Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of heteroatom-containing pyrrolidine derivatives based on Ti(O-iPr)4 and EtMgBr-catalyzed carbocyclization of allylpropargyl amines with Et2Zn†
Rita N. Kadikova *,
Ilfir R. Ramazanov,
Azat M. Gabdullin,
Oleg S. Mozgovoj and
Usein M. Dzhemilev
*,
Ilfir R. Ramazanov,
Azat M. Gabdullin,
Oleg S. Mozgovoj and
Usein M. Dzhemilev
Institute of Petrochemistry and Catalysis of Russian Academy of Sciences, 141 Prospekt Oktyabrya, Ufa 450075, Russian Federation. E-mail: kadikritan@gmail.com
First published on 7th May 2020
Abstract
The Ti(O-iPr)4 and EtMgBr-catalyzed regio and stereoselective carbocyclization of N-allyl-substituted 2-alkynylamines with Et2Zn, followed by deuterolysis or hydrolysis, affords the corresponding methylenepyrrolidine derivatives in high yields. It was found that Ti–Mg-catalyzed carbocyclization of N-allyl-substituted 2-alkynylamines with Et2Zn is equally selective in dichloromethane, hexane, toluene, and diethyl ether. The reaction was tolerant to the presence of aryl, alkyl, trimethylsilyl, methoxymethyl and aminomethyl substituents on the alkyne. A selective method was proposed for the preparation of bis-pyrrolidine derivatives using Ti–Mg-catalyzed carbocyclization of bis-allylpropargyl amines with Et2Zn.
Introduction
Five- and six-membered nitrogen-containing heterocycles are frequently encountered structural elements of lots of natural and biologically active compounds,1–4 such as pyrrolizidine alkaloids5,6 and carbapenems.7,8 For example, nitrogen heterocycles with an aryl moiety in the β- or γ-position relative to nitrogen are of considerable interest as neuroactive compounds, as they are conformational constrained analogues of neurotransmitters such as serotonin and dopamine and opiate receptor ligands.9,10 Of particular interest are 3-benzylpyrrolidine derivatives exhibiting biological activities such as protein kinase C inhibitors,11,12 NK-3 receptor antagonists,13 and dopamine receptor antagonists.14 Thus, development of new efficient synthetic routes to five- and six-membered heterocycles is an important task. Transition metal-catalyzed cyclization of enynes is an efficient tool for the design of carbo and heterocyclic compounds.15–17 One of the earliest approach to intramolecular cyclization of enynes and substituted N-allylpropargylamines consists in the use of low-valent zirconium complexes generated by treating Cp2ZrCl2 with Mg and HgCl2, or 2 equiv. of alkyllithium, such as n-BuLi, or a Grignard reagent, such as EtMgBr.18,19 Low-valent iron complexes generated using Et2Zn were also active towards carbocyclization of activated enynes.20 A known method for the preparation of acyl-substituted pyrrolidines is Me2Zn/Ni(0)-promoted cyclization of nitrogen-containing alkynyl enones.21 The cyclization of N-allyl-N-benzyl-3-(trimethylsilyl)-2-propynylamine on treatment with triallylmanganate is not stereoselective and gives a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Z/E isomers in 55% overall yield.22 Meanwhile, the reaction of N-benzyl-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine with four equivalents of methallylmagnesium chloride in the presence of catalytic amounts of CrCl3 is stereoselective, giving the E-isomer in 73% yield.23 The synthesis of acyl-substituted pyrrolidine derivatives with an exocyclic double bond by treatment of allyl-substituted propargylamines with methylmanganese carbonyl complex was reported.24 An unusual Pd(II)-catalyzed carbocyclization of nitrogen-containing 1,6-enynes accompanied by Pd-initiated migration of β-hydrogen to give 1,3- and 1,4-dienes was described.25 Palladium-catalyzed intramolecular cyclization of nitrogen-containing enynyl acetate in the presence of ZnCl2 followed by cross-coupling with alkenylstannane results in the selective formation of pyrrolidine derivative.26 A pyrrolidine derivative with a conjugated double bond is formed in a similar way upon Pd-catalyzed reaction of nitrogen-containing acetoxyenyne with tris(isopropenyl)indium.27 An example involving low-valent titanium complex is the reaction of the Sato reagent, (η2-propene)Ti(O-i-Pr)2 (obtained from Ti(O-i-Pr)4 and i-PrMgCl in 1
1 mixture of Z/E isomers in 55% overall yield.22 Meanwhile, the reaction of N-benzyl-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine with four equivalents of methallylmagnesium chloride in the presence of catalytic amounts of CrCl3 is stereoselective, giving the E-isomer in 73% yield.23 The synthesis of acyl-substituted pyrrolidine derivatives with an exocyclic double bond by treatment of allyl-substituted propargylamines with methylmanganese carbonyl complex was reported.24 An unusual Pd(II)-catalyzed carbocyclization of nitrogen-containing 1,6-enynes accompanied by Pd-initiated migration of β-hydrogen to give 1,3- and 1,4-dienes was described.25 Palladium-catalyzed intramolecular cyclization of nitrogen-containing enynyl acetate in the presence of ZnCl2 followed by cross-coupling with alkenylstannane results in the selective formation of pyrrolidine derivative.26 A pyrrolidine derivative with a conjugated double bond is formed in a similar way upon Pd-catalyzed reaction of nitrogen-containing acetoxyenyne with tris(isopropenyl)indium.27 An example involving low-valent titanium complex is the reaction of the Sato reagent, (η2-propene)Ti(O-i-Pr)2 (obtained from Ti(O-i-Pr)4 and i-PrMgCl in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio), with N-(4-methylbenzyl)-N-(prop-2-yn-1-yl)prop-2-en-1-amine with a terminal double bond giving the cyclization product in 53% yield.28 Now we demonstrate that the reaction of N,N-dialkyl-substituted 2-alkynylamines with Et2Zn catalyzed by the Ti(i-OPr)4–EtMgBr system results in the selective formation of 2-zincoethylzincation products.28,29 Relying on analysis of the above literature, we assumed that Ti–Mg-catalyzed carbozincation of functionalized allyl-substituted propargylamines could serve for the development of selective one-pot methods for the synthesis of various methylenepyrrolidine derivatives. As a continuation of research on the Ti–Mg-catalyzed carbozincation of functionally substituted alkyne substrates, we studied here carbocyclization of a variety of allyl-substituted propargylamines.
2 ratio), with N-(4-methylbenzyl)-N-(prop-2-yn-1-yl)prop-2-en-1-amine with a terminal double bond giving the cyclization product in 53% yield.28 Now we demonstrate that the reaction of N,N-dialkyl-substituted 2-alkynylamines with Et2Zn catalyzed by the Ti(i-OPr)4–EtMgBr system results in the selective formation of 2-zincoethylzincation products.28,29 Relying on analysis of the above literature, we assumed that Ti–Mg-catalyzed carbozincation of functionalized allyl-substituted propargylamines could serve for the development of selective one-pot methods for the synthesis of various methylenepyrrolidine derivatives. As a continuation of research on the Ti–Mg-catalyzed carbozincation of functionally substituted alkyne substrates, we studied here carbocyclization of a variety of allyl-substituted propargylamines.
Previously, we have showed that Zr-catalyzed carbocyclization of dialkyl-substituted propargylamines results in the selective formation of products of 2-aluminum ethylalumination in high yields.30 We were interested in studying the behavior of allyl-substituted propargylamines towards Zr-catalyzed cycloalumination. However, after 24 hours of the reaction of N-(4-methoxybenzyl)-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine, prepared from ethynyltrimethylsilane and N-(4-methoxybenzyl)prop-2-en-1-amine,31 with 3 equivalents of Et3Al in the presence of 20 mol% Cp2ZrCl2 in hexane at 40 °C, the yield of the carbocyclization product did not exceed 5–10% (Scheme 1).
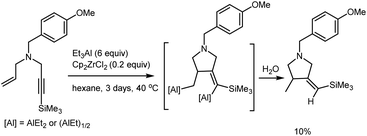 | ||
| Scheme 1 Ti–Mg-catalyzed reaction of N-(4-methoxybenzyl)-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine with Et2Zn. | ||
The increase in the amount of Et3Al taken in the reaction to 6 equivalents and increase in the reaction time to 3 days did not result in higher yields of the target product. The observed low conversion of the nitrogen-containing substrate may be attributable to steric and electronic factors involved in the coordination of low-valent Cp2Zr to a enyne molecule.30 It should be borne in mind that coordination of the Et3Al aluminum atom to the nitrogen lone pair may not only decrease the triple bond nucleophilicity, but also generate additional steric hindrance for coupling of the olefinic and acetylenic moieties of the enyne substrate with the zirconium atom bearing bulky cyclopentadienyl ligand. The steric hindrance arising at the stage of coupling of the olefinic and acetylenic enyne moieties with the low-valent zirconocene may also be enhanced due to the presence of bulky benzyl substituent at the nitrogen atom.
