Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Two decades of the synthesis of mono- and bis-aminomercapto[1,2,4]triazoles
Sayed M. Riyadhab and
Sobhi M. Gomha *bc
*bc
aDepartment of Chemistry, Faculty of Science, Taibah University, Al-Madinah Al-Munawarah, 30002, Saudi Arabia
bDepartment of Chemistry, Faculty of Science, Cairo University, Giza, 12613, Egypt. E-mail: s.m.gomha@gmail.com
cDepartment of Chemistry, Faculty of Science, Islamic University in Al-Madinah Al-Munawarah, 42351, Saudi Arabia
First published on 1st July 2020
Abstract
4-Amino-5-mercapto[1,2,4]triazole and its 3-substituted derivatives have proven to be of biological interest and provide access to a new class of biologically active heterocyclic compounds for biomedical applications. This study will be helpful for scientific researchers interested in the chemistry of bifunctional versatile compounds as it provides a collection of all the methods for the preparation of 3-substituted-4-amino-5-mercapto[1,2,4]triazoles with aliphatic, aromatic, and heterocyclic moieties during the period from 2000 to mid-2020.
1. Introduction
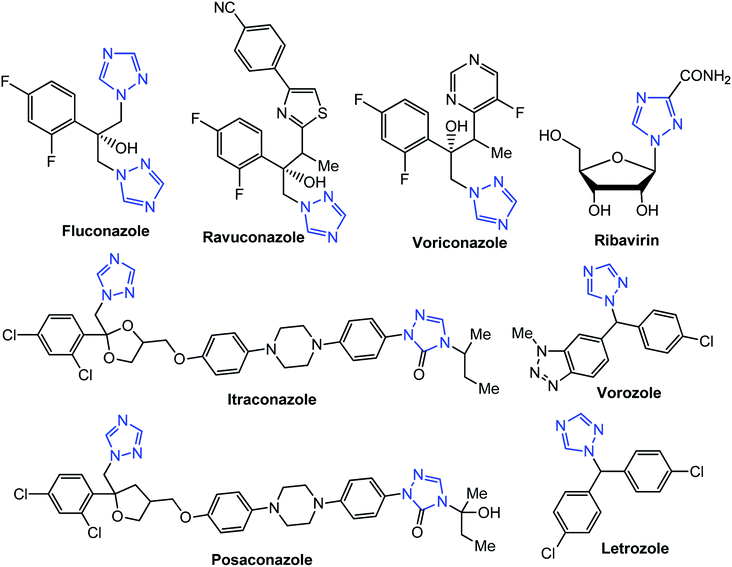
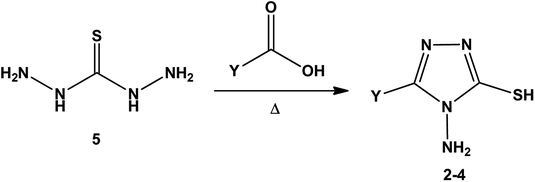
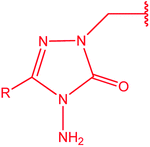
[1,2,4]Triazoles and their fused heterocyclic derivatives have occupied a unique position as novel biologically active agents with remarkably diverse pharmacological properties such as antimicrobial, antifungal, anticancer, anticonvulsant, antiviral, anti-inflammatory, anti-HIV, and anti-mycobacterial activities.1–8 A large number of ring systems containing [1,2,4]triazoles have been incorporated into a wide variety of therapeutically interesting drug candidates such as fluconazole, ravuconazole, itraconazole, voriconazole, posaconazole, vorozole, letrozole, ribavirin, triazolam, alprazolam, etizolam, furacylin, hexaconazole, triadimefon, myclobutanil, rizatriptan, propiconazole, and fluotrimazole (Chart 1).9 Moreover, the synthesis of bis-heterocyclic compounds containing triazole rings has attracted attention due to the diverse applications of these compounds in numerous pharmacological and biological fields.10–13Bis-[4-amino-5-mercapto[1,2,4]triazoles] (1) and 3-substituted-4-amino-5-mercapto[1,2,4] triazoles (2–4) (Chart 2) contain both amino and mercapto groups as ready-made nucleophilic centers for the synthesis of condensed heterocyclic rings. The introduction of these groups in different nuclei enhances their biological activities. Accordingly, the objective of the present review is to highlight the synthetic methods used to obtain 3-substituted-4-amino-5-mercapto[1,2,4]triazoles and bis-[4-amino-5-mercapto[1,2,4]triazoles] from 2000 until mid-2020.
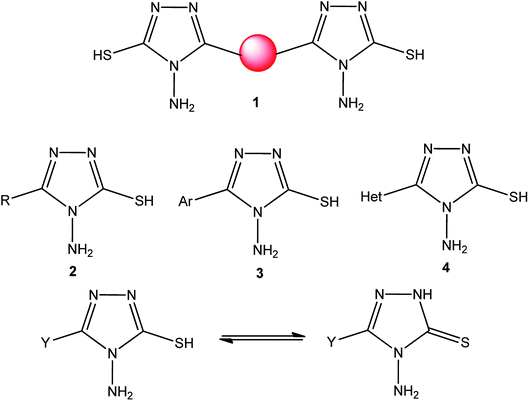 | ||
| Chart 2 Structures of bis-[4-amino-5-mercapto[1,2,4]triazoles] (1) and 3-substituted-4-amino-5-mercapto[1,2,4]triazoles (2–4). | ||
2. Synthetic routes using thiocarbohydrazide as the precursor
2.1. Reactions with carboxylic acids
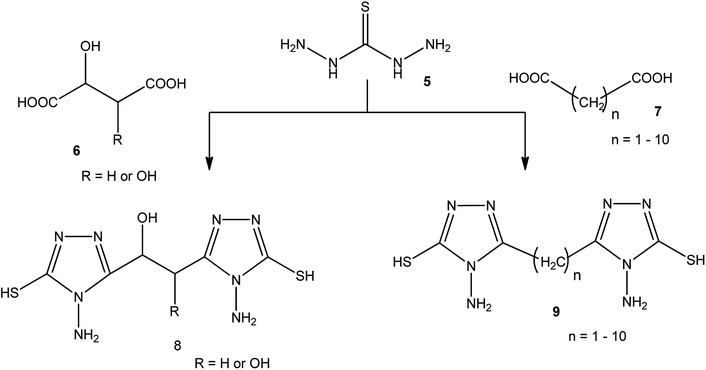
3-Substituted-4-amino-5-mercapto[1,2,4]triazoles 2–4 were prepared from the treatment of thiocarbohydrazide (5) with carboxylic acids (Scheme 1) (Table 1).A series of dicarboxylic acids such as tartaric, malic,41–43 succinic,44 glutaric,45 and others46 were treated with thiocarbohydrazide (5) to afford the respective series of bis-(4-amino-5-mercapto[1,2,4]triazoles) 8, 9 (Scheme 2).
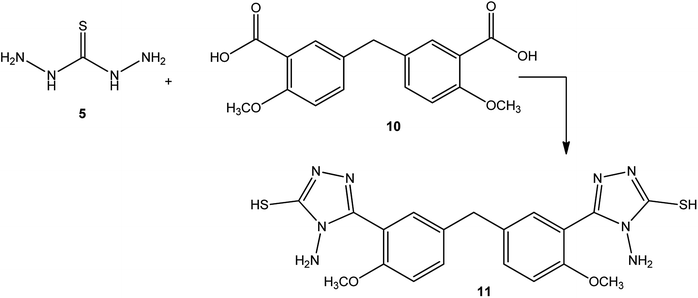
Similarly, a condensation reaction between 5-(3-formyl-4-methoxybenzyl)-2-methoxybenzoic acid (10) and thiocarbohydrazide (5) at the melt temperature afforded bis[4-methoxy-3-[4-amino-5-sulfanyl-4H-1,2,4-triazol-3-yl]phenyl]methane (11) (Scheme 3).47
2.2. Reactions with esters
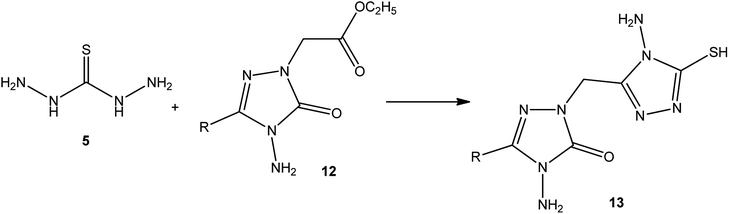
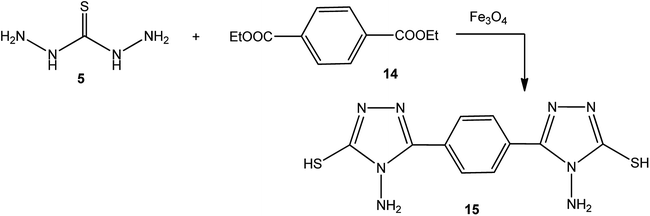
In addition, Demirbas et al.48 reported the treatment of ethyl(3-alkyl-4-amino-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl) acetates (12) with thiocarbohydrazide (5), which furnished 5-alkyl-4-amino-2-[(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)methyl]-2,4-dihydro-3H-1,2,4-triazol-3-ones (13) (Scheme 4).Moreover, refluxing thiocarbohydrazide (5) with diethyl terephthalate 14 using magnetic iron oxide (Fe3O4) nanoparticles as an eco-friendly catalyst yielded the respective 3,3′-(1,4-phenylene)bis(4-amino-1H-1,2,4-triazole-5(4H)-thione) (15) (Scheme 5).49
2.3. Reactions with lactones
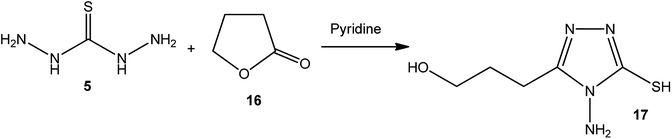
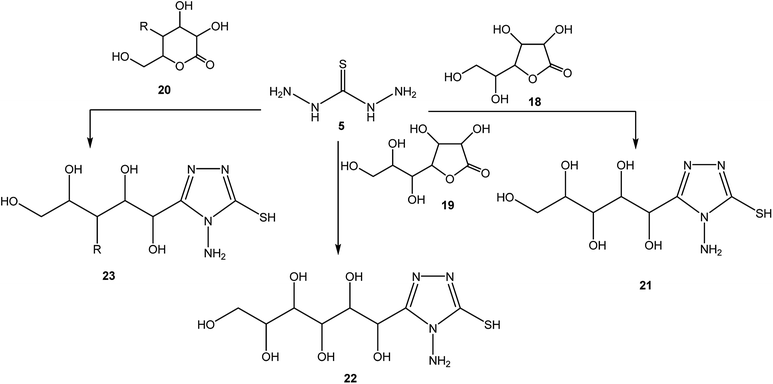
4-Amino-3-(3-hydroxypropyl)-5-mercapto[1,2,4]triazole (17) was prepared via the treatment of thiocarbohydrazide (5) with lactone 16, as reported by Zhang et al.50 [Scheme 6].The synthetic routes for the preparation of 4-amino-3-(D-galactopentitol-1-yl)-5-mercapto[1,2,4]triazole (21),51 4-amino-3-(D-glucoheptonic-hexitol-1-yl)-1H-[1,2,4]triazole-5-thione (22),52 and 3-(D-alditol-1-yl)-4-amino-5-mercapto-[1,2,4]triazole (23)53 were reported through reactions of thiocarbohydrazide (5) with D(−)galactono-1,4-lactone (18), D-glucoheptonic-γ-lactone (19), and D-galactono-1,5-lactones (20), respectively (Scheme 7).
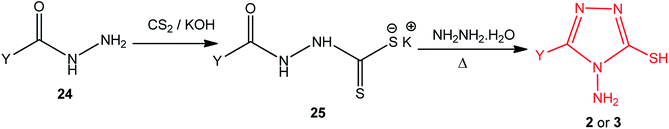
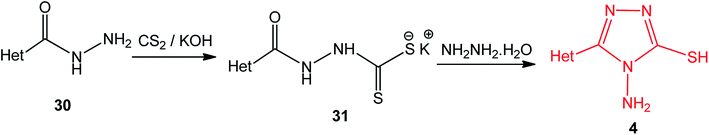
3. Use of potassium acyldithiocarbazates with hydrazine hydrate
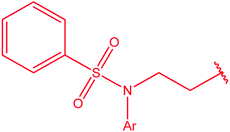
Potassium acyldithiocarbazates 25 is usually prepared by a reaction between the corresponding acid hydrazides 24 and carbon disulfide in an ethanolic potassium hydroxide solution. This method was extensively used in the synthesis of numerous derivatives of 4-amino-5-mercapto[1,2,4]triazoles 2 (3) upon treatment with hydrazine hydrate (Scheme 8) (Table 2).| Y | Ref. |
|---|---|
| –CH3, –C2H5, –C3H7 | 54 |
| CH3–(CH2)13–CH2– | 55 |
| CH3–(CH2)15–CH(SO3Na)– | 56 |
| C6H5– | 57–60 |
| 3-ClC6H4– | 8 |
| 4-CH3OC6H4– | 61 |
| 2-HOC6H4– | 62 |
| 2-CH3C6H4– & 2-CH3-4-ClC6H3– | 63 |
| C6H5– & 2-HOC6H4– | 64 |
| 2-C2H5OC6H4– | 65 |
| 3-Br-4-CH3OC6H3– | 66 |
| 2-HOC6H4– & 4-HOC6H4– & 4-C2H5OC6H4– & 2-HO-5-ClC6H3– & 4-HOC6H4–CH2– & 4-C2H5OC6H4–CH2– | 67 |
| 2-FC6H4–CH2– & 2-BrC6H4–CH2– & 4-HOC6H4–CH2– & 2-CH3OC6H4–CH2– & 4-NO2C6H4–CH2– | 68 |
| C6H5– & 2-ClC6H4– & 2-NO2C6H4– & 2-HOC6H4– & 2-furyl | 69 |
| C6H5– & 4-ClC6H4– & 4-BrC6H4– & 4-CH3OC6H4– & 2-naphthyl–CH2– | 70 |
| 2-HOC6H4– & 4-HOC6H4– & 2-NH2C6H4– & 4-NH2C6H4– & 3,4,5-(HO)3C6H2– | 71 |
| C6H5–CH2–CH2– | 72 |
| 2-(CH3)2NC6H4– & 4-CH3NHC6H4– & 1-naphthyl–CH2– | 73 |
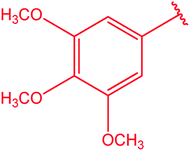
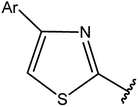
 |
74 |
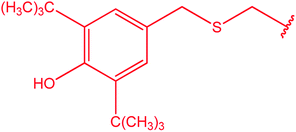
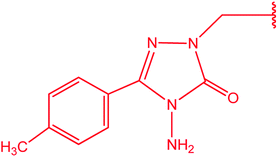
 |
75 |
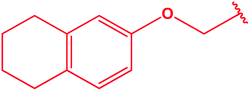
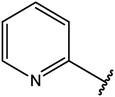
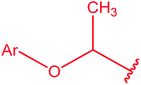
 |
76 |
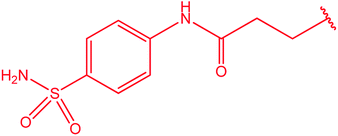
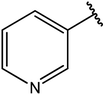
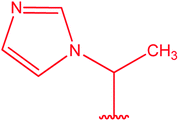
 |
77 |
 |
77 |
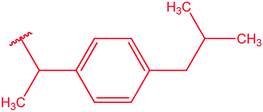 |
78 and 79 |
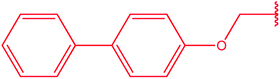 |
79 |
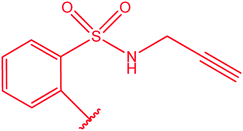 |
80 |
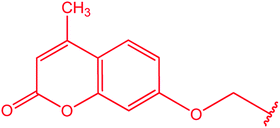 |
81 |
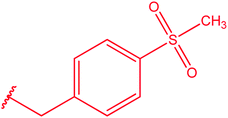 |
82 |
 |
83 |
 |
84 |
 |
85 |
 |
85 and 86 |
 |
87 |
 |
88 |
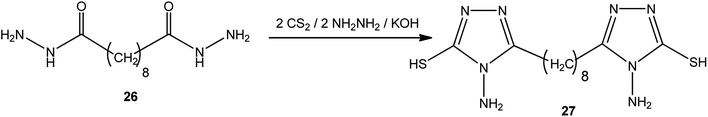
1,8-Bis-(3-mercapto-4-amino-[1,2,4]-triazol-5-yl)-octane (27) was achieved via the reaction of sebacic acid dihydrazide (26) with carbon disulfide and hydrazine hydrate in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in the presence of potassium hydroxide89 (Scheme 9).
2 in the presence of potassium hydroxide89 (Scheme 9).
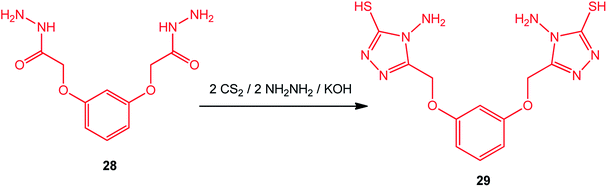
Bis-(3-mercapto-4-amino-[1,2,4]-triazole) with an aromatic moiety was prepared under similar conditions by Zhao et al.90 Thus, the reaction of 2,2′-[1,3-phenylenebis(oxy)]bis-acetic hydrazide (28) with CS2/NH2NH2 afforded 2,2′-[1,3-phenylenebis(oxymethylene)]bis-(4-amino-3-mercapto-[1,2,4]triazole) (29) (Scheme 10).
3-Heteroaryl-4-amino-5-mercapto[1,2,4]triazoles (4) were synthesized by the treatment of the corresponding dithiocarbazate 31 with hydrazine hydrate (Scheme 11) (Table 3).
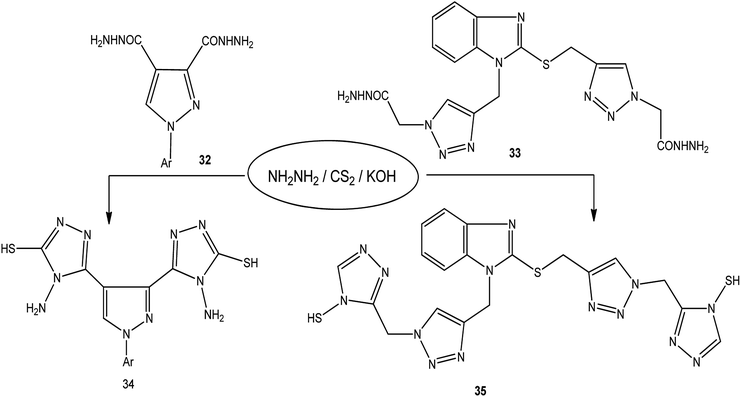
The treatment of dicarbohydrazides 32 (ref. 121) and 33 (ref. 122) with CS2/NH2NH2 in the presence of KOH proceeded smoothly to afford the respective bis-triazoles 34 and 35 (Scheme 12).
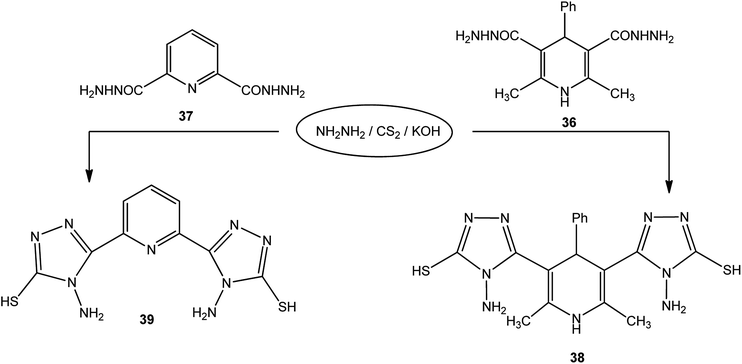
In addition, pyridine dicarbohydrazide derivatives 36 (ref. 123) and 37 (ref. 124 and 125) were reacted with the above reagents under similar conditions to give 38 and 39, respectively (Scheme 13).
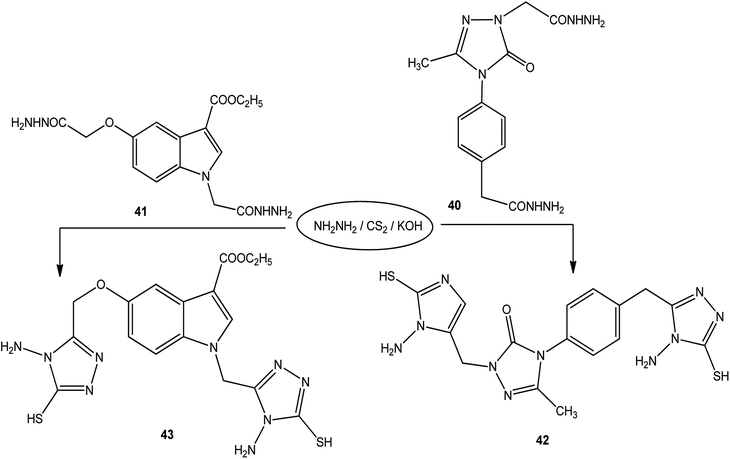
Moreover, the reactions of dicarbohydrazide of triazole 40 (ref. 126) or indole derivatives 41 (ref. 127) with the same reagents in an alkaline solution furnished 42 or 43, respectively (Scheme 14).
4. Synthesis of 5-mercapto[1,3,4]oxadiazoles with hydrazine hydrate via ring transformation reactions
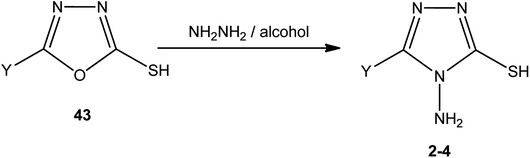
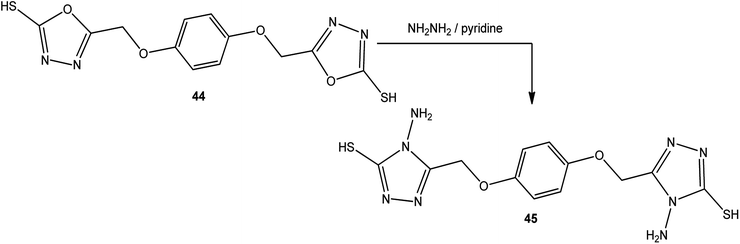
An alcoholic solution of hydrazine hydrate achieves the ring transformation of 3-substituted-5-mercapto[1,3,4]oxadiazoles (43) to 3-substituted-4-amino-5-mercapto[1,3,4]triazoles 2–4 (Scheme 15) (Table 4).5,5′-[1,4-Phenylenebis(oxymethylene)]-bis(1,3,4-oxadiazole-2-thiol) (44) was converted into 5,5′-[(1,4-phenylenebis(oxymethylene)]-bis(4-amino-4H-1,2,4-triazole-3-thiol) (45) upon treatment with hydrazine hydrate in dry pyridine under thermal conditions (Scheme 16).142
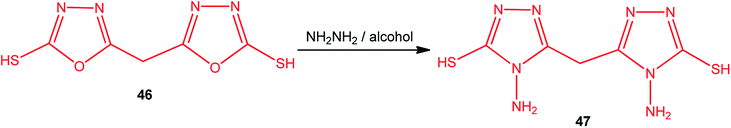
Similarly, the conversion of 5,5′-methylenebis(1,3,4-oxadiazole-2-thiol) (46) into 5,5′-methylenebis(4-amino-4H-1,2,4-triazole-3-thiol) (47) was achieved using an alcoholic hydrazine solution under refluxing conditions (Scheme 17).1
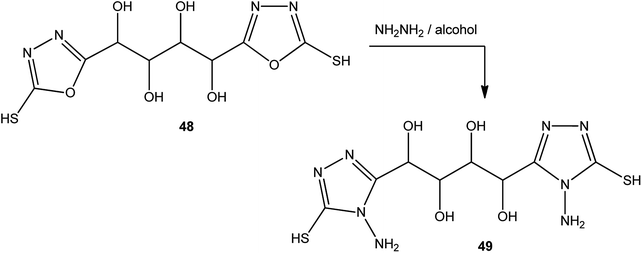
In addition, the same procedure (alcoholic hydrazine solution) was applied to the conversion of 1,4-bis(2-mercapto-1,3,4-oxadiazol-5-yl)butane-1,2,3,4-tetrol (48) to 1,4-bis(4-amino-5-mercapto-4H-1,2,4-triazol-3-yl)butane-1,2,3,4-tetrol (49) (Scheme 18).143
4-Amino-5-mercapto[1,2,4]triazole 1 and its 3-substituted derivatives 2–4 (Chart 2) contain both amino and mercapto groups as ready-made nucleophilic centers for the synthesis of condensed heterocyclic rings.
5. Conclusions and future directions
The reports in this review clearly demonstrate the elevated synthetic potential of 3-substituted-4-amino-5-mercapto[1,2,4]triazoles and bis-[4-amino-5-mercapto[1,2,4]triazoles]. Numerous scientific researchers in the fields of chemistry and pharmaceutical science are interested in the study and utilization of these compounds as building blocks in the synthesis of important bioactive compounds.Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.References
- S. Rani, B. Agaiah and M. Sarangapani, Int. J. Pharm. Technol., 2010, 2, 366 Search PubMed.
- S. M. Gomha, H. M. Abdel-aziz, T. Z. Abolibda, S. A. Hassan and M. M. Abdalla, J. Heterocycl. Chem., 2020, 57, 1034 CrossRef CAS.
- M. A. Abdallah, S. M. Riyadh, I. M. Abbas and S. M. Gomha, J. Chin. Chem. Soc., 2005, 52, 987 CrossRef CAS.
- S. M. Gomha and S. M. Riyadh, Molecules, 2011, 16, 8244 CrossRef CAS PubMed.
- S. M. Sondhi, S. Arya, R. Rani, N. Kumar and P. Roy, Med. Chem. Res., 2012, 21, 3620 CrossRef CAS.
- X. Q. Deng, M. X. Song, B. Shu, Sh. B. Wang, Z. Sh. Quan and Z. Dong, Arch. Pharmazie, 2012, 345, 565 CrossRef CAS PubMed.
- B. Jiang, X. Huang, H. Yao, J. Jiang, X. Wu, S. Jiang, Q. Wang, T. Lu and J. Xu, Org. Biomol. Chem., 2014, 12, 2114 RSC.
- S. M. Gomha, Int. J. Pharm. Pharmaceut. Sci., 2013, 5, 42 CAS.
- D. J. Prasad, M. Ashok, P. Karegoudar, B. Poojary, B. S. Holla and N. S. Kumari, Eur. J. Med. Chem., 2009, 44, 551 CrossRef CAS.
- D. K. Kim, J. Kim and H. J. Park, Bioorg. Med. Chem. Lett., 2004, 14, 2401 CAS.
- B. M. Banachiewicz, J. Banachiewicz, A. Chodkowska, E. J. Wojtowicz and L. Mazur, Eur. J. Med. Chem., 2004, 39, 873 CrossRef.
- L. Tian, Y. Sun and H. Li, J. Inorg. Biochem., 2005, 99, 1646 CrossRef CAS PubMed.
- J. Liu, L. Li, H. Dai, Z. Liu and J. Fang, J. Organomet. Chem., 2006, 691, 2686 CrossRef CAS.
- A. Dandia, S. L. Gupta, M. Sudheer and A. Quraishi, J. Mater. Environ. Sci., 2012, 3, 993 CAS.
- F. M. Abdelrazek, S. M. Gomha, M. E. B. Shaaban, A. I. lotfi and H. N. El-Shemy, Synth. Commun., 2018, 34, 32 CrossRef.
- P. A. Castelino, J. P. Dasappa, K. G. Bhat, S. A. Joshi and S. Jalalpure, Med. Chem. Res., 2016, 25, 83 CrossRef CAS.
- B.-L. Wang, Y.-Z. Zhan, L.-Y. Zhang, Y. Zhang, X. Zhang and Z.-M. Li, Phosphorus, Sulfur, Silicon Relat. Elem., 2016, 191, 48 Search PubMed.
- R. Jin, J. Liu, G. Zhang, J. Li, S. Zhang and H. Guo, Chem. Biodiversity, 2018, 15, e1800263 CrossRef PubMed.
- R. Liu, Z. Hu, G. Liu, Y. Huang and Z. Zhang, Miner. Process. Extr. Metall. Rev., 2020, 41, 96 CrossRef.
- L. Liu, J. Ye, M. Xiao, K. Yuan, M. He, A. Hu, H. Jia and A. Liu, J. Heterocycl. Chem., 2019, 56, 2192 CrossRef CAS.
- N. Kaushik, N. Kumar and A. Kumar, Int. J. Pharm. Pharmaceut. Sci., 2015, 7, 120 CAS.
- M. R. Aouad, Molecules, 2014, 19, 18897 CrossRef PubMed.
- A. K. Gupta, S. P. Prachand, A. Patel and S. Jain, International Journal of Pharmaceutical and Life Science, 2012, 3, 1848 CAS.
- M. K. Altıntopa, Z. A. Kaplancıkl, G. Turan-Zitouni, A. Özdemir, G. Iscan, G. Akalın and S. U. Yıldırım, Eur. J. Med. Chem., 2011, 46, 5562 CrossRef PubMed.
- M. M. Ghorab, A. M. S. El-Sharief, Y. A. Ammar and S. I. Mohamed, Phosphorus, Sulfur, Silicon Relat. Elem., 2001, 173, 223 CrossRef CAS.
- P. S. Aytaç, I. Durmaz, D. R. Houston, R. Cetin-Atalay and B. Tozkoparan, Bioorg. Med. Chem., 2016, 24, 858 CrossRef PubMed.
- A. Nithinchandra, B. Kalluraya, S. Aamir and A. R. Shabaraya, Eur. J. Med. Chem., 2012, 54, 597 CrossRef PubMed.
- A. Saeed, F. Larik, P. A. Channar, H. Mehfooz, M. H. Ashraf, Q. Abbas and S.-Y. Seo, Chem. Biol. Drug Des., 2017, 90, 764 CrossRef CAS PubMed.
- A. Ameril, G. Khodarahmi, F. Hassanzadeh, H. Forootanfar and G.-H. Hakimelahi, Arch. Pharm. Chem. Life Sci., 2016, 349, 662 CrossRef.
- W. Yehye, N. Abdul Rahman, O. Saad, A. Ariffin, S. B. Abd Hamid, A. A. Alhadi, F. A. Kadir, M. Yaeghoobi and A. Matlob, Molecules, 2016, 21, 847 CrossRef.
- I. Khan, A. Ibrar and N. Abbas, Eur. J. Med. Chem., 2013, 63, 854 CrossRef CAS PubMed.
- A. G. M. Al-Sehemi, Phosphorus, Sulfur, Silicon Relat. Elem., 2009, 148, 1991 CrossRef.
- K.-M. Sim, P.-Q. Chan, X.-L. Boo, K.-S. Heng, K.-W. Lye and K.-C. Teo, Lett. Org. Chem., 2018, 15, 575 CrossRef CAS.
- B. Lingappa, K. S. Girisha, B. Kalluraya and N. S. Rai, Indian J. Chem., Sect. B, 2008, 47, 1858 Search PubMed.
- A. Y. Hassan, Phosphorus, Sulfur, Silicon Relat. Elem., 2009, 184, 2759 CrossRef CAS.
- T. S. F. Al-Mathkuri, H. M. S. Al-Jubori and A. Saleh, Orient. J. Chem., 2018, 34, 2031 CAS.
- B. Sever, M. D. Altintop, G. Kus, M. Ozkurt, A. Ozdemir and Z. A. Kaplancikli, Eur. J. Med. Chem., 2016, 113, 179 CrossRef CAS PubMed.
- C. R. Prakash, S. Raja and G. Saravanan, Int. J. Pharm. Pharmaceut. Sci., 2014, 6, 539 CAS.
- Chandramouli, M. R. Shivanand, T. B. Nayanbhai, Bheemachari and R. H. Udupi, J. Chem. Pharm. Res., 2012, 4, 1151 CAS.
- B. Kalluraya, B. Lingappa and S. R. Nooji, Phosphorus, Sulfur, Silicon Relat. Elem., 2007, 182, 1393 CrossRef CAS.
- S. Pavurala, K. Vaarl, R. Kesharwani, L. Naesens, S. Liekens and R. R. Vedula, Synth. Commun., 2018, 48, 1494 CrossRef CAS.
- A. H. Moustafa, R. A. Haggam, M. E. Younes and E. S. H. El-Ashry, Phosphorus, Sulfur, Silicon Relat. Elem., 2006, 181, 2361 CrossRef CAS.
- A. H. Moustafa, R. A. Haggam, M. E. Younes and E. S. H. El-Ashry, Nucleosides, Nucleotides Nucleic Acids, 2005, 24, 1885 CrossRef CAS PubMed.
- R. A. Haggam, Res. Chem. Intermed., 2016, 42, 7313 CrossRef CAS.
- S. Subashchandrabose, V. Thanikachalam, G. Manikandan, H. Saleem and Y. Erdogdu, Spectrochim. Acta Mol. Biomol. Spectrosc., 2016, 157, 96 CrossRef CAS.
- S. A. Chavan, A. G. Ulhe, S. A. Gharad and B. N. Berad, Phosphorus, Sulfur, Silicon Relat. Elem., 2015, 190, 2315 CrossRef CAS.
- A. Srinivas, Acta Chim. Slov., 2016, 63, 173 CrossRef CAS PubMed.
- N. Demirbas, A. Demirbas, S. A. Karaoglu and E. Celik, Arkivoc, 2005, i, 75 Search PubMed.
- N. Nami, D. Zareyee, M. Ghasemi, A. Asgharzadeh, M. Forouzani, S. Mirzad and S. M. Hashemi, J. Sulfur Chem., 2017, 38, 279 Search PubMed.
- J.-Y. Jin, L.-X. Zhang, X.-X. Chen and A.-J. Zhang, Molecules, 2007, 12, 297 Search PubMed.
- J.-Y. Jin, L.-X. Zhang, A.-J. Zhang, X.-X. Lie and J.-H. Zhu, Molecules, 2007, 12, 1596 Search PubMed.
- X. Ye, Z. Chen, A. Zhang and L. Zhang, J. Chem. Res., 2007, 244 Search PubMed.
- E. S. H. El-Ashry, L. F. Awad and H. M. Abdel-Hamid, Nucleos Nucleot. Nucleic Acids, 2006, 25, 325 Search PubMed.
- W. Xie, J. Zhang, X. Ma, W. Yang, Y. Zhou, X. Tang, Y. Zou, H. Li, J. He, S. Xie, Y. Zhao and F. Liu, Chem. Biol. Drug Des., 2015, 86, 1087 CrossRef CAS PubMed.
- M. Chehrouri and A. A. Othman, Synth. Commun., 2019, 49, 1301 CrossRef CAS.
- R. J. Singh, J. Chem. Soc. Pak., 2011, 33, 485 CAS.
- P. K. Sahoo, R. Sharma and P. Pattanayak, Med. Chem. Res., 2010, 19, 127 CrossRef CAS.
- A. K. Singh and K. R. Kandel, J. Nepal Chem. Soc., 2012, 30, 174 CrossRef.
- A. A. Hamed and F. Hassan, J. Appl. Sci. Technol., 2014, 4, 202 Search PubMed.
- K. Parmar, S. Prajapati, R. Patel, S. Joshi and R. Patel, Int. J. Chemtech. Res., 2011, 3, 761 CAS.
- N. Upmanyu, J. K. Gupta, K. Shah and P. Mishra, J. Pharm. BioAllied Sci., 2011, 3, 259 CrossRef CAS PubMed.
- N. Lechani, M. Hamdi, B. Kheddis-Boutemeur, O. Talhi, Y. Laichi, K. Bachari and A. M. S. Silva, Synlett, 2018, 29, 1502 CrossRef CAS.
- C. Aswathanarayanappa, E. Bheemappa, Y. D. Bodke, P. S. Krishnegowda, S. P. Venkata and R. Ningegowda, Arch. Pharm. Chem. Life Sci., 2013, 346, 922 CrossRef CAS PubMed.
- P. K. Sahoo, R. Sharma and P. Pattanayak, Med. Chem. Res., 2010, 19, 127 CrossRef CAS.
- S.-N. Zhou, L.-X. Zhang, A.-J. Zhang, J.-S. Sheng and H.-L. J. Zhang, Heterocycl. Chem., 2007, 44, 1019 CrossRef CAS.
- K. C. Patel and J. A. Maroliwala, J. Chem. Pharm. Res., 2010, 2, 392 Search PubMed.
- K. Colanceska-Ragenovic, V. Dimova, D. G. Molnar and A. Buzarovska, Molecules, 2001, 6, 815 CrossRef CAS.
- M. Rafiq, M. Saleem, M. Hanif, S. K. Kang, S.-Y. Seo and K. H. Lee, Arch. Pharm. Res., 2016, 39, 161 CrossRef CAS PubMed.
- J. Khalafy, M. Mohammadlou, M. Mahmoody, F. Salami and A. P. Marjani, Tetrahedron Lett., 2015, 56, 1528 CrossRef CAS.
- Z. Li, X. Bai, Q. Deng, G. Zhang, L. Zhou, Y. Liu, J. Wang and Y. Wang, Med. Chem., 2017, 25, 213 CAS.
- V.-N. Bercean, A.-A. Creanga, V. Badea, C. Deleanu and C. Csunderlik, (New) Revista de Chim., 2011, 62, 47 CAS.
- A. F. Alghamdi and N. Rezki, J. Taibah Univ. Sci., 2017, 11, 759 CrossRef.
- V. Mathew, D. Giles, J. Keshavayya and V. P. Vaidya, Arch. Pharm. Chem. Life Sci., 2009, 342, 210 CrossRef CAS PubMed.
- P. Puthiyapurayil, B. Poojary, S. Kumar and R. Hunnur, J. Heterocycl. Chem., 2011, 48, 998 CrossRef CAS.
- R. H. Udupi and C. J. Manjunath, J. Pharmaceut. Sci. Res., 2019, 11, 44 CAS.
- A. V. Patel, J. H. Tailor and G. M. Malik, Int. J. Pharma Bio Sci., 2014, 5, 552 CAS.
- K. Sujatha, B. Kalluraya and S. D. Joshi, Rasayan J. Chem., 2019, 12, 1405 CrossRef CAS.
- K. Raviprabha, B. Poojary, K. Manjunatha, K. Vasantha, N. J. Fernandes and N. S. Kumari, Der Pharma Chem., 2016, 8, 1 CAS.
- M. Amir, H. Kumar and S. Javed, Eur. J. Med. Chem., 2008, 43, 2056 CrossRef CAS PubMed.
- A. W. Naser, M. S. Farhan and K. A. Abdulqader, Der Pharma Chem., 2018, 10, 145 CAS.
- M. A. I. Elbastawesy, B. G. M. Youssif, M. H. Abdelrahman and A. M. Hayallah, Chem, 2015, 7, 337 CAS.
- V. Sumangala, B. Poojary, N. Chidananda, T. Arulmoli and S. Shenoy, Eur. J. Med. Chem., 2012, 54, 59 CrossRef CAS.
- M. Amir, H. Kumar and S. A. Javed, Bioorg. Med. Chem. Lett, 2007, 17, 4504 CrossRef CAS.
- G. Mustafa, A. Angeli, M. Zia-ur-Rehman, N. Akbar, S. Ishtiaq and C. T. Supuran, Bioorg. Chem., 2019, 91, 103110 CrossRef CAS.
- K. Ilango and P. Valentina, Eur. J. Chem., 2010, 1, 50 CrossRef CAS.
- K. Ilango and P. Valentina, Der Pharma Chem., 2010, 2, 16 CAS.
- S. R. Desai, U. Laddi, R. S. Bennur, P. A. Patil and A. S. Bennur, Indian J. Pharm. Sci., 2011, 73, 115 CrossRef CAS PubMed.
- M. A. Al-Omar, E. S. Al-Abdullah, I. A. Shehata, E. E. Habib, T. M. Ibrahim and A. A. El-Emam, Molecules, 2010, 15, 2526 CrossRef CAS PubMed.
- R. M. Kharate, P. P. Deohate and B. N. Berad, Der Pharma Chem., 2012, 4, 2434 CAS.
- Q. Mei, J. Yang, Y. Peng and Z. Zhao, J. Chem. Res., 2011, 35, 386 CrossRef CAS.
- J. Wu, X. Liu, X. Cheng, Y. Cao, D. Wang, Z. Li, W. Xu, C. Pannecouque, M. Witvrouw and E. De-Clercq, Molecules, 2007, 12, 2003 CrossRef CAS PubMed.
- N. B. Saidov, V. A. Georgiyants and E. Y. Lipakova, Pharm. Chem. J., 2017, 51, 26 CrossRef CAS.
- A. M. Dhiman, K. N. Wadodkar and S. D. Patil, Indian J. Chem., Sect. B, 2001, 40, 640 Search PubMed.
- A. Stana, A. Enache, D. C. Vodnar, C. Nastasa, D. Benedec, I. Ionut, C. Login, G. Marc, O. Oniga and B. Tiperciuc, Molecules, 2016, 21, 1595 CrossRef PubMed.
- B. A. Baviskar, M. R. Shiradkar, S. S. Khadabadi, S. L. Deore and K. G. Bothara, Indian J. Chem., Sect. B, 2011, 50, 321 Search PubMed.
- M. Kakadiya, B. Parmar, G. H. Chethan, S. Deka, J. Saravanan and S. Mohan, Int. J. PharmTech Res., 2013, 3, 633 CAS.
- A. Demirbas, D. Ahin, N. Demirbas, S. A. Karaoglu and H. Bektas, J. Cheminf., 2010, 34, 347 CAS.
- A. Mandal, T. K. Dutta and R. L. Gupta, Indian J. Chem., Sect. B, 2015, 54, 228 Search PubMed.
- S. Vijayaraghavan and P. Y. Shirodkar, Indian J. Chem., Sect. B, 2015, 54, 1149 Search PubMed.
- S. Majumder, B. M. Bashyal and R. L. Gupta, Indian J. Chem., Sect. B, 2015, 54, 1260 Search PubMed.
- M. E. Bhanojirao and V. G. Rajurkar, Asian J. Chem., 2009, 21, 4733 CAS.
- P. Pathak, P. K. Shukla, V. Naumovich, M. Grishina, A. Verma and V. Potemkin, Arch. Pharm. Chem. Life Sci., 2020, 353, 1900233 CrossRef CAS PubMed.
- S. Garrepalli, S. Katherasala, B. Yamini and A. Mounika, J. Pharm. Res., 2014, 3, 20 Search PubMed.
- I. Khan, S. Zaib, A. Ibrar, N. H. Rama, J. Simpson and J. Iqbal, Eur. J. Med. Chem., 2014, 78, 167 CrossRef CAS PubMed.
- M. M. Kamel and N. Y. M. Abdo, Eur. J. Med. Chem., 2014, 86, 75 CrossRef CAS PubMed.
- P. P. Deohate, Der Pharma Chem., 2012, 4, 2042 CAS.
- N. Nami and M. Hosseinzadeh, Heterocycl. Commun., 2007, 13, 403 CAS.
- D. S. Donawade, A. V. Raghu and G. S. Gadaginamath, Indian J. Chem., Sect. B, 2006, 45, 689 Search PubMed.
- E. S. H. El Ashry, A. A. Kassem, H. Abdel-Hamid, F. F. Louis, S. A. N. Khattab and M. R. Aouad, Arkivoc, 2006, XIV, 119 Search PubMed.
- A. Singh, V. Parmar and S. K. Saraf, Der Pharma Chem., 2016, 8, 39 Search PubMed.
- R. D. Dighe, M. R. Shiradkar, S. S. Rohom and P. D. Dighe, Chem. Sin., 2011, 2, 70 Search PubMed.
- L. Jiang, M.-Y. Wang, F.-X. Wan and Z.-Q. Qu, Phosphorus, Sulfur, Silicon Relat. Elem., 2015, 190, 1599 CrossRef CAS.
- Y. J. Li, L. J. Liu, K. Jin, Y. T. Xu and S. Q. Sun, Chin. Chem. Lett., 2010, 21, 293 CrossRef CAS.
- M. A. Raslan and M. A. Khalil, Heteroat. Chem., 2003, 14, 114 CrossRef CAS.
- N. Aggarwal, R. Kumar, P. Dureja and J. M. Khurana, Eur. J. Med. Chem., 2011, 46, 4089 CrossRef CAS PubMed.
- S. Wagle, A. V. Adhikari and N. S. Kumari, Asian J. Chem., 2008, 20, 629 CAS.
- T. R. Devi, E. Laxminarayana and T. Chary, J. Heterocycl. Chem., 2019, 29, 125 CAS.
- A. S. N. Formagio, L. T. D. Tonin, M. A. Foglio, C. Madjarof, J. E. de Carvalho, W. F. da Costa, F. P. Cardoso and M. H. Sarragiotto, Med. Chem., 2008, 16, 9660 CAS.
- K. Nagaraju, Y. Kotaiah, C. Sampath, N. Harikrishna and C. V. Rao, J. Sulfur Chem., 2013, 34, 264 CrossRef CAS.
- B. Hegazi, H. Abdel-Gawad, H. A. Mohamed, F. A. Badria and A. M. Farag, Chem. Inf., 2013, 50, 355 CAS.
- Asma, B. Kalluraya and N. Manju, Heterocycl. Lett., 2018, 8, 69 CAS.
- N. Rezki, Org. Prep. Proced. Int., 2017, 49, 525 CrossRef CAS.
- S. M. Gomha, M. M. Edrees, Z. A. Muhammad, N. A. Kheder, S. Abu-Melha and A. M. Saad, Polycyclic Aromat. Compd., 2020 DOI:10.1080/10406638.2020.1720751.
- H. Xiao, P. Li, D. Guo, J. Hu, Y. Chai and W. He, Med. Chem. Res., 2014, 23, 1941 CrossRef CAS.
- H. Xiao, P. Li, J. Hu, R. Li, L. Wu and D. Guo, Appl. Biochem. Biotechnol., 2013, 172, 2188 CrossRef.
- M. Ozil, O. Bodur, S. Ulker and B. Kahveci, Chem. Heterocycl. Compd., 2015, 51, 88 CrossRef CAS.
- M. G. Bhovi and G. S. Gadaginamath, Asian J. Chem., 2005, 17, 518 CAS.
- A. Hasan, N. F. Thomas and S. Gapil, Molecules, 2011, 16, 1297 CrossRef CAS.
- M. Kalhor and A. Dadras, J. Heterocycl. Chem., 2013, 50, 220 CrossRef CAS.
- W. M. El-Husseiny, M. A. A. El-Sayed, N. I. Abdel-Aziz, A. S. El-Azab, Y. A. Asiri and A. A. M. Abdel-Aziz, Eur. J. Med. Chem., 2018, 158, 134 CrossRef CAS.
- C. S. Reddy, L. S. Rao, G. R. Kumar and A. Nagaraj, Chem. Pharm. Bull., 2010, 58, 1328 CrossRef PubMed.
- D.-Q. Qi, C.-M. Yu, J.-Z. You, G.-H. Yang, X.-J. Wang and Y.-P. Zhang, Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 70 CrossRef CAS.
- D. Szulczyk, P. Tomaszewski, M. Jozwiak, A. E. Koziol, T. Lis, D. Collu, D. Iuliano and M. Struga, Molecules, 2009, 22, 409 CrossRef PubMed.
- A. M. Manikrao, P. N. Khatale, T. Sivakumar, D. R. Chaple, P. M. Sable and R. D. Jawarkar, Der Pharma Chem., 2011, 3, 334 CAS.
- K. Siddoju and J. K. Ega, J. Pharm. Chem. Biol. Sci., 2018, 8, 139–146 CAS.
- S. B. Ozdemir, N. Demirbas, A. Demirbas, F. A. Ayaz and N. Çolak, J. Heterocycl. Chem., 2018, 55, 2744 CrossRef.
- R. D. Patil and J. S. Biradar, Indian J. Chem., Sect. B, 2000, 39, 929 Search PubMed.
- P. K. Dubey and B. Babu, Indian J. Heterocycl. Chem., 2007, 16, 357 CAS.
- T. A. Farghaly, J. Chin. Chem. Soc., 2004, 51, 147–156 CrossRef.
- A. H. El-masry, H. H. Fahmy and S. H. A. Abdelwahed, Molecules, 2000, 5, 1429 CrossRef CAS.
- A. Nema and S. K. Srivastava, J. Indian Chem. Soc., 2007, 84, 1037 CAS.
- S. Dwivedi and P. K. Singh, Asian J. Chem., 2017, 29, 19 CrossRef CAS.
- S. Amara and A. A. Othman, Arab. J. Chem., 2016, 9, S1840 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |