Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new protocol for the preparation of superconducting KBi2
Huan Lia,
Yanan Wanga,
Yutaro Aokia,
Saki Nishiyamaa,
Xiaofan Yanga,
Tomoya Taguchia,
Akari Miuraa,
Ai Suzukia,
Lei Zhia,
Hidenori Gotoa,
Ritsuko Eguchia,
Takashi Kambeb,
Yen-Fa Liaoc,
Hirofumi Ishiic and
Yoshihiro Kubozono *a
*a
aResearch Institute for Interdisciplinary Science, Okayama University, Okayama 700-8530, Japan. E-mail: kubozono@cc.okayama-u.ac.jp
bDepartment of Physics, Okayama University, Okayama 700-8530, Japan
cNational Synchrotron Radiation Research Center, Hsinchu 30076, Taiwan
First published on 16th July 2020
Abstract
A superconducting KBi2 sample was successfully prepared using a liquid ammonia (NH3) technique. The temperature dependence of the magnetic susceptibility (M/H) showed a superconducting transition temperature (Tc) as high as 3.6 K. In addition, the shielding fraction at 2.0 K was evaluated to be 87%, i.e., a bulk superconductor was realized using the above method. The Tc value was the same as that reported for the KBi2 sample prepared using a high-temperature annealing method. An X-ray diffraction pattern measured based on the synchrotron X-ray radiation was analyzed using the Rietveld method, with a lattice constant, a, of 9.5010(1) Å under the space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (face-centered cubic, no. 227). The lattice constant and space group found for the KBi2 sample using a liquid NH3 technique were the same as those reported for KBi2 through a high-temperature annealing method. Thus, the superconducting behavior and crystal structure of the KBi2 sample obtained in this study are almost the same as those for the KBi2 sample reported previously. Strictly speaking, the magnetic behavior of the superconductivity was different from that of a KBi2 sample reported previously, i.e., the KBi2 sample prepared using a liquid NH3 technique was a type-II like superconductor, contrary to that prepared using a high-temperature annealing method, the reason for which is fully discussed. These results indicate that the liquid NH3 technique is effective and simple for the preparation of a superconducting KBi2. In addition, the topological nature of the superconductivity for KBi2 was not confirmed.
m (face-centered cubic, no. 227). The lattice constant and space group found for the KBi2 sample using a liquid NH3 technique were the same as those reported for KBi2 through a high-temperature annealing method. Thus, the superconducting behavior and crystal structure of the KBi2 sample obtained in this study are almost the same as those for the KBi2 sample reported previously. Strictly speaking, the magnetic behavior of the superconductivity was different from that of a KBi2 sample reported previously, i.e., the KBi2 sample prepared using a liquid NH3 technique was a type-II like superconductor, contrary to that prepared using a high-temperature annealing method, the reason for which is fully discussed. These results indicate that the liquid NH3 technique is effective and simple for the preparation of a superconducting KBi2. In addition, the topological nature of the superconductivity for KBi2 was not confirmed.
1. Introduction
Topological materials such as a topological insulators, Dirac and Weyl semimetals, and axion insulators have attracted significant attention from physicists and chemists owing to their exciting and fascinating physical properties.1–29 Gapless surface states (Dirac-like linear dispersions) in a topological insulator are protected through a time reversal symmetry, which show a spin polarization to lock the spin along a direction perpendicular to the momentum (spin-momentum locking).1–5 Here, it is noted that the bulk of a topological insulator possesses a band gap, as in a traditional insulator. The representative topological insulators are Bi1−xSbx, Bi2Se3, Bi2Te3, and Sb2Te3,6–10 with Bi2Se3 being the most popular three-dimensional (3D) topological insulator, having a single Dirac cone inside a band gap at the center of the Brillouin Zone (BZ).7,8Moreover, Dirac and Weyl semimetals have also elicited attention from physicists and chemists owing to their unique electronic structures, in which linear band crossings are in fourfold/twofold degenerated points.11–15 A Dirac semimetal appears when both time-reversal symmetry and special inversion symmetry are maintained, whereas a Weyl semimetal appears when either the time-reversal symmetry or special inversion symmetry is broken. Namely, by breaking either the time-reversal symmetry or the special inversion symmetry, one Dirac fermion will transform into two Weyl fermions with opposite chirality in the BZ. The above semimetals are categorized as type-I or type-II depending on whether the Lorentz invariance is preserved. With type-I Dirac/Weyl semimetals, massless Dirac fermions (or Weyl fermions) should emerge at the Dirac and Weyl points,15–19 whereas in type-II Dirac/Weyl semimetals, they emerge at the topologically protected crossing points of the electron and hole pockets.17,20–22 Currently, many researchers are working on the condensed matter physics of Dirac/Weyl semimetals, owing to not only the above unique electronic properties, but also interesting physical properties such as a negative magnetoresistance,23,24 chiral magnetic effects,25 and quantum anomalous Hall (QAH) effect.26 Furthermore, recent interest in the complex interplay between topology and magnetism has led to a topological quantum state, which is indicated as an axion insulator.27,28 The attempt to realize an axion insulator began in the heterostructures of QAH effect films,27 and is currently realized in a stoichiometric material.28
Alkali–Bi based compounds (ABi3 (A = Na, K, or Rb)) have recently attracted significant attention because crystalline ABi3 has been proposed as a Dirac semimetal in which bulk 3D Dirac points are protected by a crystal symmetry.15,29 Moreover, these materials were predicted to possess a nontrivial Fermi arc on the surface. In addition, various novel physical properties (giant diamagnetism, linear quantum magnetoresistance, and the QAH effect) were expected for ABi3.29 Since the above reports, the physical properties of a family of materials (alkaline and alkaline-earth metal–Bi compounds) have been investigated owing to an interest in the coupling of a Dirac semimetal and the superconductivity, i.e., under the topological nature of the superconductivity, the particular superconducting properties of BaBi3 were fully investigated.30 In 2016, the superconductivity of the KBi2 sample, which was a traditional superconductor,31 was fully studied.32 The detailed data of the superconductivity in KBi2 were collected in this study. The motivation of this study is the interplay of the topological nature and the superconductivity. In addition, the strong spin orbit coupling (SOC) may affect the superconductivity in a metal–Bi compound because Bi is a heavy atom (atomic no. 83). All metal–Bi compounds were prepared using a high-temperature annealing method,30–35 for instance, a stoichiometric amount of Bi and K were mixed and sealed in a quartz tube. The tube was heated at more than 500 °C for several hours and slowly cooled. Finally, the crystals of KBi2 were isolated using a centrifuge. Thus, the heating of metal and Bi is indispensable for the preparation of metal–Bi compounds, and isolation using a centrifuge is frequently required.
In this study, we report a new protocol for the preparation of KBi2 in which a sample heating is not employed, i.e., the liquid ammonia (NH3) technique was applied to the sample preparation of a superconducting KBi2. The KBi2 sample obtained using liquid NH3 showed a bulk superconductivity, and a clear crystalline powder was prepared. To characterize its superconductivity and crystal structure, the magnetic susceptibility (M/H) and X-ray diffraction (XRD) pattern of the obtained sample were measured; M and H refer to the magnetization and applied magnetic field, respectively. The superconducting transition temperature, Tc, and crystal structure of the KBi2 sample prepared using liquid NH3 were consistent with those of the sample prepared through a high-temperature annealing method. However, the magnetic behavior of the superconductivity was significantly different from that of the KBi2 sample reported previously,32 which is fully discussed herein.
2. Experimental
Stoichiometric amounts of K and Bi were inserted into a glass tube in an Ar-filled glove box, and the space inside the glass tube was pumped to 10−2 torr. NH3 gas was then collected in a glass tube and frozen. The K was dissolved in liquid NH3 at less than −50 °C, and K atoms were introduced into the Bi crystallites, forming KBi2. Finally, NH3 was removed through vacuum distillation, and the removal of NH3 was then carried out through pumping under 10−2 torr at room temperature. Because the KBi2 sample is quite sensitive to air, the handling of the sample was achieved without air exposure. Details of the reaction process are described later.The temperature dependence of M/H of the obtained KBi2 sample was measured using a SQUID magnetometer (Quantum Design, MPMS2 or MPMS–SQUID–VSM). The powder XRD pattern of the sample was measured at room temperature, using synchrotron radiation at BL12B2 of SPring-8, and the wavelength λ of the X-ray beam was 0.6859610 Å. The XRD pattern was analyzed using a Rietveld refinement.
3. Results and discussion
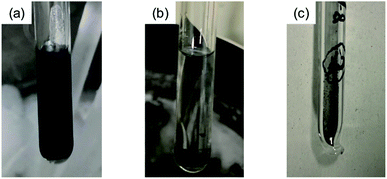
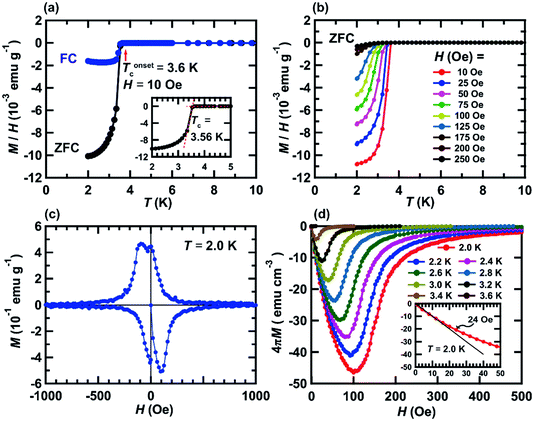
Fig. 1 shows the process of the sample preparation of KBi2. A photograph of the initial stage of the reaction between K and Bi is shown in Fig. 1(a), and K is dissolved in liquid NH3. The color of the K-dissolved NH3 solution is dark blue. After 3 days, the solution became transparent and black precipitate was obtained, as shown in Fig. 1(b). After removal of NH3, a black powder sample was obtained, as shown in Fig. 1(c). The sample was then introduced into a quartz cell and glass capillary for M/H – T and XRD measurements, respectively, in an Ar-filled glove box. Here, it should be stressed that the most important point in the sample preparation of KBi2 is to handle the sample without exposure to air to avoid a degradation of the sample.Fig. 2(a) shows M/H – T plots of the KBi2 sample obtained using the liquid NH3 technique in zero-field cooling (ZFC) and field-cooling (FC) measurement modes; the value of H was 10 Oe. As shown in the inset of Fig. 2(a), the superconducting transition temperature, Tc, was determined to be 3.56 K from the crossing point between the dropped M/H – T plot and that under a normal state. In addition, the onset superconducting transition temperature, Tonsetc, was 3.6 K (Fig. 2(a)). The values of Tc and Tonsetc determined from the M/H – T plot in ZFC mode were the same as those from the M/H – T plot in FC mode. The values of Tc and Tonsetc were the same as those of the KBi2 sample prepared using a high-temperature heating method.32 The shielding fraction at 2.0 K determined from the M/H – T plot in ZFC mode was evaluated to be 87%, indicating a bulk superconductivity.
The M/H – T plots at H = 10–250 Oe in ZFC mode are shown in Fig. 2(b), exhibiting a clear suppression of the superconducting transition by applying H. The Tonsetc value against each H is employed for depicting the H – T phase diagram (Fig. 3(c)), as described later. Fig. 2(c) shows the M – H loop of a KBi2 sample prepared using a liquid NH3 technique, recorded at 2.0 K, showing a clear hysteresis loop. This behavior indicates a type-II superconductor, which is different from that (type-I superconductor) reported previously for a KBi2 crystal,32 which was prepared using a high-temperature annealing method. However, the M – H loop of the KBi2 sample prepared using a liquid NH3 technique (Fig. 2(c)) is similar to that of a α-PdBi2 compound (type-II superconductor).36 As described later, the values of the lower critical field, Hc1, and the upper critical field, Hc2, were too low, similar to those of KBi2.32
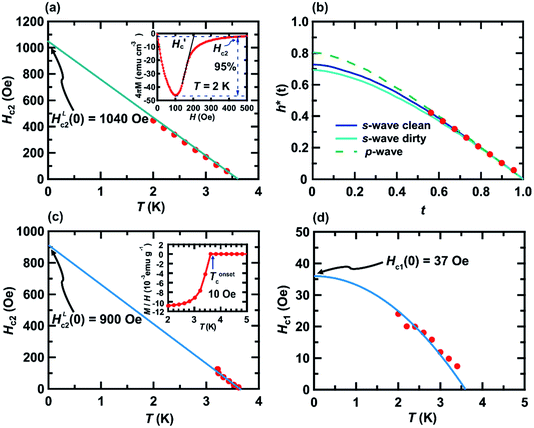 | ||
| Fig. 3 (a) Hc2 – T, (b) h* – t, (c) Hc2 – T, and (d) Hc1 – T plots of KBi2 sample prepared using liquid NH3 technique. Definition of all parameters in (a)–(d) are fully shown in the text. The method for determining the Hc2 value at each T is shown in the inset of (a). Inset of (c), the M/H – T plot at 10 Oe shown in Fig. 2(b) is provided to show the Tonsetc value, as an example. The value of Tonsetc was utilized to depict the Hc2 – T plot in (c). | ||
Fig. 2(d) shows 4πM – H plots of a KBi2 sample prepared using a liquid NH3 technique, and recorded at 2.0–3.6 K. As shown in the inset of Fig. 2(d), the lower critical field, Hc1, was evaluated to be 24 Oe at 2.0 K, whereas the Hc2 value was determined to be >500 Oe at 2.0 K; the value of Hc1 was determined from the deviation from the linear line with a slope of −1.0 (as shown in the inset of Fig. 2(d)). A previous report concluded that the KBi2 prepared using an annealing method was presumably a type-I superconductor.32 Actually, the M – H loop with a small hysteresis for the KBi2 sample32 was similar to other type-I superconductors such as YbSb2 and LaRhSi3.37–39 However, the M – H loop of KBi2 prepared in this study (Fig. 2(c)) seems to be different from those of YbSb2 and LaRhSi3.
Herein, we should discuss why a type-II like superconducting behavior can be seen in the KBi2 sample prepared using a liquid NH3 technique. The first scenario is based on a type of impurity effect, and the second is based on KBi2 prepared using liquid NH3, creating an intrinsically bulk type-II superconductor. In a type-II superconductor, 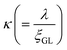 is larger than 1.0
is larger than 1.0 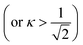 , where
, where 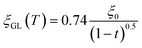 for a clean limit, and
for a clean limit, and 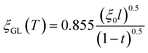 for a dirty limit, at near Tc; in addition, t = T/Tc. Here, ξGL, λ, ξ0, and l refer to the Ginzburg–Landau coherence length, magnetic penetration depth, Pippard coherence length, and mean-free path of a conduction electron, respectively.40 Moreover, the magnetic penetration depth under a dirty limit, λeff (or λ), is expressed as
for a dirty limit, at near Tc; in addition, t = T/Tc. Here, ξGL, λ, ξ0, and l refer to the Ginzburg–Landau coherence length, magnetic penetration depth, Pippard coherence length, and mean-free path of a conduction electron, respectively.40 Moreover, the magnetic penetration depth under a dirty limit, λeff (or λ), is expressed as 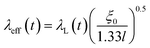 ; in addition, λL(t) is London's penetration depth. Therefore, under a dirty limit, a smaller l must lead to a smaller ξGL and greater λ, yielding a greater κ, i.e., a type-II superconductor. Actually, l is governed by the impurity, as indicated by the fact that the conversion of a type-I superconductor into a type-II superconductor is found in the In doping of Pb.41 To summarize, the polycrystalline powder sample of KBi2 obtained using a liquid NH3 technique may result in a small l because of the impurity effect. In addition, more detailed experiments such as specific heat measurements at H = 0 and H ≠ 0 will be significant for determining either a type-I or type-II superconductor, as was achieved for a single crystal of KBi2.32 In addition, the experiment results will simultaneously indicate whether the KBi2 prepared using liquid NH3 is a true bulk type-II superconductor, or the result of an impurity effect.
; in addition, λL(t) is London's penetration depth. Therefore, under a dirty limit, a smaller l must lead to a smaller ξGL and greater λ, yielding a greater κ, i.e., a type-II superconductor. Actually, l is governed by the impurity, as indicated by the fact that the conversion of a type-I superconductor into a type-II superconductor is found in the In doping of Pb.41 To summarize, the polycrystalline powder sample of KBi2 obtained using a liquid NH3 technique may result in a small l because of the impurity effect. In addition, more detailed experiments such as specific heat measurements at H = 0 and H ≠ 0 will be significant for determining either a type-I or type-II superconductor, as was achieved for a single crystal of KBi2.32 In addition, the experiment results will simultaneously indicate whether the KBi2 prepared using liquid NH3 is a true bulk type-II superconductor, or the result of an impurity effect.
Fig. 3(a) shows the value of upper critical filed, Hc2, versus T plot in which Hc2 value at each temperature was defined as H value providing 5% of the absolute value of minimum M, as indicated in inset of Fig. 3(a). In addition, the experimental M – H plots for the KBi2 sample prepared in this study using high-temperature annealing substantially followed a linear line,32 and the value of Hc (∼160 Oe)32 for the KBi2 sample at 2.0 K is close to the critical field, 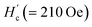 , for KBi2 at 2.0 K (see the inset of Fig. 3(a)).
, for KBi2 at 2.0 K (see the inset of Fig. 3(a)).
Fig. 3(b) shows the normalized temperature (t = T/Tc) dependence of the reduced critical field, h*, where h*(T) at any temperature (T) is given by the equation, 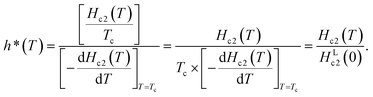 The value of Hc2(T) corresponds to that shown in Fig. 3(a), and the value of 1040 Oe was utilized for HLc2(0); here, HLc2(0) corresponds to
The value of Hc2(T) corresponds to that shown in Fig. 3(a), and the value of 1040 Oe was utilized for HLc2(0); here, HLc2(0) corresponds to 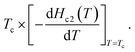 It should be noted that the dirty limit superconductivity of an s-wave corresponds to the Werthamer–Helfand–Hohenberg (WHH) model (h*(0) = 0.69).42,43 In addition, the h*(0) value should reach 0.80–0.85 in the case of p-wave pairing.44–49 As shown in Fig. 3(b), it is difficult to uniquely conclude which model follows the h* – t plot between the s-wave and p-wave. The Hc2 data at a temperature of lower than 2.0 K are required to achieve a unique conclusion. However, the p-wave Cooper pair coupling of KBi2 seems to lack a positive support. Thus, the superconductivity of KBi2 may not comprise a topologically non-trivial nature because the Cooper pair symmetry will be a p-wave (with an odd parity) in the case of a topologically non-trivial superconductor.46–49 A more detailed study, including a theoretical approach, may be indispensable for pursuing its topological nature. In this study, we further discuss the Hc2 value of the KBi2 sample within the framework of an p-wave dirty limit model.42,43 The Hc2 value was determined to be 720 Oe based on the WHH model,
It should be noted that the dirty limit superconductivity of an s-wave corresponds to the Werthamer–Helfand–Hohenberg (WHH) model (h*(0) = 0.69).42,43 In addition, the h*(0) value should reach 0.80–0.85 in the case of p-wave pairing.44–49 As shown in Fig. 3(b), it is difficult to uniquely conclude which model follows the h* – t plot between the s-wave and p-wave. The Hc2 data at a temperature of lower than 2.0 K are required to achieve a unique conclusion. However, the p-wave Cooper pair coupling of KBi2 seems to lack a positive support. Thus, the superconductivity of KBi2 may not comprise a topologically non-trivial nature because the Cooper pair symmetry will be a p-wave (with an odd parity) in the case of a topologically non-trivial superconductor.46–49 A more detailed study, including a theoretical approach, may be indispensable for pursuing its topological nature. In this study, we further discuss the Hc2 value of the KBi2 sample within the framework of an p-wave dirty limit model.42,43 The Hc2 value was determined to be 720 Oe based on the WHH model, 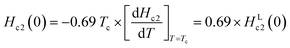 , using an HLc2(0) value of 1040 Oe (Fig. 3(a)).
, using an HLc2(0) value of 1040 Oe (Fig. 3(a)).
Moreover, although we tried to determine the value of Hc2 at each temperature, Hc2(T), from Tonsetc at each H (see Fig. 2(b)), and to depict an h* – t plot, it was difficult to evaluate Tonsetc at high H. Therefore, an h* – t plot was not created. Based on the WHH model42,43 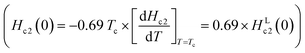 , the Hc2(0) value was determined to be 620 Oe from a linear fitting at near Tc for the Hc2 – T plot (HLc2(0) = 900 Oe in Fig. 3(c)). An Hc2(0) value of 620 Oe may be reasonable owing to an agreement with 720 Oe, as evaluated from the Hc2 – T plot shown in Fig. 3(a). As shown in Fig. 3(d), the lower critical field (Hc1(0)) was 37 Oe, as determined from the fitting of the conventional formula,
, the Hc2(0) value was determined to be 620 Oe from a linear fitting at near Tc for the Hc2 – T plot (HLc2(0) = 900 Oe in Fig. 3(c)). An Hc2(0) value of 620 Oe may be reasonable owing to an agreement with 720 Oe, as evaluated from the Hc2 – T plot shown in Fig. 3(a). As shown in Fig. 3(d), the lower critical field (Hc1(0)) was 37 Oe, as determined from the fitting of the conventional formula, 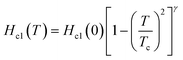 , in which γ is ∼1.0. This empirical formula was employed for an evaluation of Hc1 of various superconducting materials including BaBi3, CeRu2, CaIr2, and SrIr2.30,50–52
, in which γ is ∼1.0. This empirical formula was employed for an evaluation of Hc1 of various superconducting materials including BaBi3, CeRu2, CaIr2, and SrIr2.30,50–52
In addition, we evaluated ξGL of KBi2 to have a value of 73 nm using the expression,41 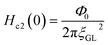 and the real Hc2(0) to be 620 Oe, where Φ0 is 2.0678 × 10−7 G cm2. By contrast, the λ value was evaluated as 220 nm using the formula,
and the real Hc2(0) to be 620 Oe, where Φ0 is 2.0678 × 10−7 G cm2. By contrast, the λ value was evaluated as 220 nm using the formula,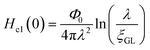 ,40 and a Hc1(0) value of 37.0 Oe. The value of
,40 and a Hc1(0) value of 37.0 Oe. The value of 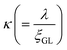 was ∼3, which is much larger than
was ∼3, which is much larger than 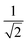 , indicating a type-II superconductor, as described above.
, indicating a type-II superconductor, as described above.
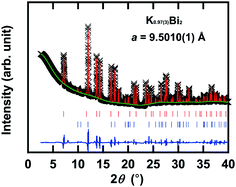
Fig. 4 shows an XRD pattern of the KBi2 sample prepared using a liquid NH3 technique, along with the XRD pattern reproduced through a Rietveld refinement with a space group of Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (face-centered cubic, no. 227). The lattice constant, a, was determined to be 9.5010(1) Å, which is consistent with that (9.5017(1) Å) from a Le Bail analysis (not shown) and a = 9.5233(2) Å of KBi2 prepared using a high-temperature annealing method.32 This phase can be assigned to KBi2. Table 1 lists the atomic coordinates of KBi2 as determined through a Rietveld refinement. The actual stoichiometry of KBi2 determined by a Rietveld refinement was K0.97(3)Bi2, which was evaluated from the occupancy of K and Bi atoms (see Table 1). The actual stoichiometry is the same as the nominal stoichiometry. In a Rietveld refinement, an additional minor phase that can be assigned to Bi (R
m (face-centered cubic, no. 227). The lattice constant, a, was determined to be 9.5010(1) Å, which is consistent with that (9.5017(1) Å) from a Le Bail analysis (not shown) and a = 9.5233(2) Å of KBi2 prepared using a high-temperature annealing method.32 This phase can be assigned to KBi2. Table 1 lists the atomic coordinates of KBi2 as determined through a Rietveld refinement. The actual stoichiometry of KBi2 determined by a Rietveld refinement was K0.97(3)Bi2, which was evaluated from the occupancy of K and Bi atoms (see Table 1). The actual stoichiometry is the same as the nominal stoichiometry. In a Rietveld refinement, an additional minor phase that can be assigned to Bi (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (rhombohedral, no. 166) and a = 4.53440(8) Å and c = 11.8310(5) Å in a hexagonal structure) was included. The lattice constants obtained are the same as those of the Bi metal.53 In the Rietveld refinement, the experimental and calculated XRD patterns (Fig. 4) showed a good fit. The values of Rp and wRp were 1.55% and 2.87%, respectively.
m (rhombohedral, no. 166) and a = 4.53440(8) Å and c = 11.8310(5) Å in a hexagonal structure) was included. The lattice constants obtained are the same as those of the Bi metal.53 In the Rietveld refinement, the experimental and calculated XRD patterns (Fig. 4) showed a good fit. The values of Rp and wRp were 1.55% and 2.87%, respectively.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 227, choice 2). Lattice constant a = 9.5010(1) Å
m (no. 227, choice 2). Lattice constant a = 9.5010(1) Å
| Occupancy | x | y | z | B (Å2) | |
|---|---|---|---|---|---|
| 16c Bi | 1.0 | 0 | 0 | 0 | 0.72(5) |
| 8b K | 0.97(3) | 0.375 | 0.375 | 0.375 | 1.5(6) |
Herein, we discuss whether NH3 is included in the KBi2 sample prepared using a liquid NH3 technique. The a value of KBi2 prepared in this study is consistent with that of the KBi2 sample (a = 9.5233(2) Å) prepared using a high-temperature annealing method.32 For instance, the c value of ammoniated K-doped FeSe ((NH3)yKxFeSe) was 14.84–16.2 Å,54,55 which is larger than that of KxFeSe, i.e., 14.0367(7) Å, prepared using a high-temperature annealing method.56 As a consequence, the KBi2 sample prepared using the liquid NH3 technique is exactly KBi2 without NH3. It can be concluded that the liquid NH3 technique can exactly yield a KBi2 sample in the same manner as a high-temperature annealing method.
A schematic representation of the crystal structure of KBi2 depicted with the atomic coordinates determined from the Rietveld refinement for an XRD pattern (Fig. 4) is shown in Fig. 5. As shown in the projection from the [111] direction, the Bi atoms form a Kagomé net, which is the same as a previously reported crystal structure.32 The atoms of K are linked to the Bi atoms in a 3D network, as shown in Fig. 5. The crystal structure obtained is the same as that reported previously.32 As a consequence, throughout this study, it was demonstrated that the liquid NH3 technique can easily produce a crystalline powder sample of KBi2 in the same manner as used in a high-temperature annealing method. The value of Tc and the crystal structure are consistent with those previously reported.32
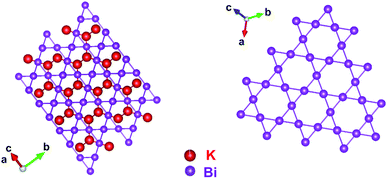 | ||
| Fig. 5 Schematic representation of crystal structure of KBi2, which was determined using a Rietveld refinement (Fig. 4(b)). | ||
4. Conclusions
A new protocol for the preparation of superconducting KBi2 was completely verified through this study, i.e., a liquid NH3 technique is available for the preparation of a crystalline powder of superconducting KBi2. The Tc value of KBi2 prepared in this study was consistent with that reported previously.32 The crystal structure was also the same as that described elsewhere.32 Regardless of the preparation when using liquid NH3, the inclusion of NH3 or amide was not confirmed in the KBi2 sample. Actually, the magnetic properties of a polycrystalline KBi2 sample prepared using a liquid NH3 technique were different from those of the single-crystal KBi2 prepared through a high-temperature annealing method.32 The reason why the former is a type-II superconductor and the latter is type-I remains questionable, although pursuing it may be extremely significant from the chemistry viewpoint of material synthesis as well as the physics perspective of a superconductor.Because the liquid NH3 technique is extremely effective and simple in terms of the preparation of KBi2, various metal–Bi compounds may easily be fabricated using the technique, leading to an elucidation of their superconducting behavior and quantum/topological properties. Admittedly, this type of material is interesting owing to the suggested topological nature such as a Dirac semimetal.29,30 At the present stage, the topological nature of the superconducting KBi2 sample was definitely not demonstrated. The pursuit of a topological nature in metal–Bi compounds must be continued; our approach regarding the preparation of a Bi-rich compound using a liquid NH3 technique is currently being used to produce various alkali–Bi compounds such as LiBi, NaBi, RbBi2, and CsBi2 as superconductors.57
To summarize, the development of an effective, simple, and exact preparation of metal–Bi compounds as described in this paper is significant for a detailed study on the superconducting behaviors of their compounds. The next target is to synthesize new superconducting metal–Bi compounds that have yet to be prepared using a high-temperature annealing method.
Author contributions
Huan Li, Yanan Wang, and Yutaro Aoki prepared the KBi2 sample using a liquid NH3 technique. In addition, Huan Li, Yanan Wang, and Yutaro Aoki carefully measured the magnetic behavior of the KBi2 sample, under cooperation with Takashi Kambe. Huan Li, Yanan Wang, Xiaofan Yang, Tomoya Taguchi, Akari Miura, Ai Suzuki, Lei Zhi, and R. Eguchi measured the X-ray diffraction patterns (XRDs) of the KBi2 sample and analyzed the data using Le Bail and Rietveld analysis methods. The XRD measurement system using synchrotron radiation was designed by Yen-Fa Liao and Hirofumi Ishii, which allowed the suitable results of this study to be achieved. Yoshihiro Kubozono supervised all of the measurements and data analyses conducted in this study and prepared the manuscript under discussions with Hidenori Goto and Huan Li. Saki Nishiyama suggested the idea of this study, and the preliminary experiment was conducted by Yoshihiro Kubozono. All coauthors finally reviewed the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This study was partly supported by a Grant-in-Aid (17K05500, 18K04940, 18K18736, and 19H02676) from MEXT, and by the Program for Promoting the Enhancement of Research Universities. The XRD measurements at SPring-8 were supported by 2018B4140 and 2019A4131.References
- C. L. Kane and E. J. Mele, Phys. Rev. Lett., 2005, 95, 146802 CrossRef CAS PubMed.
- C. L. Kane and E. J. Mele, Phys. Rev. Lett., 2005, 95, 226801 CrossRef CAS PubMed.
- J. E. Moore and L. Balents, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 121306 CrossRef.
- L. Fu and C. L. Kane, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 045302 CrossRef.
- M. Z. Hasan and C. L. Kane, Rev. Mod. Phys., 2010, 82, 3045 CrossRef CAS.
- A. A. Schafgans, K. W. Post, A. A. Taskin, Y. Ando, X.-L. Qi, B. C. Chapler and D. N. Basov, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 195440 CrossRef.
- H. Zhang, C.-X. Liu, X.-L. Qi, X. Dai, Z. Fang and S.-C. Zhang, Nat. Phys., 2009, 5, 438 Search PubMed.
- Y. Xia, D. Qian, D. Hsieh, L. Wray, A. Pal, H. Lin, A. Bansil, D. Grauer, Y. S. Hor, R. J. Cava and M. Z. Hasan, Nat. Phys., 2009, 5, 398 Search PubMed.
- Y. L. Chen, J. G. Analytis, J.-H. Chu, Z. K. Liu, S.-K. Mo, X. L. Qi, H. J. Zhang, D. H. Lu, X. Dai, Z. Fang, S. C. Zhang, I. R. Fisher, Z. Hussain and Z.-X. Shen, Science, 2009, 325, 178 CrossRef CAS PubMed.
- D. Hsieh, Y. Xia, D. Qian, L. Wray, F. Meier, J. H. Dil, J. Osterwalder, L. Patthey, A. V. Fedorov, H. Lin, A. Bansil, D. Grauer, Y. S. Hor, R. J. Cava and M. Z. Hasan, Phys. Rev. Lett., 2009, 103, 146401 CrossRef CAS PubMed.
- X. Wan, A. M. Turner, A. Vishwanath and S. Y. Savrasov, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 205101 CrossRef.
- A. A. Burkov, M. D. Hook and L. Balents, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 235126 CrossRef.
- H. Weng, X. Dai and Z. Fang, J. Phys.: Condens. Matter, 2016, 28, 303001 CrossRef PubMed.
- A. Bansil, H. Lin and T. Das, Rev. Mod. Phys., 2016, 88, 021004 CrossRef.
- Z. K. Liu, B. Zhou, Y. Zhang, Z. J. Wang, H. M. Weng, D. Prabhakaran, S.-K. Mo, Z. X. Shen, Z. Fang, X. Dai, Z. Hussain and Y. L. Chen, Science, 2014, 343, 864 CrossRef CAS PubMed.
- M. Neupane, S.-Y. Xu, R. Sankar, N. Alidoust, G. Bian, C. Liu, I. Belopolski, T.-R. Chang, H.-T. Jeng, H. Liu, A. Bansil, F. Chou and M. Z. Hasan, Nat. Commun., 2014, 5, 3786 CrossRef CAS PubMed.
- R. C. Xiao, P. L. Gong, Q. S. Wu, W. J. Lu, M. J. Wei, J. Y. Li, H. Y. Lv, X. Luo, P. Tong, X. B. Zhu and Y. P. Sun, Phys. Rev. B, 2017, 96, 075101 CrossRef.
- H. Weng, C. Fang, Z. Fang, B. A. Bernevig and X. Dai, Phys. Rev. X, 2015, 5, 011029 Search PubMed.
- S.-Y. Xu, I. Belopolski, N. Alidoust, M. Neupane, G. Bian, C. Zhang, R. Sankar, G. Chang, Z. Yuan, C.-C. Lee, S.-M. Huang, H. Zheng, J. Ma, D. S. Sanchez, B. Wang, A. Bansil, F. Chou, P. P. Shibayev, H. Lin, S. Jia and M. Z. Hasan, Science, 2015, 349, 613 CrossRef CAS PubMed.
- A. A. Soluyanov, D. Gresch, Z. Wang, Q. Wu, M. Troyer, X. Dai and B. A. Bernevig, Nature, 2015, 527, 495 CrossRef CAS PubMed.
- K. Deng, G. Wan, P. Deng, K. Zhang, S. Ding, E. Wang, M. Yan, H. Huang, H. Zhang, Z. Xu, J. Denlinger, A. Fedorov, H. Yang, W. Duan, H. Yao, Y. Wu, S. Fan, H. Zhang, X. Chen and S. Zhou, Nat. Phys., 2016, 12, 1105 Search PubMed.
- K. Zhang, M. Yan, H. Zhang, H. Huang, M. Arita, Z. Sun, W. Duan, Y. Wu and S. Zhou, Phys. Rev. B, 2017, 96, 125102 CrossRef.
- J. Xiong, S. K. Kushwaha, T. Liang, J. W. Krizan, M. Hirschberger, W. Wang, R. J. Cava and N. P. Ong, Science, 2015, 350, 413 CrossRef CAS PubMed.
- T. Liang, Q. Gibson, M. N. Ali, M. Liu, R. J. Cava and N. P. Ong, Nat. Mater., 2014, 14, 280 CrossRef PubMed.
- A. A. Zyuzin and A. A. Burkov, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 115133 CrossRef.
- C.-X. Liu, P. Ye and X.-L. Qi, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 235306 CrossRef.
- M. Mogi, M. Kawamura, R. Yoshimi, A. Tsukazaki, Y. Kozuka, N. Shirakawa, K. S. Takahashi, M. Kawasaki and Y. Tokura, Nat. Mater., 2017, 16, 516 CrossRef CAS PubMed.
- C. Liu, Y. Wang, H. Li, Y. Wu, Y. li, J. Li, K. He, Y. Xu, J. Zhang and Y. Wang, Nat. Mater., 2020, 19, 522–527 CrossRef CAS PubMed.
- Z. Wang, Y. Sun, X.-Q. Chen, C. Franchini, G. Xu, H. Weng, X. Dai and Z. Fang, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 195320 CrossRef.
- N. Haldolaarachchige, S. K. Kushwaha, Q. Gibson and R. J. Cava, Supercond. Sci. Technol., 2014, 27, 105001 CrossRef.
- J. M. Reynolds and C. T. Lane, Phys. Rev., 1950, 79, 405 CrossRef CAS.
- S. Sun, K. Liu and H. Lei, J. Phys.: Condens. Matter, 2016, 28, 085701 CrossRef PubMed.
- B. T. Mattias and J. K. Hulm, Phys. Rev., 1952, 87, 799 CrossRef.
- K. Nishimura, T. Yasukawa and K. Mori, Phys. B, 2003, 329–333, 1399–1400 CrossRef CAS.
- K. Górnicka, S. Gutowska, M. J. Winiarski, B. Wiendlocha, W. Xie, R. J. Cava and T. Klimczuk, Chem. Mater., 2020, 32, 3150–3159 CrossRef.
- Y. Zhou, X. Chen, C. An, Y. Zhou, L. Ling, J. Yang, C. Chen, L. Zhang, M. Tian, Z. Zhang and Z. Yang, Phys. Rev. B, 2019, 99, 054501 CrossRef.
- L. L. Zhao, S. Lausberg, H. Kim, M. A. Tanatar, M. Brando, R. Prozorov and E. Morosan, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 214526 CrossRef.
- Y. Yamaguchi, S. Waki and K. Mitsugi, J. Phys. Soc. Jpn., 1987, 56, 419–420 CrossRef CAS.
- V. K. Anand, A. D. Hillier and D. T. Adroja, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 064522 CrossRef.
- M. Thinkham, Introduction to Superconductivity, McGraw-Hill Inc., New York, 1975 Search PubMed.
- C. Kittel, Introduction to Solid State Physics, John Willey & Sons, Inc., New York, 2005 Search PubMed.
- E. Helfand and N. R. Werthamer, Phys. Rev., 1966, 147, 288 CrossRef CAS.
- N. R. Werthamer, E. Helfand and P. C. Hohenberg, Phys. Rev., 1966, 147, 295 CrossRef CAS.
- K. Scharnberg and R. A. Klemm, Phys. Rev. B: Condens. Matter Mater. Phys., 1980, 22, 5233 CrossRef CAS.
- K. Maki, E. Puchkaryov, G.-F. Wang and H. Won, Chin. J. Phys., 2000, 38, 386 CAS.
- K. Kirshenbaum, P. S. Syers, A. P. Hope, N. P. Butch, J. R. Jeffries, S. T. Weir, J. J. Hamlin, M. B. Maple, Y. K. Vohra and J. Paglione, Phys. Rev. Lett., 2013, 111, 087001 CrossRef PubMed.
- Y. Zhou, X. Chen, R. Zhang, J. Shao, X. Wang, C. An, Y. Zhou, C. Park, W. Tong, L. Pi, Z. Yang, C. Zhang and Y. Zhang, Phys. Rev. B, 2016, 93, 144514 CrossRef.
- T. He, X. Yang, T. Terao, T. Uchiyama, T. Ueno, K. Kobayashi, J. Akimitsu, T. Miyazaki, T. Nishioka, K. Kimura, K. Hayashi, N. Happo, H. Yamaoka, H. Ishii, Y.-F. Liao, H. Ota, H. Goto and Y. Kubozono, Phys. Rev. B, 2018, 97, 104503 CrossRef CAS.
- T. He, X. Yang, T. Taguchi, T. Ueno, K. Kobayashi, J. Akimitsu, H. Yamaoka, H. Ishii, Y.-F. Liao, H. Ota, H. Goto, R. Eguchi, K. Terashima, T. Yokoya, H. O. Jeschke, X. Wu and Y. Kubozono, Phys. Rev. B, 2019, 100, 094525 CrossRef CAS.
- M. Hedo, Y. Inada, E. Yamamoto, Y. Haga, Y. Onuki, Y. Aoki, T. D. Matsuda, H. Sato and S. Takahashi, J. Phys. Soc. Jpn., 1998, 67, 272–279 CrossRef CAS.
- N. Haldolaarachchige, Q. Gibson, L. M. Schoop, H. Luo and R. J. Cava, J. Phys.: Condens. Matter, 2015, 27, 185701 CrossRef PubMed.
- R. Horie, K. Horigane, S. Nishiyama, M. Akimitsu, K. Kobayashi, S. Onari, T. Kambe, Y. Kubozono and J. Akimitsu, J. Phys.: Condens. Matter, 2020, 32, 175703 CrossRef CAS PubMed.
- B. P. Cucka and C. S. Barrett, Acta Crystallogr., 1962, 15, 865 CrossRef.
- T. Ying, X. Chen, G. Wang, S. Jin, X. Lai, T. Zhou, H. Zhang, S. Shen and W. Wang, J. Am. Chem. Soc., 2013, 135, 2951–2954 CrossRef CAS PubMed.
- L. Zheng, M. Izumi, Y. Sakai, R. Eguchi, H. Goto, Y. Takabayashi, T. Kambe, T. Onji, S. Araki, T. C. Kobayashi, J. Kim, A. Fujiwara and Y. Kubozono, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 094521 CrossRef.
- J. Guo, S. Jin, G. Wang, S. Wang, K. Zhu, T. Zhou, M. He and X. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 180520 CrossRef.
- H. Li, Y. Wang, A. Suzuki, T. Taguchi, H. Goto, R. Eguchi and Y. Kubozono, to be submitted.
| This journal is © The Royal Society of Chemistry 2020 |