Open Access Article
Open Access ArticleDechlorination of fly ash by hydrolysate of municipal solid waste leachate
Ming Gaoa,
Menglu Wanga,
Chuanfu Wu*a,
Xiaona Wanga,
Yufei Yangb,
Shu Liuc,
Takayuki Shimaokad and
Qunhui Wang *a
*a
aDepartment of Environmental Science and Engineering, School of Energy and Environmental Engineering, University of Science and Technology Beijing, Beijing Key Laboratory on Resource-oriented Treatment of Industrial Pollutants, 30 Xueyuan Road, Haidian District, Beijing 100083, PR China. E-mail: wucf@ustb.edu.cn; wangqh59@sina.com; Fax: +86-10-6233-2778; Tel: +86-10-6233-2778
bState Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, PR China
cDepartment of Environmental Science and Engineering, School of Space and Environment, Beihang University, Beijing 100191, PR China
dDepartment of Urban and Environmental Engineering, Faculty of Engineering, Kyushu University, 744 Motooka, Nishi-ku, Fukuoka 819-0395, Japan
First published on 15th July 2020
Abstract
Municipal solid waste incineration fly ash (referred to as the fly ash) presents an important environmental problem in China today, but strategies for its treatment have yet to be widely studied and implemented. The currently available methods for the dechlorination of fly ash are not sufficient, given the amounts of fly ash produced each year. To increase the reuse fraction of fly ash as raw material for cement production, we propose an improved dechlorination method. Specifically, fly ash was leached with the hydrolysate of municipal solid waste leachate (HMSWL) to remove the water-insoluble chlorine. Three-step HMSWL leaching removed 94.3% of the total chlorine in fly ash, much more than the 82.7% that was removed through three-step ultrapure water (UW) leaching. X-ray diffraction indicated that three-step UW leaching could remove Cl mainly in the forms of KCl, NaCl, CaClOH and AlOCl, whereas three-step HMSWL leaching could further remove more water-insoluble Cl in the forms of AlOCl. In addition, the experimental results further suggested that the low pH of HMSWL (4.9) contributed little to the water-insoluble Cl removal, whereas the displacement of organic acid radicals (especially by the butyrate radical) was the major cause of water-insoluble Cl removal. Therefore, HMSWL rich in butyrate radical could be an ideal water substitute for fly ash dechlorination.
1. Introduction
It has been estimated that approximately 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 500
000 to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of municipal solid waste incineration fly ash (referred to as the fly ash) are produced in China each year.1 It is therefore crucial that fly ash treatment strategies are established and implemented. At present, fly ash is mostly treated by cement curing,2 chemical agent stabilisation,3 heat treatment,4 immersion5 and bioleaching.6 Moreover, it is investigated as a promising raw material for cement production, because its composition is similar to that of cement raw materials.4,7 However, the biggest problem with the utilisation of fly ash in cement kilns is its high chlorine (Cl) content (i.e. 2.9–33.9%).7–10
000 tonnes of municipal solid waste incineration fly ash (referred to as the fly ash) are produced in China each year.1 It is therefore crucial that fly ash treatment strategies are established and implemented. At present, fly ash is mostly treated by cement curing,2 chemical agent stabilisation,3 heat treatment,4 immersion5 and bioleaching.6 Moreover, it is investigated as a promising raw material for cement production, because its composition is similar to that of cement raw materials.4,7 However, the biggest problem with the utilisation of fly ash in cement kilns is its high chlorine (Cl) content (i.e. 2.9–33.9%).7–10
In general, total Cl in fly ash can be divided into water-soluble Cl and water-insoluble Cl. Water-soluble Cl mainly exists in the form of CaCl2, KCl, NaCl and CaClOH,7,11 accounting for about 59–93% of the total Cl in fly ash.12 At present, water-soluble Cl is mainly removed through water leaching.13–15 However, the Cl content of the leached residue of fly ash is still 1–4%, even after multistep water leaching, due to the presence of water-insoluble Cl in fly ash.16 In this context, the additional fraction of fly ash should not be higher than 0.5–2.1% of the raw materials entering the cement kiln (according to the Chinese standard HJ662-2013). Considering the current issue of cement overcapacity, it would be difficult to recycle a large amount of fly ash. Therefore, it is necessary to develop a deeper dechlorination technology.
Water-insoluble Cl in fly ash mainly exists in the forms of Friedel's salt (3CaO·Al2O3·CaCl2·10H2O) and AlOCl.7,12 Since most chlorides have a low melting point, more than 52% of water-insoluble Cl can be removed by calcining the water leaching residue of fly ash.17 However, the high processing cost of leaching and calcining hinders the wide application of this process. On the other hand, during the calcining of raw ash without water leaching, the water-insoluble Cl content in the ash remains relatively stable because Friedel's salt that is contained in the ash is converted into calcium chloroaluminate (11CaO·7Al2O3·CaCl2).18 In addition to the calcination method, lowering the pH value of the leaching solvent is also beneficial for the removal of water-insoluble Cl.14 For instance, ash dechlorination by adding sulphuric acid to reduce the pH of the leaching solvent could remove most water-insoluble Cl, reducing the Cl content in the residue to less than 0.1%.19 However, the high price of sulphuric acid has to be taken into account in the application of this technology. Therefore, finding a suitable acidic waste liquid is crucial to the reduction of the dechlorination cost of fly ash.
Municipal solid waste is usually piled up for 5–7 days before incineration to reduce the moisture and improve the heat value of the waste. Municipal solid waste leachate produced in the process of garbage piling is acidic and rich in ammonium nitrogen, COD, heavy metals, organic matters, etc.20–22 Traditionally, municipal solid waste leachate may be treated through physical, chemical and/or biological methods.23,24 Aromatic organic compounds in municipal solid waste leachate are extremely difficult to be biodegraded, resulting in poor destruction efficiency of pollutants by conventional biological methods. High ammonium nitrogen (NH4+–N) concentration is another troublesome issue. Conventional physical and chemical methods can effectively remove heavy metals from municipal solid waste leachate, but they are expensive, and multiple organic matters in municipal solid waste leachate cannot be removed sufficiently at the same time.25 Studies have shown that heavy metals and organics can be removed from municipal solid waste leachate simultaneously by selecting suitable adsorbents for adsorption.26–28 Due to their obvious advantages of large specific surface area and large production, using fly ash could be an effective and low-cost alternative approach.29
When fly ash is dechlorinated through leaching with municipal solid waste leachate, the following benefits are expected. First, the dosage of water or acidic solvent used in the dechlorination process of fly ash is reduced. Second, the pH values of the original liquid of the municipal solid waste leachate (OMSWL) and the hydrolysate of the municipal solid waste leachate (HMSWL) are expected to reach the level of water-insoluble Cl removal. Therefore, the fly ash dechlorination degree is higher.30 Finally, fly ash can adsorb some organic matter in municipal solid waste leachate, which is beneficial to the subsequent treatment of municipal solid waste leachate.31–33 In this study, we compare the dechlorination effects of ultrapure water (UW), OMSWL and HMSWL on fly ash. The physicochemical property changes of the leaching solvent before and after the experiment and the changes of the Cl forms in the mineral phase of fly ash are also analysed. The results of this study are expected to provide technical support for the cooperative disposal of fly ash and municipal solid waste leachate.
2. Materials and methods
2.1. Experimental materials
The fly ash used in this research was taken from a municipal solid waste incineration plant in Beijing. The plant adopts a mechanical grate incinerator design and uses Ca(OH)2 to remove acid gases from flue gas. To obtain a representative sample, the fly ash generated from five different batches was collected from the discharged port of the bag filters. Six kilograms fly ash from each batch were then sampled randomly with a shovel and mixed thoroughly. Totally 30 kg fly ash were taken back to our laboratory. After grinding and homogenisation, the fly ash was divided into small bags and sealed for storage. The fly ash used for the leaching experiments was taken from the same bag. The physicochemical properties of fly ash are shown in Table 1. On the other hand, the OMSWL used for the experiments was collected from a leachate storage tank in the same municipal solid waste incineration plant as aforementioned. The leachate storage tank was used to temporary store the leachate generated from the raw municipal solid waste during the dewatering stage. Totally 20 L OMSWL were taken back to the laboratory and were in turn stored in a refrigerator at −20 °C. The frozen OMSWL was thawed at 4 °C and shaken homogenously before used.| Parameters | Value | Parameters | Value |
|---|---|---|---|
| pH | 11.82 | Total Cl (g g−1) | 0.30 |
| Moisture content (%) | 0.90 | Mn (mg g−1) | 0.16 |
| Loss on ignition (%) | 5.57 | Cr (mg g−1) | 0.74 |
| Surface area (m2 g−1) | 17.07 | As (mg g−1) | 0.01 |
| Pore volume (cm3 g−1) | 0.03 | Cu (mg g−1) | 0.33 |
| Average pore width (nm) | 8.06 | Pb (mg g−1) | 0.50 |
| Median pore width (nm) | 1.44 | Cd (mg g−1) | 0.05 |
| Water-soluble Cl (g g−1) | 0.21 | Zn (mg g−1) | 0.93 |
Furthermore, in order to investigate the influence of the pH value of municipal solid waste leachate on the dechlorination effect of fly ash, HMSWL was also used as a leaching solvent of fly ash. The HMSWL preparation process was divided into two steps: sludge acclimation and OMSWL hydrolysis. Sludge acclimation was achieved as follows: anaerobic sludge was taken from anaerobic digester at a biogas station in Shunyi District, Beijing. The biogas station uses a medium-temperature (37–38 °C) single-phase anaerobic fermentation process, and the fermentation raw material is pig manure. Totally 10 L of sludge were taken back to the laboratory for acclimation. After natural sedimentation for 5 days, the supernatant of the sludge was poured out. Glucose solution was added to the remaining sludge substrate daily. The COD load of the sludge was 10 g COD/(L d) and the HRT was 7 days.34 The sludge was acclimated at 35 ± 1 °C under micro-oxygen conditions.35 On the premise that the daily COD load of the sludge remained unchanged, the volume of the glucose solution added was reduced gradually, and the volume of the OMSWL added was increased. Sludge acclimation was achieved when the pH value remained stable with further daily addition of OMSWL to the sludge. OMSWL hydrolysis was achieved as follows: OMSWL was continuously added into the acclimatised sludge as described above, so that the COD load of sludge remained 10 g COD/(L d) and the HRT was 7 days. OMSWL was hydrolysed at 35 ± 1 °C under micro-oxygen conditions. When the pH dropped to 4.8–4.9 and remained stable at that level, the obtained solution was the HMSWL used in this study. The physicochemical properties of OMSWL and HMSWL are shown in Table 2.
| Parameters | UW | UW I | UW IIIa | OMSWL | OMSWL IIIa | HMSWL | HMSWL IIIa |
|---|---|---|---|---|---|---|---|
| a The properties of the leaching solvent were measured after each leaching step, and the mean value of three-step leaching was presented. | |||||||
| pH (−) | 6.57 | 12.32 | — | 5.81 | — | 4.90 | — |
| TOC (g L−1) | 0 | 0 | 0 | 25.92 | 15.59 | 20.72 | 11.68 |
| CODCr (g L−1) | 0 | 0 | 0 | 58.76 | 43.67 | 44.31 | 29.70 |
| NH4+–N (g L−1) | 0 | 0 | 0 | 1.64 | 1.43 | 1.20 | 1.20 |
| NO3−–N (g L−1) | 0 | 0 | 0 | 0.13 | 0.10 | 0.10 | 0.09 |
| NO2−–N (mg L−1) | 0 | 0 | 0 | 0.25 | 0.25 | 0.11 | 0.05 |
| Cl−(g L−1) | 0 | 20.65 | 24.82 | 4.57 | 31.45 | 3.93 | 32.26 |
| Mn (mg L−1) | 0 | 0 | 0 | 9.69 | 1.91 | 13.91 | 1.60 |
| Cr (mg L−1) | 0 | 0.11 | 0.02 | 0.20 | 0.06 | 0.33 | 0.13 |
| As (mg L−1) | 0 | 0.03 | 0.04 | 0.46 | 0.25 | 0.46 | 0.12 |
| Cu (mg L−1) | 0 | 0.11 | 0.35 | 1.28 | 20.56 | 0.75 | 27.37 |
| Pb (mg L−1) | 0 | 20.03 | 17.44 | 0.35 | 2.01 | 0.40 | 7.30 |
| Cd (mg L−1) | 0 | 0.02 | 0.01 | 0.01 | 0.31 | 0.01 | 0.70 |
| Zn (mg L−1) | 0 | 2.89 | 1.16 | 1.05 | 38.19 | 3.12 | 31.65 |
| Lactic acid (mmol L−1) | 0 | 0 | 0 | 27.98 | 26.52 | 266.50 | 251.18 |
| Formic acid mmol L−1) | 0 | 0 | 0 | 5.95 | 3.58 | 115.66 | 112.93 |
| Acetic acid (mmol L−1) | 0 | 0 | 0 | 0 | 0 | 52.62 | 7.35 |
| Propionic acid (mmol L−1) | 0 | 0 | 0 | 0 | 0 | 17.34 | 3.43 |
| Isobutyric acid (mmol L−1) | 0 | 0 | 0 | 1.78 | 0.55 | 7.91 | 0 |
| Butyric acid (mmol L−1) | 0 | 0 | 0 | 3.57 | 1.37 | 45.82 | 7.10 |
| Isovaleric acid (mmol L−1) | 0 | 0 | 0 | 0 | 0 | 7.73 | 0.26 |
| Valeric acid (mmol L−1) | 0 | 0 | 0 | 1.44 | 0.23 | 28.09 | 4.69 |
| Hexanoic acid (mmol L−1) | 0 | 0 | 0 | 5.08 | 2.34 | 58.66 | 9.18 |
2.2. Experimental methods
The leaching experiments of fly ash were carried out in a series of 250 mL conical flasks. In order to compare the dechlorination effect of one-step and three-step leaching on fly ash, UW was first used as the leaching solvent. The one-step leaching experiment was conducted as follows: 15 g fly ash was weighed and placed in a conical flask, which was placed in a shaker after 150 mL UW was added. The temperature in the shaker was adjusted to 37 °C, and the rotation speed was adjusted to 200 rpm. After shaking for 3 h, the mixture was poured into a centrifuge tube and centrifuged at 4000 rpm for 15 min. The supernatant was filtered with a nylon filter membrane with a pore diameter of 0.45 μm to measure its Cl-concentration, heavy metals concentrations, pH value and other physicochemical properties. The remaining solid matter in the centrifuge tube (referred to as the residue) was dried at 105 °C for 24 h before further measurement. Compared with the one-step leaching experiment, the three-step leaching experiment had the following differences: 50 mL UW was first mixed with 15 g fly ash in the conical flask and shook for 1 h. The suspension was then centrifuged, and the supernatant was removed. Another fresh 50 mL UW was added into the conical flask, and the shaking and centrifugation operations were repeated. Finally, the last fresh 50 mL UW was added, and the shaking and centrifugation were repeated. The residue left in the centrifuge tube after the three steps of the leaching process was dried at 105 °C for 24 h before measurement. Comparing the dechlorination effects of the two leaching methods, the method with better dechlorination effect was adopted. In this method, the fly ash was leached by OMSWL and HMSWL. The experiments were conducted three times, in parallel, for replication, and the average values of the test indices (e.g. pH, heavy metals, organic acids concentrations) taken into account for the final evaluation were obtained after discarding the outliers.2.3. Analytical methods
The pH value of fly ash was measured according to the Chinese standard HJ962-2018. The moisture content and the loss on ignition of fly ash were measured using the gravimetric method. Surface area, pore volume, average pore width and median pore width were measured using fully automatic specific surface and porosity analyzer (ASAP 2020, Micromeritics (Shanghai) Instrument Co., Ltd.) After the fly ash was treated according to the Japanese standard JIS A1154-2003, the total Cl content was measured using intelligent ion chromatograph (YC3000, Qingdao Allen Chromatography Technology Co., Ltd) and converted into solid mass concentration. The water-soluble Cl content was measured according to the method provided in Appendix F of the Chinese standard GB5085.3-2007 (the solid-to-liquid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, and the ultrasonic extraction time was 30 min). The fly ash was digested with HCl, HNO3, HF and HClO4 according to the Chinese standard HJ781-2016. Then, the heavy metals concentrations were determined through atomic absorption spectrometry (AA6860, Shimadzu International Trade Shanghai Co., Ltd.). The mineral phase of the fly ash before and after leaching was analysed using X-ray diffraction (XRD, LabX XRD-6000, Shimadzu International Trade Shanghai Co., Ltd.).
16, and the ultrasonic extraction time was 30 min). The fly ash was digested with HCl, HNO3, HF and HClO4 according to the Chinese standard HJ781-2016. Then, the heavy metals concentrations were determined through atomic absorption spectrometry (AA6860, Shimadzu International Trade Shanghai Co., Ltd.). The mineral phase of the fly ash before and after leaching was analysed using X-ray diffraction (XRD, LabX XRD-6000, Shimadzu International Trade Shanghai Co., Ltd.).
The relevant physicochemical indexes (TOC, CODCr, NH4+–N, NO3−–N, NO2−–N) of the leaching solvent were measured according to the Chinese standards HJ501-2009, GB11914-89, GB7479-87, HJ/T346-2007, GB7493-87. The concentrations of lactic acid and formic acid in the leaching solvent were measured using a high-performance liquid chromatograph (LC-20AT, Shimadzu International Trade Shanghai Co., Ltd.) equipped with an SH1011 chromatographic column. The concentrations of acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid and hexanoic acid in the leaching solvent were measured using a high-performance gas chromatograph (GC-2010 plus, Shimadzu International Trade Shanghai Co., Ltd.) equipped with a hydrogen flame ionisation detector and a 30 m capillary column DB-FFAP (i.d. 0.53 mm; 125–3237; Agilent Technologies).
3. Results
3.1. Dechlorination effect and mass reduction of fly ash under different leaching solvents
Generally, the dechlorination effect of the fly ash may affect by multiple factors, such as temperature, the solid-to-liquid ratio in the leaching process, the pH value of the leaching solvent.13–15 In our preliminary experiments, we explored these optimum leaching parameters for the specific fly ash used. In brief, the investigated temperatures were 15, 25, 35, 37, 45 and 55 °C, respectively, whereas the trial solid–liquid ratio were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 1
8, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 1
15, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, respectively. The leaching time we examined were 30, 60, 90, 120, 240, 480 minutes, respectively. The experimental data (data not shown) revealed that Cl removal rate increased with the increase of temperature, solid–liquid ratio and leaching time. The Cl removal rate exceeded 80% when the leaching temperature, solid–liquid ratio and the leaching time were 37 °C, 1
20, respectively. The leaching time we examined were 30, 60, 90, 120, 240, 480 minutes, respectively. The experimental data (data not shown) revealed that Cl removal rate increased with the increase of temperature, solid–liquid ratio and leaching time. The Cl removal rate exceeded 80% when the leaching temperature, solid–liquid ratio and the leaching time were 37 °C, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 60 minutes, respectively and it did not increased significantly with further rising temperature, solid–liquid ratio and the prolonging leaching time. Therefore, we used these leaching parameters to conduct the following experiments.
10 and 60 minutes, respectively and it did not increased significantly with further rising temperature, solid–liquid ratio and the prolonging leaching time. Therefore, we used these leaching parameters to conduct the following experiments.
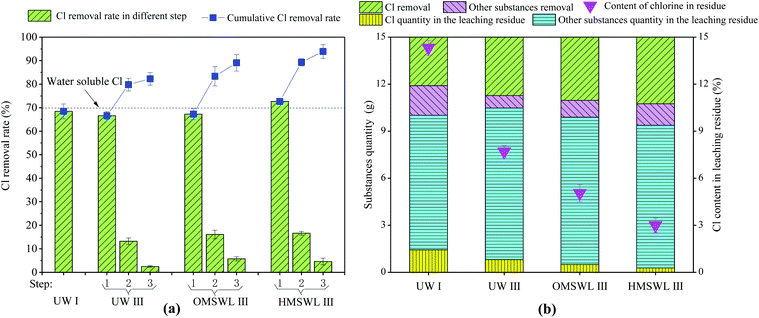
In present study, we explore the influence of different leaching solvents with different physicochemical properties (pH value, organic acids, etc.) on the fly ash dechlorination. The total Cl, water-soluble Cl and water-insoluble Cl contents are 0.30 g g−1 fly ash, 0.21 g g−1 fly ash and 0.09 g g−1 fly ash, respectively, and the water-soluble Cl quantity accounts for 70% of the total Cl quantity. The dechlorination effect of these three leaching solvents on the fly ash can be observed in Fig. 1(a). When UW is used as the leaching solvent, Cl removal rate through one-step leaching and three-step leaching is 68.7% and 82.7%, respectively. After three-step leaching, some water-insoluble Cl in the fly ash is removed, so the dechlorination effect of three-step leaching is better than that of one-step leaching. In view of this, three-step leaching is adopted for both OMSWL and HMSWL. As shown in Fig. 1(a), in three-step leaching, more Cl is removed when the initial pH is lower; specifically, 82.7%, 89.7% and 94.3% of the total Cl quantity are removed by UW, OMSWL and HMSWL, respectively. Furthermore, Cl removal through three-step OMSWL leaching (OMSWL III) and three-step HMSWL leaching (HMSWL III) is 8.5% and 14.1% higher, respectively, than the Cl removal through three-step UW leaching (UW III) and 30.6% and 37.4% higher, respectively, than the Cl removal through one-step UW leaching (UW I). Water-insoluble Cl removal through UW III, OMSWL III and HMSWL III accounts for 42.2%, 65.5% and 81.1% of the water-insoluble Cl quantity, respectively.
After the leaching experiment with different leaching solvents, the fly ash mass is reduced, as shown in Fig. 1(b). The Cl content in the residue of fly ash is determined by the Cl quantity in the residue and the total quantity of the residue. As can be seen from Fig. 1(a), the Cl quantity removed in UW I, UW III, OMSWL III and HMSWL III is 0.206, 0.248, 0.269 and 0.283 g g−1 fly ash, respectively. The total Cl content is 0.30 g g−1 fly ash. Therefore, the Cl content in the residue is 0.094, 0.052, 0.031 and 0.017 g g−1 fly ash, respectively. As can be seen from Fig. 1(b), for 15 g fly ash, the residue quantity after leaching with UW I, UW III, OMSWL III and HMSWL III is 10.01, 10.48, 9.89 and 9.37 g, respectively. Therefore, the Cl content in the residue (i.e. the ratio of the Cl quantity in the residue to the total quantity of the residue) after leaching with UW I, UW III, OMSWL III and HMSWL III is 14.1%, 7.4%, 4.7% and 2.7%, respectively.
3.2. Changes of organic matter and nitrogen in leaching solvent
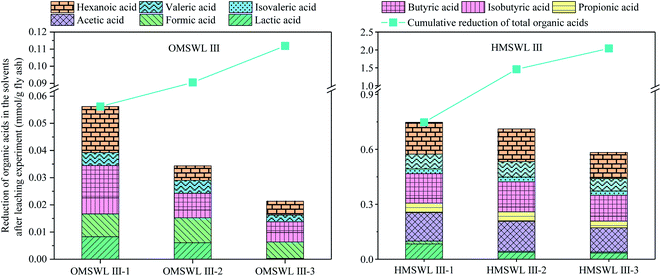
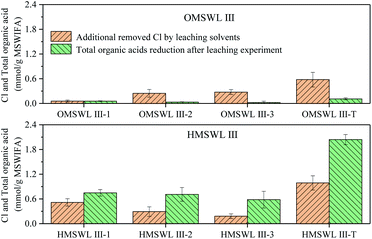
After sludge is added to OMSWL, the degradable organic matter in OMSWL is hydrolysed into ethanol and organic acids, such as lactic acid, formic acid and acetic acid. According to the different types of organic matter mainly produced in the hydrolysis process, hydrolysis can be divided into ethanol hydrolysis, mixed acid hydrolysis and butyric acid hydrolysis. These take place in the pH value ranges of 4.0–4.5, 4.5–5.5 and 5.5–6.5, respectively.36–38 The pH value of HMSWL in this study is finally stabilised around 4.9 at the end of OMSWL hydrolysis process, so OMSWL passes through mixed acid hydrolysis and becomes HMSWL. The concentrations of organic acids in OMSWL and HMSWL are shown in Table 2. The five acids with the highest concentrations in OMSWL are lactic acid, formic acid, hexanoic acid, butyric acid and isobutyric acid (in decreasing concentration order). The five acids with the highest concentrations in HMSWL are lactic acid, formic acid, hexanoic acid, acetic acid and butyric acid (in decreasing concentration order).The reduction of the organic acid content in OMSWL and HMSWL during the leaching experiments is shown in Fig. 2. The ratios of the reductions in the contents of specific organic acid to the reductions in the contents of total organic acids in each leaching step of OMSWL and HMSWL is shown in Table 3. For 1 g fly ash, the reduction in the total organic acids concentration (i.e. the sum of lactic acid, formic acid, acetic acid, propionic acid, isobutyric acid, butyric acid, isovaleric acid, valeric acid and hexanoic acid) at each leaching step is 1 > 2 > 3. After the three-step leaching, the reduced content of total organic acids in OMSWL is 0.11 mmol g−1 fly ash, accounting for 23.91% of the content of total organic acids in OMSWL (0.46 mmol g−1 fly ash). The acids with the highestest reduction are hexanoic acid, formic acid and butyric acid, accounting for 24.43%, 21.17% and 19.65% of the reduced content of total organic acids in OMSWL, respectively. After three-step leaching, the reduced content of total organic acids in HMSWL is 2.04 mmol g−1 fly ash, accounting for 34.00% of the content of total organic acids in HMSWL (6.00 mmol g−1 fly ash). The acids with the greatest reduction are hexanoic acid, acetic acid and butyric acid, accounting for 24.23%, 22.17% and 18.96% of the reduced content of total organic acids in HMSWL, respectively. Therefore, the concentration of organic acid in the leaching solvent is not necessarily proportional to the reduced content in the experimental process. For example, lactic acid is the organic acid with the highest molar concentration in OMSWL and HMSWL (61.09% and 44.39%, respectively), but the cumulative reduced content of lactic acid only accounts for 13.00% and 7.50% of the cumulative reduced content of total organic acids in OMSWL and HMSWL, respectively, after three-step leaching.
