Open Access Article
Open Access ArticleStructural control of magnetite nanoparticles for hyperthermia by modification with organic polymers: effect of molecular weight
Toshiki Miyazaki *a,
Takayuki Tangea,
Masakazu Kawashita
*a,
Takayuki Tangea,
Masakazu Kawashita b and
Balachandran Jeyadevan
b and
Balachandran Jeyadevan c
c
aGraduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, 2-4 Hibikino, Wakamatsu-ku, Kitakyushu, Japan. E-mail: tmiya@life.kyutech.ac.jp
bInstitute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Tokyo, Japan
cDepartment of Material Science, The University of Shiga Prefecture, Hikone, Shiga, Japan
First published on 14th July 2020
Abstract
Hyperthermia treatment using appropriate magnetic materials in an alternating magnetic field to generate heat has been recently proposed as a low-invasive cancer treatment method. Magnetite (Fe3O4) nanoparticles are expected to be an appropriate type of magnetic thermal seed for this purpose, and the addition of organic substances during the synthesis process has been studied for controlling particle size and improving biological functions. However, the role of the properties of the organic polymer chosen as the modifier in the physical properties of the thermal seed has not yet been comprehensively revealed. Therefore, this study clarifies these points in terms of the molecular weight and the charge of the functional groups of the added polymers. Excepting polyethyleneimine, the Fe3O4 crystallite size decreased with increasing polymer molecular weight. Neutral polymers did not suppress the Fe3O4 formation regardless of the difference in molecular weight, while suppression of the Fe3O4 formation was observed for low molecular weight anionic polymers and high molecular weight cationic polymers. Samples with a small amount of Fe3O4 or with crystallite size less than 10 nm induced low heat generation under an alternating magnetic field.
1. Introduction
In cancer tissues, blood perfusion at 41 to 43 °C is typical, which is lower than in normal tissues.1 Therefore, cancer cells can be killed at lower temperatures than would be necessary to kill normal cells. Based on this phenomenon, hyperthermia is attracting increasing attention as a novel cancer treatment to treat deep-seated cancers with minimal invasion.2 Magnetic materials such as magnetite (Fe3O4) and maghemite (γ-Fe2O3) with appropriate physical properties achieve heat generation under an alternating magnetic field. When the size of Fe3O4 particles reaches the nanometer level, they become superparamagnetic, where Néel relaxation and Brownian relaxation are the primary heat generation mechanisms. Jeyadevan has theoretically studied the dependence of the specific absorption rate (SAR) upon particle size under a magnetic field strength and frequencies of 3.2 kA m−1 (40 Oe) and 600 kHz, respectively, and reported that SAR is maximized at a magnetite particle size around 12 nm.3 However, improvement of cellular uptake of the Fe3O4 nanoparticles by surface modification is desired to enhance efficiency for the hyperthermia.4Organic modification is a useful technique for enhancing the biological functionality of magnetic nanoparticles for hyperthermia such as the target drug delivery, gene therapy, sensing and so on.5,6 In particular, the G250 antibody, capable of binding to the MN antigen in a tumor, has been conjugated with a magnetic liposome to impart a targeting function to the affected area. It was found that its uptake into the tumor increased six-fold compared with an un-conjugated magnetic liposome.7 In addition, the molecular weight of the polymer modifier affects the viscosity, the aggregation state of the nanoparticles in liquid, and the immobilization density of their surfaces, besides changing biological functions. For example, modification of Fe3O4 nanoparticles with polyethylene glycol (PEG) of varying molecular weight exhibits a varying stability of the resulting conjugates in liquid.8 It was also revealed that the cytotoxicity of Fe3O4 nanoparticles varies depending on the type of the polymer coating and the number of block copolymer units.9,10
Fe3O4 can be conventionally synthesized by an aqueous solution process.11 If organic substances can be simultaneously incorporated during this process, a relatively simple one-pot manufacturing process can be established. For example, the size of spherical aggregates of Fe3O4 nanoparticles prepared by the hydrothermal method in the presence of polyacrylic acid (PAA) varies with the molecular weight of the PAA used during the synthesis, where higher molecular weight corresponds to a smaller aggregate size.12 Authors have found that the size and magnetic properties of the Fe3O4 nanoparticles vary depending on the functional groups of the added organic molecules added during the aqueous synthesis of Fe3O4, and that other types of iron hydroxide will form depending on the synthetic conditions.13,14 However, the effects that the polymer molecular weight and functional groups have on the crystalline phase and the heat generation characteristics of the resulting conjugates have not been comprehensively investigated. Therefore, the purpose of this study was to clarify these points in terms of the molecular weight and the charge of the functional groups of the added polymers.
2. Materials and methods
2.1 Sample preparation
An aqueous co-precipitation method was used for the preparation of the samples. Specifically, FeCl2·4H2O (Wako Pure Chemical Industries Ltd., Osaka, Japan) and FeCl3·6H2O (Wako Pure Chemical Industries Ltd., Osaka, Japan) were dissolved in ultrapure water to prepare 50 mL of solution containing 2.5 mM Fe2+ and 4.9 mM Fe3+ while bubbling with N2 gas. Separately, 100 mL of 2.0 mM polymer solution was prepared, using one of the four polymers PEG (molecular weight: 200, 600 and 6000, Wako Pure Chemical Industries Ltd., Osaka, Japan), PAA (molecular weight: 5000 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Wako Pure Chemical Industries Ltd., Osaka, Japan), polystyrene sulfonate sodium salt (PSSS; molecular weight: 300
000, Wako Pure Chemical Industries Ltd., Osaka, Japan), polystyrene sulfonate sodium salt (PSSS; molecular weight: 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 500
000 and 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Alfa Aesar, Lancashire, UK), or polyethyleneimine (PEI; molecular weight: 600, 1800, and 10
000, Alfa Aesar, Lancashire, UK), or polyethyleneimine (PEI; molecular weight: 600, 1800, and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Wako Pure Chemical Industries Ltd., Osaka, Japan). For sample nomenclature, the polymer abbreviation is followed by the polymer molecular weight used. For example, for the sample incorporating PEG with a molecular weight of 600, the solution containing polymer was mixed with the metallic ion solution at 75 °C while bubbling N2 gas, and 1 M-NH3 aqueous solution was added dropwise till a pH of 7 was achieved and stirred for 1 hour. Then it was packed in a cellulose Visking tube (Nihon Medical Science Inc., Osaka, Japan), immersed in ultrapure water, and dialyzed for 1 day to remove water-soluble salts in the solution. After 1 day of dialysis, the ultrapure water used for the dialysis was replaced and dialysis was performed again for an extra day. After the dialysis, the sample was dried at 60 °C for 3 days in an oven (NDO-700, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and then ground by a mortar.
000, Wako Pure Chemical Industries Ltd., Osaka, Japan). For sample nomenclature, the polymer abbreviation is followed by the polymer molecular weight used. For example, for the sample incorporating PEG with a molecular weight of 600, the solution containing polymer was mixed with the metallic ion solution at 75 °C while bubbling N2 gas, and 1 M-NH3 aqueous solution was added dropwise till a pH of 7 was achieved and stirred for 1 hour. Then it was packed in a cellulose Visking tube (Nihon Medical Science Inc., Osaka, Japan), immersed in ultrapure water, and dialyzed for 1 day to remove water-soluble salts in the solution. After 1 day of dialysis, the ultrapure water used for the dialysis was replaced and dialysis was performed again for an extra day. After the dialysis, the sample was dried at 60 °C for 3 days in an oven (NDO-700, Tokyo Rikakikai Co., Ltd., Tokyo, Japan) and then ground by a mortar.
2.2 Characterization
The crystalline structure of the obtained samples was characterized by powder X-ray diffraction (XRD; M03XHF22, Mac Science Co., Ltd., Yokohama, Japan). In XRD, a CuKα X-ray was used as a source; the voltage and of the current of the Cu tube were fixed at 40 kV and 30 mA, respectively; the step width was 0.020°; and the counting time was 1 s. The crystallite size of the obtained particles (t) was calculated by using Scherrer's equation:15
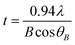 | (1) |
The surface zeta potential of the sample in ultrapure water was measured with a zeta potential analyzer (ELS-Z, Otsuka Electronics Co., Ltd., Osaka, Japan). A quartz cell was used for the measurement.
2.3 Measurement of Fe2+ concentration in aqueous solution in the presence of polymer
The change in Fe2+ concentration owing to the addition of the polymer was examined by an assay using 4,7-diphenyl-1,10-phenanthrolinedisulfonic acid, and disodium salt (Bathophenanthroline, Dojindo Laboratories, Kumamoto, Japan). The bathophenanthroline–Fe2+ complex exhibits visible-light absorption at 535 nm, while Fe3+ does not.16 Herein, 10 mL of an aqueous solution containing 0.072 mM of iron chloride(II) and 0.020 mM of each polymer was prepared. Then, 0.0022 mmol of bathophenanthroline was added to the solution, and the mixture was stirred for 1 min to stabilize the coloring. The absorbance of the obtained solutions at 535 nm was subsequently measured by ultraviolet-visible spectroscopy (UV-Vis; V-630, JASCO Co., Tokyo, Japan).2.4 Heat generation measurement
The temperature change the samples exhibited in an alternating magnetic field was measured, and the SAR of the samples was calculated. First, 100 mg of the sample was dispersed in 1 mL of 1 mass% agar boiling solution and naturally cooled. Sample concentration was higher than the previous study (4 mg mL−1),17 because magnetic field intensity was lower. The sample, now embedded in the agar phantom, was then placed in an alternating magnetic field generator consisting of a high frequency power supply (T162-5723A, Thamway Co. Ltd., Shizuoka, Japan), an impedance matching box (T020-5723C, Thamway Co. Ltd., Shizuoka, Japan), and a work coil. The alternating magnetic field application conditions were a frequency of 600 kHz, a magnetic field intensity of 3.2 kA m−1 (40 Oe), and an application time of 10 min. The temperature change of the sample was obtained using a fiber optic thermometer (OTG-MPK5, Opsens Inc., Québec, Canada) attached to a signal conditioner (TempSens, Opsens Inc., Québec, Canada). The SAR value was calculated from the temperature rise of the sample in an applied alternating magnetic field; using the relation
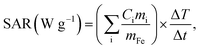 | (2) |
Magnetization behavior of the sample without polymer addition, PEG 200, PEG 6000 and PEI-added samples was measured by a superconducting quantum interference device (SQUID) (MPMS-XL7AC, Quantum Design Inc., San Diego, California, USA) in an applied DC magnetic field of ±2389 kA m−1 (30 kOe) at 27 °C.
3. Results
Fig. 1 shows the XRD patterns of the samples prepared by addition of the polymers having various molecular weights. Although diffraction peaks from Fe3O4 (JCPDS # 19-0629) were detected from all samples, the peaks were notably weak for PAA 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, PEI 1800 and PEI 10
000, PEI 1800 and PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Additionally, the presence of a peak allocated to γ-FeOOH (JCPDS # 44-1415) was observed for PSSS 300
000. Additionally, the presence of a peak allocated to γ-FeOOH (JCPDS # 44-1415) was observed for PSSS 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and PAA 5000 and α-FeOOH (JCPDS # 29-0713) for PEI 1800 and PEI 10
000 and PAA 5000 and α-FeOOH (JCPDS # 29-0713) for PEI 1800 and PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Table 1 summarizes crystallite sizes of the Fe3O4 particles determined from the XRD patterns. The crystallite sizes of all the samples were smaller than the sample without polymer addition. It tended to decrease with increase in molecular weight of the polymer.
000. Table 1 summarizes crystallite sizes of the Fe3O4 particles determined from the XRD patterns. The crystallite sizes of all the samples were smaller than the sample without polymer addition. It tended to decrease with increase in molecular weight of the polymer.
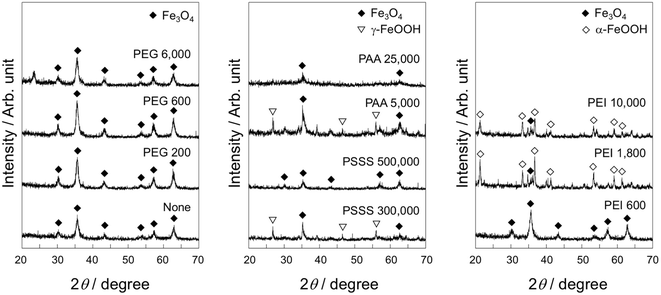 | ||
| Fig. 1 XRD patterns of the samples prepared by addition of the polymers having various molecular weights. | ||
| Sample | Crystallite size/nm |
|---|---|
| None | 21.3 |
| PEG 200 | 15.5 |
| PEG 600 | 13.8 |
| PEG 6000 | 11.0 |
PSSS 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18.3 |
PSSS 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14.4 |
| PAA 5000 | 11.4 |
PAA 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8.5 |
| PEI 600 | 19.3 |
| PEI 1800 | Not measured |
PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Not measured |
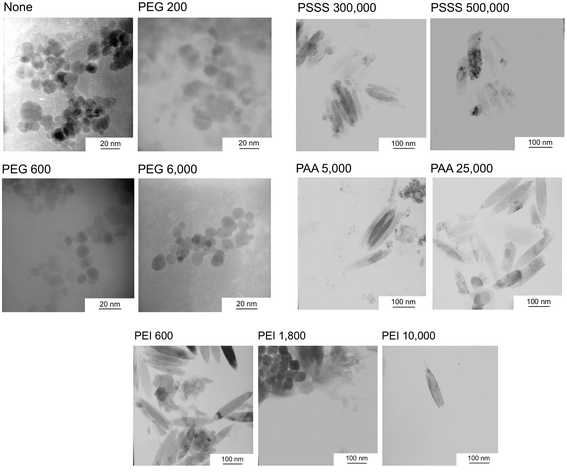
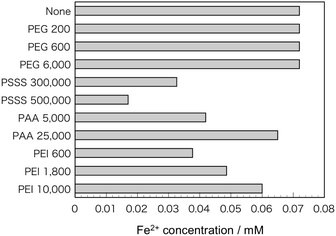
Fig. 2 shows TEM micrographs of the samples. With the addition of PEG, spherical particles derived from Fe3O4 about 10–20 nm in size were observed. Although some spherical particles were observed with the addition of PSSS and PAA, many particles were needle-shaped with a major axis of 100–200 nm. The addition of PEI yielded a mix of spherical particles and needle-like particles derived from FeOOH in PEI 600 and PEI 1800, where the size of the spherical particles in the latter sample of several tens of nanometers was much larger than those in the other samples. In PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, only needle-shaped particles were observed. Fig. 3 shows the Fe2+ concentration of the FeCl2 solutions including polymers with various molecular weights. When PEG was added, the Fe2+ concentration was almost the same as that without polymer addition. With the addition of the other polymers, however, the Fe2+ concentration tended to decrease. In particular, PSSS addition significantly decreased the Fe2+ concentration in the solutions.
000, only needle-shaped particles were observed. Fig. 3 shows the Fe2+ concentration of the FeCl2 solutions including polymers with various molecular weights. When PEG was added, the Fe2+ concentration was almost the same as that without polymer addition. With the addition of the other polymers, however, the Fe2+ concentration tended to decrease. In particular, PSSS addition significantly decreased the Fe2+ concentration in the solutions.
Fig. 4 shows the time-dependent temperature change of samples embedded in agar phantom under an alternating magnetic field. The temperature increase was about 23 °C without polymer addition, whereas that of the PEG 6000 sample was as high as about 38 °C. In addition, the temperature increase was 17 to 22 °C for the other PEG samples and the PSSS samples, and about 12 °C for PEI 600 and PAA 5000. Finally, the temperature increased by only about 2 °C for the remaining PEI samples and for PAA 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Table 2 shows the SAR value of all samples calculated from the temperature–time curve, where a higher temperature increase corresponds to a higher SAR value.
000. Table 2 shows the SAR value of all samples calculated from the temperature–time curve, where a higher temperature increase corresponds to a higher SAR value.
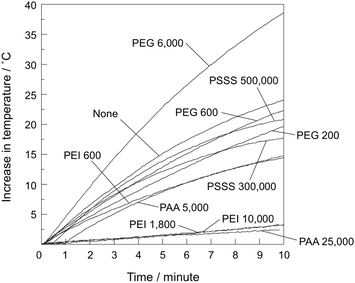 | ||
| Fig. 4 Time-dependent temperature variation of the samples embedded in agar phantom under an alternating magnetic field. | ||
| Sample | SAR (W gFe−1) |
|---|---|
| None | 3.0 |
| PEG 200 | 2.3 |
| PEG 600 | 2.7 |
| PEG 6000 | 4.5 |
PSSS 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.0 |
PSSS 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.2 |
| PAA 5000 | 0.20 |
PAA 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.49 |
| PEI 600 | 2.3 |
| PEI 1800 | 0.29 |
PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.49 |
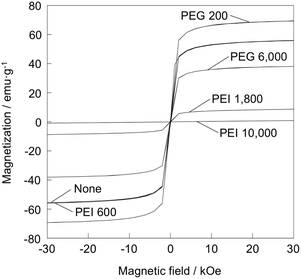
Fig. 5 shows magnetization curves of the samples. Saturation magnetization increased in the order: PEI 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (0.885 emu g−1) < PEI 1800 (8.73 emu g−1) < PEG 6000 (38.1 emu g−1) < none (55.8 emu g−1) < PEI 600 (55.9 emu g−1) < PEG 200 (69.5 emu g−1).
000 (0.885 emu g−1) < PEI 1800 (8.73 emu g−1) < PEG 6000 (38.1 emu g−1) < none (55.8 emu g−1) < PEI 600 (55.9 emu g−1) < PEG 200 (69.5 emu g−1).
4. Discussion
It was found that the size and crystalline phase of the formed iron oxide greatly varied depending on the type and molecular weight of the added polymer. In the case of non-ionic PEG, Fe3O4 was formed regardless of its molecular weight, whereas the crystallite size of the formed nanoparticles decreased with increased PEG molecular weight. This size decrease can be attributed to the suppression of iron ion diffusion owing to the increased viscosity of the surrounding solution with increased molecular weight, with a consequent suppression of the crystal growth. However, PEG did not have a site capable of tight chelation with the iron ions, thus nucleation of Fe3O4 was not suppressed. This premise is supported by the results that the Fe2+ concentration in the PEG-containing solution decreased little compared with that of the solution without the polymer (Fig. 3).When the anionic polymers PSSS and PAA were added, the crystal growth of Fe3O4 also tended to be suppressed. In the case of PSSS, the crystallite size decreased with increased molecular weight, similar to the results of PEG. A previous study showed that PSSS with a molecular weight of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 resulted in an average Fe3O4 particle size of 6.9 nm,13 which is consistent with the trend in this study. This result is attributed to the chelating ability of PSSS with iron ions, supported by the results in Fig. 3. The crystal growth inhibition by PAA was stronger than PSSS. The stability constant of the Fe(CH3COO)2+ complex (3.38), comprising Fe3+ and a carboxyl group, is greater than that of the FeSO4+ complex (2.56), comprising Fe3+ and sulfonic group18,19 In addition, the molecular weight of PAA is 1/10 or less that of PSSS. Therefore, it is considered that the polymer molecules can easily adsorb on the Fe3O4 nuclei owing to their high mobility and chelating ability. The addition of anionic polymers resulted in FeOOH as well as Fe3O4 (Fig. 1), indicating that the nucleation of Fe3O4 is also suppressed by the formation of the complex. Furthermore, the amount of Fe2+ required for Fe3O4 formation decreases owing to oxidation of the Fe2+–polymer complex via dissolved O2. A previous work reported no adverse effect on the crystalline structure of Fe3O4 synthesized by the reaction of Fe(OH)2 and NaNO3 in the presence of 0.0125 mM of PAA.20 This absence of the effect observed in that previous work is attributed to the lower PAA concentration therein than used in the present study (2.0 mM).
000 resulted in an average Fe3O4 particle size of 6.9 nm,13 which is consistent with the trend in this study. This result is attributed to the chelating ability of PSSS with iron ions, supported by the results in Fig. 3. The crystal growth inhibition by PAA was stronger than PSSS. The stability constant of the Fe(CH3COO)2+ complex (3.38), comprising Fe3+ and a carboxyl group, is greater than that of the FeSO4+ complex (2.56), comprising Fe3+ and sulfonic group18,19 In addition, the molecular weight of PAA is 1/10 or less that of PSSS. Therefore, it is considered that the polymer molecules can easily adsorb on the Fe3O4 nuclei owing to their high mobility and chelating ability. The addition of anionic polymers resulted in FeOOH as well as Fe3O4 (Fig. 1), indicating that the nucleation of Fe3O4 is also suppressed by the formation of the complex. Furthermore, the amount of Fe2+ required for Fe3O4 formation decreases owing to oxidation of the Fe2+–polymer complex via dissolved O2. A previous work reported no adverse effect on the crystalline structure of Fe3O4 synthesized by the reaction of Fe(OH)2 and NaNO3 in the presence of 0.0125 mM of PAA.20 This absence of the effect observed in that previous work is attributed to the lower PAA concentration therein than used in the present study (2.0 mM).
A larger amount of FeOOH was formed by the addition of cationic PEI than the other three polymer types, and a remarkable inhibition of Fe3O4 nucleation was observed (Fig. 1). The amount of ionic functional groups in PEI is lower than that in most anionic polymers because of its lower molecular weight. Nevertheless, the decrease in Fe2+ with the presence of PEI was comparable to most anionic polymers (Fig. 3). In addition, PEI has an amino group in the main chain capable of chelating, indicating that PEI is less susceptible to its conformation. Therefore, PEI is considered to have a high chelating ability. The stability constant of the triethylenetetramine ((CH2NHCH2CH2NH2)2) complex, possessing an atomic arrangement similar to PEI, against Fe3+ is reported to be around 8, which is greater than FeSO4+ and Fe(CH3COO)2+.21
In the present study, suppression of Fe3O4 formation tended to occur with anionic polymers of low molecular weight and cationic polymers of high molecular weight. This signifies that the relationship between the molecular weight of the added polymers and the crystalline phase of Fe3O4 varied depending on the charge of the polymer. The molecular weight,21,22 conformation,23 steric hindrance,24 and side chain length25 are the factors known to effect the stability constant of the polymer complex, meaning that the mineralization behavior in the presence of polymers is highly complicated. In this regard, further investigation using polymers having the same functional groups but different conformation is needed.
The crystalline structure of FeOOH as a byproduct varied depending on the added polymer. This can be attributed to the difference in the pH of the solution, as revealed in a previous investigation of the relationship between pH of the precursor solution containing Fe2+ and Cl− and the crystalline phase of the produced FeOOH.26 According to that work, γ-FeOOH is the primary crystalline phase for pH 4–7, a mixture of γ-FeOOH and α-FeOOH is present for higher pH, and primarily the α-FeOOH phase for pH > 12. The pH herein ranged from 3.5–5.8 for PAA and PSSS, and from 11.3–12.8 for PEI. Therefore, the present results are consistent with the earlier report.
Heat generation under an alternating magnetic field also varied greatly with the type and molecular weight of the added polymer (Fig. 4). Samples with a small amount of Fe3O4 or with crystallite sizes less than 10 nm tended to exhibit a low heat generation. Yanase et al. reported that heating at about 45 °C induces evolution of heat shock proteins that activate immune cells.27 Considering that the initial temperature of all samples in this study was about 25 °C, the samples exhibiting a temperature increase of 20 °C or more are considered desirable for hyperthermia applications.
Heat generation of PEG 6000 was rather higher than that of the particles without polymer addition. The particle size of the former was smaller than the latter. Jeyadevan reports that the heat generation drastically decreases when particle size of Fe3O4 is less than 10 nm.3 This is opposite tendency to the present results. Saturation magnetization of PEG 6000 was rather lower than the sample without polymer addition (Fig. 5), meaning that magnetization ability is not likely a main governing factor. Zeta potential of the sample without polymer addition and PEG 6000 was 39.6 and −17.4 mV, respectively, while that of agarose is negative value.28 Therefore, it is assumed that PEG 6000 well disperses in agar matrix by electrostatic repulsion and exhibited high Brownian relaxation. It is reported that the state of aggregation, apparent hydrodynamic size and resultant SAR vary depending on the type of polymer coated on the Fe2O3 nanoparticles, because the state of Brownian relaxation may be affected by the aggregation.29
Suto et al. investigated heat generation of Fe3O4 nanoparticles treated with a surfactant and embedded in polyvinyl alcohol hydrogels, and reported that SAR of the nanoparticles is 16.8 W gFe3O4−1 at maximum.30 The authors prepared Fe3O4 nanoparticles by NaOH treatment of chitosan hydrogels dispersed with Fe2+ and measured the heat generation at 24 kA m−1 (300 Oe) and 100 kHz.31 Its effective specific absorption rate normalized by strength of the applied alternating magnetic field and frequency is 1.24× 10−8 W gFe−1 Oe−2 Hz−1 at maximum, whereas that of PEG 6000 in this study is 4.7 × 10−9 W gFe−1 Oe−2 Hz−1. These facts mean that SAR of the present samples is lower than the above described Fe3O4 nanoparticles. There is also possibility for difference in dispersion in hydrogel matrix. So further detailed investigation is necessary in the future.
Among the added polymers, PEI and PAA especially tended to suppress heat generation. This is attributed to the formation of non-magnetic FeOOH and the decrease in the size of Fe3O4. In the case of PEI 1800, heat generation was extremely small despite the fact that spherical particles of 50–60 nm size were observed by TEM (Fig. 2). This is supported by the result that its magnetization was also strongly suppressed (Fig. 5). Li et al. examined the particle size dependence of the heat generation characteristics of Fe3O4 nanoparticles, reporting that particles with an average size of 8 nm exhibited the highest heat generation.32 They also reported that the heat generation tended to decrease with increasing particle size owing to loss of superparamagnetism. Additionally, amount of the formed Fe3O4 was small (Fig. 1). Therefore, the low heat generation ability of PEI 1800 can be explained by the above findings.
The crystallite sizes of both PEG 6000 and PAA 5000 were around 11 nm (Table 1), which is in the range of excellent heat generation reported by Jeyadevan.3 Nevertheless, the temperature increase of the former was significantly higher than the latter (Fig. 4). Low heat generation of the latter would be attributed to reduced Fe3O4 content by formation of γ-FeOOH.
5. Conclusions
We investigated the effects that the molecular weight of various polymer additives in the aqueous synthesis of Fe3O4 nanoparticles had on their structure and their heat generation under an alternating magnetic field. The Fe3O4 crystallite size tended to decrease with increasing molecular weight. Furthermore, ionic polymers suppressed nucleation of Fe3O4, while the molecular weight dependence for suppression of Fe3O4 formation was different for anionic and cationic polymers. Heat generation was suppressed in the conditions where crystal growth of Fe3O4 was suppressed or where a large amount of the byproduct FeOOH was formed. Although the effect that the polymer structure has on the Fe2+–polymer complex formation and on the resultant Fe3O4 nucleation is complicated and further investigation is needed in future, the findings in this study can provide fundamental guidelines for organic modification of magnetic nanoparticles for hyperthermia.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
We acknowledge Dr Masashi Tanaka and Prof. Masaki Mito, from Faculty of Engineering, Kyushu Institute of Technology, for SQUID measurement. We also thank Sara Maccagnano-Zacher, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.References
- C. W. Song, Cancer Res., 1984, 44, 4721S CAS.
- T. Kobayashi, Biotechnol. J., 2011, 6, 1342 CrossRef CAS PubMed.
- B. Jeyadevan, J. Ceram. Soc. Jpn., 2010, 118, 391 CrossRef CAS.
- A. K. Gupta and M. Gupta, Biomaterials, 2005, 26, 1565–1573 CrossRef CAS PubMed.
- N. Zhu, H. Ji, P. Yu, J. Niu, M. U. Farooq, M. W. Akram, I. O. Udego, H. Li and X. Niu, Nanomaterials, 2018, 8, 810 CrossRef PubMed.
- S. O. Aisida, P. A. Akpa, I. Ahmad, T. Zhao, M. Maaza and F. I. Ezema, Eur. Polym. J., 2020, 122, 109371 CrossRef.
- M. Shinkai, B. Le, H. Honda, K. Yoshikawa, K. Shimizu, S. Saga, T. Wakabayashi, J. Yoshida and T. Kobayashi, Jpn. J. Cancer Res., 2001, 92, 1138 CrossRef CAS PubMed.
- J. D. Goff, P. P. Huffstetler, W. C. Miles, N. Pothayee, C. M. Reinholz, S. Ball, R. M. Davis and J. S. Riffle, Chem. Mater., 2009, 21, 4784 CrossRef CAS.
- U. O. Häfeli, J. S. Riffle, L. Harris-Shekhawat, A. Carmichael-Baranauskas, F. Mark, J. P. Dailey and D. Bardenstein, Mol. Pharm., 2009, 6, 1417 CrossRef PubMed.
- V. Zavisova, M. Koneracka, A. Gabelova, B. Svitkova, M. Ursinyova, M. Kubovcikova, I. Antal, I. Khmara, A. Jurikova, M. Molcan, M. Ognjanović, B. Antić and P. Kopcansky, J. Magn. Magn. Mater., 2019, 472, 66 CrossRef CAS.
- K. Petcharoena and A. Sirivat, Mater. Sci. Eng., B, 2012, 177, 421–427 CrossRef.
- Y. Song, Y. Li, Z. Teng, Y. Huang, X. Chen and Q. Wang, ACS Omega, 2018, 3, 17904 CrossRef CAS.
- Y. Kuwahara, T. Miyazaki, Y. Shirosaki and M. Kawashita, RSC Adv., 2014, 4, 23359 RSC.
- Y. Kuwahara, T. Miyazaki, Y. Shirosaki, G. Liu and M. Kawashita, Ceram. Int., 2016, 42, 6000 CrossRef CAS.
- B. D. Cullity and S. R. Stock, Elements of X-ray diffraction, Prentice Hall, New Jersey, 3rd edn, 2001 Search PubMed.
- L. Montás-Ramírez, N. Claassen and A. M. Moawad, J. Plant Nutr., 2003, 26, 2023 CrossRef.
- T. Miyazaki, A. Miyaoka, E. Ishida, Z. Li, M. Kawashita and M. Hiraoka, Mater. Sci. Eng., C, 2012, 32, 692 CrossRef CAS.
- A. E. Martell and R. M. Smith, Critical Stability Constants, Plenum Press, New York, 1975 Search PubMed.
- Y. Kanroji, Yakugaku Zasshi, 1963, 83, 424 CrossRef CAS (in Japanese).
- E. Baumgartner and M. Mijalchik, J. Colloid Interface Sci., 1991, 145, 274 CrossRef CAS.
- Stability Constants of Metal-ion Complexes, ed. L. G. Sillén and A. E. Martell, The Chemical Society, London, 1964 Search PubMed.
- O. D. Kochkodan, V. M. Kochkodan and V. K. Sharma, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2018, 53, 33 CrossRef CAS PubMed.
- H. P. Gregor, L. B. Luttinger and E. M. Loebl, J. Phys. Chem., 1955, 59, 34 CrossRef CAS.
- M. Feng, L. van der Does and A. Bantjes, J. Appl. Polym. Sci., 1995, 56, 1231 CrossRef CAS.
- A. Winston, in Bioactive Polymeric Systems: An Overview, ed. C. G. Gebelein and C. E. Carraher Jr, Plenum Press, New York, 1985, pp. 621–649 Search PubMed.
- H. Tamura, K. Takahashi and M. Nagayama, Bull. Fac. Eng., Hokkaido Univ., 1977, 85, 93 CAS (in Japanese).
- M. Yanase, M. Shinkai, H. Honda, T. Wakabayashi, J. Yoshida and T. Kobayashi, Jpn. J. Cancer Res., 1998, 89, 775 CrossRef CAS PubMed.
- F. G. Adivi, P. Hashemi and A. D. Tehrani, Polym. Bull., 2019, 76, 1239 CrossRef.
- M. Babič, D. Horák, M. Molčan and M. Timko, J. Phys. D: Appl. Phys., 2017, 50, 345002 CrossRef.
- M. Suto, Y. Hirota, H. Mamiya, A. Fujita, R. Kasuya, K. Tohji and B. Jeyadevan, J. Magn. Magn. Mater., 2009, 321, 1493 CrossRef CAS.
- T. Miyazaki, A. Iwanaga, Y. Shirosaki and M. Kawashita, Colloids Surf., B, 2019, 179, 334 CrossRef CAS PubMed.
- Z. Li, M. Kawashita, N. Araki, M. Mitsumori, M. Hiraoka and M. Doi, Mater. Sci. Eng., C, 2010, 30, 990 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |