. Ma, C. Lu, X.-M. Li, Y.-C. Luo and L. Kang, New J. Chem., 2012, 36, 1713 RSC.
- S. Vijayakumar, S.-H. Lee and K.-S. Ryu, Electrochim. Acta, 2015, 182, 979–986 CrossRef CAS.
- M. Toupin, T. Brousse and D. Bélanger, Chem. Mater., 2002, 14, 3946–3952 CrossRef CAS.
- S. G. Mohamed, S. Y. Attia and N. K. Allam, Mater. Today Energy, 2017, 4, 97–104 CrossRef.
- S. H. Aboutalebi, A. T. Chidembo, M. Salari, K. Konstantinov, D. Wexler, H. Kun Liu and S. Xue Dou, Energy Environ. Sci., 2011, 4, 1855–1865 RSC.
| This journal is © The Royal Society of Chemistry 2020 |