Open Access Article
Open Access ArticleStudy of near-infrared light-induced excitation of upconversion nanoparticles as a vector for non-viral DNA delivery†
Jen-Hsuan Wang a,
Hsin-Yu Chena,
Ching-Cheng Chuangab and
Jung-Chih Chen
a,
Hsin-Yu Chena,
Ching-Cheng Chuangab and
Jung-Chih Chen *abc
*abc
aInstitute of Biomedical Engineering, National Chiao Tung University, HsinChu, 30010 Taiwan, Republic of China. E-mail: george@nctu.edu.tw
bDepartment of Electrical and Computer Engineering, National Chiao Tung University, HsinChu, 30010 Taiwan, Republic of China
cDepartment of Biological Science and Technology, National Chiao Tung University, HsinChu, 30010 Taiwan, Republic of China
First published on 11th November 2020
Abstract
Clinical requirements have necessitated the development of biomedical nanomaterials that can be implanted into tissues or bodies. Physiological regulation can be achieved in these nanomaterials through external light. The combination of nanomaterials with infrared optics can be termed optogenetics. The low autofluorescence of upconversion nanoparticles (UCNPs) has several applications in the biological field. For optogenetics applications, UCNPs with high fluorescence performance and photostability can solve the penetration depth problem. NaYF4:Yb,Tm nanocrystals with controllable sizes, shapes, and compositions were synthesized using a rapid coprecipitation method in organic solvent. UCNPs using single crystal nanoparticles provide higher chemical stability than those using amorphous phase. However, because UCNPs are usually capped with hydrophobic ligands, it is particularly important to prepare biocompatible UCNPs with specific molecular recognition capabilities. Surface modification and subsequent functionalization are essential for the application of inorganic nanomaterials in the biological environment and are arousing increasing research interest. Due to the high biocompatibility and high loading of materials, mesoporous silica and amine groups were selected as the best candidates. Expression of plasmid DNA in vivo and transfection efficiency were determined by fluorescence microscopy and flow cytometry. The MTT assay was used to evaluate the particle biocompatibility; the results showed that UCNP@mSiO2 has great biocompatibility. Additionally, at neutral pH, the cell surface is negatively charged. Therefore, the surface is functionalized with amino groups and can be electrostatically bound to DNA. Finally, UCNP@mSiO2-NH2 as a vector was applied in live cells by loading DNA; according to the results, DNA-UCNPs were successfully transfected in the primary cells, and NaYF4:Yb,Tm@mSiO2-NH2-DNA were observed to have good transfection efficiency by flow cytometry. It is expected that this work will provide a different method from the traditional adenovirus method and improve the immune response and side effects caused by adenovirus.
Introduction
Lanthanide-doped rare-earth upconversion is a popular research field because nanocrystals exhibit unique properties; they can convert low-energy near-infrared (NIR) light to high-energy visible light,1–5 particularly when the nanocrystals are doped with sensitizers and activators. The sensitizers can absorb the NIR light, and the energy is transferred to the activators.6–8 NIR light has better penetration depth than visible light and can be used as a non-invasive treatment. UCNPs can be excited by the high penetration depth of NIR light; also, they possess a low-autofluorescence background9–11 and high biocompatibility12–14 for various biological applications.15–19One of the most advanced technologies in neuroscience, optogenetics combines optical and genetic techniques.20–25 Using this technique, a light can be used to control a single neuron cell. Optogenetics technology can be spatially regulated to single-cell size and millisecond-level precision, unlike current techniques that only use voltage and current to control the neuron membrane potential. For example, during the process of isolation of light-sensitive ion channel proteins from cyanobacteria,26–28 ion channel channelrhodopsin-2 (ChR2) dominates through blue light at the 470 nm band. However, visible light cannot attain the required penetration depth to achieve non-invasive isolation. Therefore, it is necessary to implant an invasive fiber as a light source to excite the channel proteins. However, the installation of most invasive systems is highly complex. Therefore, the visible-light penetration depth problem should be resolved. Nanoparticles with special features provide opportunities and challenges. The nanoparticles currently in clinical trials represent promising non-invasive technology for appropriate applications (e.g., thermal heating29 and DNA carriers30) and can provide better penetration ability than visible light.31–34 However, NIR cannot regulate light-sensitive channel proteins. Therefore, we developed nanocrystals that can convert NIR light into blue light to isolate channel proteins using a non-invasive method.
Most DNA transfection methods use liposomes or nanoparticles as a vector. After the DNA is delivered into the cell, the liposome or nanoparticle has no effect. However, we used UCNPs as a vector to carry DNA with ChR2 (a channel protein) into the cell. When the DNA is successfully transfected, a light-sensitive protein ion channel will be formed on the cell membrane. When irradiated with 808 nm NIR light, the UCNPs will emit blue light, causing ChR2 on the cell membrane to be stimulated by blue light to open non-specific cation channels, causing cell depolarization.35,36
NaYF4 is the main body of a common upconversion nanoparticle matrix material; it has two different NaYF4 phase structures (hexagonal phase (β-phase) and cubic phase (α-phase)). The different phases of the UCNPs can be switched by annealing between the cubic phase and the hexagonal phase. Hexagonal NaYF4-based upconversion nanocrystals with sensitizers and activators are doped into the host to achieve efficient photon upconversion.37–39 We used X-ray diffraction (XRD) and transmission electron microscopy (TEM) to identify the structure of the particle; it is a hexagonal phase structure. Yb3+ is commonly selected as a sensitizer to absorb the excitation energy from NIR light. Finally, the energy is transferred to the activator through the energy transfer upconversion method.40 Theoretically, increasing the sensitizer concentration can enhance the absorption of energy from the excitation source, thus increasing the fluorescence intensity. However, with increasing Yb3+ concentration, the size of the nanocrystals can increase to 100 nm. Because of the nucleation rate,41–43 the Yb3+ concentration-induced increase in the size of the nanocrystals hinders the upconversion of the nanocrystals. Therefore, small-sized UCNPs with excellent lifetimes are preferred, especially in the optogenetics field. The coprecipitation method is a typical treatment to obtain various morphologies and particle sizes in different organic solvents. This method is suitable to synthesize NaYF4 rare-earth inorganic materials with different ion concentrations.44,45 NaYF4 nanocrystals are synthesized using the coprecipitation method in organic solvents. Thus, the surface of the nanocrystals is covered by hydrophobic groups (such as oleic acid and 1-octadecene), which restrict their optogenetic and cell applications.
Surface hydrophilic modification is necessary before biological application, and nanocrystal stability is crucial. The use of mesoporous silica-coated lanthanide-doped upconversion nanoparticles (UCNP@mSiO2) results in simultaneous gene delivery and optogenetic application of nanocomposites.46,47 This improves the surface properties of the nanocomposites for functionalization, bioconjugation, and dispersibility in water.30 Mesoporous silica with monodispersed sizes, uniform shapes, and high biocompatibility can minimize fluorescence quenching with surface absorbent organic ligands.48
After Yb3+ absorbs 980 nm near-infrared light, it uses energy conversion to cause Tm3+ to absorb energy and emit blue light. Photoluminescence spectrometry (PL) and UV/Vis spectrophotometry (UV-Vis) are used to observe the influence of the light emission spectra and absorption spectra of mesoporous silica-coated lanthanide-doped upconversion nanoparticles.
Jie Yu's research team systematically studied the biological distribution, excretion, and toxicity of PEI@UCNPs through different administration routes. Intravenous injection of PEI@UCNPs is mainly concentrated in the spleen, and its excretion rate is slower than those of other organs; meanwhile, after intramuscular injection of PEI@UCNPs, the UCNPs are continuously removed from the liver and spleen within 30 days. This study shows that PEI@UCNPs have low toxicity, reduced accumulation in the RES (reticuloendothelial system), and gradual excretion through feces after intravenous injection. In terms of stability testing, PEI@UCNPs were shaken at 37 °C for 24 h, and no change in the crystal phase was observed. Therefore, it was concluded that PEI@UCNPs have good stability in the physiological environment.49
Currently, the most popular means of delivering optogenetics tools is viral vectors.50 When the virus successfully transfects the target cell, the light-sensitive channel protein is expressed. However, one serious problem with adenovirus transfection is the immune response in the body, which will eliminate the virus-infected cells, resulting in relatively short gene expression. Therefore, when an organism needs to express a specific gene, a very large amount of adenovirus must be transfected, which is highly likely to cause a serious inflammatory reaction or side effect.51 Compared with viral systems, non-viral systems show the advantages of low toxicity, low immunogenicity, and the ability to deliver larger DNA fragments multiple times. However, improving transfection efficiency is a substantial challenge for non-viral transfection vectors.30,52 Owing to these factors, non-viral transfection is currently popular. However, transfection efficiency is a great challenge to non-viral transfection vectors.
In this study, we systemically report the fluorescence mechanisms of various rare-earth ion molar ratios. The details of the sizes and morphologies of the synthesized single crystals of β-NaYF4 were verified through transmission electron microscopy (TEM) with selected area diffraction. The performance of single-crystal β-NaYF4 was examined. Additionally, mesoporous silica and amino groups were synthesized for application in optogenetics in the future; NaYF4:Yb,Tm@mSiO2-NH2 upconversion nanoparticles were used and correlated with pcDNA3.1/hChR2(H134R)-mCherry. Biocompatibility in the primary cell was examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method, and the efficiency of the DNA-UCNPs delivery system in primary cells was studied through fluorescence microscopy and flow cytometry. The current research demonstrates the potential of UCNPs to deliver DNA safely and efficiently.
Materials and methods
Materials
Yttrium(III) acetate hydrate (99.9%, ALFA Aesar), ytterbium(III) acetate hydrate (99.9%, ALFA Aesar), thulium(III) acetate hydrate (99.9%, ALFA Aesar), oleic acid (90%, Sigma-Aldrich), 1-octadecene (90%, ACROS), ammonium fluoride (98%, Merck), sodium hydroxide (98%, Merck), cyclohexane (99%, JT Baker), tetraethyl orthosilicate (99.0%, Sigma-Aldrich), methanol (99.9%, Fisher), hexadecyltrimethylammonium chloride (≥98.0%, Sigma-Aldrich), hydrochloric acid (37%, Sigma-Aldrich), polyethylenimine (branched, Mw 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 30% w/v aq., Polysciences), phosphate buffered saline (powder, pH 7.4, Sigma-Aldrich), trypsin–EDTA (0.5%, no phenol red, Thermo Fisher Scientific), and ethanol (99.5%, Sigma-Aldrich) were used in the experiments. All chemicals were of analytical grade and were used as received without further purification.
000, 30% w/v aq., Polysciences), phosphate buffered saline (powder, pH 7.4, Sigma-Aldrich), trypsin–EDTA (0.5%, no phenol red, Thermo Fisher Scientific), and ethanol (99.5%, Sigma-Aldrich) were used in the experiments. All chemicals were of analytical grade and were used as received without further purification.
Synthesis of NaYF4:Yb,Tm as a core structure
Core UCNPs were synthesized using the coprecipitation method. In this study, 4 mL aqueous solution containing yttrium acetate hydrate (88%), ytterbium acetate hydrate (20%), and thulium acetate hydrate (2%) was mixed with oleic acid and 1-octadecene and stirred for 30 min. The mixture was heated to 150 °C for 1 h to form a yellow solution. After cooling the solution, 2 mL of sodium hydroxide and 7.9 mL of ammonium fluoride were added to the solution and stirred for 1 h. Then, the solution was heated at 110 °C for 20 min to remove methanol. Next, the solution was heated to 310 °C for 25 min under argon atmosphere. Finally, the solution was collected through centrifugation for 10 min and stocked in cyclohexane.Synthesis of NaYF4:Yb,Tm@NaYF4:Yb,Nd as a core/shell structure
Core/shell UCNPs were synthesized using the coprecipitation method. In this study, 3.4 mL aqueous solution containing yttrium acetate hydrate (88%), ytterbium acetate hydrate (20%), and neodymium acetate hydrate (20%) was mixed with oleic acid and 1-octadecene and stirred for 30 min. The mixture was heated to 150 °C for 1 h. After cooling the solution, 1.7 mL of sodium hydroxide, 6.8 mL of ammonium fluoride, and all of NaYF4:Yb,Tm solution (around 230–240 μg) were added to the solution and stirred for 1 h. Then, the solution was heated at 110 °C for 20 min to remove methanol. Next, the solution was heated to 305 °C for 90 min under argon atmosphere. Finally, the solution was collected through centrifugation for 10 min and stocked in cyclohexane.Synthesis of the mesoporous silica shell of the UCNPs
Mesoporous shells of the UCNPs were synthesized using tetraethyl orthosilicate (TEOS) and hexadecyltrimethylammonium chloride (CTAC) as templates. UCNPs with cyclohexane (1 mL) mixed with 8 mL CTAC (0.06 M) was stirred for 1 h. The solution was heated to 80 °C for 30 min to remove cyclohexane and sonicated for 30 min with 30 mL deionized water. The resultant solution was mixed with 1.4 mL ethanol, and the pH value was adjusted to pH 10 by adding ammonia water. TEOS was added dropwise to the solution at 35 °C for 24 h. Finally, the solution was collected through centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and stocked in deionized water. Hydrochloric acid was used to remove the surfactant CTAC to form mesoporous silica shells, and the as-synthesized UCNP@mSiO2 (10 mg) was transferred to 40 mL methanol containing 200 μL hydrochloric acid at 50 °C for 24 h. Finally, the resultant solution was collected through centrifugation at 10
000 rpm for 10 min and stocked in deionized water. Hydrochloric acid was used to remove the surfactant CTAC to form mesoporous silica shells, and the as-synthesized UCNP@mSiO2 (10 mg) was transferred to 40 mL methanol containing 200 μL hydrochloric acid at 50 °C for 24 h. Finally, the resultant solution was collected through centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and dispersed in ethanol.
000 rpm for 10 min and dispersed in ethanol.
Synthesis of the amino groups of UCNP@mSiO2
UCNP@mSiO2 was sonicated for 20 min with 20 mL ethanol. 2 mL of polyethylenimine was added, and the mixture was heated to 80 °C for 3 h. Finally, the resultant solution was collected through centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and dispersed in ethanol.
000 rpm for 10 min and dispersed in ethanol.
Synthesis of UCNP@mSiO2-NH2-DNA
UCNP@mSiO2-NH2 and plasmid DNA were mixed in phosphate buffered saline (PBS) (1 mg UCNPs per mL PBS) at different ratios (w/w) at room temperature for 1 h.Isolation of the primary cardiomyocytes
The hearts of new-born of Sprague-Dawley (SD) rats (about 2–3 days old) were removed. The hearts were placed in trypsin and shaken at 80 rpm at 4 °C for 15–20 h. The trypsin was removed, 10% medium was added, and the mixture was shaken at 75 rpm for 10 min at 37 °C. The medium was removed. HBSS containing collagenase II was added and shaken for 1 minute. The cells were pipetted and filtered with a filter, then centrifuged at 500 rpm at 4 °C for 5 min. 10% medium was added, and the cells were placed in T75 flasks in a 37 °C incubator for 1 h. The cells were centrifuged at 500 rpm at 4 °C for 5 min. The supernatant was removed, 10% medium was added, the cells were counted, the cell concentration was adjusted to about 2 × 105 cells per mL, and the cells were added to a 24-well plate and placed in the 37 °C incubator.Transfection of primary cells
pcDNA3.1/hChR2(H134R)-mCherry was used for the transfection of cardiomyocyte cells with the amino-modified UCNPs. The transfection of primary cells with the UCNP@mSiO2-NH2-DNA complex solutions remained at the UCNPs/DNA weight ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). Transfection was cultured in an incubator with 5% CO2 at 37 °C for 48 h. At the end of the incubation, the cells were washed with PBS. The cells were imaged immediately to determine the mCherry fluorescence under a fluorescence microscope (Olympus).
1 (w/w). Transfection was cultured in an incubator with 5% CO2 at 37 °C for 48 h. At the end of the incubation, the cells were washed with PBS. The cells were imaged immediately to determine the mCherry fluorescence under a fluorescence microscope (Olympus).
Characterization
Field emission electron microscopy was conducted using a JEOL 7401 field emission electron microscope at an acceleration voltage of 5 kV. High-resolution TEM (HRTEM) was conducted using a JEOL 2010 at an acceleration voltage of 200 kV. Powder X-ray diffraction (XRD) data were recorded using a Bruker D8 Advance X-ray diffractometer with Cu kα radiation (λ = 1.5406 Å). The binding energy was analyzed using a Thermo Fisher Scientific ESCALAB Xi+ X-ray photon electron and Auger electron spectroscope. UV-visible-near-infrared spectra were measured using a ChromTech CT-2800 equipped with a Hitachi F-7000 fluorescence spectrophotometer to record the upconversion fluorescence.Cytotoxicity measurements
The in vitro cytotoxicities of UCNP@mSiO2 were tested through the MTT assay. Primary cardiac myoblast cells were seeded in 96-well cell culture plates (4 × 104 cells per well). UCNP@mSiO2 concentrations of 1000, 750, 500, 250, and 0 μg mL−1 in 100 μL fresh medium were added to the wells, and the 96-well plate was cultured in an incubator with 5% CO2 at 37 °C for 48 h. After washing with the previous solution, the MTT reagent (0.5 mg mL−1 in 100 μL fresh medium) was added to the plate, which was cultured for another 4 h in an incubator with 5% CO2 at 37 °C. Finally, the supernatant was removed and 100 μL dimethyl sulfoxide was added to the wells. The MTT absorbance (λ = 570 nm) of each well was measured by the reader. The cell metabolic activity was computed using the optical contrast-to-control value.Flow cytometry analysis
The cells after transfection were washed with PBS and trypsinized by trypsin–EDTA, than centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The cellular transfection effectiveness of recombinant plasmid pcDNA3.1/hChR2(H134R)-mCherry was quantified using flow cytometry (ImageStreamX Mark II) under 561 nm at 200 mW. Flow cytometry measurements were performed with an ISX MkII using ISX500 software.
000 rpm. The cellular transfection effectiveness of recombinant plasmid pcDNA3.1/hChR2(H134R)-mCherry was quantified using flow cytometry (ImageStreamX Mark II) under 561 nm at 200 mW. Flow cytometry measurements were performed with an ISX MkII using ISX500 software.
Data analysis
Data analysis was carried out using Microsoft® Excel and SPSS. Statistical significance was determined using the paired sample t-test, with P < 0.05 deemed significant.Results and discussion
Preparation and characterization of β-NaYF4 nanocrystals: in order to examine the application possibilities of UCNPs in optogenetics and biological imaging, oleic acid was used as a stabilizer and was synthesized by the thermal co-precipitation method to observe whether the NaYF4:Yb,Tm nanocrystals are β-NaYF4:Yb,Tm with a pure hexagonal structure at different Yb3+ and Tm3+ concentrations. The final products indicated that the transfer of α-NaYF4 (cubic phase) to β-NaYF4 (hexagonal phase) nanocrystals requires additional Na+ and F− ion doping with high reaction temperature. Therefore, NaOH and NH4F were used as precursors.To verify the appropriate delta between the sensitizer and the activator, we compared the intervals of the blue emission intensities at different Yb3+ and Tm3+ concentrations. The intensity for 478 nm from NaYF4:Yb,Tm (20%/2%) increased 1.5 and 1.6 times from those of NaYF4:Yb,Tm (15%/2%) and NaYF4:Yb,Tm (25%/2%), respectively (Fig. 1). Fig. 1 shows the 450 nm and 475 nm emissions resulting from the 1D2 → 3F4 and 1G4 → 3H6 transitions, respectively. This was attributed to the increase in the amount of the Tm3+ doping, which led to a serious cross-relaxation effect among the activators that resulted in the collapse of the 450 nm and 475 nm emissions.
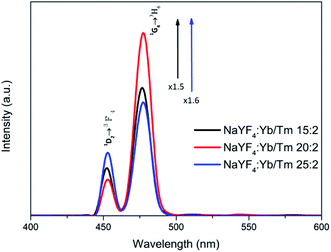 | ||
| Fig. 1 Photoluminescence spectra of UCNPs with different Yb3+ and Tm3+ concentrations in cyclohexane under 980 nm excitation (1 W cm−2). | ||
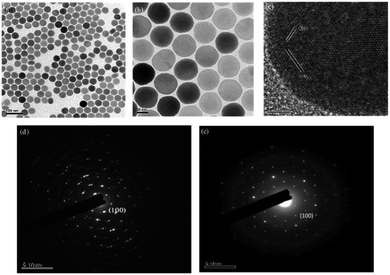
From the SEM results, it can be seen that when Y3+ ion is added to 88 mol% (Fig. 2a and b), the particle sizes and shapes are uniform and the hexagonal edges of the nanocrystals appear and sharpen. The luminous efficiency of this structure is much higher than that of the cubic phase (α-phase). The size of the particles will also affect the final light emission efficiency and subsequent DNA transfection efficiency. The crystal size is about 45 nm. After modification, nanoparticles of this size can more easily enter the cell through endocytosis for transfection. The crystal lattice of the nanoplates was revealed through HRTEM to be hexagonal phase with the P63/m space group and interplanar spacings of 5.2 and 2.0 Å, corresponding to the (100) and (201) planes, respectively; this revealed the highly crystalline nature of the NaYF4:Yb,Tm nanocrystals (Fig. 2c). The selection area electron diffraction (SAED) pattern (Fig. 2e) also confirmed the crystal structure. The SAED pattern illustrated a regular arrangement of single crystals. Nanoparticles with 78% Y3+ were defined as polycrystalline (Fig. 2d). Thus, upconversion nanoparticles can be synthesized with 88 mol% of Y3+ ion without impurity.
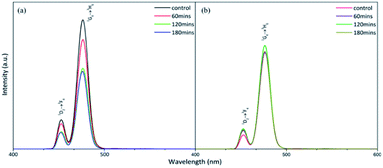
To compare the fluorescence of the crystalline and polycrystalline materials, we used a photoluminescence spectrometer (PL) to record the patterns (Fig. 3). The results demonstrated that the fluorescence of NaYF4:Yb,Tm (78%/20%/2%) decayed considerably after 60 minute irradiation by a 980 nm diode laser (Fig. 3a). In contrast, the fluorescence of NaYF4:Yb,Tm (88%/20%/2%) did not decrease significantly after being irradiated with a 980 nm diode laser for 180 min (Fig. 3b). It can be seen that high-quality pure hexagonal NaYF4:Yb,Tm nanocrystals can extend the luminous lifetime of UCNPs.
In order to effectively avoid the thermal damage caused by the 980 nm NIR irradiation of the organism, the surface of the NaYF4:Yb,Tm upconversion nanoparticles was coated with a layer of neodymium (Nd) element in this experiment so that the upconversion nanoparticles can be excited with an 808 nm laser to reduce thermal damage. However, this method increased the particle size of our upconversion nanoparticles to 95 nm. Fang Lu's team confirmed that the uptake of cells with a particle size of 50 nm is about 5 times that of a carrier with a particle size of 100 nm.53 Thus, large size is not conducive to subsequent transfection efficiency. In the future, we hope to directly synthesize NaYF4:Yb,Tm,Nd upconversion nanoparticles, which can not only reduce thermal damage but also improve transfection efficiency.
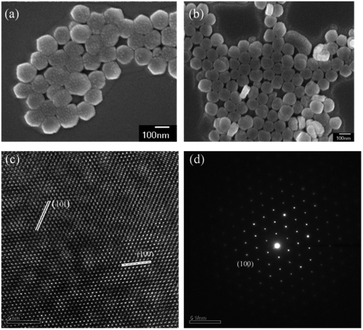
The SEM observation of the NaYF4:Yb,Tm@NaYF4:Yb,Nd upconversion nanoparticles showed a particle size of about 95 nm (Fig. 4a and b) and hexagonal phase with the P63/m space group and interplanar spacings of 5.2 and 2.9 Å, which correspond to the (100) and (101) planes, respectively; this revealed the highly crystalline nature of the NaYF4:Yb,Tm@NaYF4:Yb,Nd upconversion nanoparticles (Fig. 4c). The selection area electron diffraction (SAED) pattern (Fig. 4d) also confirmed the crystal structure. The SAED pattern illustrated a regular arrangement of single crystals. Thus confirmed again that upconversion nanoparticles can be synthesized with 88 mol% of Y3+ ion without impurity.
At present, there are three common modification methods for upconversion nanoparticles: the silylation method of coating the particle surface with a silica shell layer, the ligand exchange modification of substituting oleic acid, and the addition of other hydrophilic surface functional groups after oleic acid can all modify hydrophobic particles into hydrophilic particles, which can effectively reduce the surface quenching of UCNPs and increase their biocompatibility. In this experiment, we used surface modification to modify hydrophobic UCNPs into hydrophilic UCNPs.54,55
The mesoporous silica shell structure obtained by the sol–gel two-phase transfer strategy via surfactant modification can be obviously seen in Fig. 5. The oleic acid-capped NaYF4 nanocrystal cores reacted with CTAB and TEOS. The TEM observation of the NaYF4:Yb,Tm@mSiO2 upconversion nanoparticles shows a particle size of about 50 nm (Fig. 5a), and the TEM observation of the NaYF4:Yb,Tm@NaYF4:Yb,Nd@mSiO2 upconversion nanoparticles shows a particle size of about 100 nm (Fig. 5b).
The nanosphere materials were successfully transferred from organic to aqueous solutions. Images indicate that the nanocrystals exhibit uniform distributions, sizes, and shapes. The aggregation was attributed to the concentration of the surfactant and the volume of TEOS. Furthermore, the decrease of the CTAB concentration resulted in NaYF4 nanocrystal aggregation. However, after silication, the luminescence decreased. Thus, the organic groups influenced the luminescence intensity of the nanocrystals through photon relaxation.
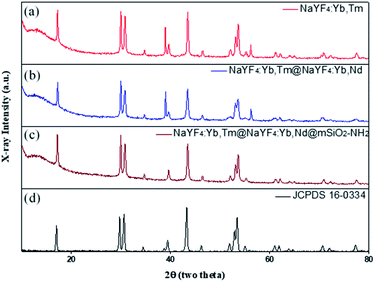
The crystal structure of NaYF4 was determined through XRD. The powder XRD patterns in Fig. 6 are consistent with JCPDS card# 16-0334; this result proved that the diffraction patterns indicated pure β-NaYF4 hexagonal phase and no other impurity diffraction peak. Therefore, it can be determined that the upconversion nanoparticles synthesized in this way are all single-crystal structures.
 | ||
| Fig. 6 XRD patterns of (a) NaYF4:Yb,Tm, (b) NaYF4:Yb,Tm@NaYF4:Yb,Nd, (c) NaYF4:Yb,Tm@NaYF4:Yb,Nd@mSiO2-NH2, and (d) hexagonal phase (JCPDS standard card no. 16-0334). | ||
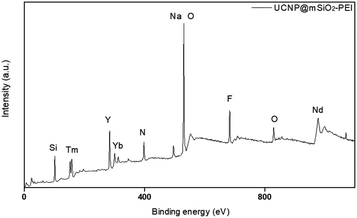
In addition, in order for the upconversion nanoparticles to successfully link with DNA, we modified a layer of an amine group on the outer layer of UCNP@SiO2 to endow the surface of the upconversion nanoparticles with a positive charge and successfully link the negatively charged DNA. The element composition of an amine group was measured through XPS (Fig. 7). Also, XRD was used to confirm the crystal structures of the upconversion nanoparticles with modified amine groups. It can be confirmed from Fig. 6 that the modification process did not affect the crystal structure of NaYF4.
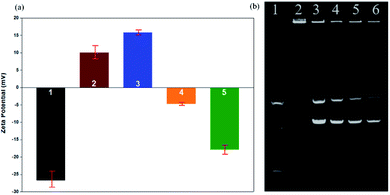
We used zeta potential measurements to confirm the potentials of the modified upconversion nanoparticles. From Fig. 8a, we can see that the upconversion nanoparticles were negatively charged after being modified on the silicon shell. After modifying the amine groups, the positive charge of the mesoporous silica shell was higher than that of the silicon shell. In addition, because DNA is negatively charged, UCNPs modified with DNA are slightly negatively charged. As a result of the zeta potential changing from a positive potential to a negative potential, it was confirmed that DNA was successfully linked to the UCNPs.
Gel electrophoresis is affected by charge, molecular weight, and isoelectric point. In the gel electrophoresis, as shown in Fig. 8b, when the UCNPs/DNA ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 10
3 or 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/w), there were two bright bands in addition to the first bright band; we judged these bands to be free circular DNA and supercoiled DNA, which indicates that some DNA was not connected to the UCNPs. When the UCNP/DNA ratio was 10
4 (w/w), there were two bright bands in addition to the first bright band; we judged these bands to be free circular DNA and supercoiled DNA, which indicates that some DNA was not connected to the UCNPs. When the UCNP/DNA ratio was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w), these two bright bands did not appear. We judged that no free DNA flowed out from the sample well, and only DNA-UCNPs remained in the sample well. Therefore, the optimal ratio of UCNPs/DNA is 10
2 (w/w), these two bright bands did not appear. We judged that no free DNA flowed out from the sample well, and only DNA-UCNPs remained in the sample well. Therefore, the optimal ratio of UCNPs/DNA is 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (w/w).
2 (w/w).
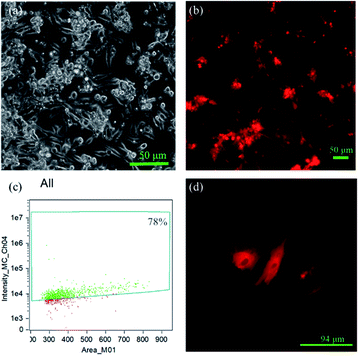
In order to evaluate the transfection efficiency of the upconversion nanoparticles, we used a fluorescence microscope and flow cytometry to observe transfection efficiency of the amino group and plasmid DNA modifications in the mesoporous silica shell of the particles. Channelrhodopsin-2 (ChR2) light-sensitive channel protein is a membrane protein. Under microscopy, a layer of membrane can be found around the periphery of cardiomyocytes (Fig. 9d). pcDNA3.1/hChR2(H134R)-mCherry contains mCherry fragments; thus, it will emit red fluorescence under microscopy.
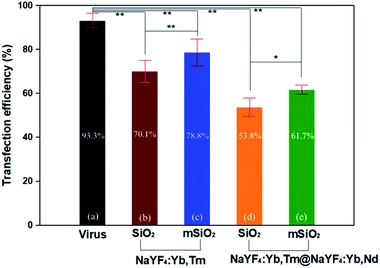
By this result, it can be determined that the DNA successfully entered the cells and was transfected. The results showed that the mCherry signal from the DNA was detected in the primary cells by fluorescence microscopy (Fig. 9b), indicating that the DNA-UCNPs have transfection ability. From the results of the flow cytometry (Fig. 9c), we can observe that the transfection efficiency of NaYF4:Yb,Tm@mSiO2-NH2-DNA is very high. Compared with traditional linear virus transfection, adenovirus transfection efficiency is as high as 93% (Fig. 10). However, DNA-UCNPs can also achieve 78% efficiency (Fig. 10). Therefore, the current research proves that UCNPs are very effective in delivering DNA safely and effectively. This is expected to replace adenovirus transfection methods in the future.
It can be seen in Fig. 10 that the transfection efficiency of the mesoporous silica shell of UCNPs is higher than that of the silica shell of UCNPs. In addition, the transfection efficiency of the NaYF4:Yb,Tm upconversion nanoparticles is also higher than the larger NaYF4:Yb,Tm@NaYF4:Yb,Nd upconversion nanoparticles. These results indicate the particle size and whether the etching will affect the transfection efficiency of the final upconversion nanoparticles.
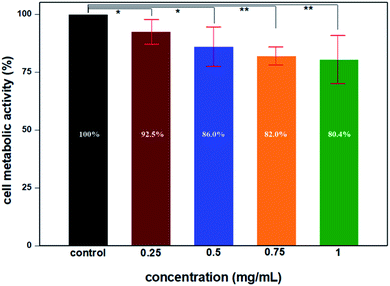
To evaluate the possible cytotoxicity of UCNP@mSiO2, the cell metabolic activity was verified using the MTT method in primary cardiac myoblast cells; the results indicated that the upconversion nanoparticles have lower cytotoxicity after modification. The cell metabolic activity was not hindered by UCNP@mSiO2 at high concentrations. The results depicted in Fig. 11 indicate that the cell metabolic activities of different concentrations of UCNP@mSiO2 were more than 80% after incubation for 48 h, which shows that UCNP@mSiO2 has great biocompatibility at high concentrations and long incubation times.
Conclusions
In this study, we used a user-friendly coprecipitation method and increased the amount of Y3+ ion to 88 mol% to synthesize high-quality pure hexagonal-phase NaYF4 nanoparticles with controllable shapes and strong fluorescence. β-NaYF4 can not only improve the luminous intensity of the upconversion nanoparticles but can also effectively extend their luminous lifetime. In order to effectively improve the biological application of upconversion nanoparticles, modifying a silicon shell on the surface of the upconversion nanoparticles not only effectively improves the hydrophobicity of the upconversion nanoparticles but also endows them with good biocompatibility.Upconversion nanoparticles modified with amine groups can effectively bind to DNA, and DNA-UCNPs with smaller particles have good transfection efficiency. The DNA-UCNPs we developed provide an alternative method to the traditional adenovirus for transfection and can provide the best UCNPs for materials science and biology.
Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of National Chiao Tung University and approved by the Animal Ethics Committee of Institutional Animal Care and Use Committee (IACUC).Conflicts of interest
There are no conflicts to declare.Notes and references
- Q. Q. Dou, H. C. Guo and E. Ye, Near-infrared upconversion nanoparticles for bio-applications, Mater. Sci. Eng., C, 2014, 45, 635–643 CrossRef CAS.
- H. Zou, F. Jin, X. Song and J. Xing, Singlet oxygen generation of photosensitizers effectively activated by Nd3+-doped upconversion nanoparticles of luminescence intensity enhancing with shell thickness decreasing, Appl. Surf. Sci., 2017, 400, 81–89 CrossRef CAS.
- X. Wu, P. Hu, S. Hu, Z. Chen, H. Yan, Z. Tang, Z. Xi, Y. Yu, G. Dai and Y. Liu, Upconversion nanoparticles for differential imaging of plant cells and detection of fluorescent dyes, J. Rare Earths, 2016, 34(2), 208–220 CrossRef CAS.
- E. Hemmer, P. Acosta-Mora, J. Méndez-Ramos and S. Fischer, Optical nanoprobes for biomedical applications: shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy, J. Mater. Chem. B, 2017, 5(23), 4365–4392 RSC.
- X. Liu, M. Liu, J. Chen, Z. Li and Q. Yuan, Rational design and biomedical applications of DNA-functionalized upconversion nanoparticles, Chin. Chem. Lett., 2018, 29(9), 1321–1332 CrossRef CAS.
- V. Muhr, C. Würth, M. Kraft, M. Buchner, A. J. Baeumner, U. Resch-Genger and T. Hirsch, Particle-size-dependent Forster resonance energy transfer from upconversion nanoparticles to organic dyes, Anal. Chem., 2017, 89(9), 4868–4874 CrossRef CAS.
- S. Wilhelm, Perspectives for upconverting nanoparticles, ACS Nano, 2017, 11(11), 10644–10653 CrossRef CAS.
- H. Dong, L.-D. Sun and C.-H. Yan, Energy transfer in lanthanide upconversion studies for extended optical applications, Chem. Soc. Rev., 2015, 44(6), 1608–1634 RSC.
- Z. Zhu, D. Tian and X. Shu, Auto-phase-locked time-gated luminescence detection for background-free upconversion spectra measurement and true-color biological imaging, Sens. Actuators, B, 2018, 260, 289–294 CrossRef CAS.
- H. LiáJo, Y. HanáSong and K. TaekáLee, Fast and background-free three-dimensional (3D) live-cell imaging with lanthanide-doped upconverting nanoparticles, Nanoscale, 2015, 7(46), 19397–19402 RSC.
- A. Bagheri, H. Arandiyan, C. Boyer and M. Lim, Lanthanide-Doped Upconversion Nanoparticles: Emerging Intelligent Light-Activated Drug Delivery Systems, Adv. Sci., 2016, 3(7), 1500437 CrossRef.
- S. S. Lucky, N. M. Idris, K. Huang, J. Kim, Z. Li, P. S. P. Thong, R. Xu, K. C. Soo and Y. Zhang, In vivo biocompatibility, biodistribution and therapeutic efficiency of titania coated upconversion nanoparticles for photodynamic therapy of solid oral cancers, Theranostics, 2016, 6(11), 1844 CrossRef.
- J. Peng, W. Xu, C. L. Teoh, S. Han, B. Kim, A. Samanta, J. C. Er, L. Wang, L. Yuan and X. Liu, High-efficiency in vitro and in vivo detection of Zn2+ by dye-assembled upconversion nanoparticles, J. Am. Chem. Soc., 2015, 137(6), 2336–2342 CrossRef CAS.
- X. Bai, S. Xu, J. Liu and L. Wang, Upconversion luminescence tracking of gene delivery via multifunctional nanocapsules, Talanta, 2016, 150, 118–124 CrossRef CAS.
- T. Cao, Y. Yang, Y. Gao, J. Zhou, Z. Li and F. Li, High-quality water-soluble and surface-functionalized upconversion nanocrystals as luminescent probes for bioimaging, Biomaterials, 2011, 32(11), 2959–2968 CrossRef CAS.
- M. H. Chan, Y. T. Pan, I. J. Lee, C. W. Chen, Y. C. Chan, M. Hsiao, F. Wang, L. Sun, X. Chen and R. S. Liu, Minimizing the Heat Effect of Photodynamic Therapy Based on Inorganic Nanocomposites Mediated by 808 nm Near-Infrared Light, Small, 2017, 13, 1700038 CrossRef.
- A. Sedlmeier and H. H. Gorris, Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications, Chem. Soc. Rev., 2015, 44(6), 1526–1560 RSC.
- C. Liu, Z. Wang, X. Wang and Z. Li, Surface modification of hydrophobic NaYF4:Yb,Er upconversion nanophosphors and their applications for immunoassay, Sci. China: Chem., 2011, 54(8), 1292 CrossRef CAS.
- Y. Tang, W. Di, X. Zhai, R. Yang and W. Qin, NIR-responsive photocatalytic activity and mechanism of NaYF4:Yb,Tm@ TiO2 core–shell nanoparticles, ACS Catal., 2013, 3(3), 405–412 CrossRef CAS.
- L. Fenno, O. Yizhar and K. Deisseroth, The development and application of optogenetics, Annu. Rev. Neurosci., 2011, 34, 389–412 CrossRef CAS.
- A. Ahmad, S. Ashraf and S. Komai, Optogenetics applications for treating spinal cord injury, Asian Spine J., 2015, 9(2), 299–305 CrossRef.
- C. M. Ambrosi, P. M. Boyle, K. Chen, N. A. Trayanova and E. Entcheva, Optogenetics-enabled assessment of viral gene and cell therapy for restoration of cardiac excitability, Sci. Rep., 2015, 5, 17350 CrossRef CAS.
- U. Nussinovitch and L. Gepstein, Optogenetics for in vivo cardiac pacing and resynchronization therapies, Nat. Biotechnol., 2015, 33(7), 750–754 CrossRef CAS.
- S. A. Park, S.-R. Lee, L. Tung and D. T. Yue, Optical mapping of optogenetically shaped cardiac action potentials, Sci. Rep., 2014, 4, 6125 CrossRef CAS.
- Q. Li, R. Ni, H. Hong, K. Y. Goh, M. Rossi, V. G. Fast and L. Zhou, Electrophysiological Properties and Viability of Neonatal Rat Ventricular Myocyte Cultures with Inducible ChR2 Expression, Sci. Rep., 2017, 7(1), 1531 CrossRef.
- K. P. Gerhardt, E. J. Olson, S. M. Castillo-Hair, L. A. Hartsough, B. P. Landry, F. Ekness, R. Yokoo, E. J. Gomez, P. Ramakrishnan and J. Suh, An open-hardware platform for optogenetics and photobiology, Sci. Rep., 2016, 6, 35363 CrossRef CAS.
- G. P. Pathak, J. D. Vrana and C. L. Tucker, Optogenetic control of cell function using engineered photoreceptors, Biol. Cell, 2013, 105(2), 59–72 CrossRef CAS.
- D. M. Shcherbakova, A. A. Shemetov, A. A. Kaberniuk and V. V. Verkhusha, Natural photoreceptors as a source of fluorescent proteins, biosensors, and optogenetic tools, Annu. Rev. Biochem., 2015, 84, 519–550 CrossRef CAS.
- Y.-C. Chen, W.-T. Chiu, J.-C. Chen, C.-S. Chang, L. H.-C. Wang, H.-P. Lin and H.-C. Chang, The photothermal effect of silica–carbon hollow sphere–concanavalin A on liver cancer cells, J. Mater. Chem. B, 2015, 3(12), 2447–2454 RSC.
- H. Guo, R. Hao, H. Qian, S. Sun, D. Sun, H. Yin, Z. Liu and X. Liu, Upconversion nanoparticles modified with aminosilanes as carriers of DNA vaccine for foot-and-mouth disease, Appl. Microbiol. Biotechnol., 2012, 95(5), 1253–1263 CrossRef CAS.
- R. Lv, D. Yang, P. Yang, J. Xu, F. He, S. Gai, C. Li, Y. Dai, G. Yang and J. Lin, Integration of upconversion nanoparticles and ultrathin black phosphorus for efficient photodynamic theranostics under 808 nm near-infrared light irradiation, Chem. Mater., 2016, 28(13), 4724–4734 CrossRef CAS.
- S. Stolik, J. Delgado, A. Perez and L. Anasagasti, Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues, J. Photochem. Photobiol., B, 2000, 57(2–3), 90–93 CrossRef CAS.
- S. Wu and H. J. Butt, Near-infrared-sensitive materials based on upconverting nanoparticles, Adv. Mater., 2016, 28(6), 1208–1226 CrossRef CAS.
- S. S. Lucky, N. Muhammad Idris, Z. Li, K. Huang, K. C. Soo and Y. Zhang, Titania coated upconversion nanoparticles for near-infrared light triggered photodynamic therapy, ACS Nano, 2015, 9(1), 191–205 CrossRef CAS.
- N. G. A. Tan, W. Wu and A. M. Seifalian, Optogenetics: lights, camera, action! A ray of light, a shadow unmasked, in Applications of Nanoscience in Photomedicine, Chandos Publishing, 2015, pp. 185–203 Search PubMed.
- S. Kasparov, Optogenetics, in Primer on the Autonomic Nervous System, Academic Press, 2012, pp. 689–691 Search PubMed.
- X. Huang, Realizing efficient upconversion and down-shifting dual-mode luminescence in lanthanide-doped NaGdF4 core–shell–shell nanoparticles through gadolinium sublattice-mediated energy migration, Dyes Pigm., 2016, 130, 99–105 CrossRef CAS.
- H. Dong, L.-D. Sun, Y.-F. Wang, J. Ke, R. Si, J.-W. Xiao, G.-M. Lyu, S. Shi and C.-H. Yan, Efficient Tailoring of Upconversion Selectivity by Engineering Local Structure of Lanthanides in NaxREF3+ x Nanocrystals, J. Am. Chem. Soc., 2015, 137(20), 6569–6576 CrossRef CAS.
- S. Wen, J. Zhou, K. Zheng, A. Bednarkiewicz, X. Liu and D. Jin, Advances in highly doped upconversion nanoparticles, Nat. Commun., 2018, 9(1), 2415 CrossRef.
- E. M. Chan, E. S. Levy and B. E. Cohen, Rationally designed energy transfer in upconverting nanoparticles, Adv. Mater., 2015, 27(38), 5753–5761 CrossRef CAS.
- D. T. Klier and M. U. Kumke, Analysing the effect of the crystal structure on upconversion luminescence in Yb3+, Er3+-co-doped NaYF4 nanomaterials, J. Mater. Chem. C, 2015, 3(42), 11228–11238 RSC.
- J. Suyver, A. Aebischer, D. Biner, P. Gerner, J. Grimm, S. Heer, K. Krämer, C. Reinhard and H.-U. Güdel, Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion, Opt. Mater., 2005, 27(6), 1111–1130 CrossRef CAS.
- Y. Tian, B. Tian, P. Huang, L. Wang and B. Chen, Size-dependent upconversion luminescence and temperature sensing behavior of spherical Gd2O3: Yb3+/Er3+ phosphor, RSC Adv., 2015, 5(19), 14123–14128 RSC.
- H. Lu, G. Yi, S. Zhao, D. Chen, L.-H. Guo and J. Cheng, Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties, J. Mater. Chem., 2004, 14(8), 1336–1341 RSC.
- H. S. Mader, P. Kele, S. M. Saleh and O. S. Wolfbeis, Upconverting luminescent nanoparticles for use in bioconjugation and bioimaging, Curr. Opin. Chem. Biol., 2010, 14(5), 582–596 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, Nonionic triblock and star diblock copolymer and oligomeric surfactant syntheses of highly ordered, hydrothermally stable, mesoporous silica structures, J. Am. Chem. Soc., 1998, 120(24), 6024–6036 CrossRef CAS.
- I. I. Slowing, J. L. Vivero-Escoto, C.-W. Wu and V. S.-Y. Lin, Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers, Adv. Drug Delivery Rev., 2008, 60(11), 1278–1288 CrossRef CAS.
- F. Wang, J. Wang and X. Liu, Direct evidence of a surface quenching effect on size-dependent luminescence of upconversion nanoparticles, Angew. Chem., Int. Ed., 2010, 49(41), 7456–7460 CrossRef CAS.
- J. Yu, W. Yin, T. Peng, Y. N. Chang, Y. Zu, J. Li and Y. Zhao, Biodistribution, excretion, and toxicity of polyethyleneimine modified NaYF4:Yb,Er upconversion nanoparticles in mice via different administration routes, Nanoscale, 2017, 9(13), 4497–4507 RSC.
- O. Yizhar, L. E. Fenno, T. J. Davidson, M. Mogri and K. Deisseroth, Optogenetics in neural systems, Neuron, 2011, 71(1), 9–34 CrossRef CAS.
- B. Sibbald, Death but one unintended consequence of gene-therapy trial. Canadian Medical Association, Journal, 2001, 164(11), 1612 CAS.
- K. L. Douglas, Toward development of artificial viruses for gene therapy: A comparative evaluation of viral and non-viral transfection, Biotechnol. Prog., 2008, 24(4), 871–883 CAS.
- F. Lu, S. H. Wu, Y. Hung and C. Y. Mou, Size effect on cell uptake in well-suspended, uniform mesoporous silica nanoparticles, Small, 2009, 5(12), 1408–1413 CrossRef CAS.
- K. Yadav, A. C. Chou, R. K. Ulaganathan, H. D. Gao, H. M. Lee, C. Y. Pan and Y. T. Chen, Targeted and efficient activation of channel rhodopsins expressed in living cells via specifically-bound upconversion nanoparticles, Nanoscale, 2017, 9(27), 9457–9466 RSC.
- A. Sedlmeier and H. H. Gorris, Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications, Chem. Soc. Rev., 2015, 44(6), 1526–1560 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05385f |
| This journal is © The Royal Society of Chemistry 2020 |