Results and discussion
We found that the reaction of N-allyl-substituted propargylamines 1 with 2.5 equivalents of Et2Zn (1 M in hexane) in the presence of 15 mol% of Ti(O-iPr)4 (0.5 M in hexane) and 20 mol% of EtMgBr (2.5 M in Et2O) conducted in dichloromethane at room temperature for 18 hours gives, after deuterolysis or hydrolysis, methylenepyrrolidine derivatives 3 and 4 with Z-configuration of the double bond (Scheme 2). The structure of the resulting methylenepyrrolidine derivatives was established by 1D and 2D NMR spectroscopy of products of their hydrolysis 3a–g,i and deuterolysis 4h,f (Scheme 2). The Z-configuration of the double bond of 3-methyl-4-methylenepyrrolidines 3a–3g, 3i, 4h,f was established by NOESY experiments. For example, the NOESY spectra of 3a and 3b clearly show the cross-peaks between the hydrogen atom at the double bond (δ = 5.31 and δ = 6.32, respectively) and the hydrogen atoms of the methyl substituent (δ = 1.09 and δ = 1.30, respectively) of the pyrrolidine ring, that indicates the formation of Z-diastereomers in the reaction mixtures. The selective formation of methylenepyrrolidine derivatives 3 and 4 with the Z-configuration of the double bond is supported by the mechanism of the Ti–Mg-catalyzed allylpropargyl amines carbometallation reaction using Et2Zn (the mechanism is described below in Scheme 7).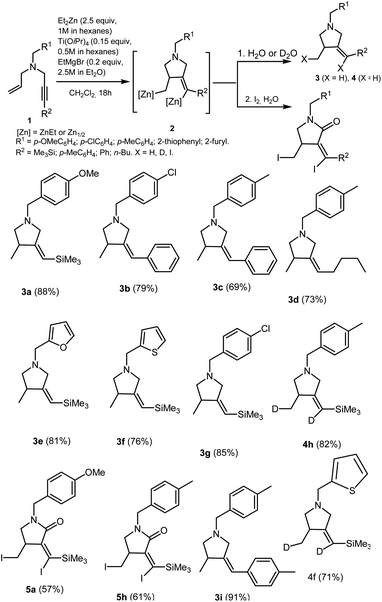 | ||
| Scheme 2 Ti–Mg-catalyzed carbocyclization of allyl substituted propargylamines with Et2Zn in CH2Cl2. | ||
Our study demonstrated that the presence of furan (3e), thiophene (3f), para-methoxybenzyl (3a), and para-chlorobenzyl (3b) substituents at nitrogen atom does not prevent regio and stereoselective carbocyclization of enynes on treatment with Et2Zn in the presence of catalytic amounts of Ti(O-iPr)4 and EtMgBr.
The carbocyclization of enynes with an aryl (3b, 3c, 3i), trimethylsilyl (3a, 3e, 3f, 3g, 4h, 5a), or butyl (3d) substituent at the triple bond is equally regio and stereoselective. The presence of two deuterium atoms (4h) or two iodine atoms (5a,h) in the methylenepyrrolidine molecules obtained after deuterolysis and iodinolysis attests to the oganometallic nature of intermediate 2. Similarly, Ti–Mg-catalyzed carbocyclization of non-activated and oxygen-containing enynes with Et2Zn proceeds.32 To our surprise, the reaction of the organozinc intermediate 2 with I2 leads to the selective formation of diiodo-substituted pyrrolidin-2-ones – cyclic amides 5a,h. It should be noted that tertiary cyclic amides are widespread structural units of many pharmaceuticals, such as lenalidomide, piperine, evodiamine, diazepam, etc.33–37 It is known that the alpha-carbon atom of tertiary amines can by easily oxidized to a carbonyl group under the action of such oxidizing agents as iodosobenzene, PhCO3tBu, tBuOOH and RuO2/NaIO4,38–42 as well as under the action of O2 in the presence of Ru-, Au-, Fe- and Cu-containing catalysts.43–47 However, the mechanism of the formation of the substituted pyrrolidin-2-ones 5a,h in our case will be the subject of our futher study. This issue deserves special consideration, as the obtained transformation is selective method for the preparation of cyclic amides of different structures. Thus, Ti–Mg-catalyzed carbocyclization of nitrogen-containing enynes on treatment with Et2Zn has benefits such as tolerance to bulky groups in the unsaturated substrate molecule and the possibility of carbometallation of enynes with a variety of heterofunctional substituents.
It is known that the reaction of Sato reagent (η2-propene)Ti(Oi-Pr)2 (obtained from Ti(Oi-Pr)4 and i-PrMgCl in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with N-(4-methylbenzyl)-N-(prop-2-yn-1-yl)prop-2-en-1-amine that has a terminal triple bond, gives a cyclization product with 53% yield.25 However, our attempts to cyclize the 1,6-enynes described in the article using one equivalent of Ti(Oi-Pr)4 and two equivalents of EtMgBr in the absence of Et2Zn led to the non-selective formation of a mixture of unidentified reaction products. The reaction of 1,6-enynes with 2 equivalents of EtMgBr in the presence of catalytic amounts of Ti(Oi-Pr)4 does not occur. Using a stoichiometric amount of Et2Zn and catalytic amounts of Ti(Oi-Pr)4 and EtMgBr the reaction gave only one reaction product in high yield. The question of the reasons for this selectivity is complex and we do not have enough data to provide a convincing theory. However coordination effects play a significant role in the reaction under study. So, the conversion of 2-alkynylamines and 1-alkynylphosphines28 is excellent, but the reaction with decyne-5 proceeds poorly. Further, the reaction goes well in Et2O, CH2Cl2, hexane, toluene, benzene and anisole but does not proceed in 1,4-dioxane, tetrahydrofuran, 1,2-dichloroethane, 1,2-dimethoxyethane, chloroform, and triethylamine.29
2) with N-(4-methylbenzyl)-N-(prop-2-yn-1-yl)prop-2-en-1-amine that has a terminal triple bond, gives a cyclization product with 53% yield.25 However, our attempts to cyclize the 1,6-enynes described in the article using one equivalent of Ti(Oi-Pr)4 and two equivalents of EtMgBr in the absence of Et2Zn led to the non-selective formation of a mixture of unidentified reaction products. The reaction of 1,6-enynes with 2 equivalents of EtMgBr in the presence of catalytic amounts of Ti(Oi-Pr)4 does not occur. Using a stoichiometric amount of Et2Zn and catalytic amounts of Ti(Oi-Pr)4 and EtMgBr the reaction gave only one reaction product in high yield. The question of the reasons for this selectivity is complex and we do not have enough data to provide a convincing theory. However coordination effects play a significant role in the reaction under study. So, the conversion of 2-alkynylamines and 1-alkynylphosphines28 is excellent, but the reaction with decyne-5 proceeds poorly. Further, the reaction goes well in Et2O, CH2Cl2, hexane, toluene, benzene and anisole but does not proceed in 1,4-dioxane, tetrahydrofuran, 1,2-dichloroethane, 1,2-dimethoxyethane, chloroform, and triethylamine.29
We studied carbocyclization of nitrogen-containing enynes in various solvents. At the same time, it must be taken into account that hexane (Et2Zn (1 M in hexane) and Ti(O-iPr)4 (0.5 M in hexane)), and Et2O (EtMgBr (2.5 M in Et2O)) are always present in the reaction mixture. Quite recently, we reported that Ti–Mg-catalyzed 2-zincoethylzincation of substituted 2-alkynylamines with Et2Zn proceeds equally selectively in diethyl ether, anisole, dichloromethane, hexane, benzene, and toluene.29 In this study, we found that the formation of carbocyclization product 4f upon the reaction of N-(thiophen-2-ylmethyl)-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine 1f with 2.5 equivalents of Et2Zn in the presence of 15 mol% of Ti(O-iPr)4, (0.5 M in hexane) and 20 mol% of EtMgBr (2.5 M in Et2O) is regio and stereoselective not only in dichloromethane (as shown in Scheme 2), but also in diethyl ether, hexane, and toluene (Scheme 3).
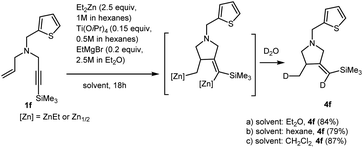 | ||
| Scheme 3 Ti–Mg-catalyzed carbocyclization reactions of N-(4-methylbenzyl)-N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine with Et2Zn in various solvents. | ||
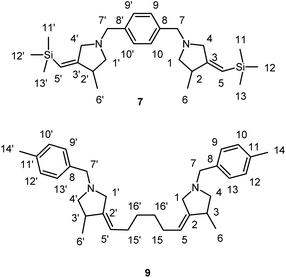
We obtained bis-methylenepyrrolidine derivative 7 by the reaction of N,N′-(1,4-phenylenebis(methylene))bis(N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine) 6 with 5 equivalents of Et2Zn in the presence of 30 mol% of Ti(O-iPr)4, (0.5 M in hexane) and 40 mol% of EtMgBr (2.5 M in Et2O) in dichloromethane (Scheme 4).
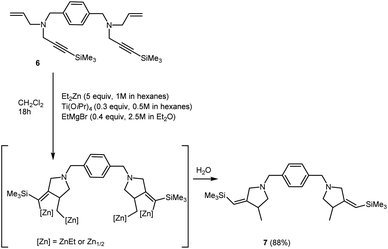 | ||
| Scheme 4 Ti–Mg-catalyzed carbocyclization of N,N′-(1,4-phenylenebis(methylene))bis(N-(3-(trimethylsilyl)prop-2-yn-1-yl)prop-2-en-1-amine) with Et2Zn in CH2Cl2. | ||
We also succeeded to perform the carbocyclization of N1,N10-diallyl-N1,N10-bis(4-methylbenzyl)deca-2,8-diyne-1,10-diamine 8, prepared from 1,7-octadiyne, to give bis-methylenepyrrolidine derivative 9 (1,6-bis(4-methyl-1-(4-methylbenzyl)pyrrolidin-3-ylidene)hexane) (Scheme 5).
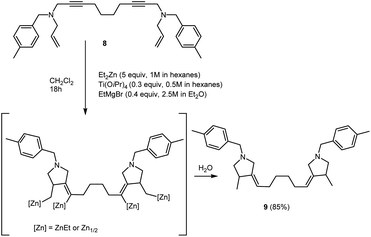 | ||
| Scheme 5 Ti–Mg-catalyzed carbocyclization of N1,N10-diallyl-N1,N10-bis(4-methylbenzyl)deca-2,8-diyne-1,10-diamine with Et2Zn in CH2Cl2. | ||
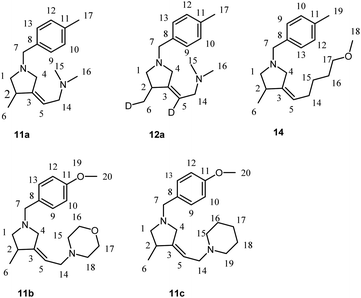
This study indicates that Ti–Mg-catalyzed carbocyclization of nitrogen-containing enynes with Et2Zn is equally efficient both for enynes with alkyl substituent at the triple bond (e.g., 3d and 9) and enynes with aryl and trimethylsilyl substituents at the triple bond (e.g., 3a–c, 3e–g, 3i, 4h,f, 5a,h). For example, it is known that cyclization of unfunctionalized enynes in the course of the Cp2ZrCl2-catalyzed cycloalumination occurs selectively if directing groups such as phenyl or trimethylsilyl group are present at the triple bond.48 Perhaps, the agostic interaction between the ortho-hydrogen atom of the phenyl group,49 or the trimethylsilyl group, and the zirconium atom may be a favorable factor promoting the cyclization of enynes in the course of cycloalumination. From this standpoint, it was of interest to study the Ti–Mg-catalyzed reaction of Et2Zn with nitrogen-containing 1,6-enynes containing additional heterofunctional substituents at the triple bond. Carbocyclization of enynes with a bifunctionally substituted triple bond may serve, in the future, for the development of one-pot syntheses of polyfunctionalized pyrrolidine derivatives. Additionally, study of the behavior of these enyne substrates towards carbocyclization would shed light on the electronic and steric effects of substituents at the triple bond in the reaction. We found that the reaction of N-allyl-substituted but-2-yn-1,4-diamines 10 with 2.5 equivalents of Et2Zn in the presence of 15 mol% of Ti(O-iPr)4, (0.5 M in hexane) and 20 mol% of EtMgBr (2.5 M in Et2O) in dichloromethane results in the regio and stereoselective formation of carbocyclization products 11 and 12 in high yields (Scheme 6). Thus, the presence of the second aminomethyl group at the triple bond in the nitrogen-containing enyne molecules 10 does not prevent the intramolecular cyclization.
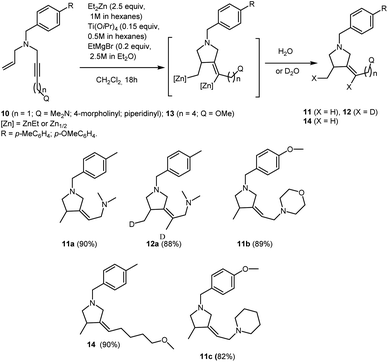 | ||
| Scheme 6 Ti–Mg-catalyzed carbocyclization of allyl substituted but-2-yne-1,4-diamines and N-allyl-substituted of oxygen-containing but-2-yn-1-amine with Et2Zn in CH2Cl2. | ||
The reaction of acetylenic ether 13 with 2.5 equivalents of Et2Zn in the presence of 15 mol% of Ti(O-iPr)4, (0.5 M in hexane) and 20 mol% of EtMgBr (2.5 M in Et2O) in dichloromethane results in regio and stereoselective formation of methoxy-substituted pyrrolidine derivative 14 (Scheme 6). It is worth noting that, unlike N,N-dialkyl substituted 2-alkynylamines, substituted acetylenic alcohols and their ethers are unreactive towards Ti–Mg-catalyzed 2-zincoethylzincation.28,29
According to the reaction scheme that we proposed (Scheme 7), ligand exchange between Ti(O-iPr)4 and EtMgBr gives (O-iPr)2TiEt2, which is converted to titanium(II)–ethylene complex (titanacyclopropane intermediate). The displacement of ethylene from the titanium coordination sphere by a enyne molecule affords intermediate complex A. The subsequent coupling of the acetylene and ethylene moieties of the enyne molecule gives titanacyclopentene intermediate B, which undergoes transmetallation with Et2Zn to be converted to organozinc intermediate C. The deuterolysis (or hydrolysis) of the latter furnishes the pyrrolidine derivative.
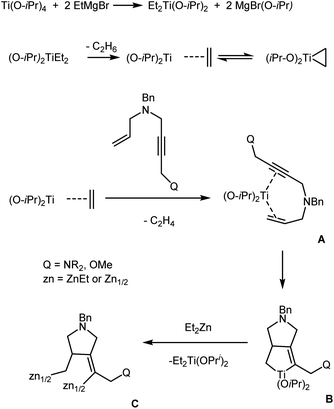 | ||
| Scheme 7 Putative mechanism of Ti–Mg-catalyzed reaction of allyl substituted but-2-yn-1,4-diamines with Et2Zn. | ||
Conclusions
We report a regio and stereoselective Ti(O-iPr)4 and EtMgBr-catalyzed carbocyclization of allyl propargyl amines with Et2Zn. It was demonstrated that the presence of amine and ether groups at the enyne triple bond does not prevent carbocyclization of allyl-substituted 2-alkynylamines. The study resulted in the development of an efficient method for the synthesis of a variety of heteroatom-containing pyrrolidine derivatives via organozinc synthesis. The observed tolerance of the Ti–Mg-catalyzed carbocyclization of enynes to the presence of various heterofunctional substituents opens prospects for further use of organozinc synthesis to develop one-pot syntheses of polyfunctional pyrrolidine derivatives. In connection with the obtained carbocyclization reaction we plan to develop an effective method for preparing methylenepiperidine derivatives based on Ti–Mg-catalyzed carbocyclization of N-homoallyl substituted 2-alkynylamines.Experimental section
General information
The reagents were obtained from Sigma-Aldrich or Acros. Hexane and dichloromethane were distilled over P2O5. Diethyl ether, tetrahydrofuran, 1,4-dioxane, toluene, benzene and anisole were dried over sodium. Dried 1,2-dimethoxyethane was obtained from Sigma-Aldrich. 2-Alkynylamines 1a–i and 6, 8 were prepared by aminomethylation of terminal alkynes with aqueous formaldehyde and secondary N-aryl-substituted allyl amines under CuBr catalysis.50 Nitrogen-containing 1,6-enynes with terminal propargyl and allyl groups were prepared by alkylation of N-aryl-substituted allyl amines with propargyl bromide under NaH.51 Allyl substituted but-2-yne-1,4-diamines 10 were prepared by aminomethylation of nitrogen-containing 1,6-enynes (with terminal propargyl and allyl groups) by bisamine.52 Acetylenic ethers 13 were prepared by aminomethylation of ethers of acetylenic alcohols with aqueous formaldehyde and secondary N-aryl-substituted allyl amines under CuBr catalysis.50 Nuclear magnetic resonance spectroscopy was performed on a Bruker Avance 500. The 1H NMR spectra were recorded at 500 MHz and 13C–{1H} NMR spectra at 100 MHz in CDCl3. The chemical shifts are reported in ppm relative to tetramethylsilane (TMS) as the internal standard. The numbering of atoms in the 13C–{1H} and 1H NMR spectra of the compounds 3a–g, 3i, 4h,f, 5a, 5h, 7, 9, 11a–c, 12a, 14 is shown in Fig. 1–3. Elemental analysis was performed using a Carlo-Erba CHN 1106 elemental analyser. Mass spectra were obtained on a Finnigan 4021 instrument. The yields were calculated from the isolated amount of pyrrolidine and pyrrolidone derivatives obtained from starting nitrogen-containing 1,6-enynes.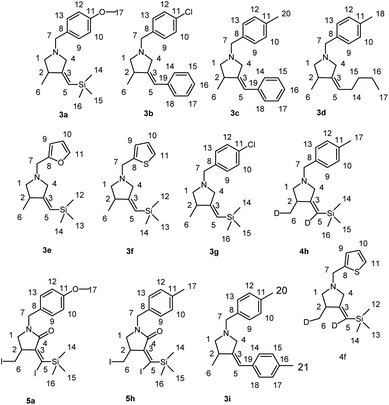 | ||
| Fig. 1 The numbering of atoms in the 13C- and 1H-NMR spectra of the compounds 3a–g, 3i, 4h,f and 5a,h. | ||
Preparation of 3-methyl-4-methylenepyrrolidines 3a–g, 3i, 4h,f and 5a,h via Ti–Mg-catalyzed carbocyclization of N-allyl substituted propargylamines with Et2Zn in CH2Cl2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) gave 3a (509 mg, 88%) as colorless oil. Rf 0.70.
8) gave 3a (509 mg, 88%) as colorless oil. Rf 0.70.1H NMR (500 MHz, CDCl3): δ = 0.09 (s, 9H, C(14, 15, 16)H3), 1.09 (d, J = 7 Hz, 3H, C(6)H3), 1.99 (t, J = 8 Hz, 1H(A), C(1)H2), 2.68 (q, J = 7 Hz, 1H, C(2)H), 2.98 (t, J = 8 Hz, 1H(B), C(1)H2), 3.03 (dt, J = 14 Hz, J = 2 Hz, 2H, C(4)H2), 3.56 (d, J = 12 Hz, 1H(A), C(7)H2), 3.63 (d, J = 12 Hz, 1H(B), C(7)H2), 3.82 (s, 3H, C(17)H3), 5.31 (q, J = 2 Hz, 1H, C(5)H), 6.89 (d, J = 8 Hz, 2H, C(10, 12)H), 7.28 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.40 (C(14, 15, 16)), 17.34 (C(6)), 40.28 (C(2)), 55.22 (C(17)), 59.32 (C(4)), 60.12 (C(7)), 61.15 (C(1)), 113.63 (C(10, 12)), 116.74 (C(5)), 130.04 (C(9, 13)), 131.66 (C(8)), 158.68 (C(11)), 162.68 (C(3)).
MS (EI): m/z, % = 289 (1) [M+], 287 (11), 214 (11), 166 (8), 121 (100).
Anal. calcd for C17H27NOSi, (%): C, 70.53; H, 9.40; N, 4.84. Found, %: C, 70.76; H, 9.57; N, 5.07.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3b (471 mg, 79%) as colorless oil. Rf 0.59.
8) to afford 3b (471 mg, 79%) as colorless oil. Rf 0.59.1H NMR (500 MHz, CDCl3): δ = 1.30 (t, J = 6 Hz, 3H, C(6)H3), 2.19 (m, 1H(A), C(1)H2), 2.97 (s, 1H, C(2)H), 3.05 (m, 1H(B), C(1)H2), 3.40 (d, J = 14 Hz, 1H(A), C(4)H2), 3.70 (s, 2H, C(7)H2), 3.82 (d, J = 14 Hz, 1H(B), C(4)H2), 6.32 (s, 1H, C(5)H), 7.25 (m, 1H, C(16)H), 7.26 (m, 2H, C(14, 18)H), 7.36 (m, 2H, C(9, 13)H), 7.38 (m, 2H, C(10, 12)H), 7.40 (m, 2H, C(15, 17)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 18.10 (C(6)), 39.20 (C(2)), 58.43 (C(4)), 59.93 (C(7)), 61.23 (C(1)), 120.69 (C(5)), 126.27 (C(16)), 127.97 (C(14, 18)), 128.45 (C(15, 17)), 128.54 (C(10, 12)), 130.12 (C(9, 13)), 132.79 (C(11)), 137.40 (C(8)), 138.05 (C(19)), 146.98 (C(3)).
Anal. calcd for C19H20ClN, (%): C, 76.62; H, 6.77; N, 4.70. Found, %: C, 76.45; H, 6.91; N, 4.75.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3c (382 mg, 69%) as colorless oil. Rf 0.61.
8) to afford 3c (382 mg, 69%) as colorless oil. Rf 0.61.1H NMR (500 MHz, CDCl3): δ = 1.33 (d, J = 7 Hz, 3H, C(6)H3), 2.23 (t, J = 8 Hz, 1H(A), C(1)H2), 2.45 (s, 3H, C(20)H3), 3.02 (q, J = 7 Hz, 1HC(2)H), 3.11 (t, J = 8 Hz, 1H(B), C(1)H2), 3.46 (d, J = 15 Hz, 1H(A), C(4)H2), 3.77 (s, 2H, C(7)H), 3.92 (d, J = 15 Hz, 1H(B), C(4)H2), 6.34 (s, 1H, C(5)H), 7.25 (d, J = 8 Hz, 2H, C(10, 12)H), 7.27 (m, 1H, C(16)H), 7.30 (d, J = 8 Hz, 2H, C(14, 18)H), 7.37 (d, J = 8 Hz, 2H, C(9, 13)H), 7.42 (d, J = 8 Hz, 2H, C(15, 17)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 18.13 (C(6)), 21.23 (C(20)), 39.31 (C(2)), 58.59 (C(4)), 60.46 (C(7)), 61.32 (C(1)), 120.45 (C(5)), 126.14 (C(16)), 128.00 (C(14, 18)), 128.41 (C(15, 17)), 128.75 (C(9, 13)), 129.09 (C(10, 11)), 136.00 (C(8)), 136.58 (C(11)), 138.23 (C(19)), 147.59 (C(3)).
MS (m/z, %): 277 (41) [M]+, 262 (19), 172 (10), 129 (13), 105 (100).
Anal. calcd for C20H23N, (%): C, 86.59; H, 8.36; N, 5.05. Found, %: C, 86.62; H, 8.43; N, 4.85.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3d (494 mg, 73%). Rf 0.68.
8) to afford 3d (494 mg, 73%). Rf 0.68.1H NMR (500 MHz, CDCl3): δ = 0.91 (m, 3H, C(17)H3), 1.08 (d, J = 7 Hz, 3H, C(6)H3), 1.31 (m, 2H, C(16)H2), 1.33 (m, 2H, C(14)H2), 1.92 (q, J = 7 Hz, 2H, C(15)H2), 2.05 (m, 1H(A), C(1)H2), 2.37 (s, 3H, C(18)H3), 2.69 (q, J = 7 Hz, 1H, C(2)H), 2.98 (d, J = 14 Hz, 1H(A), C(4)H2), 3.01 (t, J = 8 Hz, 1H(B), C(1)H2), 3.50 (d, J = 14H, 1H(B), C(4)H2), 3.62 (d, J = 13 Hz, 1H(A), C(7)H2), 3.66 (d, J = 13 Hz, 1H(B), C(7)H2), 5.15 (m, 1H, C(5)H), 7.26 (d, J = 8 Hz, 2H, C(9, 13)H), 7.16 (d, J = 8 Hz, 2H, C(10, 12)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 14.03 (C(17)), 17.59 (C(6)), 21.12 (C(18)), 22.35 (C(16)), 29.14 (C(15)), 31.75 (C(14)), 37.17 (C(2)), 56.59 (C(4)), 60.43 (C(7)), 62.07 (C(1)), 120.05 (C(5)), 136.61 (C(8)), 128.87 (C(9, 13)), 128.96 (C(10, 12)), 143.76 (C(3)).
MS (m/z, %): 257 (14) [M]+, 200 (25), 152 (10), 105 (100).
Anal. calcd for C18H27N, (%): C, 83.99; H, 10.57; N, 5.44. Found, %: C, 84.28; H, 10.73; N, 5.30.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3e (403 mg, 81%). Rf 0.74.
8) to afford 3e (403 mg, 81%). Rf 0.74.1H NMR (500 MHz, CDCl3): δ = 0.09 (s, 9H, C(12, 13, 14)H3), 1.09 (d, J = 7 Hz, 3H, C(6)H3), 2.05 (t, J = 9 Hz, 1H(A), C(1)H2), 2.70 (q, J = 7 Hz, C(2)H), 3.04 (m, 1H(B), C(1)H2), 3.06 (m, 1H(A), C(4)H2), 3.59 (dd, J = 14 Hz, J = 2 Hz, 1H(B), C(4)H2), 3.65 (d, J = 14 Hz, 1H(A), C(7)H2), 3.68 (d, J = 14 Hz, 1H(B), C(7)H2), 5.30 (m, 1H, C(5)H), 6.22 (d, J = 3 Hz, 1H, C(9)H), 6.34 (m, 1H, C(10)H), 7.39 (dd, J = 2 Hz, J = 1 Hz, 1H, C(11)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.45 (C(12, 13, 14)), 17.17 (C(6)), 40.25 (C(2)), 52.19 (C(7)), 58.96 (C(4)), 107.86 (C(9)), 110.06 (C(10)), 116.84 (C(5)), 141.97 (C(11)), 152.45 (C(8)), 162.29 (C(3)).
MS (m/z, %): 249 (16) [M]+, 176 (76), 152 (9), 81 (100).
Anal. calcd for C14H23NOSi, (%): C, 67.42; H, 9.29; N, 5.62. Found, %: C, 67.07; H, 9.14; N, 5.39.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3f (403 mg, 76%). Rf 0.80.
8) to afford 3f (403 mg, 76%). Rf 0.80.1H NMR (500 MHz, CDCl3): δ = 0.09 (s, 9H, C(12, 13, 14)H3), 1.10 (d, J = 7 Hz, 3H, C(6)H3), 2.07 (t, J = 8 Hz, 1H(A), C(1)H2), 2.70 (m, 1H, C(2)H), 3.04 (t, J = 8 Hz, 1H(B), C(1)H2), 3.09 (dt, J = 14 Hz, J = 2 Hz, 1H(A), C(4)H2), 3.61 (dd, J = 14 Hz, J = 2 Hz, 1H(B), C(4)H2), 3.84 (d, J = 14 Hz, 1H(A), C(7)H2), 3.88 (d, J = 14 Hz, 1H(B), C(7)H2), 5.32 (m, 1H, C(5)H), 6.96 (m, 1H, C(11)H), 6.98 (t, J = 3 Hz, 1H, C(10)H), 7.25 (dd, J = 5 Hz, J = 1 Hz, 1H, C(9)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.42 (C(12, 13, 14)), 17.34 (C(6)), 40.35 (C(2)), 54.69 (C(7)), 59.06 (C(4)), 61.04 (C(1)), 116.89 (C(5)), 124.79 (C(9)), 125.50 (C(11)), 126.41 (C(10)), 142.10 (C(8)), 162.44 (C(3)).
MS (m/z, %): 265 (4) [M]+, 192 (31), 97 (100), 73 (20).
Anal. calcd for C14H23NSSi, (%): C, 63.34; H, 8.73; N, 5.28. Found, %: C, 63.39; H, 8.64; N, 5.11.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3g (500 mg, 85%). Rf 0.68.
8) to afford 3g (500 mg, 85%). Rf 0.68.1H NMR (500 MHz, CDCl3): δ = 0.08 (s, 9H, C(14, 15, 16)H3), 1.09 (d, J = 7 Hz, 3H, C(6)H3), 2.01 (t, J = 8 Hz, 1H(A), C(1)H2), 2.67 (p, J = 7 Hz, 1H, C(2)H), 2.95 (t, J = 8 Hz, 1H(B), C(1)H2), 3.03 (dt, J = 14 Hz, J = 2 Hz, 1H(A), C(4)H2), 3.52 (dd, J = 14 Hz, J = 2 Hz, 1H(B), C(4)H2), 3.58 (d, J = 13 Hz, 1H(A), C(7)H2), 3.63 (d, J = 13 Hz, 1H(B), C(7)H2), 5.32 (m, 1H, C(5)H), 7.30 (d, J = 3 Hz, 4H, C(9, 10, 12, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.43 (C(14, 15, 16)), 17.45 (C(6)), 40.36 (C(2)), 59.32 (C(4)), 60.01 (C(7)), 61.26 (C(1)), 116.96 (C(5)), 128.39 (C(10, 12)), 130.06 (C(9, 13)), 132.63 (C(11)), 137.46 (C(8)), 162.45 (C(3)).
MS (m/z, %): 294 (4) [M]+, 293 (9), 220 (73), 168 (13), 125 (100), 89 (13), 73 (29).
Anal. calcd for C16H24ClNSi, (%): C, 65.39; H, 8.23; N, 4.77. Found, %: C, 65.43; H, 8.27; N, 5.01.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 4h (226 mg, 82%). Rf 0.63.
8) to afford 4h (226 mg, 82%). Rf 0.63.1H NMR (500 MHz, CDCl3): δ = 0.10 (s, 1H, C(14, 15, 16)H3), 1.09 (t, J = 8 Hz, 2H, C(6)DH2), 2.01 (t, J = 8 Hz, 1H(A), C(1)H2), 2.38 (s, 3H, C(17)H3), 2.68 (p, J = 7 Hz, 1H, C(2)H), 2.99 (t, J = 8 Hz, 1H(B), C(1)H2), 3.06 (d, J = 14 Hz, 2H, C(4)H2), 3.58 (d, J = 13 Hz, 1H(A), C(7)H2), 3.67 (d, J = 13 Hz, 1H(B), C(7)H2), 7.17 (d, J = 8 Hz, 2H, C(10, 12)H), 7.26 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.41 (C(14, 15, 16)), 17.07 (t, J = 20 Hz, C(6)), 21.14 (C(17)), 40.22 (C(2)), 59.39 (C(4)), 60.49 (C(7)), 61.17 (C(1)), 116.70 (C(5)), 128.82 (C(9, 13)), 128.96 (C(10, 12)), 135.67 (C(8)), 136.53 (C(11)), 162.69 (C(3)).
MS (m/z, %): 276 (<1) [M]+, 275 (<1), 258 (6), 200 (41), 105 (100), 73 (15).
Anal. calcd for C17H25D2NSi, (%): C, 74.11; N, 5.08. Found, %: C, 74.53; N, 5.30.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 4f (400 mg, 71%). Rf 0.80.
8) to afford 4f (400 mg, 71%). Rf 0.80.1H NMR (500 MHz, CDCl3): δ = 0.09 (s, 9H, C(12, 13, 14)H3), 1.08 (d, J = 7 Hz, 3H, C(6)DH2), 2.06 (t, J = 8 Hz, 1H(A), C(1)H2), 2.69 (m, 1H, C(2)H), 3.04 (t, J = 8 Hz, 1H(B), C(1)H2), 3.09 (dt, J = 14 Hz, J = 2 Hz, 1H(A), C(4)H2), 3.61 (d, J = 14 Hz, 1H(B), C(4)H2), 3.84 (d, J = 14 Hz, 1H(A), C(7)H2), 3.88 (d, J = 14 Hz, 1H(B), C(7)H2), 6.95 (m, 1H, C(11)H), 6.97 (t, J = 3 Hz, 1H, C(10)H), 7.25 (d, J = 5 Hz, 1H, C(9)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.44 (C(12, 13, 14)), 17.03 (t, J = 19 Hz, C(6)), 40.23 (C(2)), 54.68 (C(7)), 59.01 (C(4)), 61.00 (C(1)), 116.51 (t, C(5)), 124.79 (C(9)), 125.53 (C(11)), 126.41 (C(10)), 142.07 (C(8)), 162.37 (C(3)).
MS (m/z, %): 268 (2) [M]+, 267 (7), 252 (6), 194 (62), 97 (100), 73 (40).
Anal. calcd for C14H21D2NSSi, (%): C, 62.86; N, 5.24. Found, %: C, 62.54; N, 5.20.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 3i (265 mg, 91%). Rf 0.54.
8) to afford 3i (265 mg, 91%). Rf 0.54.1H NMR (500 MHz, CDCl3): δ = 1.27 (d, J = 7 Hz, 3H, C(6)H3), 2.17 (t, J = 8 Hz, 1H(A), C(1)H2), 2.39 (s, 3H, C(21)H3), 2.41 (s, 3H, C(20)H3), 2.96 (q, J = 7 Hz, 1H, C(2)H), 3.07 (t, J = 8 Hz, 1H(B), C(1)H2), 3.39 (d, J = 15 Hz, 1H(A), C(4)H2), 3.73 (s, 2H, C(7)H2), 3.86 (d, J = 15 Hz, 1H(B), C(4)H2), 6.26 (s, 1H, C(5)H), 7.17 (d, J = 5 Hz, 2H, C(14, 18)H), 7.18 (m, 2H, C(15, 17)H), 7.20 (d, J = 8 Hz, 2H, C(10, 12)H), 7.32 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 18.04 (C(6)), 21.17 (C(20, 21)), 39.17 (C(2)), 58.54 (C(4)), 60.45 (C(7)), 61.33 (C(1)), 120.19 (C(5)), 127.86 (C(14, 18)), 128.72 (C(9, 13)), 129.03 (C(15, 17)), 129.07 (C(10, 12)), 135.38 (C(19)), 135.71 (C(16)), 136.54 (C(11)), 137.97 (C(8)).
MS (m/z, %): 291 (77) [M]+, 276 (30), 186 (11), 143 (15), 105 (100).
Anal. calcd for C21H25N, (%): C, 86.55; H, 8.65; N, 4.81. Found, %: C, 86.37; H, 8.60; N, 4.79.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 5a (617 mg, 57%). Rf 0.85.
8) to afford 5a (617 mg, 57%). Rf 0.85.1H NMR (500 MHz, CDCl3): δ = 0.40 (s, 9H, C(14, 15, 16)H3), 3.17 (m, 1H(A), C(6)IH2), 3.18 (m, 1H(A), C(1)H2), 3.27 (m, 1H, C(2)H), 3.45 (m, 1H(B), C(1)H2), 3.55 (dd, J = 10 Hz, J = 3 Hz, 1H(B), C(6)IH2), 3.83 (s, 3H, C(17)H3), 4.27 (d, J = 14 Hz, 1H(A), C(7)H2), 4.62 (d, J = 14 Hz, 1H(B), C(7)H2), 6.89 (d, J = 9 Hz, 2H, C(10, 12)H), 7.20 (d, J = 9 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 2.21 (C(14, 15, 16)), 7.77 (C(6)), 47.03 (C(7)), 48.98 (C(2)), 49.22 (C(1)), 55.31 (C(17)), 114.20 (C(10, 12)), 127.77 (C(8)), 129.72 (C(9, 13)), 153.11 (C(3)), 159.31 (C(11)), 162.60 (C(4)).
Anal. calcd for C17H23I2NO2Si, (%): C, 36.77; H, 4.18; N, 2.52. Found, %: C, 36.21; H, 4.42; N, 2.39.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 5h (641 mg, 61%). Rf 0.87.
8) to afford 5h (641 mg, 61%). Rf 0.87.1H NMR (500 MHz, CDCl3): δ = 0.41 (s, 9H, C(14, 15, 16)H3), 2.36 (s, 3H, C(17)H3), 3.17 (m, 1H(A), C(6)IH2), 3.19 (m, 1H(A), C(1)H2), 3.27 (m, 1H, C(2)H), 3.46 (m, 1H(B), C(1)H2), 3.56 (dd, J = 10 Hz, J = 3 Hz, 1H(B), C(6)IH2), 4.28 (d, J = 14 Hz, 1H(A), C(7)H2), 4.66 (d, J = 14 Hz, 1H(B), C(7)H2), 7.17 (s, 4H, C(9, 10, 12, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 2.22 (C(14, 15, 16)), 7.78 (C(6)), 21.17 (C(17)), 47.37 (C(7)), 49.09 (C(2)), 49.29 (C(1)), 125.63 (C(5)), 128.36 (C(9, 13)), 129.52 (C(10, 12)), 132.63 (C(8)), 137.64 (C(11)), 153.08 (C(3)), 162.66 (C(4)).
MS (m/z, %): 539 (4) [M]+, 420 (8), 396 (8), 292 (8), 105 (100), 79 (15).
Anal. calcd for C17H23I2NOSi, (%): C, 37.86; H, 4.30; N, 2.60. Found, %: C, 38.08; H, 4.27; N, 2.44.
Preparation of bis-3-methyl-4-methylenepyrrolidines 7 and 9 via Ti–Mg-catalyzed carbocyclization of bis-N-allyl substituted propargylamines with Et2Zn in CH2Cl2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) gave 7 (776 mg, 88%) as colorless oil. Rf 0.52.
8) gave 7 (776 mg, 88%) as colorless oil. Rf 0.52.1H NMR (500 MHz, CDCl3): δ = 0.08 (s, 18H, C(11, 12, 13, 11′, 12′, 13′)H3), 1.09 (d, J = 7 Hz, 6H, C(6, 6′)H3), 2.01 (t, J = 9 Hz, 2H(A), C(1, 1′)H2), 2.67 (q, J = 7 Hz, 2H, C(2, 2′)H), 2.98 (t, J = 8 Hz, 2H(B), C(1, 1′)H2), 3.03 (d, J = 14 Hz, 2H(A), C(4, 4′)H2), 3.55 (d, J = 14 Hz, 2H(B), C(4, 4′)H2), 3.60 (d, J = 13 Hz, 2H(A), C(7, 7′)H2), 3.66 (d, J = 13 Hz, 2H(B), C(7, 7′)H2), 5.30 (s, 2H, C(5, 5′)H), 7.31 (s, 4H, C(9, 10, 9′, 10′)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = −0.43 (C(11, 12, 13, 11′, 12′, 13′)), 17.36 (C(6, 6′)), 40.32 (2, 2′), 59.42 (C(4, 4′)), 60.55 (C(7, 7′)), 61.31 (C(1, 1′)), 116.69 (C(5, 5′)), 128.78 (C(9, 10, 9′, 10′)), 137.65 (C(8, 8′)), 162.77 (C(3, 3′)).
MS (EI): m/z, % = 441 (16) [M]+, 440 (39), 367 (100), 272 (66), 207 (44), 168 (34), 104 (85), 73 (67), 44 (47).
Anal. calcd for C26H44N2Si2, (%): C, 70.84; H, 10.06; N, 6.35. Found, %: C, 71.07; H, 9.95; N, 6.39.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 9 (872 mg, 85%). Rf 0.80.
8) to afford 9 (872 mg, 85%). Rf 0.80.1H NMR (500 MHz, CDCl3): δ = 1.10 (d, J = 7 Hz, 6H, C(6, 6′)H3), 1.36 (s, 4H, C(16, 16′)H2), 1.92 (d, J = 5 Hz, 4H, C(15, 15′)H2), 2.04 (t, J = 9 Hz, 2H(A), C(4, 4′)H2), 2.38 (s, 6H, C(14, 14′)), 2.70 (q, J = 7 Hz, 2H, C(3, 3′)H), 2.97 (d, J = 14 Hz, 2H(A), C(1, 1′)H2), 3.00 (t, J = 8 Hz, 2H(B), C(4, 4′)H2), 3.48 (d, J = 14 Hz, 2H(B), C(1, 1′)H2), 3.61 (d, J = 13 Hz, 2H(A), C(7, 7′)H2), 3.65 (d, J = 13 Hz, 2H(B), C(7, 7′)H2), 5.15 (m, 2H, C(5, 5′)H), 7.17 (d, J = 8 Hz, 4H, C(10, 12, 10′,12′)H), 7.28 (d, J = 8 Hz, 4H, C(9, 13, 9′, 13′)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.75 (C(6, 6′)), 21.15 (C(14, 14′)), 29.24 (C(16, 16′)), 29.37 (C(15, 15′)), 37.33 (C(3, 3′)), 56.82 (C(1, 1′)), 60.61 (C(7, 7′)), 62.28 (C(4, 4′)), 119.69 (C(5, 5′)), 128.77 (C(9, 13, 9′, 13′)), 128.94 (C(10, 12, 10′, 12′)), 136.07 (C(8, 8′)), 136.46 (C(11, 11′)), 144.09 (C(2, 2′)).
MS (m/z, %): 457 (3) [M]+, 456 (3), 351 (1), 200 (10), 105 (100), 79 (6).
Anal. calcd for C32H44N2, (%): C, 84.16; H, 9.71; N, 6.13. Found, %: C, 83.89; H, 9.50; N, 6.17.
Preparation of 3-methyl-4-methylenepyrrolidines 11a–c, 12a and 14a via Ti–Mg-catalyzed carbocyclization of allyl substituted but-2-yne-1,4-diamines with Et2Zn in CH2Cl2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) gave 11a (464 mg, 90%) as colorless oil. Rf 0.85.
8) gave 11a (464 mg, 90%) as colorless oil. Rf 0.85.1H NMR (500 MHz, CDCl3): δ = 1.12 (d, J = 7 Hz, 3H, C(6)H3), 2.07 (t, J = 9 Hz, 1H(A), C(1)H2), 2.35 (s, 6H, C(15, 16)H3), 2.36 (s, 3H, C(17)H3), 2.75 (q, J = 7 Hz, 1H, C(2)H), 2.99 (m, 1H(A), C(4)H2), 3.00 (m, 2H, C(14)H2), 3.02 (m, 1H(B), C(1)H2), 3.50 (d, J = 14 Hz, 1H(B), C(4)H2), 3.60 (d, J = 13 Hz, 1H(A), C(7)H2), 3.65 (d, J = 12 Hz, 1H(B), C(7)H2), 5.31 (m, 1H, C(5)H), 7.15 (d, J = 8 Hz, 2H, C(10, 12)H), 7.24 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.49 (C(6)), 21.21 (C(17)), 37.69 (C(2)), 44.87 (C(15, 16)), 56.55 (C(4)), 57.66 (C(14)), 60.25 (C(7)), 61.64 (C(1)), 114.34 (C(5)), 128.77 (C(9, 13)), 129.02 (C(10, 12)), 135.36 (C(8)), 136.73 (C(11)), 149.76 (C(3)).
MS (EI): m/z, % = 258 (<1) [M+], 257 (<1), 213 (80), 198 (57), 105 (100).
Anal. calcd for C17H26N2, (%): C, 79.02; H, 10.14; N, 10.84. Found, %: C, 78.86; H, 10.09; N, 11.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 11b (534 mg, 89%). Rf 0.48.
8) to afford 11b (534 mg, 89%). Rf 0.48.1H NMR (500 MHz, CDCl3): δ = 1.09 (d, J = 7 Hz, 3H, C(6)H3), 2.01 (t, J = 8 Hz, 1H(A), C(1)H2), 2.42 (s, 4H, C(15, 18)H2), 2.71 (q, J = 8 Hz, 1H, C(2)H), 2.88 (d, J = 6 Hz, 2H, C(14)H2), 2.93 (d, J = 14 Hz, 1H(A), C(4)H2), 2.97 (t, J = 8 Hz, 1H(B), C(1)H2), 3.47 (d, J = 14 Hz, 1H(B), C(4)H2), 3.55 (d, J = 13 Hz, 1H(A), C(7)H2), 3.59 (d, J = 13 Hz, 1H(B), C(7)H2), 3.71 (s, 4H, C(16, 17)H2), 3.80 (s, 3H, C(19)H3), 5.25 (s, 1H, C(5)H), 6.87 (d, J = 8 Hz, 2H, C(10, 12)H), 7.25 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.53 (C(6)), 37.66 (C(2)), 53.61 (C(15, 18)), 55.21 (C(19)), 56.74 (C(4)), 57.82 (C(14)), 60.04 (C(7)), 61.78 (C(1)), 66.99 (C(16, 17)), 113.63 (C(10, 12)), 115.49 (C(5)), 129.89 (C(9, 13)), 130.93 (C(8)), 148.32 (C(3)), 158.66 (C(11)).
MS (EI): m/z, % = 316 (<1) [M]+, 229 (39), 121 (100), 77 (4).
Anal. calcd for C19H28N2O2, (%): C, 72.12; H, 8.92; N, 8.85. Found, %: C, 72.15; H, 8.79; N, 8.49.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 11c (515 mg, 82%). Rf 0.79.
8) to afford 11c (515 mg, 82%). Rf 0.79.1H NMR (500 MHz, CDCl3): δ = 1.04 (d, J = 7 Hz, 3H, C(6)H3), 1.44 (s, 2H, C(17)H2), 1.59 (p, J = 6 Hz, 4H, C(16, 18)), 1.99 (t, J = 9 Hz, 1H(A), C(1)H2), 2.36 (s, 4H, C(15, 19)H2), 2.71 (q, J = 8 Hz, 1H, C(2)H), 2.84 (d, J = 7 Hz, 2H, C(14)H2), 2.93 (d, J = 14 Hz, 1H(A), C(4)H2), 2.97 (t, J = 8 Hz, 1H(B), C(1)H2), 3.47 (d, J = 14 Hz, 1H(B), C(4)H2), 3.55 (d, J = 13 Hz, 1H(A), C(7)H2), 3.59 (d, J = 13 Hz, 1H(B), C(7)H2), 3.81 (s, 3H, C(20)H3), 5.29 (m, 1H, C(5)H), 6.87 (d, J = 8 Hz, 2H, C(10, 12)H), 7.26 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.49 (C(6)), 24.39 (C(17)), 25.95 (C(16, 18)), 37.62 (C(2)), 54.52 (C(15, 19)), 55.23 (C(20)), 56.77 (C(4)), 58.24 (C(14)), 60.10 (C(7)), 61.86 (C(1)), 113.61 (C(10, 12)), 116.53 (C(5)), 129.91 (C(9, 13)), 131.05 (C(8)), 147.19 (C(3)), 158.63 (C(11)).
MS (EI): m/z, % = 314 (<1) [M+], 121 (100), 77 (5).
Anal. calcd for C20H30N2O, (%): C, 76.39; H, 9.62; N, 8.91. Found, %: C, 76.44; H, 9.86; N, 8.59.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 12a (480 mg, 88%). Rf 0.85.
8) to afford 12a (480 mg, 88%). Rf 0.85.1H NMR (500 MHz, CDCl3): δ = 1.10 (m, 2H, C(6)DH2), 2.04 (t, J = 8 Hz, 1H(A), C(1)H2), 2.23 (s, 6H, C(15, 16)H3), 2.37 (s, 3H, C(17)H3), 2.73 (p, J = 7 Hz, 1H, C(2)H), 2.83 (s, 2H, C(14)H2), 2.96 (d, J = 14 Hz, 1H(A), C(4)H2), 2.99 (t, J = 8 Hz, 1H(B), C(1)H2), 3.49 (d, J = 14 Hz, 1H(B), C(4)H2), 3.59 (d, J = 13 Hz, 1H(A), C(7)H2), 3.64 (d, J = 13 Hz, 1H(B), C(7)H2), 7.15 (d, J = 8 Hz, 2H, C(10, 12)H), 7.25 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.39 (t, J = 19 Hz, C(6)), 21.12 (C(17)), 37.50 (C(2)), 45.11 (C(15, 16)), 56.69 (C(4)), 58.29 (C(14)), 60.45 (C(7)), 61.88 (C(1)), 128.72 (C(9, 13)), 128.96 (C(10, 12)), 135.84 (C(8)), 136.55 (C(11)), 147.53 (C(3)).
MS (EI): m/z, % = 260 (<1) [M]+, 215 (36), 199 (30), 105 (100), 79 (7).
Anal. calcd for C17H24D2N2, (%): C, 78.41; N, 10.76. Found, %: C, 78.48; N, 11.08.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropyl alcohol
isopropyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) to afford 14 (517 mg, 90%). Rf 0.63.
8) to afford 14 (517 mg, 90%). Rf 0.63.1H NMR (500 MHz, CDCl3): δ = 1.08 (d, J = 7 Hz, 3H, C(6)H3), 1.42 (p, J = 8 Hz, 2H, C(15)H2), 1.58 (p, J = 8 Hz, 2H, C(16)H2), 1.95 (q, J = 7 Hz, 2H, C(14)H2), 2.03 (t, J = 8 Hz, 1H(A), C(1)H2), 2.37 (s, 3H, C(19)H3), 2.96 (d, J = 13 Hz, 1H(A), C(4)H2), 2.99 (t, J = 8 Hz, C(1)H2), 3.34 (s, 3H, C(18)H3), 3.37 (t, J = 7 Hz, 2H, C(17)H2), 3.47 (d, J = 13 Hz, 1H(B), C(4)H2), 3.59 (d, J = 13 Hz, 1H(A), C(7)H2), 3.64 (d, J = 13 Hz, 1H(B), C(7)H2), 5.14 (m, 1H, C(5)H), 7.15 (d, J = 8 Hz, 2H, C(10, 12)H), 7.26 (d, J = 8 Hz, 2H, C(9, 13)H).
13C–{1H} NMR (500 MHz, CDCl3): δ = 17.68 (C(6)), 21.12 (C(19)), 26.08 (C(15)), 29.20 (C(14)), 29.25 (C(16)), 37.30 (C(2)), 56.73 (C(4)), 58.55 (C(18)), 60.54 (C(7)), 62.22 (C(1)), 119.43 (C(5)), 128.77 (C(9, 13)), 128.93 (C(10, 12)), 135.95 (C(8)), 136.47 (C(11)), 144.32 (C(3)).
MS (EI): m/z, % = 287 (18) [M+], 200 (38), 105 (100), 79 (9).
Anal. calcd for C19H29NO, (%): C, 79.39; H, 10.17; N, 4.87. Found, %: C, 79.11; H, 10.00; N, 4.53.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Russian Science Foundation (grant no. 19-73-10113).References
- B. M. Trost, Chem. Soc. Rev., 1981, 11, 141–170 RSC.
- M. Ramaiah, Synthesis, 1984, 529–570 CrossRef.
- L. A. Paquette, Top. Curr. Chem., 1984, 119, 1–158 Search PubMed.
- B. M. Trost and D. M. T. Chan, J. Am. Chem. Soc., 1983, 105, 2315–2325 CrossRef.
- F. L. Warren, Fortschr. Chem. Org. Naturst., 1966, 24, 329–406 CAS.
- D. Robins, Fortschr. Chem. Org. Naturst., 1982, 41, 115–203 CrossRef CAS.
- R. Southgate and S. Elson, Prog. Chem. Org. Nat. Prod., 1985, 47, 1–106 CrossRef CAS.
- G. Albers-Schonberg, B. H. Arison, O. D. Hensens, J. Hirshfield, K. Hoogsteen, E. A. Kaczka, R. E. Rhodes, J. S. Kahan, F. M. Kahan, R. W. Ratcliffe, E. Walton, L. J. Ruswinkle, R. B. Morin and B. G. Christensen, J. Am. Chem. Soc., 1978, 100, 6491–6499 CrossRef.
- B. E. Kane, B. Svensson and D. M. Ferguson, AAPS J., 2006, 8, E126–E137 CrossRef CAS.
- R. A. Glennon, A. M. Ismaiel, J. D. Smith, M. Yousif, M. El-Ashmawy, J. L. Herndon, J. B. Fischer, K. J. B. Howie and A. C. Server, J. Med. Chem., 1991, 34, 1855–1859 CrossRef CAS.
- J. M. Defauw, M. M. Murphy, G. E. Jagdmann, H. Hu, J. W. Lampe, S. P. Hollinshead, T. J. Mitchell, H. M. Crane, J. M. Heerding, J. S. Mendoza, J. E. Davis, J. W. Darges, F. R. Hubbard and S. E. Hall, J. Med. Chem., 1996, 39, 5215–5227 CrossRef.
- G. E. Jagdmann, J. M. Defauw, J. W. Lampe, J. W. Darges and K. Kalter, Bioorg. Med. Chem. Lett., 1996, 6, 1759–1764 CrossRef.
- D. C. Horwell, D. Naylor and H. M. G. Willems, Bioorg. Med. Chem. Lett., 1997, 7, 31–36 CrossRef.
- C. Thomas, U. Ohnmacht, M. Niger and P. Gmeiner, Bioorg. Med. Chem. Lett., 1998, 8, 2885–2890 CrossRef.
- A. Marinetti, H. Jullien and A. Voituriez, Chem. Soc. Rev., 2012, 41, 4884–4908 RSC.
- C. Aubert, O. Buisine and M. Malacria, Chem. Rev., 2002, 102, 813–834 CrossRef PubMed.
- B. M. Trost, Acc. Chem. Res., 1990, 23, 34–42 CrossRef.
- E. Negishi, S. J. Holmes, J. M. Tour, J. A. Miller, F. E. Cederbaum, D. R. Swanson and T. Takahashi, J. Am. Chem. Soc., 1989, 111, 3336–3346 CrossRef.
- R. A. Hunter, D. P. S. Macfarlane and R. J. Whitby, Synlett, 2006, 19, 3314–3318 Search PubMed.
- T. Xi, X. Chen, H. Zhang and Z. Lu, Synthesis, 2016, 48, 2837–2844 CrossRef.
- J. Montgomery, M. V. Chevliakov and H. L. Brielmann, Tetrahedron, 1997, 53, 16449–16462 CrossRef.
- J. Tang, H. Shinokubo and K. Oshima, Organometallics, 1998, 17, 290–292 CrossRef.
- T. Nishikawa, H. Shinokubo and K. Oshima, Org. Lett., 2002, 4, 2795–2797 CrossRef.
- J. E. Lee, S. H. Hong and Y. K. Chung, Tetrahedron Lett., 1997, 38, 1781–1784 CrossRef.
- H. Urabe, T. Hata and F. Sato, Tetrahedron Lett., 1995, 36, 4261–4264 CrossRef.
- B. M. Trost and S.-F. Chen, J. Am. Chem. Soc., 1986, 108, 6053–6054 CrossRef.
- J. T. Metza, R. A. Terzian and T. G. Minehan, Tetrahedron Lett., 2006, 47, 8905–8910 CrossRef.
- R. N. Kadikova, I. R. Ramazanov, O. S. Mozgovoi, A. M. Gabdullin and U. M. Dzhemilev, Synlett, 2019, 311–314 Search PubMed.
- R. N. Kadikova, I. R. Ramazanov, O. S. Mozgovoi, A. M. Gabdullin and U. M. Dzhemilev, Catalysts, 2019, 9, 1022, DOI:10.3390/catal9121022.
- J.-L. Montchamp and E. Negishi, J. Am. Chem. Soc., 1998, 120, 5345–5346 CrossRef.
- Y. Panahi, M. S. Hosseini, N. Khalili, E. Naimi, M. Majeed and A. Sahebkar, Clin. Nutr., 2015, 34, 1101–1108 CrossRef PubMed.
- Z.-J. Meng, N. Wu, Y. Liu, K.-J. Shu, X. Zou, R.-X. Zhang and Z.-L. Deng, Oncol. Rep., 2015, 34, 1388–1396 CrossRef PubMed.
- S. Wang, K. Fang, G. Dong, S. Chen, N. Liu, Z. Miao and C. Sheng, J. Med. Chem., 2015, 58, 6678–6696 CrossRef CAS PubMed.
- A. K. Stewart, S. V. Rajkumar, M. A. Dimopoulos, T. Masszi, I. Špička, A. Oriol and V. Goranova-Marinova, N. Engl. J. Med., 2015, 372, 142–152 CrossRef.
- J. Kronke, N. D. Udeshi, A. Narla, P. Grauman, S. N. Hurst, M. McConkey and C. Ciarlo, Science, 2014, 343, 301–305 CrossRef PubMed.
- C. J. Legacy, A. Wang, B. J. O'Day and M. H. Emmert, Angew. Chem., Int. Ed., 2015, 54, 14907–14910 CrossRef CAS PubMed.
- R. M. Moriarty, R. K. Vaid, M. P. Duncan, M. Ochiai, M. Inenaga and Y. Nagao, Tetrahedron Lett., 1988, 29, 6913–6916 CrossRef CAS.
- K. Tanaka, S. Yoshifuji and Y. A. Nitta, Chem. Pharm. Bull., 1988, 36, 3125–3129 CrossRef CAS.
- X.-F. Wu, C. B. Bheeter, H. Neumann, P. H. Dixneuf and M. Beller, Chem. Commun., 2012, 48, 12237–12239 RSC.
- J.-B. Feng, H. Neumann, A. Pews-Davtyan, P. Langer and M. A. Beller, Green Chem., 2013, 15, 1956–1961 RSC.
- Y.-P. Zhu, S. Sergeyev, P. Franck, R. V. Orru and B. U. Maes, Org. Lett., 2016, 18, 4602–4605 CrossRef CAS PubMed.
- J. W. Kim, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2008, 47, 9249–9251 CrossRef CAS PubMed.
- R. Ray, A. S. Hazari, S. Chandra, D. Maiti and G. K. Lahiri, Chem.–Eur. J., 2017, 24, 1067–1071 CrossRef PubMed.
- X. Jin, K. Kataoka, T. Yatabe, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2016, 55, 7212–7217 CrossRef CAS PubMed.
- J. R. Khusnutdinova, Y. Ben-David and D. Milstein, J. Am. Chem. Soc., 2014, 136, 2998–3001 CrossRef CAS PubMed.
- I. R. Ramazanov, R. N. Kadikova and U. M. Dzhemilev, Russ. Chem. Bull., 2011, 60, 99–106 CrossRef CAS.
- B. P. Zavesky, N. R. Babij and J. P. Wolfe, Org. Lett., 2014, 16, 4952–4955 CrossRef CAS PubMed.
- E. Negishi, J.-L. Montehamp, L. Anastasia, A. Elizarov and D. Choueiry, Tetrahedron Lett., 1998, 39, 2503–2506 CrossRef CAS.
- M. Brookhart, M. L. Green and G. Parkin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6908–6914 CrossRef CAS PubMed.
- Y. Shao, F. Zhang, J. Zhang and X. Zhou, Angew. Chem., Int. Ed., 2016, 55, 11485–11489 CrossRef CAS.
- H.-J. Li, R. Guillot and V. Gandon, J. Org. Chem., 2010, 75, 8435–8449 CrossRef CAS PubMed.
- M. G. Shaibakova, I. G. Titova, A. G. Ibragimov and U. M. Dzhemilev, Russ. J. Org. Chem., 2008, 44, 1126–1129 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02677h |
| This journal is © The Royal Society of Chemistry 2020 |