Open Access Article
Open Access ArticleConstruct indeno[1,2-b]oxepine or cis-cyclopropylacrylate by sulfur ylides†
Qinfang Chen,
Yihao Pan,
Dongxin Zhao,
Weiran Yang and
Jing Zheng *
*
School of Resources Environmental and Chemical Engineering, Nanchang University, 999 XuFu Road, Nangchang, 330031, China. E-mail: Zhengjing@ncu.edu.cn
First published on 8th June 2020
Abstract
For the first time, the [4 + 3] or [2 + 1] annulation of crotonate-derived sulfur ylides with arylidenemalononitrile or arylidene-1H-indene-1,3(2H)-dione is reported using Na2CO3 as the base. This protocol is advantageous as it does not require prior preparation of arylidenemalononitrile or arylidene-1H-indene-1,3(2H)-dione substrates, due to the independent participation of the base in the two reactions. This mild, operationally multicomponent process can be employed for the transformation of a wide variety of commercially available aldehydes into the corresponding indeno[1,2-b]oxepine or cyclopropyl acrylate core in moderate to excellent yields under mild conditions.
1. Introduction
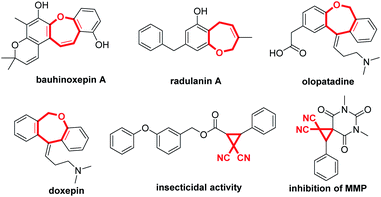
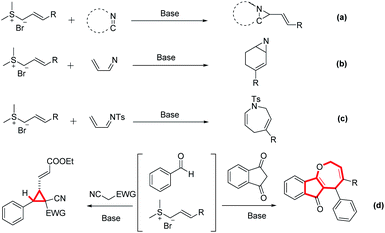
Oxygen-containing heterocyclic compounds, especially unsaturated 7-membered oxacycle (oxepine) frameworks, exhibit a wide range of biological properties, such as ion-channel blocking, antiplasmodial, antiviral, antipsychotic, and antifungal activities.1 Various natural and marine natural products containing the oxepine motif play a vital role in biological processes.2 The cyclopropyl group is also a vital structural unit in several synthetic and naturally occurring compounds, exhibiting a wide spectrum of biological properties ranging from enzyme inhibition to herbicidal, antibiotic, antitumor, and antiviral activities.3 Fig. 1 shows some representative examples of these compounds.Recently, crotonate-derived sulfur ylides have attracted attention in organic synthesis as sources of one-carbon or three-carbon synthons in the presence of an inorganic base.4 The ability of crotonate-derived sulfur ylides to undergo [m + n] cycloaddition reactions with various substrates is exploited to construct several cyclic compounds.5 Tang and co-workers have synthesized vinylaziridine by the diastereoselective annulation of crotonate-derived sulfur ylides with cyclic ketamine (Scheme 1a).6 Huang and co-workers have reported access to cyclic 2-alkenyl aziridines by sequential annulation using crotonate-derived sulfur ylides as the C3 synthon with α,β-unsaturated cyclic ketimines (Scheme 1b).5f,7 Huang has synthesized seven-membered nitrogen-heterocycles with moderate-to-excellent yields by the development of a novel [4 + 3] annulation of azadienes with crotonate sulfonium salts (Scheme 1c).8 On the other hand, multicomponent reactions (MCRs), defined as processes that combine at least three reactants in the same pot to generate a product containing most of the atoms of the starting material, have been extensively exploited to prepare small molecules with step economy, energy conservation, and emission reduction.9 As highly reactive reagents, sulfur ylides can react with various electrophiles such as aldehydes, imines, and electron-deficient alkenes.10 Therefore, the design of MCRs involving sulfur ylides in the presence of aldehydes, 1,3-indanedione, and malononitrile might lead to selectivity problems.
Despite these challenges, it is interesting to develop sulfur ylide-mediated MCRs to construct core structures with medicinal interest. Based on the aforementioned motivation and our continued interest in organocatalysis, herein, the construction of indeno[1,2-b]oxepine by the three-component [4 + 3] or [1 + 2] annulation of crotonate-derived sulfur ylides, aldehydes, and 1,3-indanedione or malononitrile (Scheme 1d).
2. Results and discussion
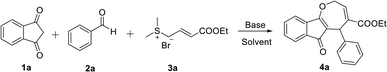
Prior to investigation of the desired MCRs and sulfur ylides, studies were first initiated by investigating reactions between crotonate sulfonium salt 3a and 2-benzylidene-1H-indene-1,3(2H)-dione, which was generated by the reaction of 1,3-indanedione (1a) and benzaldehyde (2a) (Table 1). The reaction was performed in DCM with NaHCO3 as the base at 25 °C (Table 1, entry 1). Gratifyingly, the [4 + 3] annulation product was obtained in 76% yield. The structure of 4a was confirmed by NMR and HRMS. Reaction conditions were optimized to improve the product yield. Optimization of base was first carried out. When the reaction was conducted in t-BuOK, the yield was improved to 85% (Table 1, entry 2). Moderate product yields were observed by using other bases, such as DABCO, TMAF, Cs2CO3, NaOH, Et3N, K2CO3 and K3PO4 (Table 1, entries 3–9). To our surprise, the product yield was improved to 90% when the base was used instead of Na2CO3. Although NaH was also found to afford yields similar to that obtained using Na2CO3, the cost-effective base was used for [4 + 3] cyclization (Table 1, entries 10 and 11). After the optimization of the base, the scope of different solvents for the was investigated. Common solvents (CH3CN, CH3OH, THF, toluene, DCE) failed to further improve the product yields (Table 1, entries 12–16). In addition, the reaction temperature significantly affected the reaction. By decreasing the temperature from room temperature to 0 °C, the product yield decreased from 90% to 45% even for a longer reaction time of 6 h (Table 1, entry 17). Neither the decrease nor increase the temperature led to the improvement of the yield of 4a (Table 1, entries 17 and 18).| Entry | Base | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise specified, the reactions were carried out with 1a (0.2 mmol), 2a (0.24 mmol) and 3a (0.3 mmol) in the presence of base (0.4 mmol) in a solvent (2 mL) at 25 °C.b Isolated yield. | ||||
| 1 | NaHCO3 | DCM | 25 | 76 |
| 2 | t-BuOK | DCM | 25 | 85 |
| 3 | DABCO | DCM | 25 | 75 |
| 4 | TMAF | DCM | 25 | 76 |
| 5 | Cs2CO3 | DCM | 25 | 56 |
| 6 | NaOH | DCM | 25 | 73 |
| 7 | Et3N | DCM | 25 | 70 |
| 8 | K2CO3 | DCM | 25 | 79 |
| 9 | K3PO4 | DCM | 25 | 50 |
| 10 | NaH | DCM | 25 | 90 |
| 11 | Na2CO3 | DCM | 25 | 90 |
| 12 | Na2CO3 | CH3CN | 25 | 79 |
| 13 | Na2CO3 | CH3OH | 25 | 48 |
| 14 | Na2CO3 | THF | 25 | 55 |
| 15 | Na2CO3 | Toluene | 25 | 67 |
| 16 | Na2CO3 | DCE | 25 | 70 |
| 17 | Na2CO3 | DCM | 0 | 45 |
| 18 | Na2CO3 | DCM | 60 | 88 |
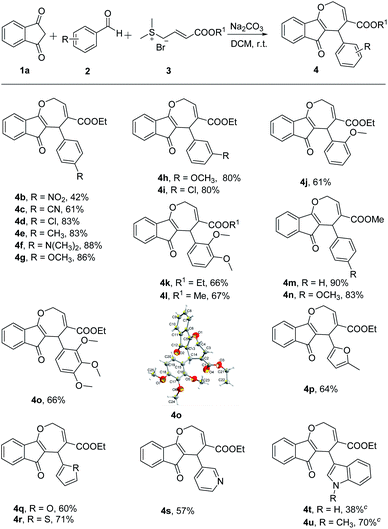
Using optimized reaction conditions (Table 1, entry 11), the substrate scope of the substituted benzaldehydes was first investigated (Scheme 2). Different benzaldehydes with a variety of functional groups such as NO2, CN, Cl, CH3, N(CH3)2, and OCH3 smoothly reacted with 1a and 3a to afford the corresponding indeno[1,2-b]oxepine (4b–4o) in moderate to excellent yields (42–90%), thus offering a broad range of opportunities for further derivatization. These results revealed that reactions of benzaldehydes bearing electron-donating and electron-withdrawing groups at the para position do not clearly affect this transformation. In particular, aromatic aldehydes bearing a functional group at the para position exhibited higher reactivity than their ortho- or meta-substituted counterpart (4g vs. 4h and 4j). However, for polysubstituted aromatic aldehydes, yields for the desired products were less (4k, 4o) than those for the monosubstituted aromatic aldehydes. In addition, the structure of 4o was further confirmed by single-crystal X-ray analysis.11 Moreover, reactions efficiently proceeded when sulfonium salt 3b was used, affording desired products in moderate to excellent yields (4l–4n). As expected, heteroaryl aldehydes, such as 5-methylfurfural, furfural, 2-thenaldehyde, and nicotinaldehyde, performed quite well in the MCRs (4p–4s). The use of indole-3-carboxaldehyde afforded low yields of the desired product (4t). However, when indole-3-carboxaldehyde was protected using iodomethane, the product yield was increased to 70% (4u).
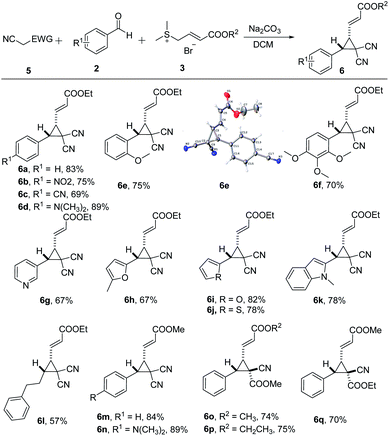
To demonstrate the generality of this transformation, malononitrile was investigated instead of 1,3-indanedione under the optimal conditions. As shown in Scheme 3, electron-withdrawing or electron-donating groups such as nitro, cyano and N,N-dimethyl, at the para-position R1 in the substrate 2 smoothly reacted with 5a and 3a to afford [2 + 1] annulation products 6a–6d (69–89%) with an excellent cis/trans ratio. To our delight, the desired cis-cyclopropylacrylate 6a was still exclusively obtained (cis/trans >99/1) in 80% yield using 1.0 mmol of substrate 5a. While the methoxy group substituted at the meta position in 6e afforded low product yield (75%), the structure of 6e was further confirmed by single-crystal X-ray analysis.12 However, for polysubstituted aromatic aldehydes, the yield of the desired product 6f was less than that of the monosubstituted aromatic aldehyde. As expected, heteroaryl aldehydes, such as 5-methylfurfural, furfural, 2-thenaldehyde, nicotinaldehyde, and 1-methyl-1H-indole-3-carbaldehyde performed quite well in the MCRs (6g–6k). In addition, aliphatic aldehydes proved to be suitable substrates to furnish the desired reaction in moderate yields (6l, 57%). Moreover, reactions proceeded efficiently proceeded when sulfonium salt 3b was used, affording desired products in moderate to excellent yields (6m–6o, 6q). Furthermore, cyanoacrylates also afforded the desired products (6o–6q). The relative configuration of 6o was defined on the basis of the phase-sensitive NOESY spectrum. No correlation between hydrogen on cyclopropane and methyl by the NOESY spectrum was observed (see the ESI†).
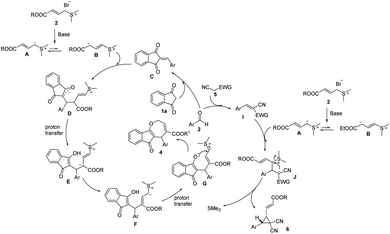
Based on the present experimental data and the results described in the literature,4f–h,5e,7,13 a plausible MCR reaction mechanism can be proposed as shown in Scheme 4. Crotonate-derived ylide 2 was treated with base (Na2CO3) to afford allylic ylide A, which resonated to B. Next, the Michael addition of B to C, which was formed by 1a and 2 in the presence of the base catalyst, afforded intermediate D. Intermediate D subsequently transformed into intermediate G via two proton-transfer processes, followed by an intramolecular SN2 nucleophilic substitution to furnish [4 + 3] annulation product 4. When we 5 was used instead of 1a, the Michael addition of A to I in the presence of the base catalyst would lead to intermediate J. Then, the subsequent intramolecular nucleophilic addition and elimination of Me2S further afforded the final product.
3. Conclusions
To the best of our knowledge, for the first time, a novel Na2CO3-promoted MCR reaction using crotonate-derived sulfonium salts, aromatic aldehydes, and 1,3-indanedione or malononitrile was developed. Various indeno[1,2-b]oxepine and cis-cyclopropylacrylate derivatives were obtained in good-to-excellent yields. The advantages of the current protocol included readily available starting materials, mild reaction conditions, good functional group tolerance, and broad substrate scope. Currently, further applications of this MCR reaction in organic synthesis, as well as investigations on the detailed mechanism, are underway in our laboratory.4. Experimental section
4.1 General information
Infrared spectra were obtained on a FTIR spectrometer. 1H NMR and 13C NMR spectra were recorded on Agilent DD2400 spectrometer. CDCl3 was used as solvent. Chemical shifts were referenced relative to residual solvent. Melting points were measured with micro melting point apparatus. Infrared spectra were recorded on a FT-IR spectrometer. 1a, 2 and 5 were prepared from Energy Chemical. 3 were prepared according to the literature procedure.144.2 Experimental procedures and characterization date
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4a as a Yellow solid (62.3 mg, 90% yield), Rf = 0.21 (EtOAc/petroleum ether 1
10) as eluent to give 4a as a Yellow solid (62.3 mg, 90% yield), Rf = 0.21 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.36 (d, J = 6.8 Hz, 1H), 7.28–7.16 (m, 6H), 7.12–7.05 (m, 3H), 5.29 (s, 1H), 4.83 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.54 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.28–4.16 (m, 2H), 1.25 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.6, 166.1, 144.5, 141.9, 139.7, 132.5, 132.1, 131.3, 130.0, 128.6, 127.1, 126.7, 121.3, 118.4, 109.6, 65.1, 61.8, 35.5, 14.3. IR(KBr) ν: 2974, 2918, 1712, 1628, 1579, 1460, 1397, 1243, 1095, 1046. HRMS(EI) (m/z): calcd for C22H18O4 (M + H)+: 347.1278; found: 347.1276.
10). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.36 (d, J = 6.8 Hz, 1H), 7.28–7.16 (m, 6H), 7.12–7.05 (m, 3H), 5.29 (s, 1H), 4.83 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.54 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.28–4.16 (m, 2H), 1.25 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.6, 166.1, 144.5, 141.9, 139.7, 132.5, 132.1, 131.3, 130.0, 128.6, 127.1, 126.7, 121.3, 118.4, 109.6, 65.1, 61.8, 35.5, 14.3. IR(KBr) ν: 2974, 2918, 1712, 1628, 1579, 1460, 1397, 1243, 1095, 1046. HRMS(EI) (m/z): calcd for C22H18O4 (M + H)+: 347.1278; found: 347.1276.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4b as a yellow solid (32.8 mg, 42% yield), Rf = 0.12 (EtOAc/petroleum ether 1
5) as eluent to give 4b as a yellow solid (32.8 mg, 42% yield), Rf = 0.12 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 8.15 (t, 1H), 8.13 (t, 1H), 7.52–7.47 (m, 3H), 7.39 (t, J = 8.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.25–7.20 (m, 2H), 5.42 (s, 1H), 4.80 (dd, J1 = 12.8 Hz, J2 = 7.2 Hz, 1H), 4.74–4.69 (m, 1H), 4.39–4.26 (m, 2H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.1, 165.8, 149.5, 146.8, 143.4, 139.4, 132.8, 132.8, 131.1, 130.5, 128.2, 123.9, 121.6, 118.9, 108.4, 65.2, 62.2, 35.9, 14.3. IR(KBr) ν: 2925, 2855, 1712, 1628, 1523, 1474, 1355, 1116, 990. HRMS(EI) (m/z): calcd for C22H17NO6 (M + H)+: 392.1129; found: 392.1128.
10). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 8.15 (t, 1H), 8.13 (t, 1H), 7.52–7.47 (m, 3H), 7.39 (t, J = 8.0 Hz, 1H), 7.33 (t, J = 8.0 Hz, 1H), 7.25–7.20 (m, 2H), 5.42 (s, 1H), 4.80 (dd, J1 = 12.8 Hz, J2 = 7.2 Hz, 1H), 4.74–4.69 (m, 1H), 4.39–4.26 (m, 2H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.1, 165.8, 149.5, 146.8, 143.4, 139.4, 132.8, 132.8, 131.1, 130.5, 128.2, 123.9, 121.6, 118.9, 108.4, 65.2, 62.2, 35.9, 14.3. IR(KBr) ν: 2925, 2855, 1712, 1628, 1523, 1474, 1355, 1116, 990. HRMS(EI) (m/z): calcd for C22H17NO6 (M + H)+: 392.1129; found: 392.1128.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4c as a yellow solid (45.3 mg, 61% yield), Rf = 0.15 (EtOAc/petroleum ether 1
10) as eluent to give 4c as a yellow solid (45.3 mg, 61% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 132 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.57 (d, 2H), 7,46–7.44 (m, 3H), 7.37 (t, J = 7.2 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.21 (t, J = 8.0 Hz, 2H), 5.38 (s, 1H), 4.81–4.76 (m, 1H), 4.72–4.67 (m, 1H), 4.37–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.1, 165.8, 147.4, 143.4, 139.4, 132.8, 132.7, 132.5, 131.0, 130.4, 128.1, 121.5, 118.9, 118.8, 110.7, 108.3, 65.2, 62.1, 35.9, 14.3. IR(KBr) ν: 2981, 2918, 2231, 1705, 1621, 1515, 1452, 1396, 1242, 1179, 1102, 1046. HRMS(EI) (m/z): calcd for C23H17NO4 (M + H)+: 372.1230; found: 372.1231.
10). Mp 132 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.57 (d, 2H), 7,46–7.44 (m, 3H), 7.37 (t, J = 7.2 Hz, 1H), 7.32 (d, J = 8.0 Hz, 1H), 7.21 (t, J = 8.0 Hz, 2H), 5.38 (s, 1H), 4.81–4.76 (m, 1H), 4.72–4.67 (m, 1H), 4.37–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.1, 165.8, 147.4, 143.4, 139.4, 132.8, 132.7, 132.5, 131.0, 130.4, 128.1, 121.5, 118.9, 118.8, 110.7, 108.3, 65.2, 62.1, 35.9, 14.3. IR(KBr) ν: 2981, 2918, 2231, 1705, 1621, 1515, 1452, 1396, 1242, 1179, 1102, 1046. HRMS(EI) (m/z): calcd for C23H17NO4 (M + H)+: 372.1230; found: 372.1231.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4d as a yellow solid (63.1 mg, 83% yield), Rf = 0.21 (EtOAc/petroleum ether 1
10) as eluent to give 4d as a yellow solid (63.1 mg, 83% yield), Rf = 0.21 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 108 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.4 Hz, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.25–7.22 (m, 4H), 7.19–7.15 (m, 2H), 5.31 (s, 1H), 4.89–4.84 (m, 1H), 4.69–4.64 (m, 1H), 4.35–4.25 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.8, 166.0, 144.1, 140.5, 139.6, 132.6, 132.6, 132.3, 131.2, 130.2, 128.7, 128.6, 121.4, 118.6, 109.1, 65.1, 62.0, 35.2, 14.3. IR(KBr) ν: 2918, 1705, 1628, 1585, 1410, 1235, 1172, 1109, 1039, 955, 906, 765, 730. HRMS(EI) (m/z): calcd for C22H17ClO4 (M + H)+: 381.0888; found: 381.0890.
10). Mp 108 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.4 Hz, 1H), 7.28 (t, J = 7.6 Hz, 1H), 7.25–7.22 (m, 4H), 7.19–7.15 (m, 2H), 5.31 (s, 1H), 4.89–4.84 (m, 1H), 4.69–4.64 (m, 1H), 4.35–4.25 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.8, 166.0, 144.1, 140.5, 139.6, 132.6, 132.6, 132.3, 131.2, 130.2, 128.7, 128.6, 121.4, 118.6, 109.1, 65.1, 62.0, 35.2, 14.3. IR(KBr) ν: 2918, 1705, 1628, 1585, 1410, 1235, 1172, 1109, 1039, 955, 906, 765, 730. HRMS(EI) (m/z): calcd for C22H17ClO4 (M + H)+: 381.0888; found: 381.0890.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4e as a Yellow oil (59.8 mg, 83% yield), Rf = 0.18 (EtOAc/petroleum ether 1
10) as eluent to give 4e as a Yellow oil (59.8 mg, 83% yield), Rf = 0.18 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.37–7.33 (m, 1H), 7.31–7.27 (m, 1H), 7.22–7.13 (m, 4H), 7.08 (d, J = 8.0 Hz, 2H), 5.33 (s, 1H), 4.95 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.36–4.24 (m, 2H), 2.29 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.6, 166.2, 144.6, 139.8, 138.9, 136.3, 132.5, 132.0, 131.3, 130.0, 129.3, 127.1, 121.3, 118.4, 109.8, 65.2, 61.8, 35.3, 21.0, 14.3. IR(KBr) ν: 2911, 2855, 1712, 1628, 1508, 1466, 1403, 1235, 1109, 1039. HRMS(EI) (m/z): calcd for C23H20O4 (M + H)+: 361.1434; found: 361.1436.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.37–7.33 (m, 1H), 7.31–7.27 (m, 1H), 7.22–7.13 (m, 4H), 7.08 (d, J = 8.0 Hz, 2H), 5.33 (s, 1H), 4.95 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.36–4.24 (m, 2H), 2.29 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.6, 166.2, 144.6, 139.8, 138.9, 136.3, 132.5, 132.0, 131.3, 130.0, 129.3, 127.1, 121.3, 118.4, 109.8, 65.2, 61.8, 35.3, 21.0, 14.3. IR(KBr) ν: 2911, 2855, 1712, 1628, 1508, 1466, 1403, 1235, 1109, 1039. HRMS(EI) (m/z): calcd for C23H20O4 (M + H)+: 361.1434; found: 361.1436.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4f as a yellow oil (68.5 mg, 88% yield), Rf = 0.15 (EtOAc/petroleum ether 1
5) as eluent to give 4f as a yellow oil (68.5 mg, 88% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.37–7.33 (m, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.21–7.09 (m, 4H), 6.65 (d, J = 8.8 Hz, 2H), 5.27 (s, 1H), 5.01 (dd, J1 = 12.4 Hz, J2 = 7.3 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 7.8 Hz, 1H), 4.35–4.23 (m, 2H), 2.89 (s, 6H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.5, 173.4, 166.4, 149.5, 144.9, 140.0, 132.5, 131.8, 131.4, 129.9, 129.7, 127.9, 121.2, 118.3, 112.8, 110.3, 65.3, 61.8, 40.8, 34.9, 14.4. IR(KBr) ν: 2911, 2841, 1705, 1628, 1515, 1473, 1403, 1340, 1228, 1109, 1046. HRMS(EI) (m/z): calcd for C24H23NO4 (M + H)+: 390.1700; found: 390.1699.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.37–7.33 (m, 1H), 7.29 (d, J = 8.0 Hz, 1H), 7.21–7.09 (m, 4H), 6.65 (d, J = 8.8 Hz, 2H), 5.27 (s, 1H), 5.01 (dd, J1 = 12.4 Hz, J2 = 7.3 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 7.8 Hz, 1H), 4.35–4.23 (m, 2H), 2.89 (s, 6H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.5, 173.4, 166.4, 149.5, 144.9, 140.0, 132.5, 131.8, 131.4, 129.9, 129.7, 127.9, 121.2, 118.3, 112.8, 110.3, 65.3, 61.8, 40.8, 34.9, 14.4. IR(KBr) ν: 2911, 2841, 1705, 1628, 1515, 1473, 1403, 1340, 1228, 1109, 1046. HRMS(EI) (m/z): calcd for C24H23NO4 (M + H)+: 390.1700; found: 390.1699.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4g as a Yellow oil (64.7 mg, 86% yield), Rf = 0.18 (EtOAc/petroleum ether 1
10) as eluent to give 4g as a Yellow oil (64.7 mg, 86% yield), Rf = 0.18 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.24 (d, J = 9.2 Hz, 2H), 7.18–7.12 (m, 2H), 6.81 (d, J = 8.8 Hz, 2H), 5.30 (s, 1H), 4.95 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.64 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.36–4.24 (m, 2H), 3.76 (s, 3H), 1.34 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.6, 166.2, 158.4, 144.7, 139.9, 134.0, 132.5, 132.0, 131.3, 130.1, 128.3, 121.3, 118.4, 114.0, 109.9, 65.2, 61.8, 55.4, 35.0, 14.3. IR(KBr) ν: 2925, 1712, 1628, 1515, 1466, 1403, 1249, 1179, 1102, 1039. HRMS(EI) (m/z): calcd for C23H20NO5 (M + H)+: 377.1384; found: 377.1385.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.24 (d, J = 9.2 Hz, 2H), 7.18–7.12 (m, 2H), 6.81 (d, J = 8.8 Hz, 2H), 5.30 (s, 1H), 4.95 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.64 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.36–4.24 (m, 2H), 3.76 (s, 3H), 1.34 (t, J = 7.1 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.6, 166.2, 158.4, 144.7, 139.9, 134.0, 132.5, 132.0, 131.3, 130.1, 128.3, 121.3, 118.4, 114.0, 109.9, 65.2, 61.8, 55.4, 35.0, 14.3. IR(KBr) ν: 2925, 1712, 1628, 1515, 1466, 1403, 1249, 1179, 1102, 1039. HRMS(EI) (m/z): calcd for C23H20NO5 (M + H)+: 377.1384; found: 377.1385.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4h as a yellow oil (60.2 mg, 80% yield), Rf = 0.18 (EtOAc/petroleum ether 1
10) as eluent to give 4h as a yellow oil (60.2 mg, 80% yield), Rf = 0.18 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.22–7.14 (m, 3H), 6.90 (d, J = 6.8 Hz, 2H), 6.75–6.72 (m, 1H), 5.34 (s, 1H), 4.97 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.37–4.24 (m, 2H), 3.76 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.7, 166.1, 159.8, 144.4, 143.6, 139.8, 132.5, 132.2, 131.3, 130.1, 129.6, 121.3, 119.6, 118.5, 113.7, 111.5, 109.5, 65.1, 61.9, 55.3, 35.5, 14.4. IR(KBr) ν: 2932, 2855, 1712, 1628, 1592, 1585, 1452, 1396, 1242, 1109, 1046. HRMS(EI) (m/z): calcd for C23H20O5 (M + H)+: 377.1384; found: 377.1387.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.22–7.14 (m, 3H), 6.90 (d, J = 6.8 Hz, 2H), 6.75–6.72 (m, 1H), 5.34 (s, 1H), 4.97 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.37–4.24 (m, 2H), 3.76 (s, 3H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.7, 166.1, 159.8, 144.4, 143.6, 139.8, 132.5, 132.2, 131.3, 130.1, 129.6, 121.3, 119.6, 118.5, 113.7, 111.5, 109.5, 65.1, 61.9, 55.3, 35.5, 14.4. IR(KBr) ν: 2932, 2855, 1712, 1628, 1592, 1585, 1452, 1396, 1242, 1109, 1046. HRMS(EI) (m/z): calcd for C23H20O5 (M + H)+: 377.1384; found: 377.1387.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4i as a yellow oil (60.8 mg, 80% yield), Rf = 0.22 (EtOAc/petroleum ether 1
10) as eluent to give 4i as a yellow oil (60.8 mg, 80% yield), Rf = 0.22 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 7.2 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 7.30 (t, 2H), 7.22–7.16 (m, 5H), 5.33 (s, 1H), 4.87 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.69–4.64 (m, 1H), 4.38–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 173.9, 165.9, 144.0, 143.9, 139.6, 134.5, 132.6, 132.4, 131.2, 130.2, 129.9, 127.3, 127.0, 125.5, 121.4, 118.6, 108.9, 65.1, 62.0, 35.3, 14.3. IR(KBr) ν: 2925, 2855, 1712, 1628, 1585, 1466, 1403, 1235, 1179, 1102, 1032, 983. HRMS(EI) (m/z): calcd for C22H17ClO4 (M + H)+: 381.0888; found: 381.0886.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 7.2 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 7.30 (t, 2H), 7.22–7.16 (m, 5H), 5.33 (s, 1H), 4.87 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.69–4.64 (m, 1H), 4.38–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 173.9, 165.9, 144.0, 143.9, 139.6, 134.5, 132.6, 132.4, 131.2, 130.2, 129.9, 127.3, 127.0, 125.5, 121.4, 118.6, 108.9, 65.1, 62.0, 35.3, 14.3. IR(KBr) ν: 2925, 2855, 1712, 1628, 1585, 1466, 1403, 1235, 1179, 1102, 1032, 983. HRMS(EI) (m/z): calcd for C22H17ClO4 (M + H)+: 381.0888; found: 381.0886.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4j as a yellow solid (45.9 mg, 61% yield), Rf = 0.19 (EtOAc/petroleum ether 1
10) as eluent to give 4j as a yellow solid (45.9 mg, 61% yield), Rf = 0.19 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 195 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.19 (t, J = 6.8 Hz, 2H), 6.88–6.81 (m, 2H), 6.70 (t, J = 8.0 Hz, 1H), 5.31 (s, 1H), 5.04 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.53–4.48 (m, 1H), 4.36–4.27 (m, 2H), 3.73 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.7, 174.5, 166.9, 156.4, 146.5, 139.8, 132.4, 131.4, 130.3, 130.0, 128.7, 128.2, 128.0, 121.2, 120.2, 118.2, 110.8, 107.8, 64.5, 61.2, 55.0, 32.5, 14.4. IR(KBr) ν: 2932, 2848, 1740, 1691, 1642, 1543, 1515, 1459, 1424, 1102, 1046, 997. HRMS(EI) (m/z): calcd for C23H20O5 (M + H)+: 377.1384; found: 377.1386.
10). Mp 195 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 6.8 Hz, 1H), 7.36 (t, J = 8.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.26 (d, J = 7.2 Hz, 1H), 7.19 (t, J = 6.8 Hz, 2H), 6.88–6.81 (m, 2H), 6.70 (t, J = 8.0 Hz, 1H), 5.31 (s, 1H), 5.04 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.53–4.48 (m, 1H), 4.36–4.27 (m, 2H), 3.73 (s, 3H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.7, 174.5, 166.9, 156.4, 146.5, 139.8, 132.4, 131.4, 130.3, 130.0, 128.7, 128.2, 128.0, 121.2, 120.2, 118.2, 110.8, 107.8, 64.5, 61.2, 55.0, 32.5, 14.4. IR(KBr) ν: 2932, 2848, 1740, 1691, 1642, 1543, 1515, 1459, 1424, 1102, 1046, 997. HRMS(EI) (m/z): calcd for C23H20O5 (M + H)+: 377.1384; found: 377.1386.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4k as a yellow solid (53.6 mg, 66% yield), Rf = 0.15 (EtOAc/petroleum ether 1
5) as eluent to give 4k as a yellow solid (53.6 mg, 66% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 172 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 7.2 Hz, 1H), 7.37 (t, 1H), 7.30 (t, 1H), 7.19 (d, J = 7.2 Hz, 1H), 6.95 (t, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.82–6.76 (m, 2H), 5.36 (s, 1H), 4.51 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.51 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.30 (q, J = 7.2 Hz, 2H), 3.83 (s, 3H), 3.79 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.7, 174.3, 166.4, 153.2, 146.3, 146.2, 139.8, 136.2, 132.4, 131.5, 130.1, 128.0, 123.5, 121.3, 121.1, 118.3, 111.5, 108.3, 64.8, 61.5, 60.3, 55.9, 32.3, 14.4. IR(KBr) ν: 2918, 2814, 1726, 1466, 1396, 1291, 1228, 1116, 1032, 983. HRMS(EI) (m/z): calcd for C24H22O6 (M + H)+: 407.1489; found: 407.1488.
10). Mp 172 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 7.2 Hz, 1H), 7.37 (t, 1H), 7.30 (t, 1H), 7.19 (d, J = 7.2 Hz, 1H), 6.95 (t, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.82–6.76 (m, 2H), 5.36 (s, 1H), 4.51 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.51 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.30 (q, J = 7.2 Hz, 2H), 3.83 (s, 3H), 3.79 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.7, 174.3, 166.4, 153.2, 146.3, 146.2, 139.8, 136.2, 132.4, 131.5, 130.1, 128.0, 123.5, 121.3, 121.1, 118.3, 111.5, 108.3, 64.8, 61.5, 60.3, 55.9, 32.3, 14.4. IR(KBr) ν: 2918, 2814, 1726, 1466, 1396, 1291, 1228, 1116, 1032, 983. HRMS(EI) (m/z): calcd for C24H22O6 (M + H)+: 407.1489; found: 407.1488.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4l as a Yellow solid (52.5 mg, 67% yield), Rf = 0.2 (EtOAc/petroleum ether 1
10) as eluent to give 4l as a Yellow solid (52.5 mg, 67% yield), Rf = 0.2 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 181 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 6.8 Hz, 1H), 7.37 (t, 1H), 7.30 (t, 1H), 7.19 (d, J = 7.2 Hz, 1H), 6.95 (t, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.79 (dd, J1 = 19.2 Hz, J2 = 8.0 Hz, 2H), 5.34 (s, 1H), 5.00 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.54–4.49 (m, 1H), 3.84 (s, 3H), 3.83 (s, 3H), 3.79 (s, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 174.4, 166.9, 153.2, 146.2, 145.9, 139.8, 136.1, 132.5, 131.5, 130.1, 128.3, 123.6, 121.3, 121.0, 118.4, 111.6, 108.1, 64.7, 60.3, 55.9, 52.5, 32.3. IR(KBr) ν: 2915, 2808, 1733, 1465, 1387, 1283, 1216, 1109, 1022, 981. HRMS(EI) (m/z): calcd for C23H20O6 (M + H)+: 393.1333; found: 393.1335.
10). Mp 181 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, J = 6.8 Hz, 1H), 7.37 (t, 1H), 7.30 (t, 1H), 7.19 (d, J = 7.2 Hz, 1H), 6.95 (t, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.79 (dd, J1 = 19.2 Hz, J2 = 8.0 Hz, 2H), 5.34 (s, 1H), 5.00 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.54–4.49 (m, 1H), 3.84 (s, 3H), 3.83 (s, 3H), 3.79 (s, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 174.4, 166.9, 153.2, 146.2, 145.9, 139.8, 136.1, 132.5, 131.5, 130.1, 128.3, 123.6, 121.3, 121.0, 118.4, 111.6, 108.1, 64.7, 60.3, 55.9, 52.5, 32.3. IR(KBr) ν: 2915, 2808, 1733, 1465, 1387, 1283, 1216, 1109, 1022, 981. HRMS(EI) (m/z): calcd for C23H20O6 (M + H)+: 393.1333; found: 393.1335.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4m as a yellow oil (59.8 mg, 90% yield), Rf = 0.22 (EtOAc/petroleum ether 1
10) as eluent to give 4m as a yellow oil (59.8 mg, 90% yield), Rf = 0.22 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 7.2 Hz, 1H), 7.37–7.27 (m, 6H), 7.21–7.15 (m, 3H), 5.37 (s, 1H), 4.91 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 3.84 (s, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.6, 166.6, 144.2, 141.7, 139.7, 132.5, 132.4, 131.2, 130.0, 128.6, 127.1, 126.7, 121.2, 118.4, 109.5, 65.1, 52.8, 35.5. IR(KBr) ν: 2925, 2848, 1719, 1628, 1459, 1403, 1242, 1102, 1039, 976. HRMS(EI) (m/z): calcd for C21H16O4 (M + H)+: 333.1121; found: 323.1124.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.45 (d, J = 7.2 Hz, 1H), 7.37–7.27 (m, 6H), 7.21–7.15 (m, 3H), 5.37 (s, 1H), 4.91 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 4.63 (dd, J1 = 12.4 Hz, J2 = 7.6 Hz, 1H), 3.84 (s, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.3, 173.6, 166.6, 144.2, 141.7, 139.7, 132.5, 132.4, 131.2, 130.0, 128.6, 127.1, 126.7, 121.2, 118.4, 109.5, 65.1, 52.8, 35.5. IR(KBr) ν: 2925, 2848, 1719, 1628, 1459, 1403, 1242, 1102, 1039, 976. HRMS(EI) (m/z): calcd for C21H16O4 (M + H)+: 333.1121; found: 323.1124.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4n as a yellow solid (60.1 mg, 83% yield), Rf = 0.18 (EtOAc/petroleum ether 1
10) as eluent to give 4n as a yellow solid (60.1 mg, 83% yield), Rf = 0.18 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 112 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.0 Hz, 1H), 7.35 (t, 1H), 7.29 (t, 1H), 7.23 (d, 2H), 7.20–7.11 (m, 2H), 6.81 (d, J = 8.8 Hz, 1H), 5.30 (s, 1H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.5, 166.7, 158.4, 144.4, 139.8, 133.9, 132.5, 132.3, 131.3, 130.1, 128.2, 121.3, 118.5, 114.0, 109.9, 65.2, 55.4, 52.8, 35.0. IR(KBr) ν: 2981, 2925, 1712, 1628, 1508, 1459, 1403, 1242, 1186, 1095, 1053. HRMS(EI) (m/z): calcd for C22H18O5 (M + H)+: 363.1227; found: 363.1225.
10). Mp 112 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.0 Hz, 1H), 7.35 (t, 1H), 7.29 (t, 1H), 7.23 (d, 2H), 7.20–7.11 (m, 2H), 6.81 (d, J = 8.8 Hz, 1H), 5.30 (s, 1H); 13C NMR (101 MHz, CDCl3), δ: 194.4, 173.5, 166.7, 158.4, 144.4, 139.8, 133.9, 132.5, 132.3, 131.3, 130.1, 128.2, 121.3, 118.5, 114.0, 109.9, 65.2, 55.4, 52.8, 35.0. IR(KBr) ν: 2981, 2925, 1712, 1628, 1508, 1459, 1403, 1242, 1186, 1095, 1053. HRMS(EI) (m/z): calcd for C22H18O5 (M + H)+: 363.1227; found: 363.1225.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4o as a Yellow solid (57.6 mg, 66% yield), Rf = 0.11 (EtOAc/petroleum ether 1
5) as eluent to give 4o as a Yellow solid (57.6 mg, 66% yield), Rf = 0.11 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 155 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.36 (t, 1H), 7.30 (t, 1H), 7.18 (d, J = 7.2 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.79 (t, 1H), 6.52 (d, J = 8.4 Hz, 1H), 5.25 (s, 1H), 4.56–4.51 (m, 1H), 4.33–4.27 (m, 1H), 4.30 (q, 2H), 3.82 (s, 3H), 3.81 (s, 3H), 3.79 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 174.3, 166.4, 153.1, 151.0, 146.3, 142.5, 139.8, 132.4, 131.4, 130.0, 128.2, 128.1, 123.1, 121.2, 118.3, 108.3, 106.1, 64.7, 61.4, 60.8, 60.4, 56.0, 32.0, 14.4. IR(KBr) ν: 2925, 2855, 1726, 1628, 1466, 1396, 1249, 1109, 1039, 983. HRMS(EI) (m/z): calcd for C25H24O7 (M + H)+: 437.1595; found: 437.1594.
10). Mp 155 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.36 (t, 1H), 7.30 (t, 1H), 7.18 (d, J = 7.2 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.79 (t, 1H), 6.52 (d, J = 8.4 Hz, 1H), 5.25 (s, 1H), 4.56–4.51 (m, 1H), 4.33–4.27 (m, 1H), 4.30 (q, 2H), 3.82 (s, 3H), 3.81 (s, 3H), 3.79 (s, 3H), 1.35 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 174.3, 166.4, 153.1, 151.0, 146.3, 142.5, 139.8, 132.4, 131.4, 130.0, 128.2, 128.1, 123.1, 121.2, 118.3, 108.3, 106.1, 64.7, 61.4, 60.8, 60.4, 56.0, 32.0, 14.4. IR(KBr) ν: 2925, 2855, 1726, 1628, 1466, 1396, 1249, 1109, 1039, 983. HRMS(EI) (m/z): calcd for C25H24O7 (M + H)+: 437.1595; found: 437.1594.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4p as a yellow oil (44.8 mg, 64% yield), Rf = 0.23 (EtOAc/petroleum ether 1
10) as eluent to give 4p as a yellow oil (44.8 mg, 64% yield), Rf = 0.23 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.19 (t, 2H), 6.05 (d, J = 2.8 Hz, 1H), 5.84 (d, J = 2.0 Hz, 1H), 5.47 (dd, J1 = 12.4 Hz, J2 = 6.8 Hz, 1H), 5.28 (s, 1H), 4.75 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.35–4.21 (m, 2H), 2.22 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.5, 165.5, 151.8, 151.4, 141.6, 139.9, 133.1, 132.5, 131.3, 130.0, 121.3, 118.5, 107.6, 107.5, 106.4, 65.4, 61.7, 31.3, 14.3, 13.8. IR(KBr) ν: 2925, 2848, 1719, 1635, 1585, 1459, 1403, 1242, 1109. HRMS(EI) (m/z): calcd for C21H18O5 (M + H)+: 351.1227; found: 351.1225.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.19 (t, 2H), 6.05 (d, J = 2.8 Hz, 1H), 5.84 (d, J = 2.0 Hz, 1H), 5.47 (dd, J1 = 12.4 Hz, J2 = 6.8 Hz, 1H), 5.28 (s, 1H), 4.75 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.35–4.21 (m, 2H), 2.22 (s, 3H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.5, 165.5, 151.8, 151.4, 141.6, 139.9, 133.1, 132.5, 131.3, 130.0, 121.3, 118.5, 107.6, 107.5, 106.4, 65.4, 61.7, 31.3, 14.3, 13.8. IR(KBr) ν: 2925, 2848, 1719, 1635, 1585, 1459, 1403, 1242, 1109. HRMS(EI) (m/z): calcd for C21H18O5 (M + H)+: 351.1227; found: 351.1225.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4q as a yellow oil (40.3 mg, 60% yield), Rf = 0.21 (EtOAc/petroleum ether 1
10) as eluent to give 4q as a yellow oil (40.3 mg, 60% yield), Rf = 0.21 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (d, J = 7.2 Hz, 1H), 7.35 (t, 1H), 7.29 (d, 2H), 7.19 (dd, J1 = 8.0 Hz, J2 = 12.0 Hz, 2H), 6.27 (dd, J1 = 3.2 Hz, J2 = 2.0 Hz, 1H), 6.18 (d, J = 3.2 Hz, 1H), 5.41–5.36 (m, 1H), 5.33 (s, 1H), 4.75 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.34–4.23 (m, 2H), 1.32 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.7, 165.4, 153.4, 142.1, 141.4, 139.8, 133.3, 132.5, 131.3, 130.1, 121.3, 118.6, 110.5, 107.3, 106.8, 65.3, 61.8, 31.3, 14.3. IR(KBr) ν: 2918, 2848, 1712, 1635, 1543, 1508, 1459, 1403, 1242, 1109, 1046, 1004. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 337.1071; found: 337.1076.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (d, J = 7.2 Hz, 1H), 7.35 (t, 1H), 7.29 (d, 2H), 7.19 (dd, J1 = 8.0 Hz, J2 = 12.0 Hz, 2H), 6.27 (dd, J1 = 3.2 Hz, J2 = 2.0 Hz, 1H), 6.18 (d, J = 3.2 Hz, 1H), 5.41–5.36 (m, 1H), 5.33 (s, 1H), 4.75 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.34–4.23 (m, 2H), 1.32 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.7, 165.4, 153.4, 142.1, 141.4, 139.8, 133.3, 132.5, 131.3, 130.1, 121.3, 118.6, 110.5, 107.3, 106.8, 65.3, 61.8, 31.3, 14.3. IR(KBr) ν: 2918, 2848, 1712, 1635, 1543, 1508, 1459, 1403, 1242, 1109, 1046, 1004. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 337.1071; found: 337.1076.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4r as a yellow oil (50 mg, 71% yield), Rf = 0.24 (EtOAc/petroleum ether 1
10) as eluent to give 4r as a yellow oil (50 mg, 71% yield), Rf = 0.24 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.29 (t, 1H), 7.19–7.13 (m, 3H), 6.91–6.88 (m, 2H), 5.46 (s, 1H), 5.19–5.15 (m, 1H) 4.71 (dd, J1 = 12.8 Hz, J2 = 8.0 Hz, 1H), 4.37–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.5, 165.5, 145.5, 143.6, 139.6, 132.7, 132.6, 131.1, 130.2, 126.8, 124.7, 124.4, 121.4, 118.7, 109.6, 65.2, 61.9, 32.4, 14.3. IR(KBr) ν: 2925, 2855, 1705, 1628, 1585, 1466, 1396, 1242, 1109, 1046. HRMS(EI) (m/z): calcd for C20H16O4S (M + H)+: 353.0842; found: 353.0845.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.44 (d, J = 7.2 Hz, 1H), 7.35 (t, J = 8.0 Hz, 1H), 7.29 (t, 1H), 7.19–7.13 (m, 3H), 6.91–6.88 (m, 2H), 5.46 (s, 1H), 5.19–5.15 (m, 1H) 4.71 (dd, J1 = 12.8 Hz, J2 = 8.0 Hz, 1H), 4.37–4.24 (m, 2H), 1.34 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 193.8, 173.5, 165.5, 145.5, 143.6, 139.6, 132.7, 132.6, 131.1, 130.2, 126.8, 124.7, 124.4, 121.4, 118.7, 109.6, 65.2, 61.9, 32.4, 14.3. IR(KBr) ν: 2925, 2855, 1705, 1628, 1585, 1466, 1396, 1242, 1109, 1046. HRMS(EI) (m/z): calcd for C20H16O4S (M + H)+: 353.0842; found: 353.0845.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as eluent to give 4s as a brown oil (39.6 mg, 57% yield), Rf = 0.18 (EtOAc/petroleum ether 1
10) as eluent to give 4s as a brown oil (39.6 mg, 57% yield), Rf = 0.18 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 8.56 (s, 1H), 8.44 (d, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 7.2 Hz, 1H), 7.36 (t, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.22–7.17 (m, 3H), 5.35 (s, 1H), 4.84 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.73–4.68 (m, 1H), 4.34–4.25 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.0, 165.8, 148.4, 148.1, 143.3, 139.5, 137.5, 135.2, 132.7, 132.7, 131.1, 130.3, 129.6, 123.5, 121.4, 118.7, 108.4, 65.2, 62.1, 33.9, 14.3. IR(KBr) ν: 2974, 2925, 1705, 1621, 1585, 1550, 1459, 1410, 1256, 1116, 1046. HRMS(EI) (m/z): calcd for C21H17NO4 (M + H)+: 348.1230; found: 348.1227.
10). 1H NMR (CDCl3, 400 MHz), δ: 8.56 (s, 1H), 8.44 (d, 1H), 7.67 (d, J = 8.0 Hz, 1H), 7.44 (d, J = 7.2 Hz, 1H), 7.36 (t, 1H), 7.29 (t, J = 7.2 Hz, 1H), 7.22–7.17 (m, 3H), 5.35 (s, 1H), 4.84 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 4.73–4.68 (m, 1H), 4.34–4.25 (m, 2H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.2, 174.0, 165.8, 148.4, 148.1, 143.3, 139.5, 137.5, 135.2, 132.7, 132.7, 131.1, 130.3, 129.6, 123.5, 121.4, 118.7, 108.4, 65.2, 62.1, 33.9, 14.3. IR(KBr) ν: 2974, 2925, 1705, 1621, 1585, 1550, 1459, 1410, 1256, 1116, 1046. HRMS(EI) (m/z): calcd for C21H17NO4 (M + H)+: 348.1230; found: 348.1227.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4t as a yellow solid (29.3 mg, 38% yield), Rf = 0.15 (EtOAc/petroleum ether 1
5) as eluent to give 4t as a yellow solid (29.3 mg, 38% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 183 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.69 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.36 (t, 1H), 7.29 (d, J = 8.0 Hz, 2H), 4.84 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 7.12–7.05 (m, 2H), 6.98 (m, 1H), 5.54 (t, J = 1.2 Hz, 1H), 5.40–5.35 (m, 1H), 4.61 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.39–4.31 (m, 2H), 1.36 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.0, 173.6, 166.1, 144.1, 139.8, 137.0, 132.4, 132.1, 131.3, 129.9, 125.8, 122.9, 122.3, 121.2, 120.1, 119.7, 118.3, 117.4, 111.2, 109.8, 64.5, 61.8, 28.5, 14.3. IR(KBr) ν: 2939, 1719, 1635, 1200, 1144. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 86.1543; found: 386.1541.
10). Mp 183 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.69 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 8.0 Hz, 1H), 7.36 (t, 1H), 7.29 (d, J = 8.0 Hz, 2H), 4.84 (dd, J1 = 12.4 Hz, J2 = 7.2 Hz, 1H), 7.12–7.05 (m, 2H), 6.98 (m, 1H), 5.54 (t, J = 1.2 Hz, 1H), 5.40–5.35 (m, 1H), 4.61 (dd, J1 = 12.4 Hz, J2 = 8.0 Hz, 1H), 4.39–4.31 (m, 2H), 1.36 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.0, 173.6, 166.1, 144.1, 139.8, 137.0, 132.4, 132.1, 131.3, 129.9, 125.8, 122.9, 122.3, 121.2, 120.1, 119.7, 118.3, 117.4, 111.2, 109.8, 64.5, 61.8, 28.5, 14.3. IR(KBr) ν: 2939, 1719, 1635, 1200, 1144. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 86.1543; found: 386.1541.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as eluent to give 4u as a yellow oil (55.9 mg, 70% yield), Rf = 0.12 (EtOAc/petroleum ether 1
5) as eluent to give 4u as a yellow oil (55.9 mg, 70% yield), Rf = 0.12 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.67 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 7.2 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.24–7.18 (m, 3H), 7.12–7.05 (m, 2H), 6.87 (s, 1H), 5.53 (s, 1H), 5.44–5.39 (m, 1H), 4.65–4.60 (m, 1H), 4.39–4.33 (m, 2H), 3.70 (s, 3H), 1.37 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.1, 173.6, 166.2, 144.4, 139.9, 137.8, 132.5, 132.1, 131.4, 130.0, 127.7, 126.3, 122.0, 121.3, 120.4, 119.3, 118.3, 115.8, 110.1, 109.3, 64.7, 61.8, 32.9, 29.8, 28.6, 14.4. IR(KBr) ν: 2925, 2855, 1719, 1628, 1585, 1473, 1403, 1235, 1109, 1039, 983. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 400.1543; found: 400.1544.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.67 (d, J = 8.0 Hz, 1H), 7.46 (d, J = 7.2 Hz, 1H), 7.37 (t, J = 8.0 Hz, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.24–7.18 (m, 3H), 7.12–7.05 (m, 2H), 6.87 (s, 1H), 5.53 (s, 1H), 5.44–5.39 (m, 1H), 4.65–4.60 (m, 1H), 4.39–4.33 (m, 2H), 3.70 (s, 3H), 1.37 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 194.1, 173.6, 166.2, 144.4, 139.9, 137.8, 132.5, 132.1, 131.4, 130.0, 127.7, 126.3, 122.0, 121.3, 120.4, 119.3, 118.3, 115.8, 110.1, 109.3, 64.7, 61.8, 32.9, 29.8, 28.6, 14.4. IR(KBr) ν: 2925, 2855, 1719, 1628, 1585, 1473, 1403, 1235, 1109, 1039, 983. HRMS(EI) (m/z): calcd for C25H21NO4 (M + H)+: 400.1543; found: 400.1544.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford compound 6a as a brown oil (44.2 mg, 83% yield), Rf = 0.17 (EtOAc/petroleum ether 1
10) to afford compound 6a as a brown oil (44.2 mg, 83% yield), Rf = 0.17 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (m, 3H), 7.32 (m, 2H), 6.43–6.34 (m, 2H), 4.23–4.18 (m, 2H), 3.57 (d, J = 9.6 Hz, 1H), 3.15–3.10 (m, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 136.1, 129.9, 129.6, 129.6, 129.1, 128.2, 114.8, 111.1, 61.2, 38.5, 36.4, 14.3, 12.3. IR(KBr) ν: 2927.97, 2253.41, 1721.18, 1646.23, 1271.47, 1162.45, 1039.80, 971.66, 740.00, 692.30. HRMS(EI) (m/z): calcd for C16H14N2O2 (M + H)+: 267.1128; found: 267.1123.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.43 (m, 3H), 7.32 (m, 2H), 6.43–6.34 (m, 2H), 4.23–4.18 (m, 2H), 3.57 (d, J = 9.6 Hz, 1H), 3.15–3.10 (m, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 136.1, 129.9, 129.6, 129.6, 129.1, 128.2, 114.8, 111.1, 61.2, 38.5, 36.4, 14.3, 12.3. IR(KBr) ν: 2927.97, 2253.41, 1721.18, 1646.23, 1271.47, 1162.45, 1039.80, 971.66, 740.00, 692.30. HRMS(EI) (m/z): calcd for C16H14N2O2 (M + H)+: 267.1128; found: 267.1123.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6b as a yellow oil (46.7 mg, 75% yield), Rf = 0.23 (EtOAc/petroleum ether 1
5) to afford compound 6b as a yellow oil (46.7 mg, 75% yield), Rf = 0.23 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).1H NMR (CDCl3, 400 MHz), δ: 8.29 (d, 2H), 7.43 (d, 2H), 7.05 (s, 1H), 4.90 (s, 1H), 4.14 (dd, J1 = 7.2 Hz, J2 = 4.0 Hz, 2H), 3.48 (m, 2H), 1.17 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 161.7, 148.6, 141.2, 139.4, 136.6, 129.3, 124.6, 115.9, 113.1, 61.8, 59.6, 44.0, 39.8, 14.1. IR(KBr) ν: 2918, 2245, 1719, 1649, 1494, 1466, 1256, 1165, 1032, 976, 751. HRMS(EI) (m/z): calcd for C16H13N3O4 (M + H)+: 312.0979; found: 312.0976.
5).1H NMR (CDCl3, 400 MHz), δ: 8.29 (d, 2H), 7.43 (d, 2H), 7.05 (s, 1H), 4.90 (s, 1H), 4.14 (dd, J1 = 7.2 Hz, J2 = 4.0 Hz, 2H), 3.48 (m, 2H), 1.17 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 161.7, 148.6, 141.2, 139.4, 136.6, 129.3, 124.6, 115.9, 113.1, 61.8, 59.6, 44.0, 39.8, 14.1. IR(KBr) ν: 2918, 2245, 1719, 1649, 1494, 1466, 1256, 1165, 1032, 976, 751. HRMS(EI) (m/z): calcd for C16H13N3O4 (M + H)+: 312.0979; found: 312.0976.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford compound 6c as a white solid (40.2 mg, 69% yield), Rf = 0.22 (EtOAc/petroleum ether 1
3) to afford compound 6c as a white solid (40.2 mg, 69% yield), Rf = 0.22 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Mp 151 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.75 (d, 2H), 7.46 (d, 2H), 6.41 (d, J = 15.2 Hz, 1H), 6.27 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 4.21 (dd, J1 = 14.0 Hz, J2 = 7.2 Hz, 2H), 3.57 (d, J = 10.0 Hz, 1H), 3.20 (t, J = 10.0 Hz, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.1, 134.8, 133.3, 133.3, 130.8, 130.1, 117.9, 114.0, 113.9, 110.7, 61.4, 37.5, 35.9, 14.3, 12.4. IR(KBr) ν: 2925, 2231, 1733, 1642, 1438, 1284, 1228, 1046. HRMS(EI) (m/z): calcd for C17H13N3O2 (M + H)+: 292.1081; found: 292.1080.
3). Mp 151 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.75 (d, 2H), 7.46 (d, 2H), 6.41 (d, J = 15.2 Hz, 1H), 6.27 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 4.21 (dd, J1 = 14.0 Hz, J2 = 7.2 Hz, 2H), 3.57 (d, J = 10.0 Hz, 1H), 3.20 (t, J = 10.0 Hz, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.1, 134.8, 133.3, 133.3, 130.8, 130.1, 117.9, 114.0, 113.9, 110.7, 61.4, 37.5, 35.9, 14.3, 12.4. IR(KBr) ν: 2925, 2231, 1733, 1642, 1438, 1284, 1228, 1046. HRMS(EI) (m/z): calcd for C17H13N3O2 (M + H)+: 292.1081; found: 292.1080.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6d as a brown oil (55 mg, 89% yield), Rf = 0.17 (EtOAc/petroleum ether 1
5) to afford compound 6d as a brown oil (55 mg, 89% yield), Rf = 0.17 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (CDCl3, 400 MHz), δ: 7.13 (d, 2H), 6.74–6.67 (m, 3H), 6.30 (d, J = 16.0 Hz, 1H), 4.28–4.22 (m, 2H), 3.27 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 2.98 (s, 6H), 1.32 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.7, 151.2, 138.4, 129.1, 128.2, 115.9, 113.3, 112.7, 112.4, 61.2, 41.1, 40.3, 35.5, 14.9, 14.3. IR(KBr) ν: 2925, 2245, 1712, 1614, 1536, 1354, 1277, 1165, 1095, 1039, 964, 822. HRMS(EI) (m/z): calcd for C18H19N3O2 (M + H)+: 310.1550; found: 310.1553.
5). 1H NMR (CDCl3, 400 MHz), δ: 7.13 (d, 2H), 6.74–6.67 (m, 3H), 6.30 (d, J = 16.0 Hz, 1H), 4.28–4.22 (m, 2H), 3.27 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 2.98 (s, 6H), 1.32 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.7, 151.2, 138.4, 129.1, 128.2, 115.9, 113.3, 112.7, 112.4, 61.2, 41.1, 40.3, 35.5, 14.9, 14.3. IR(KBr) ν: 2925, 2245, 1712, 1614, 1536, 1354, 1277, 1165, 1095, 1039, 964, 822. HRMS(EI) (m/z): calcd for C18H19N3O2 (M + H)+: 310.1550; found: 310.1553.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6e as a white solid (44.4 mg, 75% yield), Rf = 0.46 (EtOAc/petroleum ether 1
5) to afford compound 6e as a white solid (44.4 mg, 75% yield), Rf = 0.46 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Mp 118 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.40 (t, J = 8.0 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 7.01–6.94 (m, 2H), 6.45 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.35 (d, J = 15.6 Hz, 1H), 4.24–4.19 (m, 2H), 3.93 (s, 3H), 3.29 (d, J = 9.6 Hz, 1H), 3.11 (t, J = 9.2 Hz, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.6, 158.7, 137.1, 131.2, 130.3, 128.4, 120.8, 116.9, 115.1, 111.7, 111.3, 61.1, 55.8, 36.2, 35.0, 14.3, 13.1. IR(KBr) ν: 2921, 2245, 1714, 1646, 1503, 1462, 1257, 1162, 1026, 978, 753. HRMS(EI) (m/z): calcd for C17H16N2O3 (M + H)+: 297.1234; found: 297.1229.
3). Mp 118 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.40 (t, J = 8.0 Hz, 1H), 7.12 (d, J = 7.6 Hz, 1H), 7.01–6.94 (m, 2H), 6.45 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.35 (d, J = 15.6 Hz, 1H), 4.24–4.19 (m, 2H), 3.93 (s, 3H), 3.29 (d, J = 9.6 Hz, 1H), 3.11 (t, J = 9.2 Hz, 1H), 1.28 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.6, 158.7, 137.1, 131.2, 130.3, 128.4, 120.8, 116.9, 115.1, 111.7, 111.3, 61.1, 55.8, 36.2, 35.0, 14.3, 13.1. IR(KBr) ν: 2921, 2245, 1714, 1646, 1503, 1462, 1257, 1162, 1026, 978, 753. HRMS(EI) (m/z): calcd for C17H16N2O3 (M + H)+: 297.1234; found: 297.1229.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford compound 6f as a white solid (49.8 mg, 70% yield), Rf = 0.22 (EtOAc/petroleum ether 1
3) to afford compound 6f as a white solid (49.8 mg, 70% yield), Rf = 0.22 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 6.81 (d, J = 8.4 Hz, 1H), 6.61 (d, J = 8.4 Hz, 1H), 6.53–6.46 (m, 1H), 6.34 (d, J = 15.6 Hz, 1H), 4.25–4.19 (m, 2H), 4.08 (s, 3H), 3.87 (s, 3H), 3.86 (s, 3H), 3.29 (d, J = 9.2 Hz, 1H), 3.08 (t, J = 10.0 Hz, 1H), 1.29 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.6, 155.4, 153.3, 141.9, 137.1, 128.5, 124.7, 115.0, 114.0, 111.8, 106.6, 61.4, 61.1, 61.1, 56.1, 36.2, 35.3, 14.3, 13.0. IR(KBr) ν: 2925, 2848, 2252, 1719, 1656, 1607, 1494, 1466, 1424, 1312, 1263, 1158, 1095, 1039, 976. HRMS(EI) (m/z): calcd for C19H20N2O5 (M + H)+: 357.1445; found: 357.1447.
3). Mp 115 °C; 1H NMR (CDCl3, 400 MHz), δ: 6.81 (d, J = 8.4 Hz, 1H), 6.61 (d, J = 8.4 Hz, 1H), 6.53–6.46 (m, 1H), 6.34 (d, J = 15.6 Hz, 1H), 4.25–4.19 (m, 2H), 4.08 (s, 3H), 3.87 (s, 3H), 3.86 (s, 3H), 3.29 (d, J = 9.2 Hz, 1H), 3.08 (t, J = 10.0 Hz, 1H), 1.29 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.6, 155.4, 153.3, 141.9, 137.1, 128.5, 124.7, 115.0, 114.0, 111.8, 106.6, 61.4, 61.1, 61.1, 56.1, 36.2, 35.3, 14.3, 13.0. IR(KBr) ν: 2925, 2848, 2252, 1719, 1656, 1607, 1494, 1466, 1424, 1312, 1263, 1158, 1095, 1039, 976. HRMS(EI) (m/z): calcd for C19H20N2O5 (M + H)+: 357.1445; found: 357.1447.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6g as a yellow oil (35.8 mg, 67% yield), Rf = 0.24 (EtOAc/petroleum ether 1
5) to afford compound 6g as a yellow oil (35.8 mg, 67% yield), Rf = 0.24 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (CDCl3, 400 MHz), δ: 8.66 (d, J = 3.6 Hz, 1H), 8.52 (s, 1H), 7.56 (dt, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H), 7.37 (dd, J1 = 7.6 Hz, J2 = 4.8 Hz, 1H), 7.02 (dd, J1 = 4.0 Hz, J2 = 2.4 Hz, 1H), 4.81 (s, 1H), 4.19–4.08 (m, 2H), 3.51–3.39 (m, 2H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 161.8, 150.9, 149.2, 139.0, 136.6, 135.8, 130.1, 124.1, 116.1, 113.3, 61.7, 57.8, 43.8, 40.0, 14.1. IR(KBr) ν: 1712, 1642, 1249, 1102, 1025, 709. HRMS(EI) (m/z): calcd for C15H13N3O2 (M + H)+: 268.1081; found: 268.1085.
5). 1H NMR (CDCl3, 400 MHz), δ: 8.66 (d, J = 3.6 Hz, 1H), 8.52 (s, 1H), 7.56 (dt, J1 = 8.0 Hz, J2 = 2.0 Hz, 1H), 7.37 (dd, J1 = 7.6 Hz, J2 = 4.8 Hz, 1H), 7.02 (dd, J1 = 4.0 Hz, J2 = 2.4 Hz, 1H), 4.81 (s, 1H), 4.19–4.08 (m, 2H), 3.51–3.39 (m, 2H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 161.8, 150.9, 149.2, 139.0, 136.6, 135.8, 130.1, 124.1, 116.1, 113.3, 61.7, 57.8, 43.8, 40.0, 14.1. IR(KBr) ν: 1712, 1642, 1249, 1102, 1025, 709. HRMS(EI) (m/z): calcd for C15H13N3O2 (M + H)+: 268.1081; found: 268.1085.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford compound 6h as a brown oil (36.2 mg, 67% yield), Rf = 0.45 (EtOAc/petroleum ether 1
10) to afford compound 6h as a brown oil (36.2 mg, 67% yield), Rf = 0.45 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (CDCl3, 400 MHz), δ: 6.81 (dd, J1 = 15.6, J2 = 10.0 Hz, 1H), 6.33 (d, 2H), 5.99 (s, 1H), 4.27–4.21 (m, 2H), 3.43 (d, J = 9.6 Hz, 1H), 3.05 (t, J = 10.0 Hz, 1H), 2.30 (s, 3H), 1.31 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.5, 154.6, 140.5, 135.7, 129.5, 114.2, 113.6, 110.9, 107.2, 61.2, 35.4, 32.9, 14.3, 13.7, 12.7. IR(KBr) ν: 2921, 2362, 1714, 1653, 1278, 1039, 978, 571. HRMS(EI) (m/z): calcd for C15H14N2O3 (M + H)+: 271.1077; found: 277.1072.
5). 1H NMR (CDCl3, 400 MHz), δ: 6.81 (dd, J1 = 15.6, J2 = 10.0 Hz, 1H), 6.33 (d, 2H), 5.99 (s, 1H), 4.27–4.21 (m, 2H), 3.43 (d, J = 9.6 Hz, 1H), 3.05 (t, J = 10.0 Hz, 1H), 2.30 (s, 3H), 1.31 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.5, 154.6, 140.5, 135.7, 129.5, 114.2, 113.6, 110.9, 107.2, 61.2, 35.4, 32.9, 14.3, 13.7, 12.7. IR(KBr) ν: 2921, 2362, 1714, 1653, 1278, 1039, 978, 571. HRMS(EI) (m/z): calcd for C15H14N2O3 (M + H)+: 271.1077; found: 277.1072.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford compound 6i as a yellow oil (42 mg, 82% yield), Rf = 0.5 (EtOAc/petroleum ether 1
10) to afford compound 6i as a yellow oil (42 mg, 82% yield), Rf = 0.5 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, 1H), 6.69 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.48–6.39 (m, 2H), 6.32 (t, J = 17.2 Hz, 1H), 4.26–4.21 (m, 2H), 2.78 (d, J = 10.0 Hz, 1H), 1.81 (s, 1H), 1.30 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 146.5, 144.1, 143.7, 136.5, 128.9, 111.3, 111.2, 110.8, 61.1, 42.5, 37.6, 23.0, 14.2. IR(KBr) ν: 2918, 2245, 1712, 1656, 1452, 1270, 1039, 976, 744, 667. HRMS(EI) (m/z): calcd for C14H12N2O3 (M + H)+: 257.0921; found: 257.0923.
5). 1H NMR (CDCl3, 400 MHz), δ: 7.46 (d, 1H), 6.69 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.48–6.39 (m, 2H), 6.32 (t, J = 17.2 Hz, 1H), 4.26–4.21 (m, 2H), 2.78 (d, J = 10.0 Hz, 1H), 1.81 (s, 1H), 1.30 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 146.5, 144.1, 143.7, 136.5, 128.9, 111.3, 111.2, 110.8, 61.1, 42.5, 37.6, 23.0, 14.2. IR(KBr) ν: 2918, 2245, 1712, 1656, 1452, 1270, 1039, 976, 744, 667. HRMS(EI) (m/z): calcd for C14H12N2O3 (M + H)+: 257.0921; found: 257.0923.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford compound 6j as a yellow oil (42.4 mg, 78% yield), Rf = 0.24 (EtOAc/petroleum ether 1
10) to afford compound 6j as a yellow oil (42.4 mg, 78% yield), Rf = 0.24 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.39 (d, J = 5.2 Hz, 1H), 7.16–7.14 (m, 1H), 7.07–7.04 (m, 1H), 6.67–6.61 (m, 1H), 6.37 (d, J = 15.6 Hz, 1H), 4.23 (q, 2H), 3.59 (d, J = 9.6 Hz, 1H), 3.14 (t, 1H), 1.30 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 135.3, 129.9, 129.8, 129.7, 128.1, 127.8, 114.2, 110.9, 61.3, 36.8, 34.2, 14.3, 13.9. IR(KBr) ν: 2918, 2245, 1719, 1649, 1459, 1375, 1319, 1277, 1172, 1102, 1039, 969, 857, 702. HRMS(EI) (m/z): calcd for C14H12N2O2S (M + H)+: 273.0692; found: 273.0694.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.39 (d, J = 5.2 Hz, 1H), 7.16–7.14 (m, 1H), 7.07–7.04 (m, 1H), 6.67–6.61 (m, 1H), 6.37 (d, J = 15.6 Hz, 1H), 4.23 (q, 2H), 3.59 (d, J = 9.6 Hz, 1H), 3.14 (t, 1H), 1.30 (t, J = 6.8 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.4, 135.3, 129.9, 129.8, 129.7, 128.1, 127.8, 114.2, 110.9, 61.3, 36.8, 34.2, 14.3, 13.9. IR(KBr) ν: 2918, 2245, 1719, 1649, 1459, 1375, 1319, 1277, 1172, 1102, 1039, 969, 857, 702. HRMS(EI) (m/z): calcd for C14H12N2O2S (M + H)+: 273.0692; found: 273.0694.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford compound 6k as a brown yellow (49.8 mg, 78% yield), Rf = 0.21 (EtOAc/petroleum ether 1
3) to afford compound 6k as a brown yellow (49.8 mg, 78% yield), Rf = 0.21 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Mp 113 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.62 (d, J = 8.0 Hz, 1H), 7.37–7.31 (m, 2H), 7.25–7.22 (m, 1H), 7.06 (s, 1H), 6.79 (dd, J1 = 15.6 Hz, J2 = 8.8 Hz, 1H), 6.35 (d, J = 16.0 Hz, 1H), 4.30–4.24 (m, 2H), 3.81 (s, 3H), 3.45 (d, J = 8.4 Hz, 1H), 3.02 (t, J = 8.8 Hz, 1H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.7, 138.3, 137.2, 128.4, 127.5, 123.2, 120.7, 118.2, 113.2, 112.9, 110.2, 104.3, 61.3, 36.5, 33.9, 33.3, 14.9, 14.3. IR(KBr) ν: 2918, 1719, 1656, 1466, 1375, 1270, 1214, 1039, 976, 737. HRMS(EI) (m/z): calcd for C19H17N3O2 (M + H)+: 320.1394; found: 320.1389.
3). Mp 113 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.62 (d, J = 8.0 Hz, 1H), 7.37–7.31 (m, 2H), 7.25–7.22 (m, 1H), 7.06 (s, 1H), 6.79 (dd, J1 = 15.6 Hz, J2 = 8.8 Hz, 1H), 6.35 (d, J = 16.0 Hz, 1H), 4.30–4.24 (m, 2H), 3.81 (s, 3H), 3.45 (d, J = 8.4 Hz, 1H), 3.02 (t, J = 8.8 Hz, 1H), 1.33 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.7, 138.3, 137.2, 128.4, 127.5, 123.2, 120.7, 118.2, 113.2, 112.9, 110.2, 104.3, 61.3, 36.5, 33.9, 33.3, 14.9, 14.3. IR(KBr) ν: 2918, 1719, 1656, 1466, 1375, 1270, 1214, 1039, 976, 737. HRMS(EI) (m/z): calcd for C19H17N3O2 (M + H)+: 320.1394; found: 320.1389.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to afford compound 6l as a brown oil (33.5 mg, 57% yield), Rf = 0.24 (EtOAc/petroleum ether 1
10) to afford compound 6l as a brown oil (33.5 mg, 57% yield), Rf = 0.24 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.32 (t, 2H), 7.24–7.18 (m, 3H), 6.48 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.18 (d, J = 15.2 Hz, 1H), 4.26–4.20 (m, 2H), 2.91–2.78 (m, 2H), 2.73 (t, J = 10.0 Hz, 1H), 2.24–2.18 (m, 1H), 2.13–1.94 (m, 2H), 1.31 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.5, 139.2, 134.7, 129.5, 129.0, 128.7, 128.7, 128.6, 127.0, 114.6, 111.4, 61.2, 35.6, 35.1, 34.1, 27.4, 14.3, 11.1. IR(KBr) ν: 2924, 1634.18, 1454.10, 1202.00, 1036.32, 748.19, 697. HRMS(EI) (m/z): calcd for C18H18N2O2 (M + H)+: 295.1441; found: 295.1445.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.32 (t, 2H), 7.24–7.18 (m, 3H), 6.48 (dd, J1 = 15.6 Hz, J2 = 10.0 Hz, 1H), 6.18 (d, J = 15.2 Hz, 1H), 4.26–4.20 (m, 2H), 2.91–2.78 (m, 2H), 2.73 (t, J = 10.0 Hz, 1H), 2.24–2.18 (m, 1H), 2.13–1.94 (m, 2H), 1.31 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 164.5, 139.2, 134.7, 129.5, 129.0, 128.7, 128.7, 128.6, 127.0, 114.6, 111.4, 61.2, 35.6, 35.1, 34.1, 27.4, 14.3, 11.1. IR(KBr) ν: 2924, 1634.18, 1454.10, 1202.00, 1036.32, 748.19, 697. HRMS(EI) (m/z): calcd for C18H18N2O2 (M + H)+: 295.1441; found: 295.1445.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6m as a white solid (42.3 mg, 84% yield), Rf = 0.16 (EtOAc/petroleum ether 1
5) to afford compound 6m as a white solid (42.3 mg, 84% yield), Rf = 0.16 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). Mp 122 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.37 (s, 3H), 7.21–7.15 (m, 1H), 6.33–6.32 (m, 2H), 3.69 (d, J = 3.6 Hz, 3H), 3.52 (d, J = 9.6 Hz, 1H), 3.10–3.05 (m, 1H). 13C NMR (101 MHz, CDCl3), δ: 164.4, 135.3, 130.0, 129.7, 129.7, 128.1, 127.8, 114.2, 110.9, 61.3, 36.8, 34.2, 14.3, 13.9. IR(KBr) ν: 2918, 2848, 2252, 1726, 1656, 1438, 1270, 1039, 737, 695. HRMS(EI) (m/z): calcd for C15H12N2O2 (M + H)+: 253.0972; found: 253.0966.
10). Mp 122 °C; 1H NMR (CDCl3, 400 MHz), δ: 7.37 (s, 3H), 7.21–7.15 (m, 1H), 6.33–6.32 (m, 2H), 3.69 (d, J = 3.6 Hz, 3H), 3.52 (d, J = 9.6 Hz, 1H), 3.10–3.05 (m, 1H). 13C NMR (101 MHz, CDCl3), δ: 164.4, 135.3, 130.0, 129.7, 129.7, 128.1, 127.8, 114.2, 110.9, 61.3, 36.8, 34.2, 14.3, 13.9. IR(KBr) ν: 2918, 2848, 2252, 1726, 1656, 1438, 1270, 1039, 737, 695. HRMS(EI) (m/z): calcd for C15H12N2O2 (M + H)+: 253.0972; found: 253.0966.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6n as a brown oil (52.5 mg, 89% yield), Rf = 0.34 (EtOAc/petroleum ether 1
5) to afford compound 6n as a brown oil (52.5 mg, 89% yield), Rf = 0.34 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). 1H NMR (CDCl3, 400 MHz), δ: 7.13 (d, J = 8.8 Hz, 2H), 6.75–6.68 (m, 3H), 6.31 (d, J = 15.2 Hz, 1H), 3.79 (s, 3H), 3.27 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 2.98 (s, 6H); 13C NMR (101 MHz, CDCl3), δ: 165.1, 151.2, 138.8, 129.1, 127.7, 115.9, 113.3, 112.6, 112.4, 52.3, 41.2, 40.3, 35.5, 14.9. IR(KBr) ν: 2918, 1726, 1529, 1277, 1116, 1046, 983. HRMS(EI) (m/z): calcd for C17H17N3O2 (M + H)+: 296.1394; found: 296.1396.
5). 1H NMR (CDCl3, 400 MHz), δ: 7.13 (d, J = 8.8 Hz, 2H), 6.75–6.68 (m, 3H), 6.31 (d, J = 15.2 Hz, 1H), 3.79 (s, 3H), 3.27 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 2.98 (s, 6H); 13C NMR (101 MHz, CDCl3), δ: 165.1, 151.2, 138.8, 129.1, 127.7, 115.9, 113.3, 112.6, 112.4, 52.3, 41.2, 40.3, 35.5, 14.9. IR(KBr) ν: 2918, 1726, 1529, 1277, 1116, 1046, 983. HRMS(EI) (m/z): calcd for C17H17N3O2 (M + H)+: 296.1394; found: 296.1396.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6o as a white oil (42.2 mg, 74% yield), Rf = 0.15 (EtOAc/petroleum ether 1
5) to afford compound 6o as a white oil (42.2 mg, 74% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.42–7.36 (m, 3H), 7.29 (d, 2H), 6.95 (dd, J1 = 15.6 Hz, J2 = 9.6 Hz, 1H), 6.25 (d, J = 15.6 Hz, 1H), 3.88 (s, 3H), 3.76 (s, 3H), 3.53 (d, J = 8.4 Hz, 1H), 3.08 (t, 1H); 13C NMR (101 MHz, CDCl3), δ: 165.7, 165.1, 139.6, 132.0, 129.1, 129.0, 128.3, 126.6, 115.6, 54.1, 52.0, 39.6, 38.1, 29.8. IR(KBr) ν: 1726, 1649, 1438, 1277, 1249, 983, 702. HRMS(EI) (m/z): calcd for C16H15NO4 (M + H)+: 286.1074; found: 286.1070.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.42–7.36 (m, 3H), 7.29 (d, 2H), 6.95 (dd, J1 = 15.6 Hz, J2 = 9.6 Hz, 1H), 6.25 (d, J = 15.6 Hz, 1H), 3.88 (s, 3H), 3.76 (s, 3H), 3.53 (d, J = 8.4 Hz, 1H), 3.08 (t, 1H); 13C NMR (101 MHz, CDCl3), δ: 165.7, 165.1, 139.6, 132.0, 129.1, 129.0, 128.3, 126.6, 115.6, 54.1, 52.0, 39.6, 38.1, 29.8. IR(KBr) ν: 1726, 1649, 1438, 1277, 1249, 983, 702. HRMS(EI) (m/z): calcd for C16H15NO4 (M + H)+: 286.1074; found: 286.1070.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6p as a white oil (44.9 mg, 75% yield), Rf = 0.16 (EtOAc/petroleum ether 1
5) to afford compound 6p as a white oil (44.9 mg, 75% yield), Rf = 0.16 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.42–7.36 (m, 3H), 7.30 (d, J = 6.8 Hz, 2H), 6.97–6.91 (m, 1H), 6.24 (d, 1H), 4.25–4.19 (m, 2H), 3.88 (s, 3H), 3.54 (d, J = 8.8 Hz, 1H), 3.07 (t, J = 9.2 Hz, 1H), 1.30 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 165.3, 165.1, 139.3, 132.0, 129.1, 129.0, 128.3, 127.1, 115.6, 60.9, 54.1, 39.6, 38.2, 29.7, 14.3. IR(KBr) ν: 2245, 1712, 1649, 1438, 1277, 1242, 1179, 1123, 1032, 983, 695. HRMS(EI) (m/z): calcd for C17H17NO4 (M + H)+: 300.1230; found: 300.1232.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.42–7.36 (m, 3H), 7.30 (d, J = 6.8 Hz, 2H), 6.97–6.91 (m, 1H), 6.24 (d, 1H), 4.25–4.19 (m, 2H), 3.88 (s, 3H), 3.54 (d, J = 8.8 Hz, 1H), 3.07 (t, J = 9.2 Hz, 1H), 1.30 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 165.3, 165.1, 139.3, 132.0, 129.1, 129.0, 128.3, 127.1, 115.6, 60.9, 54.1, 39.6, 38.2, 29.7, 14.3. IR(KBr) ν: 2245, 1712, 1649, 1438, 1277, 1242, 1179, 1123, 1032, 983, 695. HRMS(EI) (m/z): calcd for C17H17NO4 (M + H)+: 300.1230; found: 300.1232.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE, 1
PE, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) to afford compound 6q as a yellow oil (41.9 mg, 70% yield), Rf = 0.15 (EtOAc/petroleum ether 1
5) to afford compound 6q as a yellow oil (41.9 mg, 70% yield), Rf = 0.15 (EtOAc/petroleum ether 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10). 1H NMR (CDCl3, 400 MHz), δ: 7.43–7.36 (m, 3H), 7.31–7.29 (m, 2H), 6.96 (dd, J1 = 15.6 Hz, J2 = 9.6 Hz, 1H), 6.24 (d, J = 15.6 Hz, 1H), 4.36–4.30 (m, 2H), 3.76 (s, 3H), 3.53 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 165.6, 164.5, 139.7, 132.0, 129.0, 128.9, 128.2, 126.4, 115.5, 63.5, 51.9, 39.3, 38.0, 29.9, 14.1. IR(KBr) ν: 2925, 1733, 1649, 1508, 1284, 990, 702. HRMS(EI) (m/z): calcd for C17H17NO4 (M + H)+: 300.1230; found: 300.1234.
10). 1H NMR (CDCl3, 400 MHz), δ: 7.43–7.36 (m, 3H), 7.31–7.29 (m, 2H), 6.96 (dd, J1 = 15.6 Hz, J2 = 9.6 Hz, 1H), 6.24 (d, J = 15.6 Hz, 1H), 4.36–4.30 (m, 2H), 3.76 (s, 3H), 3.53 (d, J = 8.4 Hz, 1H), 3.06 (t, J = 8.8 Hz, 1H), 1.36 (t, J = 7.2 Hz, 3H); 13C NMR (101 MHz, CDCl3), δ: 165.6, 164.5, 139.7, 132.0, 129.0, 128.9, 128.2, 126.4, 115.5, 63.5, 51.9, 39.3, 38.0, 29.9, 14.1. IR(KBr) ν: 2925, 1733, 1649, 1508, 1284, 990, 702. HRMS(EI) (m/z): calcd for C17H17NO4 (M + H)+: 300.1230; found: 300.1234.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from NSFC of China (21801114) and Jiangxi Province “1000 Talent Plan” High-Level Entrepreneurial and Innovative Program (1001-02102129).Notes and references
- (a) V. K. Tandon, M. Kumar, A. K. Awasthi, H. O. Saxena and G. K. Goswamy, Bioorg. Med. Chem. Lett., 2004, 14, 3177–3180 CrossRef CAS PubMed; (b) J. R. Vyvyan, J. M. Oaksmith, B. W. Parks and E. M. Peterson, Tetrahedron Lett., 2005, 46, 2457–2460 CrossRef CAS; (c) T. Sugaya, N. Kato, A. Sakaguchi and S. Tomioka, Synthesis, 1995, 10, 1257–1262 CrossRef; (d) L. S. Nicole, M. H. Heather and W. P. Mark, Tetrahedron, 2006, 62, 9301–9320 CrossRef; (e) C. R. Reddy, P. Ramesh, N. N. Rao and S. A. Ali, Eur. J. Org. Chem., 2011, 11, 2133–2141 CrossRef.
- (a) J. W. Blunt, B. R. Copp, W.-P. Hu, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2008, 25, 35–95 RSC; (b) T. F. Molinski, D. S. Dalisay, S. L. Lievens and J. P. Saludes, Nat. Rev. Drug Discovery, 2009, 8, 69–85 CrossRef CAS PubMed; (c) V. J. Paul and R. Ritson-Williams, Nat. Prod. Rep., 2008, 25, 662–695 RSC; (d) D. Skropeta, Nat. Prod. Rep., 2008, 25, 1131–1166 RSC.
- (a) G. Boche and H. M. Walbirsky, Cyclopropane Derived Reactive Intermediates, John Wiley and Sons, New York, NY, 1990 CrossRef; (b) Z. Rappoport, The Chemistry of the Cyclopropyl Group, Wiley and Sons, New York, NY, 1996 Search PubMed; (c) D. W. Graham, W. T. Ashton, L. Barash, J. E. Brown, R. D. Brown, L. F. Canning, A. Chen, J. P. Springer and E. F. Rogers, J. Med. Chem., 1987, 30, 1074–1090 CrossRef CAS PubMed; (d) J. Salaun and M. S. Baird, Curr. Med. Chem., 1995, 2, 511–519 CAS; (e) Y. Baba, G. Saha, S. Nakao, C. Iwata, T. Tanaka, T. Ibuka, H. Ohishi and Y. Takemoto, J. Org. Chem., 2001, 66, 81–88 CrossRef CAS PubMed; (f) D. L. Boger, T. V. Hughes and M. P. Hedrick, J. Org. Chem., 2001, 66, 2207–2216 CrossRef CAS PubMed; (g) S. Yoshida, T. C. Rosen, O. G. J. Meyer, M. J. Sloan, S. Ye, G. Haufe and K. L. Kirk, Bioorg. Med. Chem., 2004, 12, 2645–2652 CrossRef CAS PubMed; (h) K. Yamaguchi, Y. Kazuta, K. Hirano, S. Yamada, A. Matsuda and S. Shuto, Bioorg. Med. Chem., 2008, 16, 8875–8881 CrossRef CAS PubMed; (i) W. A. Donaldson, Tetrahedron, 2001, 57, 8589–8627 CrossRef CAS; (j) W. Brandt and T. Thiemann, Chem. Rev., 2003, 103, 1625–1647 CrossRef PubMed.
- (a) W. P. Deng, A. H. Li, L. X. Dai and X.-L. Hou, Tetrahedron, 2000, 56, 2967–2974 CrossRef CAS; (b) S. Ye, Z. Z. Huang, C. A. Xia, Y. Tang and L. X. Dai, J. Am. Chem. Soc., 2002, 124, 2432–2433 CrossRef CAS PubMed; (c) X. F. Yang, M. J. Zhang, X. L. Hou and L. X. Dai, J. Org. Chem., 2002, 67, 8097–8103 CrossRef CAS PubMed; (d) D. Morton, D. Pearson, R. A. Field and R. A. Stockman, Org. Lett., 2004, 6, 2377–2380 CrossRef CAS PubMed; (e) D. Morton, D. Pearson, R. A. Field and R. A. Stockman, Chem. Commun., 2006, 17, 1833–1835 RSC; (f) X. M. Deng, P. Cai, S. Ye, X. L. Sun, W. W. Liao, K. Li, Y. Tang, Y. D. Wu and L. X. Dai, J. Am. Chem. Soc., 2006, 128, 9730–9740 CrossRef CAS PubMed; (g) Y. Q. Zhang, A. M. Yu, J. R. Jia, S. S. Ma, K. Li, Y. Wei and X. T. Meng, Chem. Commun., 2017, 53, 10672–10675 RSC.
- (a) A. H. Li, L. X. Dai and X. L. Hou, Chem. Commun., 1996, 4, 491–492 RSC; (b) Y.-G. Zhou, A.-H. Li, X. L. Hou and L. X. Dai, Chem. Commun., 1996, 11, 1353–1354 RSC; (c) A.-H. Li, L.-X. Dai, X.-L. Hou and M.-B. Chen, J. Org. Chem., 1996, 61, 4641–4648 CrossRef CAS PubMed; (d) D. Morton, D. Pearson, R. A. Field and R. A. Stockman, Org. Lett., 2004, 6, 2377–2380 CrossRef CAS PubMed; (e) B.-H. Zhu, J.-C. Zheng, C.-B. Yu, X.-L. Sun, Y.-G. Zhou, Q. Shen and Y. Tang, Org. Lett., 2010, 12, 504–507 CrossRef CAS PubMed; (f) F. Gao and Y. Huang, Adv. Synth. Catal., 2014, 356, 2422–2428 CrossRef CAS; (g) Z. Chen and J. Zhang, Chem.–Asian J., 2009, 4, 1527–1529 CrossRef CAS PubMed.
- (a) Q.-G. Wang, X.-M. Deng, B.-H. Zhu, L.-W. Ye, X.-L. Sun, C.-Y. Li, C.-Y. Zhu, Q. Shen and Y. Tang, J. Am. Chem. Soc., 2008, 130, 5408–5409 CrossRef CAS PubMed; (b) B.-H. Zhu, R. Zhou, J.-C. Zheng, X.-M. Deng, X.-L. Sun, Q. Shen and Y. Tang, J. Org. Chem., 2010, 75, 3454–3457 CrossRef CAS PubMed.
- P. Jia and Y. Huang, Org. Lett., 2016, 18, 2475–2478 CrossRef CAS PubMed.
- J. L. Chen, P. H. Jia and Y. Huang, Org. Lett., 2018, 20, 6715–6718 CrossRef CAS PubMed.
- (a) S. Brauch, S. S. VanBerkel and B. Westermann, Chem. Soc. Rev., 2013, 42, 4948–4962 RSC; (b) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 RSC; (c) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46, 2427–2440 CrossRef CAS PubMed; (d) P. Wu, M. Givskov and T. Nielsen, Chem. Rev., 2019, 119, 11245–11290 CrossRef CAS PubMed.
- For selected reviews on sulfur ylides, see: (a) L.-Q. Lu, T.-R. Li, Q. Wang and W.-J. Xiao, Chem. Soc. Rev., 2017, 46, 4135–4149 RSC; (b) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301–5365 CrossRef CAS PubMed; (c) X.-L. Sun and Y. Tang, Acc. Chem. Res., 2008, 41, 937–948 CrossRef CAS PubMed; (d) A.-H. Li, L.-X. Dai and V. K. Aggarwal, Chem. Rev., 1997, 97, 2341–2372 CrossRef CAS PubMed.
- CCDC 1956080.†.
- CCDC 1956081.†.
- (a) W. H. Ding, Y. Q. Zhang, A. M. Yu, L. Zhang and X. T. Meng, J. Org. Chem., 2018, 83, 13821–13833 CrossRef CAS PubMed; (b) L. Satham and I. N. N. Namboothiri, J. Org. Chem., 2018, 83, 9471–9477 CrossRef CAS PubMed; (c) Y. H. Wang, J. R. Cao, D. I. Schuster and J. R. Wilson, Tetrahedron Lett., 1995, 36, 6843–6846 CrossRef CAS.
- W. Yin, L. Fang, Z. Wang, F. Gao, Z. Li and Z. Wang, Org. Lett., 2019, 21, 7361–7364 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of isolated compounds. CCDC 1956080 and 1956081. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03919e |
| This journal is © The Royal Society of Chemistry 2020 |