| Leaching step | Specific organic acid reduction to total organic acid reduction (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic | Formic | Acetic | Propionic | Isobutyric | Butyric | Isovaleric | Valeric | Hexanoic | |
| a ‘-T’ refers to the ratio of the reduced contents of specific organic acid to the reduced content of total organic acids after three-step leaching experiment. | |||||||||
| OMSWL III-1 | 14.68 | 14.95 | 0.00 | 0.00 | 10.53 | 21.19 | 0.00 | 8.52 | 30.12 |
| OMSWL III-2 | 17.56 | 26.78 | 0.00 | 0.00 | 8.34 | 17.86 | 0.00 | 13.95 | 15.52 |
| OMSWL III-3 | 1.21 | 28.52 | 0.00 | 0.00 | 16.29 | 18.48 | 0.00 | 11.72 | 23.79 |
| OMSWL III-Ta | 13.00 | 21.17 | 0.00 | 0.00 | 10.95 | 19.65 | 0.00 | 10.80 | 24.43 |
| HMSWL III-1 | 11.05 | 2.18 | 20.86 | 6.86 | 3.53 | 18.05 | 3.45 | 10.81 | 23.22 |
| HMSWL III-2 | 5.28 | 0.70 | 23.14 | 7.17 | 3.70 | 19.37 | 3.62 | 11.96 | 25.06 |
| HMSWL III-3 | 5.67 | 1.03 | 22.66 | 6.31 | 4.52 | 19.63 | 3.97 | 11.70 | 24.51 |
| HMSWL III-Ta | 7.50 | 1.34 | 22.17 | 6.81 | 3.87 | 18.96 | 3.66 | 11.46 | 24.23 |
We further investigate the changes in the other physicochemical properties of the leaching solvent during the experiment, in order to provide data supporting the treatment of waste liquids after leaching fly ash. As shown in Table 2, the concentrations of TOC, CODCr, NH4+–N, NO3−–N and NO2−–N in HMSWL decrease compared with OMSWL, which is mainly due to dilution caused by anaerobic sludge added in the hydrolysis process of OMSWL. Due to the adsorption of fly ash, the concentrations of TOC, CODCr, NH4+–N and NO3−–N in OMSWL after the leaching experiment decrease by 39.8%, 25.7%, 12.8% and 23.1%, respectively, whereas the concentration of NO2−–N remains unchanged. Similarly, the concentrations of TOC, CODCr, NO3−–N and NO2−–N in HMSWL after the leaching experiment decrease by 43.6%, 33.0%, 10.0% and 54.5%, respectively, whereas the concentration of NH4+–N remains unchanged.
4. Discussion
4.1. Changes of mineral phase in fly ash and the leaching residue
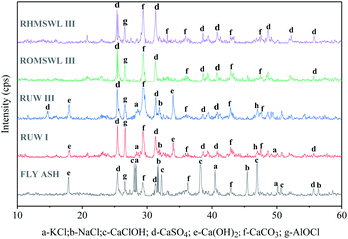
In order to explore the Cl removal mechanism in fly ash leaching using different solvents, XRD is used to analyse the mineral phases in fly ash before and after leaching. In the XRD spectrum, the relative abundance of a substance in fly ash is determined by the quantity of the substance and the total quantity of fly ash. As can be seen from Fig. 3, water-soluble Cl in fly ash mainly exists in the forms of KCl, NaCl and CaClOH, whereas water-insoluble Cl mainly exists in the forms of AlOCl (fly ash of Fig. 3). These Cl forms are similar with those met in fly ash collected in a bag filter with the injection of Ca(OH)2 for acid gas removal.7,12 As can be seen from fly ash, RUW I and RUW III of Fig. 3, most of the water-soluble Cl can be removed by UW (i.e. KCl, NaCl, CaClOH). Although the relative abundances of KCl and NaCl in RUW III are higher than that in RUW I (i.e. at 28.3° and 31.7°), the quantity of RUW III is higher than that of RUW I. So we couldn't confirm if KCl and NaCl removed more by UW III than by UW I. Compared with fly ash, the relative abundance of water-insoluble Cl–AlOCl in RUW I and RUW III increase, which may be due to a decrease in the quantity of RUW I and RUW III (Fig. 1(b)). According to the result presented in Fig. 1(a), 42.2% of water-insoluble Cl can be removed by UW III, that is to say, 42.2% of AlOCl can be removed by UW III. In addition, the XRD spectrum shows that the higher quantity of RUW III than the quantity of RUW I is mainly due to an increase in the Ca(OH)2 content. This increase may be due to the following chemical reactions during leaching:1| 2CaClOH → CaO·CaCl2 + H2O | (1) |
| CaO·CaCl2 + 5H2O → Ca(OH)2 + CaCl2·4H2O | (2) |
Compared with UW III, more Cl can be removed by OMSWL III (Fig. 1(a)), whereas the quantity of ROMSWL III is lower than that of RUW III (Fig. 1(b)). Based on the XRD spectrum (RUW III and ROMSWL III of Fig. 3), the further decrease in the quantity of ROMSWL III and Cl contained therein is mainly due to the dissolution of water-soluble Cl (KCl, NaCl) and inorganic salts (Ca(OH)2) (because their relative abundances decrease or even become zero). We couldn't confirm the quantity relationship of AlOCl between ROMSWL III and RUW III in their XRD spectrum. However, according to the result presented in Fig. 1(a), 42.2% and 65.5% of water-insoluble Cl can be removed by UW III and OMSWL III. Therefore, more AlOCl removed by OMSWL III than by UW III also contributes the decrease in the quantity of ROMSWL III and Cl contained therein.
In addition, the residue quantity and the Cl quantity contained therein can be further reduced by HMSWL III compared with OMSWL III (Fig. 1(b)). It is worth noting that compared with OMSWL III, the reduction rate of the residue quantity is lower than that of the Cl quantity removed by HMSWL III (i.e. the Cl content in the residue decreases, Fig. 1(b)). As shown for RHMSWL III and ROMSWL III of Fig. 3, the XRD spectrum of ROMSWL III is similar to that of RHMSWL III, we also couldn't confirm the quantity relationship of AlOCl between them. However, according to the result presented in Fig. 1, 81.1% and 65.5% of water-insoluble Cl can be removed by HMSWL III and OMSWL III, that is to say, more AlOCl can be removed by HMSWL III than by OMSWL III.
In summary, when UW is used as the fly ash leaching solvent, most of the water-soluble Cl (i.e. KCl, NaCl, CaClOH) in the fly ash can be removed. When OMSWL and HMSWL are used as leaching solvents, all the water-soluble Cl and some water-insoluble Cl (AlOCl) can be removed. Dechlorination is improved mainly due to the physicochemical properties (anion concentration, pH value, etc.) of OMSWL and HMSWL. The pH value and anion concentration in the leaching solvent can affect the dissolution of salts and the displacement of Cl− in fly ash. Therefore, the key to further improving fly ash dechlorination through leaching is to explore the relationship between the pH value, the anion concentration of the leaching solvent and the Cl content removed from fly ash.
4.2. Influence of organic acid concentration and types on the dechlorination of fly ash
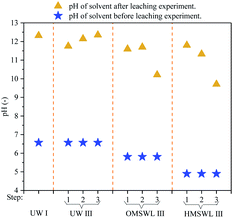
The difference in the dechlorination effect among UW, OMSWL and HMSWL is mainly caused by the difference of the pH value and the types and concentration of organic acids. Fly ash is rich in metal oxides, so when it is leached using a leaching solvent, the H+ in the leaching solvent first reacts with the metal oxides in fly ash, generating metal ions and water molecules. As is shown in Fig. 4, after three-step leaching, the pH values of the three leaching waste liquids are all higher than 9, that is to say, the concentration of H+ in the liquid is far less than the concentration of OH−. Therefore, the H+ in the three leaching solvents has mainly reacted with metal oxides, playing a limited role in the removal of water-insoluble Cl from fly ash.In the experiments conducted here, the concentration of organic acids contained in OMSWL and HMSWL decrease due to adsorption by fly ash or displacement of acid radicals with Cl−. When fly ash is leached with OMSWL III (Fig. 5), the reduced total organic acid content after each leaching step is lower than the Cl removed by OMSWL (compared with UW III-1, 2 and 3 leaching; mmol g−1 fly ash). This indicates that the greater water-insoluble Cl removal by OMSWL than UW is due to the displacement of organic acid radicals and other anions (SO42−, S2−, etc.) in OMSWL. When fly ash is leached with HMSWL III (Fig. 5), the reduced total organic acid content after each leaching step is greater than the Cl removed by HMSWL (compared with UW III-1, 2 and 3 leaching; mmol g−1 fly ash). Considering that the other anions contained in HMSWL are the same as those in OMSWL, the dechlorination improvement in HMSWL is mainly due to the displacement of organic acid radicals contained in HMSWL. As shown in Table 4, the ratios of the reductions in the contents of specific organic acids (mmol g−1 fly ash) to the additional removed Cl (compared with UW III-1, 2 and 3 leaching; mmol g−1 fly ash) in each leaching step by OMSWL are all smaller than 30%, so it is difficult to distinguish the influence of organic acids types and concentrations on the fly ash dechlorination. However, when fly ash is leached by HMSWL III, the ratio of the reductions in the contents of total organic acids (mmol g−1 fly ash) to the additional removed Cl (compared with UW III-1, 2 and 3 leaching; mmol g−1 fly ash) after the three steps of leaching by HMSWL (mmol g−1 fly ash) is 206.5%, with acetic acid, butyric acid and hexanoic acid accounting for the larger proportions. Therefore, these may be the main acids providing acid radicals to displace water-insoluble Cl.
| Leaching step | Specific organic acid reduction to additional Cl removal (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lactic | Formic | Acetic | Propionic | Isobutyric | Butyric | Isovaleric | Valeric | Hexanoic | |
| a ‘-T’ refers to the ratio of the reductions in the contents of specific organic acids to additional removed Cl after three-step leaching experiment. | |||||||||
| OMSWL III-1 | 14.51 | 14.77 | 0 | 0 | 10.41 | 20.94 | 0 | 8.42 | 29.77 |
| OMSWL III-2 | 2.45 | 3.74 | 0 | 0 | 1.16 | 2.49 | 0 | 1.95 | 2.17 |
| OMSWL III-3 | 0.09 | 2.21 | 0 | 0 | 1.26 | 1.43 | 0 | 0.91 | 1.84 |
| OMSWL III-Ta | 2.51 | 4.09 | 0 | 0 | 2.12 | 3.80 | 0 | 2.09 | 4.72 |
| HMSWL III-1 | 16.00 | 3.16 | 30.23 | 9.94 | 5.11 | 26.16 | 5.00 | 15.66 | 33.64 |
| HMSWL III-2 | 12.86 | 1.71 | 56.40 | 17.47 | 9.02 | 47.20 | 8.83 | 29.14 | 61.08 |
| HMSWL III-3 | 18.27 | 3.32 | 73.04 | 20.34 | 14.57 | 63.25 | 12.81 | 37.70 | 78.98 |
| HMSWL III-Ta | 15.49 | 2.76 | 45.79 | 14.07 | 8.00 | 39.16 | 7.56 | 23.68 | 50.04 |
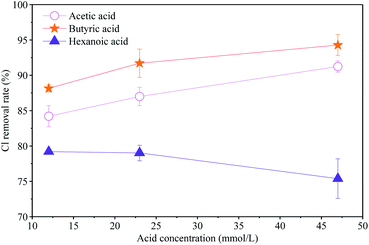
In the fly ash leaching experimental system, the decrease of organic acid concentration may be due to adsorption by fly ash or displacement of acid radicals with Cl. In order to explore further the contribution of acetic acid, butyric acid and hexanoic acid to the dechlorination process, fly ash was leached with different concentrations of these three pure acids (the highest concentration is close to their concentration in HMSWL) by one-step leaching. As can be seen in Fig. 6, the Cl content removed from fly ash increases with the concentration of butyric acid and acetic acid, and the dechlorination effect of butyric acid is greater than that of acetic acid. However, the Cl content removed in fly ash decreases with increasing hexanoic acid concentration, which means that hexanoic acid has the smallest effect on the dechlorination of fly ash. The results showed that compared with UW and OMSWL, be mainly due to the displacement of the water-insoluble Cl in fly ash by the butyrate radical contained in HMSWL. Due to the long carbon chain of hexanoic acid (which is in contact with the moisture layer when the concentration is higher), the decrease of the concentration of hexanoic acid in HMSWL after the leaching experiment may be caused by adsorption of fly ash.
4.3. Potential disposal scheme for the fly ash leaching residue and Cl-containing leaching solvents
As aforementioned, the fly ash is a promising raw material for cement production once the Cl can be sufficiently removed. The heavy metals and Cl content in leaching residues and their regulation in Chinese standard (HJ662-2013) can be found in Table 5. According to the regulation of Cl in standard HJ662-2013, the maximum addition fraction of RHMSWL III to the total raw materials entering the cement kiln is 1.5%, assuming that the main raw materials for cement kiln are chloride-free. In this context, the heavy metals contents of RHMSWL III can fully meet the corresponding regulation for raw materials of cement kiln in standard HJ662-2013. In addition, the organic matters adsorbed by the residue of HMSWL III can be transformed into CO2 in cement kiln. Therefore, utilization as raw material for cement kiln could be a promising destination for the fly ash leaching residue.| Standard and washed residues | Indexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mn (μg g−1) | Cr (μg g−1) | As (μg g−1) | Cu (μg g−1) | Pb (μg g−1) | Cd (μg g−1) | Zn (μg g−1) | Cl (wt%) | Maximum addition fractiona (%) | |
| a “Maximum addition fraction” refers to the maximum addition fraction of washing residue to the total raw materials entering the cement kiln, assuming that the main raw materials for cement kiln are chloride-free. | |||||||||
| HJ662-2013 | 3320 | 320 | 4280 | 7920 | 1590 | 40 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
0.04 | — |
| FLY ASH | 160 | 745 | 15 | 335 | 500 | 50 | 930 | 30.1 | 0.13 |
| RUW I | 160 | 744 | 15 | 334 | 300 | 50 | 901 | 14.1 | 0.28 |
| RUW III | 160 | 745 | 15 | 331 | 326 | 50 | 918 | 7.4 | 0.54 |
| ROMSWL III | 238 | 746 | 17 | 142 | 483 | 47 | 559 | 4.7 | 0.86 |
| RHMSWL III | 283 | 747 | 18 | 69 | 431 | 43 | 645 | 2.7 | 1.50 |
Disposal of Cl-containing wasted leaching solvents is considered ‘the last-mile problem’ for the application of this technology. As shown in Table 2, the concentrations of heavy metals and Cl in leaching solvents after leaching experiments are considerably high. Therefore, it could not be treated by the conventional biological wastewater treatment method. In current study, we propose a potential disposal scheme for Cl-containing leaching solvents. Firstly, to remove the heavy metals, chemical precipitation (e.g. carbonate coprecipitation) method is recommended; secondly, the remaining liquid was subjected to evaporation and crystallization step. The crystalized salts is mainly consisted by Cl salts, which can be further purified and recycled. Alternatively, the wasted leaching solvents after heavy metals removal can also be treated by electrolysis. In this scenario, the Cl− can be transformed into Cl2 for reuse; finally, the remaining waste liquid with little heavy metals, Cl salts and high concentration of organic matters can be further treated by the traditional biological treatment methods. However, the feasibility and economic efficiency of the disposal schemes need to be further evaluated before field implementation.
5. Conclusion
In this study, HMSWL was employed to leach fly ash. The results revealed that three-step HMSWL leaching removed 94.3% of the total Cl of fly ash, which was higher than that achieved through three-step UW leaching. The X-ray diffraction analysis results indicated that three-step UW leaching could remove water-soluble Cl in the forms of KCl, NaCl, CaClOH and some water-insoluble Cl in the forms of AlOCl, whereas three-step HMSWL leaching can further eliminate more water-insoluble Cl as well, in the forms of AlOCl. The data analysis further suggested that the displacement effects of organic acid radicals (especially that by the butyrate radical) were the major cause of water-insoluble Cl removal.Abbreviations
| OMSWL | Original liquid of the municipal solid waste leachate |
| HMSWL | Hydrolysate of the municipal solid waste leachate |
| UW | Ultrapure water |
| OMSWL III | Three-step OMSWL leaching |
| HMSWL III | Three-step HMSWL leaching |
| UW III | Three-step UW leaching |
| UW I | One-step UW leaching |
| ROMSWL III | Residue of the fly ash washed by OMSWL III |
| RHMSWL III | Residue of the fly ash washed by HMSWL III |
| RUW III | Residue of the fly ash washed by UW III |
| RUW I | Residue of the fly ash washed by UW I |
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The study was supported by the National Key Research and Development Program of China (2016YFE0127800 and 2019YFC1906300), the National Natural Science Foundation of China (Grant No. 51778052) and the Sino-US-Japan Joint Laboratory on Organic Solid Waste Resource and Energy Technology of USTB. Thanks to Loissi Kalakodio for his help.References
- L. Wang, Y. Jin and Y. Nie, J. Hazard. Mater., 2010, 174, 334–343 CrossRef CAS PubMed.
- K. Anastasiadou, K. Christopoulos, E. Mousios and E. Gidarakos, J. Hazard. Mater., 2012, 207–208, 165–170 CrossRef CAS PubMed.
- G. Qian, H. Zhang, X. Zhang and P. Chui, J. Hazard. Mater., 2005, 121, 251–258 CrossRef CAS PubMed.
- M. J. Quina, J. C. Bordado and R. M. Quinta-Ferreira, Waste Manag., 2008, 28, 2097–2121 CrossRef CAS PubMed.
- B. Van der Bruggen, G. Vogels, P. Van Herck and C. Vandecasteele, J. Hazard. Mater., 1998, 57, 127–144 CrossRef CAS.
- P. P. Bosshard, R. Bachofen and H. Brandl, Environ. Sci. Technol., 1996, 30, 3066–3070 CrossRef CAS.
- F. Zhu, M. Takaoka, K. Shiota, K. Oshita and Y. Kitajima, Environ. Sci. Technol., 2008, 42, 3932–3937 CrossRef CAS PubMed.
- C. Ferreira, A. Ribeiro and L. Ottosen, J. Hazard. Mater., 2003, 96, 201–216 CrossRef CAS PubMed.
- C. M. Hansson, T. Frølund and J. B. Markussen, Cem. Concr. Res., 1985, 15, 65–73 CrossRef CAS.
- M. Tyrer, Eco-Efficient Concrete, ed. F. Pacheco-Torgal, S. Jalali, J. Labrincha and V.M. John, Woodhead Publishing Limited, Sawston Cambridge, UK, 1st edn, 2013, vol. 59, pp. 273–310 Search PubMed.
- C. Chen and I. Chiou, J. Hazard. Mater., 2007, 148, 346–352 CrossRef CAS PubMed.
- B. Wu, D. Wang, X. Chai, F. Takahashi and T. Shimaoka, Front. Environ. Sci. Eng., 2016, 10, 43–51 Search PubMed.
- G. Boghetich, L. Liberti, M. Notarnicola, M. Palma and D. Petruzzelli, Waste Manage. Res., 2005, 23, 57–61 CrossRef CAS PubMed.
- I. H. Hwang, T. Matsuto and N. Tanaka, Waste Manag., 2006, 26, 571–579 CrossRef CAS PubMed.
- R. Yang, W. Liao and P. Wu, J. Environ. Manage., 2012, 104, 67–76 CrossRef CAS PubMed.
- X. Zuo, Y. Tang, G. Yin, K. Jiang and S. He, J. Mater. Civ. Eng., 2017, 29, 04017187 CrossRef.
- F. Zhu, M. Takaoka, K. Oshita and S. Morisawa, J. Air Waste Manage. Assoc., 2011, 61, 740–746 CrossRef CAS PubMed.
- S. Yang, A. Saffarzadeh, T. Shimaoka and T. Kawano, J. Hazard. Mater., 2014, 267, 214–220 CrossRef CAS PubMed.
- R. Ito, G. Dodbiba, T. Fujita and J. W. Ahn, Waste Manag., 2008, 28, 1317–1323 CrossRef CAS PubMed.
- H. Chang, X. Quan, N. Zhong, Z. Zhang, C. Lu, G. Li, Z. Cheng and L. Yang, Bioresour. Technol., 2018, 266, 374–381 CrossRef CAS PubMed.
- S. M. Iskander, J. T. Novak and Z. He, Bioresour. Technol., 2018, 255, 76–82 CrossRef CAS PubMed.
- X. Li, Y. Yuan, F. Wang, Y. Huang, Q. Qiu, Y. Yi and Z. Bi, Bioresour. Technol., 2018, 265, 357–364 CrossRef CAS PubMed.
- A. Colombo, A. N. Módenes, D. E. Góes Trigueros, S. I. Giordani Da Costa, F. H. Borba and F. R. Espinoza-Quiñones, J. Cleaner Prod., 2019, 214, 145–153 CrossRef CAS.
- K. Kaur, S. Mor and K. Ravindra, J. Colloid Interface Sci., 2016, 469, 338–343 CrossRef CAS PubMed.
- D. Zou, Y. Chi, C. Fu, J. Dong, F. Wang and M. Ni, J. Hazard. Mater., 2013, 248–249, 177–184 CrossRef CAS PubMed.
- L. Gao and J. L. Goldfarb, RSC Adv., 2019, 9, 16018–16027 RSC.
- X. Ren, L. Xiao, R. Qu, S. Liu, D. Ye, H. Song, W. Wu, C. Zheng, X. Wu and X. Gao, RSC Adv., 2018, 8, 42200–42209 RSC.
- S. Mohan and R. Gandhimathi, J. Hazard. Mater., 2009, 169, 351–359 CrossRef CAS PubMed.
- Q. Xue, J. S. Li, P. Wang, L. Liu and Z. Z. Li, Clean: Soil, Air, Water, 2015, 42, 1626–1631 Search PubMed.
- S. Chugh, D. P. Chynoweth, W. Clarke, P. Pullammanappallil and V. Rudolph, Bioresour. Technol., 1999, 69, 103–115 CrossRef CAS.
- C. J. Banks and L. Huang-Mu, Waste Manage. Res., 2003, 21, 225–234 CrossRef CAS PubMed.
- P. He, H. Pu, L. Shao and H. Zhang, Waste Manag., 2017, 69, 232–241 CrossRef CAS PubMed.
- Y. Xu, Y. Fu, W. Xia, D. Zhang, D. An and G. Qian, Environ. Technol., 2018, 39, 1949–1954 CrossRef CAS PubMed.
- H. Bouallagui, M. Torrijos, J. J. Godon, R. Moletta, R. Ben Cheikh, Y. Touhami, J. P. Delgenes and M. Hamdi, Biochem. Eng. J., 2004, 21, 193–197 CrossRef CAS.
- S. Lim, E. Kim, Y. Ahn and H. Chang, Korean J. Chem. Eng., 2008, 25, 129–133 CrossRef CAS.
- S. Begum, G. R. Anupoju, S. Sridhar, S. K. Bhargava, V. Jegatheesan and N. Eshtiaghi, Bioresour. Technol., 2018, 251, 364–373 CrossRef CAS.
- J. Horiuchi, T. Shimizu, T. Kanno and M. Kobayashi, Bioresour. Technol., 1999, 13, 155–157 CAS.
- Y. Ren, M. Yu, C. Wu, Q. Wang, M. Gao, Q. Huang and Y. Liu, Bioresour. Technol., 2018, 247, 1069–1076 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |