Open Access Article
Open Access ArticleLipofection with estrone-based luminophores featuring aggregation-induced emission properties†
Steffen Riebe‡
a,
Alexander Zimmermann‡a,
Johannes Kochb,
Cecilia Valletc,
Shirley K. Knauerc,
Andrea Sowaa,
Christoph Wölperd and
Jens Voskuhl *a
*a
aInstitute for Organic Chemistry and CENIDE, University of Duisburg-Essen, Universitätsstrasse 7, 45141 Essen, Germany. E-mail: jens.voskuhl@uni-due.de
bICCE, Center of Medical Biotechnology (ZMB), University of Duisburg-Essen, Universitätsstrasse 2, 45141 Essen, Germany
cDepartment of Molecular Biology II, Center of Medical Biotechnology (ZMB), University of Duisburg-Essen, 45117 Essen, Germany
dInstitute for Inorganic Chemistry and Center for NanoIntegration (CENIDE), University of Duisburg-Essen, Universitätsstrasse 5–7, 45117 Essen, Germany
First published on 27th May 2020
Abstract
In this communication we present the use of a novel class of luminophores with aggregation-induced emission (AIE) properties based on the steroid estrone. These molecules were equipped with cationic residues yielding amphiphiles suitable for lipofection. To this end, self-assembled luminescent structures were formed in aqueous media and mixed with a red-fluorescent protein expressing plasmid, yielding lipoplexes with increased emission intensity. These luminescent lipoplexes were able to efficiently transfect HeLa and HEK 293T cells and were able to be tracked due to the aggregation induced-emission properties.
The transport of genetic information into living cells yielding to hybridisation inside the nucleus is the key challenge in modern gene therapy. Over the past decades several approaches have been investigated to deliver specific DNA sequences and/or plasmids into nuclei to address severe diseases such as cancer1–3 or neurodegenerative diseases such as Parkinson's4,5 or Alzheimer's.6,7 A major problem in this regard is the negative surface charge of the cellular corona based on the so-called glycocalyx, which mainly consists of sialic acids and its derivatives.8 This prevents the entering of genetic information due to strong electrostatic repulsion of the phosphate backbones and the cellular surface. To this end, several approaches have been investigated to overcome this major problem. Three different compound classes have been used to form neutral to cationic complexes with DNA sequences: (A) small cationic organic compounds and cationic polymers, (B) cationic amphiphiles9,10 and (C) retroviruses,11 which are able to cross the membrane barrier and induce efficient transfection. All these potential candidates have their individual advantages and disadvantages. Small organic cationic compounds or polymers12–14 (e.g. polyethyleneimine, PEI) are known to be toxic at higher doses, which also is the case of amphiphilic structures such as Lipofectamine 2000™.15 Retroviruses are known to induce an immune response to the infected body leading to severe side effects.16,17 Especially lipofection has attracted our interest since the formation of larger self-assembled structures bearing multiple cationic charges at their surface leads to efficient DNA carriers (lipoplex). Here, several approaches for the use of amphiphiles to generate vesicles,18 micelles19 and nanotubes20 have been described in literature. Lehn21 and Huang et al.22 showed that the variation of the hydrophobic tail (e.g. cholesterol) had a huge influence on the transfection ability of cationic amphiphiles. Besides the variation of the hydrophobic part also the cationic head groups have been varied extensively. Here, especially linear or branched amines23 and oligoamines24 and oligoarginines25,26 as well as artificial binding motifs such as guanidinium–carbonylpyrroles27–29 have attracted interest and were able to efficiently induce transfection.
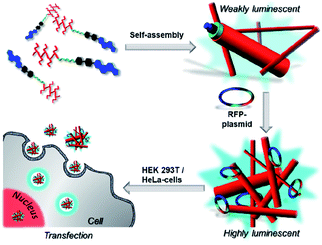
Up to now, the exact mode of action (e.g. the pathway of the vectors through the cell membrane and inside the cytosol) remained unclear in most of the above mentioned examples. Thus, we decided to use the well-known AIE-effect to follow the way of transfection. Here, luminophores show induced emission when fixed in a steric demanding (e.g. in the aggregated, bound or solid state) environment due to a restriction of intramolecular motion. First AIE-active compounds were described by Tang et al. back in 2001. Recently, we were able to synthesize a novel and easily accessible class of AIE emitters with fluorescence and phosphorescence properties. These compounds were already used for numerous applications such as luminescent micelles,30 organogels,31,32 liquid crystals33,34 and bio-imaging.35 Here, we combine our unique system with the steroid estrone, conveying luminescence properties due to its aromatic unit. Furthermore estrone was used, since it bears the characteristic steroid backbone, which is also existent in cholesterol, which is a vital part of the cellular membrane. Hence we assume a strong interaction of our transfection vectors with the cellular membrane, leading to an efficient uptake. Moreover, it is essential to mediate interactions with the cellular membrane via cationic groups such as oligoamines or tri-arginines. Subsequently, the obtained amphiphiles are able to assemble into higher ordered structures. These supra-structures should allow electrostatically binding of DNA sequences or plasmids leading to lipoplexes, which will be tested for their transfection capacity (Scheme 1). Furthermore, the self-assembled structures will be fluorescent due to the restriction of motion of the AIE active core motif upon self-assembly. Besides that, increased emission is expected upon DNA (mRFP-plasmid) binding due to cross linking of the single higher ordered structures. This behaviour furthermore enables the tracking of the lipoplexes inside the cellular environment. A further advantage of this design is the role of the luminophore as vital part – the hydrophobic residue in the amphiphile – of the transfection system, compared to the classic labelling approach of known vectors, which is often accompanied by enhanced toxicity (Scheme 2).36,37
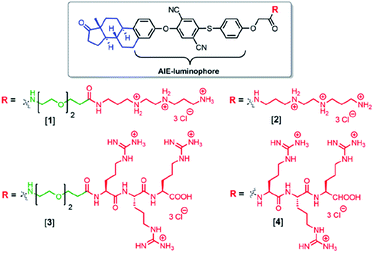 | ||
| Scheme 2 Molecular structures of the estrone-based AIE-active transfection vectors used in this study. | ||
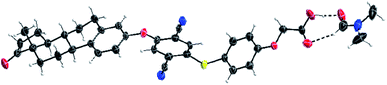
Compounds [1] and [2] were synthesized by coupling tri-(boc) protected N,N′-bis(2-aminoethyl)-1,3-propanediamine to the carboxylic acid functionalized compounds [F] (Fig. 1) and the triethyleneglycol containing analogues [H] followed by acid-assisted deprotection (see ESI†). The synthesis for compounds [3] and [4] started from a solid phase synthesis assisted route. Three arginines were coupled on the resin followed by attachment of either the AIE luminophore [F] or the AIE active compound with triethyleneglycol spacer [H] under peptide coupling conditions (see ESI†). The structure of [F] was confirmed by X-ray crystallography (see ESI†). [F] crystallizes in the tetragonal space group P41212 with one molecule plus a DMF solvent molecule in the asymmetric unit. Alternatively, a description as merohedral twin in space group P41 is possible (Fig. 1 and S17†).
From the obtained data it can be seen that all bond lengths and angles are within the expected range and the overall conformation of the estrone residue remains unchanged.
With these compounds in stock we performed an in depth investigation of the self-assembly properties of all four compounds in water in the presence and absence of plasmid, expressing a fluorescent protein (RFP), C-terminally fused to a nuclear histone protein H2B (pH2B-mRFP).
Compounds [1–4] were dissolved in water followed by vortexing, yielding self-assembled structures which were investigated by fluorescence spectroscopy (Fig. S10†).
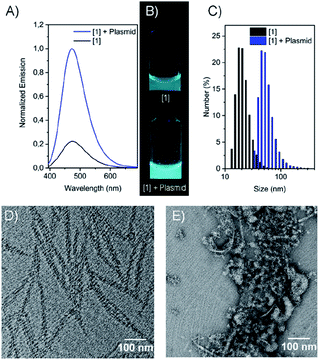
Here in all cases an emission signal at around 470–480 nm was observed which can be excited at 380 nm (see Fig. S9†), due to the formation of self-assembled structures in aqueous media. This emission cannot be observed in DMSO due to the free molecular movement, which is typical for aggregation-induced emission. The critical aggregation concentration (CAC) was determined by dilution of the aqueous samples followed by tracking of the emission wavelength, assuming a change in emission upon self-assembly due to changes in the molecular environment. Here, in all cases a change in the emission wavelength was determined for concentrations below 2 μM (Fig. S14 and S15†). To get a deeper impression of the morphology of compounds [1–4] in aqueous media, TEM imaging was performed (Fig. 2D, E and S16†). Compound [1] and [2], both revealed fibre-like structures with a thickness of around 10–15 nm, whereas [3] and [4] showed more granular and less organized morphologies in aqueous media (Fig. S16†).
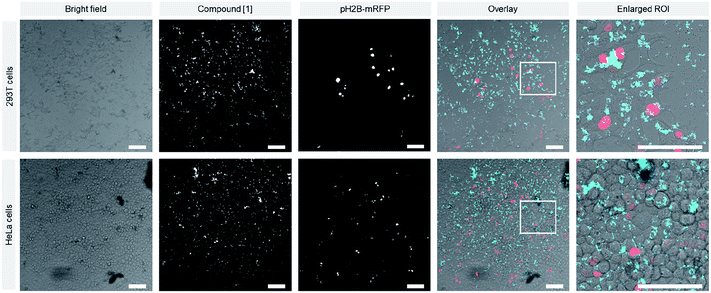
These structures showed low to moderate emission properties, which was attributed to a higher polarity and a weaker packing inside the aggregates (Fig. S10 and S12†). Besides that, ζ-potential measurements showed highly positively charged assemblies with a potential between 42–62 mV as expected for the cationic amphiphiles. Next, the influence of electrostatic binding of the RFP-plasmid was investigated in detail. The morphologies obtained were mixed with the plasmid and emission wavelengths, sizes, ζ-potentials as well as the morphologies were determined. Interestingly, in all cases a drastic fluorescence increase was observed due to the assumed formation of densely packed lipoplexes, hindering additionally the motion of the estrone-based AIE core (Fig. 2B, S12, and S13†). Besides that, for most of the compounds an increased size was determined by DLS-measurements attributed to the formation of larger structures (Fig. 2C and S6†). The decrease in ζ-potential for the lipoplexes goes along with the partial charge neutralisation (e.g. for [1] without plasmid = 62 mV and with plasmid = 49 mV) (Fig. S7 and S8†). Here compound [3] showed only marginal changes in the ζ-potential which was explained by a weak or incomplete accessibility of the cationic head groups for the plasmid DNA. TEM imaging revealed larger aggregates (lipoplexes) showing in the case of [1] (Fig. 2E) and [2] networks of thin fibres in close contact, whereas [3] and [4] showed crosslinked assemblies with worm-like morphologies (Fig. S16†). After confirmation of the electrostatically assembled lipoplexes with enhanced emission properties, first experiments to use these structures for transfection of HeLa and HEK 293T cells were conducted in which we tested the ability of the compounds [1–4] to transport plasmid DNA into cell. Therefore, HeLa und HEK 293T cells were incubated with a solution of the compounds pre-mixed with plasmid DNA. After 16 h these cells were analyzed via confocal laser scanning microscopy. Surprisingly only for compounds [1] and [2] nuclear RFP was observed, which is indicative for successful gene delivery (Fig. 3 and S20–S22†). However transfection efficiency was only marginal for [2], and treatment of cells with this compound resulted in an abnormal morphology even at low concentrations (10 μM). Compounds [3] and [4] were not able to mediate gene transfection (Fig. S23 and S24†). Thus, we focused our research on the transfection properties of [1].
The lipoplex of [1] and pH2B-mRFP was uniformly taken up by both cell lines and led to an efficient transfection rate inferior to Lipofectamine™ 2000 (Fig. S18, S19, and S26†).
The determined transfection efficiency of compound [1] in comparison with the gold standard Lipofectamine™ 2000 was determined. In the case of HEK 293T cells an efficiency of 8.4% and for HeLa cells 12.6% was found which is lower than Lipofectamine™ 2000 (31.4% (HeLa), 31.6% (HEK 293T)) (Fig. S26†).
In contrast to Lipofectamine™, localization of the lipoplex can easily be monitored by the AIE-effect of the estrone based luminophore (Fig. 3 and S18–S20†). To assess the cytotoxicity of the compounds, we performed an MTS assay, which showed a slightly decreased viability of HeLa cells treated with [1] at high concentrations compared to Lipofectamine™ 2000-treated cells (Fig. 4). Interestingly, compound [1] seemed to localize in spherical vesicles within the cells after transfection. To clarify the mechanism of uptake, we treated HeLa cells with [1] pre-mixed with plasmid DNA and stained the lysosomes with LysoTracker™ Green (Fig. S25†). We observed a partial colocalization of [1] with the lysosomes, which suggests an uptake via the endosomal pathway followed by an endosomal escape or lysosomal degradation. Although we are aware that the transfection potential of our compound so far is still inferior to the gold standard of lipofection, it opens a completely new field of transfections vectors featuring AIE-properties. These are enabling future research by varying the single parts of the vectors, e.g. cationic headgroups, linker as well as steroid anchors. Furthermore, we were able to show that the AIE luminophore is not only a luminescent tag but also key structure of the transfection system representing a completely novel approach for transfection vectors.
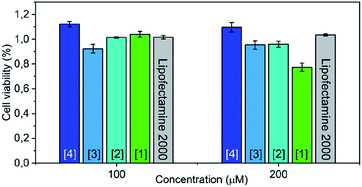 | ||
| Fig. 4 Concentration-dependent cytotoxicity as determined by an MTS assay of compounds [1–4] compared with lipofectamine™ 2000 as reference. | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Center for Nanointegration (CENIDE), Imaging Centre Campus Essen (ICCE), the Center of Medical Biotechnology (ZMB) and the Interdisciplinary Center for Analytics on the Nanoscale (ICAN).Notes and references
- F. McCormick, Nat. Rev. Cancer, 2001, 1, 130 CrossRef CAS PubMed.
- A. El-Aneed, J. Controlled Release, 2004, 94, 1–14 CrossRef CAS PubMed.
- R. G. Vile, S. J. Russell and N. R. Lemoine, Gene Ther., 2000, 7, 2 CrossRef CAS PubMed.
- M. G. Kaplitt, A. Feigin, C. Tang, H. L. Fitzsimons, P. Mattis, P. A. Lawlor, R. J. Bland, D. Young, K. Strybing, D. Eidelberg and M. J. During, Lancet, 2007, 369, 2097–2105 CrossRef CAS.
- P. A. LeWitt, A. R. Rezai, M. A. Leehey, S. G. Ojemann, A. W. Flaherty, E. N. Eskandar, S. K. Kostyk, K. Thomas, A. Sarkar, M. S. Siddiqui, S. B. Tatter, J. M. Schwalb, K. L. Poston, J. M. Henderson, R. M. Kurlan, I. H. Richard, L. Van Meter, C. V. Sapan, M. J. During, M. G. Kaplitt and A. Feigin, Lancet Neurol., 2011, 10, 309–319 CrossRef CAS PubMed.
- M. H. Tuszynski, L. Thal, M. Pay, D. P. Salmon, H. S. U, R. Bakay, P. Patel, A. Blesch, H. L. Vahlsing, G. Ho, G. Tong, S. G. Potkin, J. Fallon, L. Hansen, E. J. Mufson, J. H. Kordower, C. Gall and J. Conner, Nat. Med., 2005, 11, 551 CrossRef CAS PubMed.
- M. H. Tuszynski, Alzheimer Dis. Assoc. Disord., 2007, 21, 179–189 CrossRef CAS PubMed.
- N. M. Varki and A. Varki, Lab. Invest., 2007, 87, 851 CrossRef CAS PubMed.
- J. Zabner, A. J. Fasbender, T. Moninger, K. A. Poellinger and M. J. Welsh, J. Biol. Chem., 1995, 270, 18997–19007 CrossRef CAS PubMed.
- H. Farhood, N. Serbina and L. Huang, Biochim. Biophys. Acta, Biomembr., 1995, 1235, 289–295 CrossRef.
- W. S. Pear, G. P. Nolan, M. L. Scott and D. Baltimore, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 8392–8396 CrossRef CAS PubMed.
- D. G. Anderson, D. M. Lynn and R. Langer, Angew. Chem., 2003, 115, 3261–3266 CrossRef.
- H. Lv, S. Zhang, B. Wang, S. Cui and J. Yan, J. Controlled Release, 2006, 114, 100–109 CrossRef CAS.
- D. Fischer, T. Bieber, Y. Li, H.-P. Elsässer and T. Kissel, Pharm. Res., 1999, 16, 1273–1279 CrossRef CAS.
- A. Masotti, G. Mossa, C. Cametti, G. Ortaggi, A. Bianco, N. D. Grosso, D. Malizia and C. Esposito, Colloids Surf., B, 2009, 68, 136–144 CrossRef CAS.
- M. Mesri, N. R. Wall, J. Li, R. W. Kim and D. C. Altieri, J. Clin. Invest., 2001, 108, 981–990 CrossRef CAS.
- E. Check, Nature, 2003, 423, 573 CrossRef CAS PubMed.
- S. Oguchi, M. Kamihira, J. You, A. Tachibana and S. Iijima, J. Ferment. Bioeng., 1998, 86, 118–120 CrossRef CAS.
- C. Wölk, D. Pawlowska, S. Drescher, A. Auerswald, A. Meister, G. Hause, A. Blume, A. Langner, G. Brezesinski and B. Dobner, Langmuir, 2014, 30, 4905–4915 CrossRef.
- A. K. Salem, P. C. Searson and K. W. Leong, Nat. Mater., 2003, 2, 668 CrossRef CAS PubMed.
- J. P. Vigneron, N. Oudrhiri, M. Fauquet, L. Vergely, J. C. Bradley, M. Basseville, P. Lehn and J. M. Lehn, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 9682–9686 CrossRef CAS PubMed.
- X. Gao and L. Huang, Biochem. Biophys. Res. Commun., 1991, 179, 280–285 CrossRef CAS.
- S. Fletcher, A. Ahmad, E. Perouzel, M. R. Jorgensen and A. D. Miller, Org. Biomol. Chem., 2006, 4, 196–199 RSC.
- S. Fletcher, A. Ahmad, W. S. Price, M. R. Jorgensen and A. D. Miller, ChemBioChem, 2008, 9, 455–463 CrossRef CAS PubMed.
- V. B. Morris and C. P. Sharma, Biomaterials, 2010, 31, 8759–8769 CrossRef CAS PubMed.
- N. A. Alhakamy and C. J. Berkland, Mol. Pharmaceutics, 2013, 10, 1940–1948 CrossRef CAS.
- K. Samanta, P. Jana, S. Bäcker, S. Knauer and C. Schmuck, Chem. Commun., 2016, 52, 12446–12449 RSC.
- X.-Y. Hu, M. Ehlers, T. Wang, E. Zellermann, S. Mosel, H. Jiang, J.-E. Ostwaldt, S. K. Knauer, L. Wang and C. Schmuck, Chem.–Eur. J., 2018, 24, 9754–9759 CrossRef CAS PubMed.
- P. Jana, K. Samanta, S. Bäcker, E. Zellermann, S. Knauer and C. Schmuck, Angew. Chem., 2017, 129, 8195–8200 CrossRef.
- P. Ahlers, C. Götz, S. Riebe, M. Zirbes, M. Jochem, D. Spitzer, J. Voskuhl, T. Basché and P. Besenius, Polym. Chem., 2019, 10, 3163–3169 RSC.
- D. Aschmann, S. Riebe, T. Neumann, D. Killa, J.-E. Ostwaldt, C. Wölper, C. Schmuck and J. Voskuhl, Soft Matter, 2019, 15, 7117–7121 RSC.
- M. Externbrink, S. Riebe, C. Schmuck and J. Voskuhl, Soft Matter, 2018, 14, 6166–6170 RSC.
- S. Riebe, M. Saccone, J. Stelzer, A. Sowa, C. Wolper, K. Soloviova, C. A. Strassert, M. Giese and J. Voskuhl, Chem.–Asian J., 2019, 14, 814–820 CrossRef CAS.
- M. Saccone, M. Blanke, C. G. Daniliuc, H. Rekola, J. Stelzer, A. Priimagi, J. Voskuhl and M. Giese, ACS Mater. Lett., 2019, 1, 589–593 CrossRef CAS.
- J. Stelzer, C. Vallet, A. Sowa, D. Gonzalez-Abradelo, S. Riebe, C. G. Daniliuc, M. Ehlers, C. A. Strassert, S. K. Knauer and J. Voskuhl, ChemistrySelect, 2018, 3, 985–991 CrossRef CAS.
- R. S. Gupta and A. K. Dudani, J. Cell. Physiol., 1987, 130, 321–327 CrossRef CAS PubMed.
- J. S. Modica-Napolitano and J. R. Aprille, Cancer Res., 1987, 47, 4361–4365 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: For synthesis, photophysical properties, characterisation of the formed structures and CLSM-microscopy. CCDC 1938010. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03608k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |