Open Access Article
Open Access ArticleVisible-light promoted three-component tandem reaction to synthesize difluoromethylated oxazolidin-2-imine†
Kun Bao‡
ab,
Jun Wei‡a,
Huihui Yanab and
Rong Sheng *a
*a
aCollege of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310058, People's Republic of China. E-mail: shengr@zju.edu.cn
bCollaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals, Zhejiang University of Technology, Hangzhou, 310014, P. R. China
First published on 9th July 2020
Abstract
An effective synthetic method to achieve difluoromethylated oxazolidin-2-imine has been developed via visible-light promoted three-component tandem reaction of aryl allylamines, 2-BTSO2CF2H (BT = Benzothiazole) and isocyanates. This method features mild reaction conditions and good functional group tolerance, and the reaction mechanism was confirmed by experiments and interpreted by quantum chemical calculations.
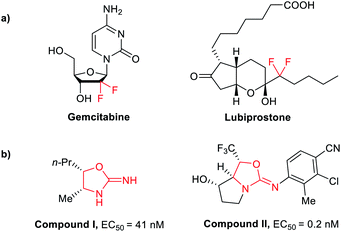
Difluoromethyl containing compounds have been widely used in the fields of pharmaceutics and agrochemistry because of their unique physical, chemical, and biological properties.1 Generally, the –CF2 moiety can act as a bioisostere of the –CH2 or –CO group and the CF2H is isopolar to the –OH and –SH group, which can be used as a lipophilic hydrogen bond donor.2,3 In recent years, much attention has been focused on the synthesis of difluoromethylated molecules.4 FDA approved medicine Gemcitabine and Lubiprostone both contain difluoromethyl group (Fig. 1a).
 | ||
| Fig. 1 (a) Structure of Gemcitabine and Lubiprostone (b) selected bioactive oxazolidin-2-imine derivatives. | ||
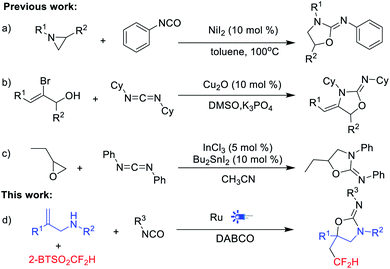
The oxazolidin-2-imine skeleton exists in many biologically active compounds. For example, compound I is a novel nitric oxide synthase inhibitor with an EC50 value of 41 nM,5 and compound II acts as a very potent selective androgen receptor modulator (SARM) with a sub-nanomolar EC50 value (Fig. 1b).6 In addition, substituted oxazolidin-2-imines are critical synthetic intermediates that can be transformed into a variety of other structures, and represent a useful chiral auxiliary for the asymmetric alkylations.7 The high value of oxazolidin-2-imine in medicinal chemistry and organic chemistry has driven successive efforts to develop effective synthetic methods. Shinichi Saito's group reported the cycloaddition of aziridines with isocyanates catalyzed by NiI2 to furnish oxazolidin-2-imines in good yields (Scheme 1a).8 Beifuss's group revealed intermolecular 1,2-addition/intramolecular N-vinylation of 2-bromo-2-propen-1-ols and dicyclohexyl carbodiimide for the synthesis of oxazolidin-2-imines (Scheme 1b).9 Recently, Bu2SnI2–InCl3 catalysed cycloaddition of propylene oxide with diphenyl carbodiimide was reported by Ikuya Shibata's group10 (Scheme 1c).
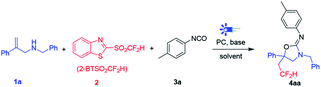
Radical-triggered cascade multi-component reactions serve as an ideal strategy in the synthesis of heterocyclic scaffolds, owing to forming multiple bonds under a single set of reaction conditions.11 Particularly, the visible-light promoted radical addition–cyclization has demonstrated its synthetic utility in the construction of compounds with various scaffold,12 including 2-oxazolidinones,13 coumarin,14 phenanthridines,15 and so on. We envisioned that the combination of difluoromethyl moiety with oxazolidin-2-imine scaffold may exert potential use in medicinal chemistry and other related fields. As part of our work on the visible-light mediated radical difluoromethylation,15 we try to explore a novel and convenient method to construct HCF2-containing oxazolidin-2-imines via visible-light promoted three-component tandem reaction (Scheme 1d).
We began our investigation by using N-benzyl-2-phenylprop-2-en-1-amine 1a, 1-isocyanato-4-methylbenzene 3a as the model substrate and 2-BTSO2CF2H (Hu's reagent) 2 as the difluoromethylation reagent. Initially, DABCO was used as base and target compound 4aa can be produced in 29% yield using Ru(bpy)3Cl2·6H2O as photocatalyst (Table 1, entry 1). Further screening of various bases, including Na2CO3, TMEDA, DBN, TEA and DIPEA, cannot get the 4aa, indicating the DABCO was the optimal base for this reaction (Table 1, entries 2–6). Then, a series of solvents, including DMF, acetonitrile, NMP, acetone and toluene were screened, and the results showed that DMF was more suitable for this reaction than others (Table 1, entries 7–11). In addition, the replacement of catalyst Ru(bpy)3Cl2·6H2O with fac-Ir(ppy)3 decreased the yield slightly (28%) (Table 1, entry 12). Interestingly, the decrease of temperature and increase the equivalence of 3a can improve the yields of 4aa obviously (entries 13 and 14). Therefore, the best result was obtained with a combination of 2 with 3a (2.5 equiv.), DABCO (1 mmol, 2.0 equiv.), Ru(bpy)3Cl2·6H2O (5 mol%) in DMF (2.0 mL) were irradiated with a 6 W blue LED for 16 h at 8∼10 °C to furnish 4aa in 75% yield (entry 15).
| Entry | Base | Solvent | 3a/equiv. | Yieldb (%) |
|---|---|---|---|---|
| a Reaction condition: 1a (0.5 mmol, 1.0 equiv.), 2 (0.6 mmol, 1.2 equiv.), PC (0.025 mmol, 5 mol%), base (1 mmol, 2.0 equiv.), solvent (2.0 mL), irradiated with a 6 W blue LED for 10 h at room temperature under N2 atmosphere.b Isolated yields.c fac-Ir(ppy)3 instead of Ru(bpy)3Cl2·6H2O.d In the ice bath (8–10 °C) for 16 h. | ||||
| 1 | DABCO | DMSO | 1.2 | 29 |
| 2 | Na2CO3 | DMSO | 1.2 | 0 |
| 3 | TMEDA | DMSO | 1.2 | 0 |
| 4 | DBN | DMSO | 1.2 | Trace |
| 5 | TEA | DMSO | 1.2 | Trace |
| 6 | DIPEA | DMSO | 1.2 | Trace |
| 7 | DABCO | DMF | 1.2 | 35 |
| 8 | DABCO | CH3CN | 1.2 | 26 |
| 9 | DABCO | NMP | 1.2 | 15 |
| 10 | DABCO | Acetone | 1.2 | 25 |
| 11 | DABCO | Toluene | 1.2 | Trace |
| 12c | DABCO | DMF | 1.2 | 28 |
| 13d | DABCO | DMF | 1.2 | 43 |
| 14d | DABCO | DMF | 1.8 | 61 |
| 15d | DABCO | DMF | 2.5 | 75 |
| 16d | DABCO | DMF | 3.0 | 74 |
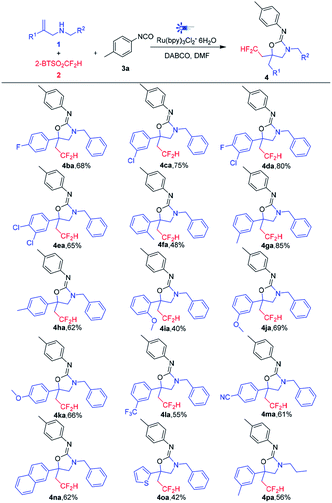
With the optimized reaction conditions in hand, the substrate scope of aryl allylamines 1 was extensively investigated. As shown in Table 2, the reaction tolerates various substituents on the phenyl ring, including halogen and alkyl (4ba∼4ha). Substrates containing both electron-donating (–OCH3) and electron-withdrawing (–CF3, –CN) groups on phenyl can proceeded this reaction smoothly and give CF2H-containing oxazolidin-2-imines in good yields (4ja, 4la). In addition, 2-naphthyl allylamine 1n and 2-thienyl allylamine 1o also can produce the corresponding CF2H-containing compounds 4na and 4oa in moderate to good yields. The relative low yields of 4fa (48%) and 4ia (40%) indicated the obvious steric effect in this reaction. More interestedly, the success of approaching 4pa in moderate yield with 1p (N-allyl-2-phenylprop-2-en-1-amine) showed the good tolerance for various substituents on amine in this reaction.
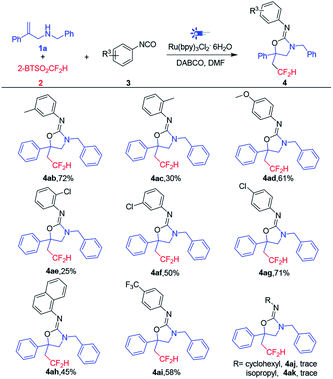
Subsequently, the substrate scope of isocyanates was also investigated (Table 3). The substituents on the phenyl ring were first evaluated, and the results showed that both electron-donating (Me, OMe) and electron-withdrawing groups (Cl, CF3) on para or meta position were tolerated with good yields of corresponding products (50–72%). While similar to aryl allylamines 1, the steric hindrance of substituent affects the yields of products obviously, for example, the ortho-methyl phenyl isocyanate 3c and ortho-chloro phenyl isocyanate 3e only gave poor yields of 4ac (30%) and 4ae (25%), respectively. The 1-naphthyl isocyanate 3h also can produce the corresponding CF2H-containing compound 4ah in 45% yield. However, the cyclohexyl-isocyanate 3j and isopropyl-isocyanate 3k cannot get target compounds in this reaction, which indicates the alkyl isocyanates are not suitable for this transformation.
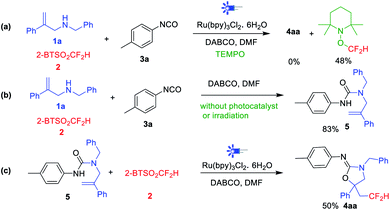
To confirm the reaction mechanism, a radical inhibition experiment was conducted. In the presence of the radical scavenger 2,2,6,6-tetramethyl-1-piperidinyl-oxy (TEMPO), the transformation was absolutely inhibited, and the TEMPO-CF2H adduct product was detected by 19F NMR spectroscopy analysis in 48% yield (Scheme 2a), which indicating a free radical pathway might be involved in this reaction. Then, condition-control experiments showed that both irradiation and photocatalyst are indispensable for this transformation (Scheme 2b). Moreover, when 1a, 2, 3a and DABCO were mixed in DMF without photocatalyst and irradiation, the urea 5 was achieved in 83% yield, and further reaction of urea 5 with 2 in the standard conditions gave 50% yield of difluoromethylated oxazolidin-2-imine 4aa (Scheme 2c). This result further confirmed that urea 5 is the key intermediate for this type of transformation. Finally, Stern–Volmer luminescence studies demonstrated that the excited state *[Ru]2+ was quenched by DABCO (Epox = +0.69 V vs. SCE, E[Ru(II)*/Ru(I)] = +0.77 V) instead of allylamines 1a, or 2-BTSO2CF2H 2, or isocyanates 3a, and the quenching effect of DABCO increased with its concentration (see the ESI for details†).
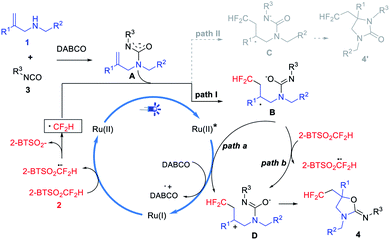
According to above studies, a plausible reaction mechanism is proposed in Scheme 3. The excited state *[Ru]2+ is formed by visible light irradiation and reduced by DABCO to get [Ru]+, which triggers 2-BTSO2CF2H to generate HCF2 radical. Then, the addition of HCF2 radical to A (formed by the condensation of 1 and 3 in situ) produces benzylic radical B, which is further oxidized by excited Ru(II) species to form carbocation D accompanying with concurrent regeneration of [Ru]+ (Path a). It is also possible that B reacts with 2-BTSO2CF2H through a SET process to generate carbocation D (Path b). Subsequent cyclization of D to achieve difluoromethylated product 4.
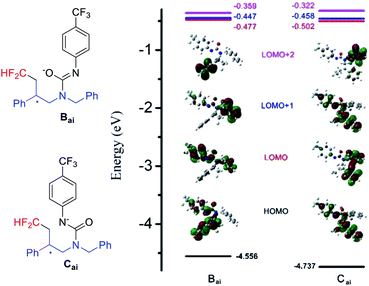
During the mechanism analysis, we found that the intermediate A and HCF2 radical may experience two cyclization pathways to achieve difluoromethylated oxazolidin-2-imine (4) or difluoromethylated imidazolinone (4′), respectively (Scheme 3). The good selectivity of this transformation prompts us to carry out quantum chemical calculations to explain the inner mechanism.16 4ai, with a single crystal structure (see the ESI for details†), was employed as the model substrate and state-of-the-art computational methods SMD-B3LYP-D3(BJ)/BS1//B3LYP/BS1 was used for calculation. As it shown in Fig. 2, the transition energies of Bai and Cai in different molecular orbitals, HOMO, LUMO, LUMO+1 and LUMO+2 were calculated. The values of intermediate Bai were −4.556 eV, −0.477 eV, −0.447 eV and −0.359 eV, respectively. And which for intermediate Cai were −4.737 eV, −0.502 eV, −0.458 eV and −0.322 eV, respectively (Fig. 2).
In photocatalytic process, it is well known that the lower energy level gap (HOMOs–LUMOs), the easier molecular will be excited.17 Therein, the energy level gaps between LOMO+2/LOMO+1/LOMO and HOMO of intermediate Bai was 4.197 eV, 4.109 eV, 4.079 eV, respectively, which were lower than that of intermediate Cai (4.415 eV, 4.279 eV, 4.235 eV, respectively). In addition, this can also be explained by a reaction between the hardest oxygen nucleophilic center with the hard carbocationic center.18 These results indicating that Path I is more favorable than Path II.
Conclusions
Overall, the one-pot synthesis of difluoromethylated oxazolidin-2-imine has been achieved by visible-light promoted three-component tandem reaction using aryl allylamines, 2-BTSO2CF2H, and isocyanates. With regard to the widely available materials, mild conditions, good tolerance of substituents on allylamines and isocyanates, this new method demonstrated potential application in medicinal chemistry, agricultural chemistry and other related fields. The quantum chemical calculations also explained the inner mechanism of this transformation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21672187), Zhejiang Natural Science Foundation (LZ17H300002).Notes and references
- (a) H. Jiang, M. Xu, W. Lu, W. Tian, W. Wan, Y. Chen, H. Deng, S. Wu and J. Hao, Direct Gem-Difluoromethylenation of sp3-Hybridized Carbon Center Through Copper-mediated Radical/Radical Cross-Coupling for the Construction of a CH2–CF2 Linkage, Chem. Commun., 2015, 51, 15756 RSC; (b) H. Xu, D. Wang, Y. Chen, W. Wan, H. Deng, K. Ma, S. Wu, J. Hao and H. Jiang, Copper-Catalyzed Gem-Difluoromethylenation of C (sp2)–H Bonds of Alkenes, Org. Chem. Front., 2017, 4, 1239 RSC; (c) J. Hu, T. J. Pu, Z. W. Xu, W. Y. Xu and Y. S. Feng, Cadmium Sulfide Quantum-Dot-Photocatalyzed Cascade Cyclization of Functionalized Difluoromethyl Chlorides with Unactivated Olefins, Adv. Synth. Catal., 2019, 361, 708 CrossRef CAS.
- (a) C. Ni, L. Zhu and J. Hu, Advances in Transition-Metal-Mediated Di-and Monofluoroalkylations, Acta Chim. Sin., 2015, 73, 90 CrossRef CAS; (b) N. A. Meanwell, Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design, J. Med. Chem., 2011, 54, 2529 CrossRef CAS PubMed.
- F. Scheidt, J. Neufeld, M. Schafer, C. Thiehoff and R. Gilmour, Catalytic Geminal Difluorination of Styrenes for the Construction of Fluorine-rich Bioisosteres, Org. Lett., 2018, 20, 8073 CrossRef CAS PubMed.
- (a) X. J. Tang and W. R. Dolbier, Efficient Cu-catalyzed atom transfer radical addition reactions of fluoroalkylsulfonyl chlorides with electron-deficient alkenes induced by visible light, Angew. Chem., Int. Ed., 2015, 54, 4246 CrossRef CAS PubMed; (b) N. Noto, T. Koike and M. Akita, Metal-free di- and tri-fluoromethylation of alkenes realized by visible-light-induced perylene photoredox catalysis, Chem. Sci., 2017, 8, 6375 RSC; (c) P. Xu, S. Guo, L. Wang and P. P. Tang, Silver-catalyzed oxidative activation of benzylic C-H bonds for the synthesis of difluoromethylated arenes, Angew. Chem., Int. Ed., 2014, 53, 5955 CrossRef CAS PubMed; (d) F. Pan, G. B. Boursalian and T. Ritter, Palladium-catalyzed decarbonylative difluoromethylation of acid chlorides at room temperature, Angew. Chem., Int. Ed., 2018, 57, 16871 CrossRef CAS PubMed.
- S. Ueda, H. Terauchi, A. Yano, M. Ido, M. Matsumoto and M. Kawasaki, 4,5-Disubstituted-1,3-oxazolidin-2-imine Derivatives: A New Class of Orally Bioavailable Nitric Oxide Synthase Inhibitor, Bioorg. Med. Chem. Lett., 2004, 14, 313 CrossRef CAS PubMed.
- A. A. Nirschl, Y. Zou, S. R. Krystek, J. C. Sutton, L. M. Simpkins, J. A. Lupisella, J. E. Kuhns, R. Seethala, R. Golla, P. G. Sleph, B. C. Beehler, G. J. Grover, D. Egan, A. Fura, V. P. Vyas, Y. X. Li, J. S. Sack, K. F. Kish, Y. An, J. A. Bryson, J. Z. Gougoutas, J. DiMarco, R. Zahler, J. Ostrowski and L. G. Hamann, N-Aryl-oxazolidin-2-imine Muscle Selective Androgen Receptor Modulators Enhance Potency through Pharmacophore Reorientation, J. Med. Chem., 2009, 52, 2794 CrossRef CAS PubMed.
- T. N. Le, Q. P. B. Nguyen, J. N. Kim and T. H. Kim, 5,5-Dimethyl-2-phenylamino-2-oxazoline as an Effective Chiral Auxiliary for Asymmetric Alkylations, Tetrahedron Lett., 2007, 48, 7834 CrossRef CAS.
- M. Takeshi, A. Isao, K. Takako, M. Hyuma and S. Shinichi, [3 + 2] Cross-Coupling Reactions of Aziridines with Isocyanates Catalyzed by Nickel(II) Iodide, Org. Lett., 2006, 8, 379 CrossRef PubMed.
- H. Weischedel, D. Schmidt, J. Conrad and U. Beifuss, Formation of Substituted 2-Iminooxazolidines via Intermolecular 1,2-Addition/Intramolecular N-vinylation Using 3-Substituted-2-Bromo-2-Propen-1-ols as Substrates, Tetrahedron, 2018, 74, 6426 CrossRef CAS.
- I. Suzuki, A. Imakuni, A. Baba and I. Shibata, Catalytic Annulation of Epoxides with Heterocumulenes by the Indium–Tin System, Molecules, 2018, 23, 782 CrossRef.
- (a) R. C. Cioc, E. Ruijter and R. V. A. Orru, Multicomponent reactions: advanced tools for sustainable organic synthesis, Green Chem., 2014, 16, 2958 RSC; (b) G. Bosica, R. Abdilla and K. Demanuele, Revisiting the Betti Synthesis: Using a Cheap, Readily Available, Recyclable Clay Catalyst under Solventless Conditions, Eur. J. Org. Chem., 2018, 44, 6127 CrossRef.
- (a) X. Ren and Z. Lu, Visible light promoted difunctionalization reactions of alkynes, Chin. J. Catal., 2019, 40, 1003 CrossRef CAS; (b) T. Koike and M. Akita, A versatile strategy for difunctionalization of carbon–carbon multiple bonds by photoredox catalysis, Org. Chem. Front., 2016, 3, 1345 RSC.
- Z. Yin, J. Ye, W. Zhou, Y. Zhang, L. Ding, Y. Gui, S. Yan, J. Li and D. G. Yu, Oxy-Difluoroalkylation of Allylamines with CO2 via Visible-Light Photoredox Catalysis, Org. Lett., 2018, 20, 190 CrossRef CAS PubMed.
- W. Fu, M. Zhu, G. Zou, C. Xu, Z. Wang and B. Ji, Visible-Light-Mediated Radical Aryldifluoroacetylation of Alkynes with Ethyl Bromodifluoroacetate for the Synthesis of 3-Difluoroacetylated Coumarins, J. Org. Chem., 2015, 80, 4766 CrossRef CAS PubMed.
- (a) J. Wei, D. Gu, S. Wang, J. Hu, X. Dong and R. Sheng, Visible-Light-Mediated Radical Arylthiodifluoromethylation of Isocyanides with Fluorinated 2-Pyridyl Sulfones, Org. Chem. Front., 2018, 5, 2568 RSC; (b) J. Rong, L. Deng, P. Tan, C. Ni, Y. Gu and J. Hu, Radical Fluoroalkylation of Isocyanides with Fluorinated Sulfones by Visible-Light Photoredox Catalysis, Angew. Chem., Int. Ed., 2016, 55, 2743 CrossRef CAS PubMed.
- (a) T. Kleine, R. Frohlich, B. Wibbeling and E. U. Wurthwein, Cyclization Reactions of 3,4-Diazaheptatrienyl Metal Compounds. Pyridines from an Anionic Analogue of the Fischer Indole Synthesis: Experiment and Theory, J. Org. Chem., 2011, 76, 4591 CrossRef CAS PubMed; (b) Y. Li, Y. Zhao, Y. Yang, W. Shi and X. Fan, Revelation solvent effects: excited state hydrogen bond and proton transfer of 2-(benzo[d]thiazol-2-yl)-3-methoxynaphthalen-1-ol, Org. Chem. Front., 2019, 6, 2780 RSC.
- H. Shahroosvand and M. Eskandari, Ultrafast Interfacial Charge Transfer from LUMO+1 in Ruthenium(II) Polypyridyl Quinoxaline-Sensitized Solar Cells, Dalton Trans., 2017, 47, 561 RSC.
- W. j. Fu, X. Han, M. Zhu, C. Xu, Z. Q. Wang, B. M. Ji, X. Q. Hao and M. P. Song, Visible-light-mediated radical oxydifluoromethylation of olefinic amides for the synthesis of CF2H-containing heterocycles, Chem. Commun., 2016, 52, 13413 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1998157. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra04729e |
| ‡ These authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2020 |