Open Access Article
Open Access ArticleThe contributions and mechanisms of iron-microbes-biochar in constructed wetlands for nitrate removal from low carbon/nitrogen ratio wastewater†
Jian Xuab,
Xiawei Liuab,
Jiaolong Huangab,
Manqi Huangab,
Tao Wangab,
Shaopan Baoa,
Wei Tanga and
Tao Fang *ab
*ab
aInstitute of Hydrobiology, Chinese Academy of Sciences, No. 7 Donghu South Road, Wuchang District, Wuhan 430072, China. E-mail: fangt@ihb.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 17th June 2020
Abstract
The removal efficiency of nitrate from low carbon/nitrogen ratio wastewater has been restricted by the lack of organics for several decades. Here, a system coupling chemical reduction, microbial denitrification and constructed wetlands (RDCWs) was developed to investigate the effect and possible mechanisms for nitrate degradation. The results showed that this coupling system could achieve a nitrate removal efficiency of 97.07 ± 1.76%, 85.91 ± 3.02% and 56.63 ± 2.88% at a hydraulic retention time of 24 h, 12 h and 6 h with feeding nitrate of 15 mg L−1, respectively. These removal efficiencies of nitrate were partly caused by microbes and biochar with a contribution rate of 31.08 ± 4.43% and 9.50 ± 3.30%. Besides, microbes were closely related to iron and biochar for the removal of nitrate. Simplicispira was able to utilize hydrogen produced by iron corrosion as an electron donor while nitrate accepted electrons to be reduced. Porous biochar could release dissolved organic matter, which provided a good living circumstance and carbon source for microbes. Therefore, the RDCW system is potential for large-scale application due to its low cost and simple operation.
1. Introduction
Nitrate in water bodies is a widespread environmental pollutant mainly from the use of a large number of artificial fertilizers, discharge of domestic sewage, output of immoderate industrial by-products, etc.1 It is universally found in groundwater, polluted surface water and the outflow of sewage treatment plants,2 and nitrate-polluted water commonly possesses a low carbon/nitrogen ratio (COD/TN lower than 3) feature.3 Nitrate contaminations can bring about eutrophication and are even likely to pose a potential risk to human health. There is, therefore, increased attention for the removal of nitrate from low carbon/nitrogen ratio wastewater.In recent years, constructed wetlands (CWs) which take advantage of the substrates, such as quartz sand, coarse gravel, ceramsite and shells, to remove suspended solid and nitrate in wastewater have become more and more popular.4 The mechanisms of the removal include filtration, adsorption, precipitation, volatilization, plant uptake and microbial processes.5 The CWs can better reflect actual nitrate removal efficiency compared to our previous batch experiments.6 Zhang et al. and Tang et al. used combined reactors including multiple substrates to degrade nitrogen.7,8 Wu et al. and Zhu et al. removed nitrate through different bioreactors.9,10 Although CWs are low cost and simple maintenance requirements,11 the removal efficiency of nitrate is limited. Jia et al. and Wu et al. reported that CWs have not well nitrate removal efficiency for the tailwater from sewage treatment plants.12,13 This lies in the fact that microbial denitrification as main role in CWs utilizes organic carbon source to degrade nitrate while there is a lack of organics in low carbon/nitrogen ratio wastewater. The conflict of two phenomena leads to low nitrate removal rate.
The most of microbial nitrate removal methods in CWs are heterotrophic denitrification, which demands organic carbon, like sucrose, methanol, ethanol and acetate,6 as carbon source and electron donor. In contrast, microbes carrying out autotrophic denitrification make use of inorganic matter as energy and electron donor. For low carbon/nitrogen ratio wastewater, its organics content is low-down or close to none. Thus, heterotrophic denitrifying microbes are inadaptable to live in this wastewater due to insufficient carbon source while autotrophic denitrifying microbes are suitable for survival through inorganic substances and conducting autotrophic denitrification. In order to generate autotrophic denitrification, denitrifying microbes in previous studies required be inoculated, cultivated and enriched. Zhang et al. purchased 28 strains that are relevant to bioremediation to be cultivated so as to facilitate nitrate removal.14 Till et al. cultivated Paracoccus denitrificans before combining with iron to carry out autotrophic denitrification.15 An et al. enriched alone Alcaligenes eutrophus from sub-superficial soil to construct iron-bacteria system.16 These ways used cause not only the difficulty of obtaining pure bacteria but also the expensive cost of bacteria cultivation. Their cultured bacteria mostly utilize hydrogen as energy owing to its nontoxic and clean by-products, but supplying indispensable hydrogen is the main problem. If hydrogen is produced through water electrolysis, the operational cost will sharply improve. Moreover, the security risk will increase when hydrogen is stored and escaped.
Consequently, to solve the trouble of enrichment of autotrophic denitrifying microbes and hydrogen supply, the substrates in CWs are replaced by a few other materials. Some researchers apply nanoscale zero-valent iron (nZVI) to enhance reaction rates17 but it is unbeneficial for the immobilization of iron powers and microbes, and later difficult to maintain long-term high efficiency of nitrate removal. Apart from this, its cost is also hard to accept. Nevertheless, iron scraps as main solid waste produced by steel mills and machinery processing plants are suitable to be used. Not only is the cost of iron scraps about one-third the price of nZVI18 but also they are as the attachments of microbes and have high-efficient nitrate removal for a long time. Wu et al. reported that iron particles were used to remove nitrate pollutants from contaminated groundwater with well eliminative effect for more than a decade.19 Iron scraps are soaked in target wastewater for some time, resulting in the large amounts of autotrophic denitrifying microbes as iron scraps can produce hydrogen as energy of denitrifying microbes through corrosion. Till et al. utilized steel wool and autotrophic denitrifying microbes to remove nitrate, reaching an average nitrate removal efficiency of 61% at hydraulic retention time (HRT) of 2.33 days.15 Lavania and Bose showed that the combination of hydrogenotrophic denitrification and steel wool provided nitrate removal rate of 75%, nevertheless, HRT reached 26 days.20 These reports reveal that efficiency of nitrate removal need to be further improved in the premise of shortening HRT and the contribution of microbes ought to be indicated for analyzing denitrification mechanisms and better cooperating with iron scraps to eliminate nitrate.
Biochar, a carbon-abundant product, is produced from pyrolyzing organic materials and has attracted increasing eyes in recent years since it can generate renewable energy, such as biogas and bio-oil,21 and absorb toxic metals and organic chemicals in soil and water.22–24 Compared with our previous work,6 the addition of biochar may improve nitrate removal. However, it has not yet been widely studied that biochar and microbes give rise to joint effect on nitrate reduction in water bodies. Coelhoso et al. suggested that activated carbon particles can help denitrification of microbes in wastewater because of their irregular surface shape and adsorptive ability.25 Beck et al. found that biochar-amended soil enhances reductive capacity of nitrogen and phosphorus in the leaching via adding the soil surface area to improve absorbency.26 Wu et al. revealed that the addition of biochar reduces the nitrogen losses to composting of organic wastes owing to its high sorption capacity.27 Oh et al. investigated that biochar is able to improve the abiotic reduction of pesticides and nitro explosives by reductants.28 To achieve better nitrate removal efficiency, it is quite essential to study the contribution of biochar to nitrate degradation and the relation between biochar and microbes.
There is, to date, little research about the contributions of iron-based both microbes and biochar to nitrate removal. Hence, considering the defects of CWs and the effect of iron and biochar on nitrate removal, a process combining chemical reduction, microbial denitrification and CWs (RDCWs) was developed in this study to remove nitrate from low carbon/nitrogen ratio wastewater. Iron scraps can eliminate nitrate through being corroded in water to generate hydrogen as energy of autotrophic denitrifying microbes. Biochar is capable of being as the site of microbial survival and reproduction for the formation of biofilm due to its unique structure and ingredient. In addition, both iron and biochar in contact with water are prone to form numerous micro-scale galvanic cells, which can facilitate electron transfer between anodes and cathodes and then accelerate the reduction of nitrate. The coupling of chemicobiological methods and CWs, therefore, can addresses the problems of the low nitrate removal efficiency and the energy supply in microbial denitrification.
What is more, when known the contributions of both microbes and biochar based on iron scraps to nitrate removal, nitrate degradation can be achieved maximization and further knew mechanisms in the reaction. Moreover, the RDCWs operated in natural conditions are capable to better reflect actual nitrate removal, and their low cost and simple operation are also great advantages for large-scale application. Therefore, this study's objectives are (1) to investigate the nitrate removal performance of iron-microbes-biochar and their respective contribution to removal; (2) to reveal the mechanisms of nitrate removal by iron-microbes-biochar.
2. Materials and methods
2.1. Construction and operation of column experiments
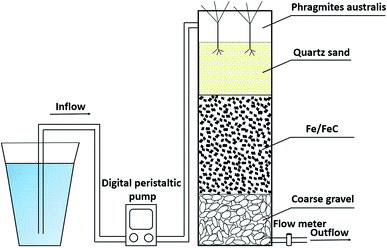
Four replicate lab-scale RDCWs (Φ100 mm × H700 mm, organic glass) were constructed to investigate influencing factors and their individual contribution for nitrate removal. The four experimental columns were named C1-1, C1-2, C2-1 and C2-2, respectively. C1-1 and C1-2 were additionally soaked in the wastewater for 40 days to make biomass enhanced while C2-1 and C2-2 were not soaked in wastewater. As illustrated in Fig. 1, the column was divided into three layers from top to bottom with length, and the both filling heights of top layer being close to the inlet and bottom layer near the outlet were 150 mm while filling height of middle layer was about 300 mm. The top and bottom parts of four columns were filled severally with quartz sand of 1700 g and coarse gravel of 1750 g while the middle part of C1-1 and C2-1 was only filled with iron scraps of 4000 g, but both iron scraps of 4000 g and biochar of 400 g (10% proportion of iron scraps weight) were regarded as the middle part of C1-2 and C2-2. Phragmites australis with a planting density of 20 stems per m2 was planted in every column for simulating constructed wetland. The volume of each column was 5.5 L and liquid volume was approximately 2.8 L. All columns were fed using synthetic water in entire operation process because it was easier to control variables compared to wastewater. Digital peristaltic pump was employed to adjust the speed of influent in the water tank and flow meter was used to control the speed of effluent. Digital peristaltic pump worked with flow meter to regulate HRT in a down-flow feeding mode. Under the natural light conditions and the natural temperature ranged from 19.2 °C to 28.3 °C, four systems were continuously performed for 70 days. The performance time of 70 days was divided into three periods on the basis of different HRT, which was concretely showed in Table 1. The statistical differences of the nitrate removal efficiencies between different experimental columns were indicated through one-way ANOVA analysis.| Influent NO3−, mg N L−1 | HRT, h | Operation time, d | |
|---|---|---|---|
| First period | 15 | 24 | 30 |
| Second period | 15 | 12 | 20 |
| Third period | 15 | 6 | 20 |
Iron scraps with 97% purity were purchased from Shuoli Machinery Limited Company (Dezhou, China). The iron scraps were sieved through 0.85 mm square hole sieve, and their particle size was 5–10 mm. Cylindrical biochar having a diameter of 4 mm and height of 10–15 mm was obtained from Green Source Activated Carbon Co., Ltd (Pingdingshan, China). The diameter of quartz sand was 2–4 mm and the size of coarse gravel was 16–32 mm. The wastewater used to soak C1-1 and C1-2 was the effluent collected from Erlang Temple sewage treatment plant in Wuhan, China. The wastewater was used for providing primordial mixed microbes and its parameter details were showed in Table S1.† Synthetic water used in entire operation process involved 15 mg L−1 NO3−–N, 0.3 g L−1 NaHCO3 and 1 mL L−1 microelement concentrated solution in order to supply nitrate, inorganic carbon and help microbes grow. Microelement concentrated solution consisted of: NaH2PO4·2H2O: 5 g L−1, CaCl2·2H2O: 8.18 g L−1, MgSO4·7H2O: 1.9 g L−1, CoCl2·6H2O: 1.61 g L−1, FeSO4·7H2O: 1.5 g L−1, H3BO3: 0.15 g L−1, KI: 0.18 g L−1, ZnSO4·7H2O: 0.12 g L−1, MnCl2·4H2O: 0.12 g L−1, CuSO4·5H2O: 0.03 g L−1 and Na2MoO4·2H2O: 0.06 g L−1.6
2.2. Construction and operation of batch experiments
To reveal the mechanisms of nitrate removal in column experiments, a series of batch systems were set up and operated. Four kinds of batch systems were designed as follows: (A) 200 mL wastewater (WC); (B) 100 g iron scraps + 200 mL wastewater (WFe); (C) 200 mL synthetic water (SC); (D) 100 g iron scraps + 200 mL synthetic water (SFe). All batch systems were firstly performed using 250 mL Erlenmeyer flasks purged with nitrogen gas for 10 min and then sealed to achieve anoxic conditions. Afterwards, four batch systems were operated continuously for 7 days. During the whole operation process, the experimental temperature was kept at 30 ± 1 °C. These systems were prepared in duplicates to make experimental errors to be estimated.Iron scraps used, wastewater collected and the component of synthetic water were consistent with those of column experiments. The potassium nitrate was added into the wastewater to make nitrate concentration up to 15 mg L−1 and the wastewater with added potassium nitrate was ultimately used in WC and WFe. Synthetic water was finally used in SC and SFe.
2.3. Chemical analysis
The aqueous samples in column experiments were collected from the outlet pipes at designed time intervals and 1 mL aqueous sample in batch experiments was withdrawn every 24 h. All aqueous samples were filtered through 0.45 μm syringe membrane filters to remove a few fine particles for further analysis. The concentrations of TN, NO3−–N, NO2−–N, NH4+–N and the CODcr value were determined according to published standard methods (State Environmental Protection Administration, China). Solution dissolved oxygen was measured using a Dissolved Oxygen Meter (DO200, YSI, USA) and solution pH was monitored using a pH Meter (FE20, METTLER TOLEDO, Switzerland).2.4. Microbial analysis
At the end of the operation of all column experiments, middle part substrate (iron scraps in C1-1 and C2-1, both iron scraps and biochar in C1-2 and C2-2) was respectively collected and put into 50 mL sterile centrifuge tubes. Later, 40 mL phosphate buffered saline (PBS, Hyclone, USA) was added to centrifuge tubes. After being shook vigorously, the liquid in centrifuge tubes was immediately transferred into 50 mL unused sterile centrifuge tubes. Finally, centrifuge tubes were centrifuged at 6000 rpm for 10 min to collect precipitates as microbial analysis samples.6 Through high-throughput sequencing, these microbial samples were analyzed and microbial DNA was extracted using the Fast DNA®SPIN Kit for Soil (MP, USA). 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primers were used for PCR amplification using the thermocycler PCR system (GeneAmp 9700, ABI, USA). The PCR reaction procedures were as follows: 3 min of denaturation at 95 °C, 27 cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C and a final extension at 72 °C for 10 min. The amplicons were purified and sequenced on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw sequence reads were looked up at the NCBI Sequence Read Archive (SRA) database using the accession number: SRP213712. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff through the UPARSE (version 7.1 http://www.drive5.com/uparse/). The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier algorithm (http://www.rdp.cme.msu.edu/) against the Silva (SSU132) 16S rRNA database at 70% confidence threshold.3. Results and discussion
3.1. Nitrate removal performance in column experiments
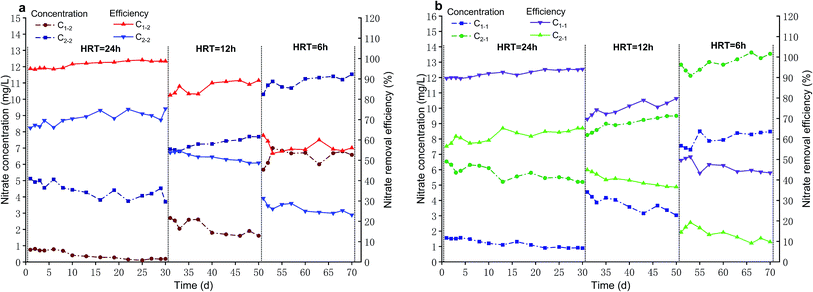
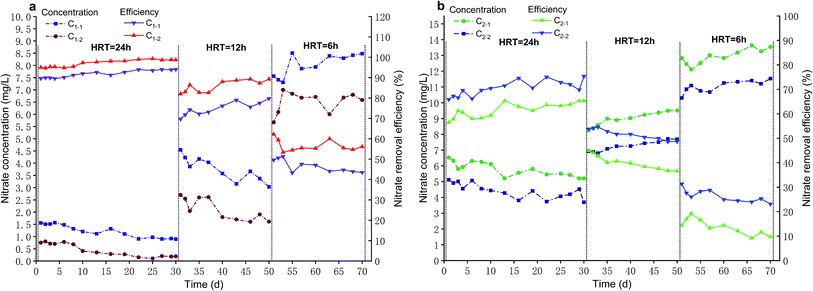
As shown in Fig. 2b, C1-1 intercompared with C2-1. At 24 h HRT, C1-1 achieved nitrate removal efficiency of 91.88 ± 1.76% with a majority of nitrate removal efficiency above 90% and its operation effect was extremely stable while nitrate removal efficiency of C2-1 was 61.50 ± 3.00%. Next, C1-1 procured nitrate removal efficiency of 74.90 ± 3.28% at 12 h HRT and C2-1 merely achieved 40.15 ± 2.96%. Eventually, when HRT was shortened to 6 h, C1-1 accomplished just nitrate removal efficiency of 46.56 ± 3.10% with a significant decline, and nitrate removal efficiency of C2-1 decreased to 13.75 ± 3.24%.
Compared with C2-2, C1-2 had a higher nitrate removal efficiency at the same level of HRT (p < 0.05), which was 26.59 ± 1.91% higher at 24 h HRT, 34.28 ± 4.80% higher at 12 h HRT and 30.24 ± 2.96% higher at 6 h HRT, respectively. Similarity, when HRT was 24 h, nitrate removal efficiency of C1-1 was 30.39 ± 1.91% higher than that of C2-1 (p < 0.05). With keeping HRT of 12 h, nitrate removal efficiency of C1-1 was 34.75 ± 6.02% higher relative to that of C2-1 (p < 0.05). At 6 h HRT, compared with nitrate removal efficiency of C2-1, that of C1-1 was 32.81 ± 2.49% higher (p < 0.05). From foregoing results, it could be seen that microbes had a positive effect on nitrate removal. Thus, for definitely knowing the contribution of microbes, microbes contribution rate (M) was calculated according to eqn (1).
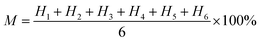 | (1) |
The nitrate removal performance of C2-1 intercompared with that of C2-2 (Fig. 3b). C2-1 procured nitrate removal efficiency of 61.50 ± 3.00% at HRT of 24 h while C2-2 achieved 70.48 ± 3.14%. When HRT was shortened to 12 h, nitrate removal efficiency of C2-1 decreased to 40.15 ± 2.96% and C2-2 reduced to 51.64 ± 2.19%. In the end, at 6 h HRT, C2-1 represented a significant decline, with nitrate removal efficiency of 13.75 ± 3.24% and C2-2 accomplished nitrate removal efficiency of 26.39 ± 2.55%.
Compared with that of C1-1, C1-2 had a higher nitrate removal efficiency at every level of HRT (p < 0.05), which was 5.19 ± 0.64% higher at 24 h HRT, 11.02 ± 1.46% higher at 12 h HRT and 10.07 ± 3.71% higher at 6 h HRT, respectively. Likewise, when HRT kept at 24 h, compared with nitrate removal efficiency of C2-1, that of C2-2 was 8.99 ± 2.19% higher (p < 0.05). Then, at 12 h HRT, nitrate removal efficiency of C2-2 was 11.48 ± 1.18% higher than that of C2-1 (p < 0.05). At last, nitrate removal efficiency of C2-2 was 12.64 ± 2.90% higher relative to that of C2-1 under the condition of 6 h HRT (p < 0.05). From above consequences, it was noticed that biochar was beneficial to nitrate removal. Therefore, biochar contribution rate (B) was calculated according to eqn (2) to clearly know the contribution of biochar.
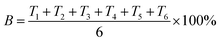 | (2) |
3.2. Nitrate removal performance in batch experiments
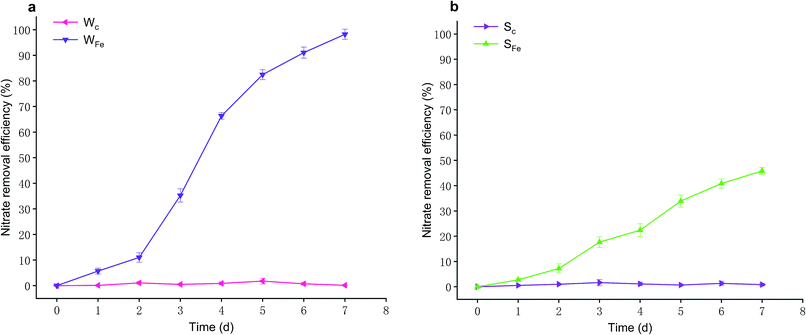
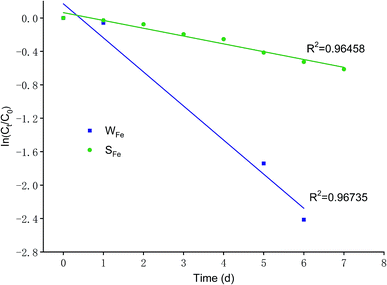
However, the operation conditions of SFe differed from that of WFe. In initial three days, nitrate removal efficiencies of SFe sustained a growth with a gradually increasing trend, which achieved efficiency of 17.66 ± 2.14% on Day 3 (Fig. 4b), but the enhanced speed of nitrate removal efficiencies of SFe declined between Day 3 and Day 4. Afterwards, in the later three days, nitrate removal efficiencies of SFe revealed the relatively quick enhancement and eventually accomplished 45.83 ± 1.36% on Day 7 (Fig. 4b). Meanwhile, nitrate removal efficiencies of SC changed hardly in seven days, and nitrate concentration maintained at 15 mg L−1 (Fig. 4b).
On account of the difference of nitrate removal efficiencies between WFe and SFe, hence, the influencing factors for nitrate removal in WFe were different from that in SFe. The nitrate removal efficiencies of SFe showed removal effect of iron scraps on nitrate. However, the reasons why the nitrate in WFe was removed were not only the presence of iron scraps but also existence of microbes, because iron scraps soaked might cause that effective microbes in wastewater propagated largely and thereby reacted with iron scraps to improve nitrate removal efficiency. In aforementioned column experiments, the system soaked in the wastewater had a better nitrate removal compared with the system not soaked. This result was accordant with that of batch experiments. Shen et al. found the abundances of denitrifying bacteria in column experiments increase through replacing biochar with irons scraps.29 In addition, Liu et al. extracted bacteria attached to the iron mixtures soaked in wastewater, indicating that bacteria-supported iron scraps have a higher nitrate removal rate than iron scraps alone.6 Fig. 4 could be noticed that the removal efficiency of sole iron scraps was near a half of complete removal of nitrate in a week while existence of the microbes made nitrate almost totally be removed. Therefore, these consequences turned out that iron scraps and microbes had synergistic effect on nitrate removal since the abundances of microbes increased by means of iron scraps, and this was also the reason why iron scraps socked in wastewater achieved greater nitrate removal efficiency.
3.3. Microbial community analysis
The microbial samples of C1-1, C1-2, C2-1 and C2-2 in column experiments were analyzed to identity what kinds of microbes probably had close correlation to nitrate removal. Fig. 6a shows the microbial community structures of each sample at the genus level, which presents the differences of four samples. Their rarefaction curves are shown in Fig. S1.† The abundance of the genus Simplicispira in the systems soaked in the wastewater (both C1-1 and C1-2) was higher than that of the systems not soaked in the wastewater (both C2-1 and C2-2). The relative abundance of Simplicispira was 13.37% in C1-1 and 35.72% in C1-2. On the contrary, Simplicispira only had the relative abundance of 0.37% in C2-2 and was hardly detected in C2-1. Moreover, there was a rule that the order of sizes of nitrate removal efficiencies in all systems was identical with that of sizes of relative abundance of Simplicispira in all systems. These results indicated that Simplicispira might contribute to the removal of nitrate. Besides, the relative abundance of Methyloversatilis in C1-1 and C1-2 was higher than that in C2-1 and C2-2. The relative abundance of Methyloversatilis was 3.30% and 1.44% in C1-1 and C1-2 while below the detection limit in C2-1 and C2-2. Kalyuzhnaya et al. verified that wide-ranging organic carbon like formate and methanol can be utilized by Methyloversatilis,31 and Zhang et al. discovered that Methyloversatilis is capable to carry out heterotrophic denitrification.32 Thus, Methyloversatilis could live through utilizing some organic carbons from biochar and low carbon/nitrogen ratio wastewater as energy source.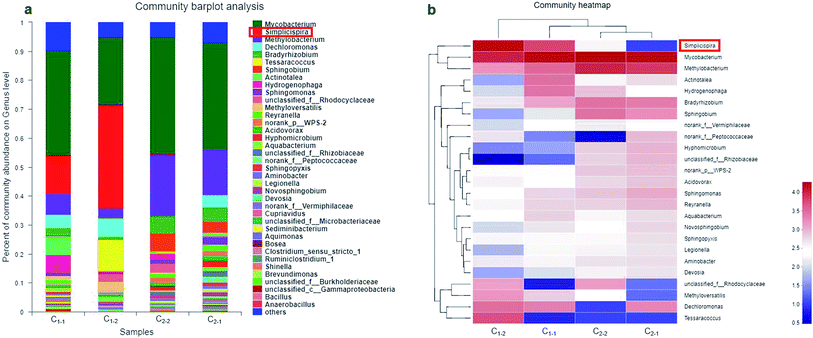 | ||
| Fig. 6 Community analysis at the genus level. (a) Microbial relative abundances in genera levels; (b) microbes heat maps of the genera (only showing the most dominant 25 genera). | ||
The abundance of Simplicispira showed sharp distinction between the systems soaked in the wastewater and the systems not soaked in the wastewater. It had close association with nitrate removal and was widely discovered in natural environments, which was reported in previous studies. In bioelectrochemical system reactor, Simplicispira is deemed to be hydrogen consumer and can grow with hydrogen to reduce nitrate.33–36 In detail, Simplicispira is responsible for offering electron using hydrogen while nitrate accepts electron. Of course, Simplicispira is not only chemolithoautotrophic but also chemoorganotrophic to survive. Zhu et al. and Ruan et al. found that Simplicispira can perform heterotrophic denitrification, and through utilizing poly (butylene succinate) (PBS) as carbon source, is dominant genera after finishing operation severally in airlift inner-loop sequencing batch reactor and anoxic denitrification reactor.37,38 Chu and Wang constructed solid-phase denitrification reactors filled with biopolymer polycaprolactone (PCL) to remove nitrate, elucidating that Simplicispira is major bacteria at genus levels under denitrifying conditions.39 In addition, there were also some studies about survival conditions of Simplicispira in anaerobic situation. Lu et al. reported that Simplicispira isolated from activated sludge are capable of reducing nitrate to nitrite under anaerobic conditions.34 Quan et al. documented the effects of aeration on microbial community, observing that aeration causes a decline of Simplicispira compared to the anaerobic control experiment and Simplicispira is able to better adapt low oxygen environment.40 In short, these experiments show that Simplicispira plays an important role for nitrate removal, and can performed both hydrogenotrophic denitrification under the condition of lacking of organics and heterotrophic denitrification, with a good resistance to oxygen.
Fig. 6b shows the community heat maps of the dominant 25 genera in all microbial samples. C2-1 and C2-2 were clustered together, signifying that the microbial community structures of these two systems were similar. Meanwhile, microbial community structures of C1-2 were different from C1-1 and both C2-1 and C2-2, which revealed that the microbial community structures of the systems soaked in the wastewater differed from that not soaked in the wastewater and biochar added changed microbial community structures of the systems soaked in the wastewater, implying further that biochar affected on nitrate removal maybe owing to the change of microbial community structures, because biochar could release dissolved organic matter to enrich denitrifying bacteria.29 This was also the reason why nitrate removal efficiencies of C1-2 were higher than that of C1-1 and that of C2-2 exceeded that of C2-1. Besides, the abundance of Dechloromonas in C1-1 and C1-2 was higher than that of C2-1 and C2-2. In contrast, the abundances of Mycobacterium, Methylobacterium, Bradyrhizobium, Sphingobium and Acidovorax were relatively low in C1-1 and C1-2 compared with C2-1 and C2-2.
3.4. Possible effective mechanisms for nitrate removal
In column experiments, efficiency of nitrate removal greatly promoted due to effect of microbes. Meanwhile, biochar had improvement effect on nitrate removal as well but the extent of removal was lower relative to microbes. These phenomena suggested that both microbes and biochar closely related to nitrate removal. Furthermore, in batch experiments, though iron scraps presented good effect on nitrate removal, the combination of iron scraps and microbes had higher nitrate removal efficiency in a week than iron scraps alone, which indicated that not only had iron scraps and microbes close relation with removal of nitrate but also iron scraps and microbes interrelated.Previous studies reported that zero-valent iron alone had been used to degrade nitrate, with abiotic reaction described in eqn (3).6,41,42 As shown in the equation, ammonium as reductive product of nitrate is unpopular pollutant in water treatment. At the same time iron is corroded in anaerobic conditions, which is illustrated in eqn (4).6,43,44 In column experiments, iron locating middle layer of column was in anaerobic situation, which was beneficial to produce hydrogen. Liu et al. verified that Hydrogenophaga can carry out autotrophic denitrification using hydrogen as electron supplier and nitrate as electron acceptor, and then produces innocuous nitrogen gas.45 Some other studies showed that Simplicispira can consume hydrogen and reduce nitrate to nitrite or nitrogen gas.33,35,36 In that way Simplicispira may utilize hydrogen produced by iron corroded in anaerobic conditions as energy, which reacts according to eqn (5) and (6).46,47 The nitrite from reductive product of nitrate temporarily accepts electron and rapidly reacts with hydrogen to produce nitrogen gas.
| NO3− + 4Fe0 + 10H+ → 4Fe2+ + NH4+ + 3H2O | (3) |
| Fe0 + 2H2O → H2 + Fe2+ + 2OH− | (4) |
| NO3− + H2 → NO2− + H2O | (5) |
| 2NO2− + 3H2 → N2 + 2H2O + 2OH− | (6) |
The relationship between biochar and microbes was close, they had a perfect cooperation to remove nitrate, which may be explained by two reasons. Primarily, biochar has porous structures which provide large amounts of sites for microbes to conduct denitrification and shelter microbes from high fluid shear forces, leading to relatively homogeneous biofilm to improve denitrification efficiency. Subsequently, biochar can release the dissolved organic matter as carbon source to facilitate the enrichment of microbes.
In general, the RDCWs degrade nitrate from low carbon/nitrogen ratio wastewater mainly through chemical reduction and microbial denitrification. Iron and nitrate can generate chemical reaction of that iron offers electron to nitrate. Meanwhile, iron corroded in anaerobic conditions produces hydrogen as microbial energy. Simplicispira can consume hydrogen in the lack of organics to carry out hydrogenotrophic denitrification. Furthermore, biochar provides good resident circumstance and carbon source like dissolved organic matter for microbes, which is beneficial to microbes to remove nitrate via heterotrophic denitrification. However, the deficiency of the RDCWs is that ammonium, by-product reduced by nitrate, is largely accumulated. The removal methods of ammonium are no worry and ammonium can be minimized through optimizing the systems. The optimized parameters, such as iron concentration, microbes concentration, biochar concentration, pH, temperature and so on, can be adjusted according to actual removal rate of nitrate and productive rate of ammonium. It turned out that iron dosage causes finally different products42 and initial biomass has obvious association with by-product reduced.21 An increase of biochar dosage leads to higher removal efficiency of nitrate in the presence of microbes and iron but the enhancement of nitrate removal does not appear without those.21 The pH can determine nitrate reduction rate via affecting iron corrosion extent.48 Temperature influences microbial activity and biomass and the removal of nitrate and ammonium.49 The respective contribution of both microbes and biochar on the basis of iron to nitrate removal was clarified in column experiments, which could be as reference to adjust the several proportion of iron, microbes and biochar, resulting in optimum composition with high removal of nitrate and low production of ammonium.
4. Conclusions
The RDCWs were applied to degrade nitrate from low carbon/nitrogen ratio wastewater. This system achieved nitrate removal efficiency of 97.07 ± 1.76%, 85.91 ± 3.02% and 56.63 ± 2.88% at 24 h, 12 h and 6 h HRT, respectively. Thereinto, microbes and biochar promoted removal of nitrate severally with contribution rate of 31.08 ± 4.43% and 9.50 ± 3.30%. The concentration of iron, biochar and microbes could be adjusted to optimize the system in order to enhance removal efficiency of nitrate. The microbes had close relation with iron and biochar for nitrate removal. Simplicispira as dominative genus was likely to contribute to elimination of nitrate. Overall, RDCWs presented a potential for large-scale application to dispose nitrate pollution in low carbon/nitrogen ratio wastewater.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China [2018YFD0900801], Natural Science Foundation of China [41907324] and Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07603-003).References
- G. C. C. Yang and L. Hsaio-Lan, Water Res., 2005, 39, 884–894 CrossRef CAS PubMed.
- T. Hosono, T. Tokunaga, A. Tsushima and J. Shimoda, Water Res., 2014, 54, 284–296 CrossRef CAS.
- S. Deng, D. Li, X. Yang, S. Zhu and J. Li, Environ. Sci. Pollut. Res. Int., 2016, 23, 6621–6630 CrossRef CAS PubMed.
- H. Wu, J. Zhang, R. Wei, S. Liang, C. Li and H. Xie, Environ. Sci. Pollut. Res. Int., 2013, 20, 443–451 CrossRef CAS PubMed.
- H. Wu, J. Zhang, H. H. Ngo, W. Guo, Z. Hu, S. Liang, J. Fan and H. Liu, Bioresour. Technol., 2015, 175, 594–601 CrossRef CAS PubMed.
- X. W. Liu, J. Xu, J. L. Huang, M. Q. Huang, T. Wang, S. P. Bao, W. Tang and T. Fang, RSC Adv., 2019, 9, 3285–3293 RSC.
- Y. Zhang, Y. Cheng, C. P. Yang, W. Luo, G. M. Zeng and L. Lu, Bioresour. Technol., 2015, 193, 424–432 CrossRef CAS PubMed.
- W. C. Tang, X. Li, H. Y. Liu, S. H. Wu, Q. Zhou, C. Du, Q. Teng, Y. Y. Zhong and C. P. Yang, Bioresour. Technol., 2020, 300, 122634 CrossRef CAS PubMed.
- Y. Wu, Z. Hu, L. Yang, B. Graham and P. G. Kerr, Bioresour. Technol., 2011, 102, 2419–2426 CrossRef CAS PubMed.
- N. Zhu, J. Tang, C. Tang, P. Duan, L. Yao, Y. Wu and D. D. Dionysiou, Chem. Eng. J., 2018, 353, 237–245 CrossRef CAS.
- H. Wu, W. Ma, Q. Kong and H. Liu, Chem. Eng. J., 2018, 350, 445–452 CrossRef CAS.
- S. Wu, P. Kuschk, H. Brix, J. Vymazal and R. Dong, Water Res., 2014, 57, 40–55 CrossRef CAS PubMed.
- L. Jia, R. Wang, L. Feng, X. Zhou, J. Lv and H. Wu, Chem. Eng. J., 2018, 345, 22–30 CrossRef CAS.
- Y. P. Zhang, G. B. Douglas, A. H. Kaksonen, L. L. Cui and Z. F. Ye, Sci. Total Environ., 2019, 646, 1195–1203 CrossRef CAS PubMed.
- B. A. Till, L. J. Weathers and P. J. J. Alvarez, Environ. Sci. Technol., 1998, 32, 634–639 CrossRef CAS.
- Y. An, T. Li, Z. Jin, M. Dong, Q. Li and S. Wang, Sci. Total Environ., 2009, 407, 5465–5470 CrossRef CAS PubMed.
- K. H. Shin and D. K. Cha, Chemosphere, 2008, 72, 257–262 CrossRef CAS PubMed.
- S. H. Deng, D. S. Li, X. Yang, W. Xing, J. L. Li and Q. Zhang, Bioresour. Technol., 2016, 219, 677–686 CrossRef CAS PubMed.
- Q. H. Wu, C. M. Zheng, J. F. Zhang and F. W. Zhang, J. Earth Sci., 2017, 28, 447–456 CrossRef CAS.
- A. Lavania and P. Bose, J. Environ. Eng., 2006, 132, 994–1000 CrossRef CAS.
- S. Y. Oh, Y. D. Seo, B. Kim, I. Y. Kim and D. K. Cha, Bioresour. Technol., 2016, 200, 891–896 CrossRef CAS PubMed.
- D. Mohan, A. Sarswat, Y. S. Ok and C. U. Pittman Jr, Bioresour. Technol., 2014, 160, 191–202 CrossRef CAS PubMed.
- A. U. Rajapaksha, M. Vithanage, M. Zhang, M. Ahmad, D. Mohan, S. X. Chang and Y. S. Ok, Bioresour. Technol., 2014, 166, 303–308 CrossRef CAS PubMed.
- V. A. Loganathan, Y. Feng, G. D. Sheng and T. P. Clement, Soil Sci. Soc. Am. J., 2009, 73, 967 CrossRef CAS.
- I. Coelhoso, R. Boaventura and A. Rodrigues, Biotechnol. Bioeng., 1992, 40, 625–633 CrossRef CAS PubMed.
- D. A. Beck, G. R. Johnson and G. A. Spolek, Environ. Pollut., 2011, 159, 2111–2118 CrossRef CAS PubMed.
- S. H. Wu, H. J. He, X. Inthapanya, C. P. Yang, L. Lu, G. M. Zeng and Z. F. Han, Environ. Sci. Pollut. Res., 2017, 24, 16560–16577 CrossRef CAS PubMed.
- S. Y. Oh and Y. D. Seo, J. Environ. Qual., 2015, 44, 833–840 CrossRef CAS PubMed.
- Y. H. Shen, L. L. Zhuang, J. Zhang, J. L. Fan, T. Yang and S. Sun, Chem. Eng. J., 2019, 359, 706–712 CrossRef CAS.
- W. Zhang, X. Li, Q. Yang, D. Wang, Y. Wu, X. Zhu, J. Wei, Y. Liu, L. Hou and C. Chen, Chemosphere, 2019, 216, 749–756 CrossRef CAS PubMed.
- M. G. Kalyuzhnaya, P. De Marco, S. Bowerman, C. C. Pacheco, J. C. Lara, M. E. Lidstrom and L. Chistoserdova, Int. J. Syst. Evol. Microbiol., 2006, 56, 2517–2522 CrossRef CAS PubMed.
- L. Zhang, C. Zhang, C. Hu, H. Liu, Y. Bai and J. Qu, Water Res., 2015, 85, 422–431 CrossRef CAS PubMed.
- C. Zhu, H. Wang, Q. Yan, H. Rong and G. Zhang, Chem. Eng. J., 2017, 312, 360–366 CrossRef CAS.
- S. Lu, S. H. Ryu, B. S. Chung, Y. R. Chung, W. Park and C. O. Jeon, Int. J. Syst. Evol. Microbiol., 2007, 57, 31–34 CrossRef CAS PubMed.
- H. Liu, Q. Yan and W. Shen, Bioresour. Technol., 2014, 171, 187–192 CrossRef CAS PubMed.
- L. Chu and J. Wang, RSC Adv., 2017, 7, 53454–53462 RSC.
- S. M. Zhu, Y. L. Deng, Y. J. Ruan, X. S. Guo, M. M. Shi and J. Z. Shen, Bioresour. Technol., 2015, 192, 603–610 CrossRef CAS PubMed.
- Y. J. Ruan, Y. L. Deng, X. S. Guo, M. B. Timmons, H. F. Lu, Z. Y. Han, Z. Y. Ye, M. M. Shi and S. M. Zhu, Bioresour. Technol., 2016, 216, 1004–1013 CrossRef CAS PubMed.
- L. Chu and J. Wang, Chemosphere, 2013, 91, 1310–1316 CrossRef CAS PubMed.
- X. C. Quan, Y. P. Quan and K. Tao, Chem. Eng. J., 2012, 210, 150–156 CrossRef CAS.
- M. J. Alowitz and M. M. Scherer, Environ. Sci. Technol., 2002, 36, 299–306 CrossRef CAS PubMed.
- S. Comba, M. Martin, D. Marchisio, R. Sethi and E. Barberis, Water, Air, Soil Pollut., 2012, 223, 1079–1089 CrossRef CAS.
- B. T. Oh, C. L. Just and P. J. J. Alvarez, Environ. Sci. Technol., 2001, 35, 4341–4346 CrossRef CAS PubMed.
- X. Yu, C. Amrhein, M. A. Deshusses and M. R. Matsumoto, Environ. Sci. Technol., 2006, 40, 1328–1334 CrossRef CAS PubMed.
- X. Liu, M. Huang, S. Bao, W. Tang and T. Fang, Bioresour. Technol., 2020, 301, 122731 CrossRef CAS PubMed.
- M. Kurt, I. J. Dunn and J. R. Bourne, Biotechnol. Bioeng., 1987, 29, 493–501 CrossRef CAS PubMed.
- S. Dadang, T. Kawanishi, N. Shimizu and Y. Hayashi, Water Sci. Technol., 2002, 46, 39–44 CrossRef CAS.
- J. Kielemoes, P. De Boever and W. Verstraete, Environ. Sci. Technol., 2000, 34, 663–671 CrossRef CAS.
- Q. Zhou, H. Zhu, G. Banuelos, B. Yan, Y. Liang, J. Yu and H. Li, Int. J. Phytorem., 2017, 19, 915–924 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra03609a |
| This journal is © The Royal Society of Chemistry 2020 |