DOI:
10.1039/D0RA05405D
(Paper)
RSC Adv., 2020,
10, 28213-28224
Optical properties of 3-substituted indoles†
Received
20th June 2020
, Accepted 23rd July 2020
First published on 29th July 2020
Abstract
The optical properties of various donor or acceptor p-phenyl substituted ethenyl indoles were studied in solvents of varying polarity using absorption, fluorescence and TDDFT methods. Ethenyl indole exhibits non-linear optical properties (NLO) in a substituent dependent manner. Compound with a strong electron-attracting substituent, shows large NLO properties with charge transfer behavior, whereas ethenyls with moderate electron withdrawing or electron donating substituent exhibit lower NLO properties with non polar excited state. A highly dipolar excited state for p-nitro phenyl substituted ethenyl indoles (μe: 18.2–27.1 debye; Δμ: 9.4–17.8 debye) is observed as compared to other ethenyls (μe: 6.6–9.5 debye; Δμ: 4.2–6.2 debye). From TDDFT study, it is shown that the HOMO–LUMO energy of ethenyl is increased with increasing the electron donating ability of the p-phenyl substitution. The optical band gap of ethenyl 3 without substitution, is decreased upon p-phenyl substitution either with an electron withdrawing (Cl, NO2) or an electron donating (OCH3, OH, NH2) substituent. The compound with a strong electron accepting, p-nitrophenyl ethenyl indole 1 shows 12 times better NLO response as compared to the reference ethenyl indole 3 (β: 1: 115 × 10−30 esu−1 cm5, 3: 9 × 10−30 esu−1 cm5). Ethenyls 2–6 bearing a weak or moderately electron withdrawing or electron accepting substituent, exhibit lower NLO response. The β of ethenyl is increased with increasing the order of electron withdrawing nature of phenyl ring. Overall, a correlation of β with the optical band gap, ground state dipole moment, % of charge transfer in the ground and excited state is found.
1. Introduction
Donor–acceptor substituted conjugated molecules have a wide range of applications in chemistry, biology1–6 and organic electronics7,8 like photoswitches,9–12 organic light-emitting diodes (OLEDS),13,14 dye-sensitized solar cell (DSSC),15–25 nonlinear optics (NLO).7,13,14,26–37 In particular, NLO materials with varied shape and size (e.g. dipolar, quadrupolar, octupolar) were developed and extensively studied using various testing methods such as electric field induced second harmonic (EFISH) generation, hyper Rayleigh scattering (HRS),26–37 solvatochromic methods38–46 and computational based density functional theory (DFT).39,42 Among all the methods, the solvatochromic method is most suitable, easy and cost effective method, in which the NLO response, first hyperpolarizability coefficient (β) of small dipolar molecule can be obtained accurately.38–46 The large second order NLO response, first hyperpolarizability can be achieved through extended conjugation as well as by tuning the donor–acceptor length in electron donor–acceptor substituted molecule.7,8 NLO response of 6-substituted indole derivatives were tested previously.47,48 These includes indole with tricyano furan acceptor based conjugated molecules and studied their thermal and electro-optic properties. It is shown that indole can act as a donor in developing nonlinear optical material. In such system, increasing the electron donating ability of indole moiety leads to decrease in thermal stability and increased in NLO responsive electro-optical properties.47 Similarly, as compared to aniline, the 6-(pyrrolidin-1-yl)-1H-indole based donor system exhibits enhance in its macro NLO response electro-optic properties.48 In such system, the NLO properties is increased with increasing the electron donating ability of indole moiety. This could be due to favorable intermolecular dipole interaction forces.49 In comparison to earlier report, the 3-substituted indole derivatives are more sensitive to medium polarity due to (i) the formation of stable resonating structure at indolyl-3-position and (ii) the donating ability of indole moiety can also be tuned through various N-substitution.50,51
The advantage of these molecules is due to their substituent induced varied optical properties such as absorption, fluorescence, extinction coefficient, HOMO–LUMO energy gap, excited state dipole moment and transition energy, which provide most valuable information in designing future molecules. In order to gain more insight into the NLO response of 3-substituted indole based conjugated molecules, we have studied the substituent dependent first hyperpolarizability (β) of various electron donor/acceptor substituted p-phenyl and N-substituted ethenyl indoles (1–9) using solvatochromic method. The ethenyls (1–9) with varied electron withdrawing and donating p-phenyl substitution (NO2, Cl, H, OCH3, OH, NH2, N–SO2C6H5, N–COCH3, N–Et) were synthesized (Scheme 1) and the effect of substitution on the optical properties of indole compounds were evaluated.
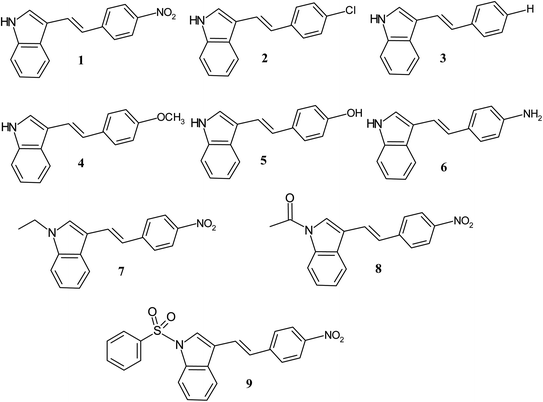 |
| | Scheme 1 Structure of indole compounds 1–9. | |
The excited state of nitro substituted ethenyls (1, 7, 8, 9) is highly dipolar and exhibit charge transfer excited state. A higher β value is found for these ethenyls bearing a strong electron withdrawing substituent. On the other hand, excited state of compounds 2–6 is non-polar and exhibit a lower β value. Overall, the 2nd order NLO properties is proportional to the ground state dipole moment, polarizability, ionic character and % of charge separation in the molecule. On the other hand, β value is inversely proportional to the optical band gap of the ethenyl indole. The above substituent dependent studies on p-phenyl and N-substituted ethenyl indole provide most valuable information in understanding the optical properties in conjugated molecules.
2. Experimental section
2.1 Materials and analytical equipments
The starting materials and reagents for the synthesis of ethenyl indoles were purchased from the local suppliers (Ms E. Merck, Sisco Research Laboratory, Sigma-Aldrich). UV grade solvents are used for spectroscopy study. Compounds are synthesized using Carousel 6 plus reaction station, Radleys make. Synthetic compounds are characterized by 1H and 13C nuclear magnetic resonance (NMR), Fourier-transform infrared (FTIR), mass spectrometry (MS) using electron impact (EI) method and carbon, hydrogen, nitrogen and sulfur (CHNS) analysis. The absorption spectra are recorded on a PerkinElmer Lambda 25 UV-Vis and Lambda 750 UV/VIS/near infrared (NIR) spectrophotometer. The fluorescence spectra are recorded on a PerkinElmer LS-55 fluorescence spectrophotometer using a red photomultiplier tubes (PMT) detector system. FTIR spectra are recorded on a Impact Nicolet-400 spectrophotometer using KBr discs. The 1H and 13C NMR spectra are recorded on a JEOL 500 MHz FTNMR instrument in CDCl3 as solvent and using tetramethylsilane (TMS) as an internal standard. MS spectra are measured on a GCD 1800A Hewlett Packard gas chromatography (GC)–mass spectrometer. CHNS analyses are recorded on a Theoquest CE instrument 1112 series CHNS auto analyzer. Melting points are recorded on a Lab India make melting point apparatus.
2.2 Synthesis of compounds 1–9
The substituted p-phenyl ethenyl-E-indoles (1–5) are synthesized through condensation of 2 molar ratio of p-substituted phenyl acetic acid with respect to 3-formylindole in pyridine–piperidine mixture as described earlier6,45,46,50,51 and the routes are shown in Scheme 2, e.g. for obtaining compound 1, the typical synthetic protocol is as follows: 2-formyl indole (1.45 g, 0.01 M) is taken with p-nitrophenyl acetic acid (3.62 g, 0.02 mol) along with in freshly distilled pyridine (10 mL) and piperidine (0.6 mL) in a round bottom flask. The reaction mixture was refluxed at 100 °C for six hours. The progress of the reaction is monitored by thin layer chromatography (TLC). After cooling the reaction mixture, the excess of pyridine was remove from the reaction mixture by treating with 100 mL of diluted hydrochloric acid. A red colored product is collected after extracting the crude product in dichloromethane. The product was further purified by column chromatography using 2% ethyl acetate in petroleum ether as the eluting solvent. The yield of the desired compound is obtained in 31%. Similarly, compounds 2–5 were obtained with yield 56%, 45%, 34% and 33% respectively. Compound 6 was prepared with 47% yield through reduction reaction of 1. For this purpose, the alcoholic solution of ethenyl indole 1 (0.2 g, 0.001 M) is refluxed in ferrous sulfate (1.5 g, 0.01 M) and aqueous ammonia solution at 100 °C for 3 h. Compound 7 was obtained with 80% yield through N-alkylation of compound 1 (0.5 g, 0.002 M) with ethyl bromide (2 mL, 0.01 mol) in presence of potassium-t-butoxide (0.2 g, 0.002 M) and t-butyl alcohol (20 mL).52 Compound 8 was obtained with 83% yield through N-acetylation of compound 1 (0.1 g, 0.0004 M) using acetic anhydride (10 mL) in presence of sodium acetate (0.1 g, 0.0012 M).53 Compound 9 was obtained with 80% yield through N-sulphonation of compound 1 (0.2 g, 0.001 M) using benzene sulphonyl chloride (1 mL, 0.01 M) and potassium carbonate (1.0 g, 0.01 M) in acetone54 as shown in Scheme 2. The products are purified by column chromatography using 2–5% ethyl acetate in petroleum ether (60–80 °C) as the eluting solvent. The characterization of compounds were carried out satisfactory using 1H and 13C NMR, MS, FTIR, CHNS analysis.
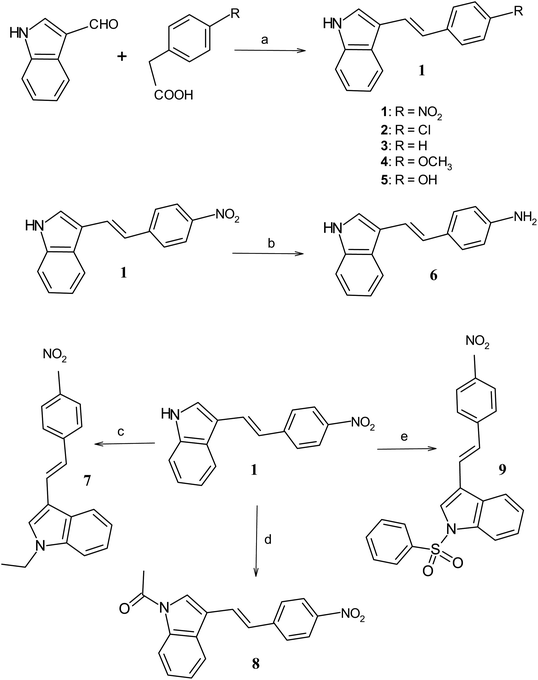 |
| | Scheme 2 Synthetic routes and reaction conditions for obtaining indole compounds, (a) pyridine, piperidine, 100 °C, reflux, 6 h; (b) FeSO4, aqu. NH3, ethanol, 100 °C, 3 h; (c) ethyl bromide, potassium-t-butoxide, t-butyl alcohol, reflux, 4 h; (d) acetic anhydride, sodium acetate, reflux, 1 h; (e) benzene sulphonyl chloride, potassium carbonate, acetone, 0 °C, 2 h. | |
2.3 Absorption and fluorescence studies
For all absorption and fluorescence measurements, UV grade solvents were used. For absorption, (1–4) × 10−5 M solution and for fluorescence studies, 0.5 × 10−5 M solution of compounds were prepared in different solvents and recorded using a 1 cm × 1 cm, light path length quartz cuvette. Fluorescence spectra were recorded by exciting the sample at their absorption maximum (λabs max). The ground and excited state energy (E) of the compounds are calculated using absorption wavelength (λabs max), fluorescence wavelength (λem max) maximum and eqn (1). Where,| | |
Eabs = (hc/λabs max) = (1.24/λabs max) (in KeV)
| (1a) |
| | |
Eem = (hc/λem max)= (1.24/λem max) (in Kev)
| (1b) |
The energy band gap (ΔE) of 1–9 is obtained from the intersection of excitation and fluorescence spectrum, Tauc plot and TDDFT computation method.
2.4 Dipole moment calculation
Change of excited state dipole moment of compounds is calculated using McRay eqn (2).41,55| | |
νabs − νem = (δabs + δem) + {(2Δμ2/hca3)F(ε,n)} = constant + mF(ε,n)
| (2) |
| F(ε,n) = [(ε − 1)/(ε + 2) − (n2 − 1)/(n2 + 2)], m = (2Δμ2/hca3) |
where, νabs is absorption maximum wave number, νem fluorescence maximum wave number, νabs − νem is the Stokes' shift, δabs and δem are difference in vibrational energy of molecule (in cm−1) in excited and ground state for absorption and emission respectively, μe and μg are the excited state and ground state dipole moments respectively, μe − μg = Δμ is the change in dipole moment, h is the Planck constant (6.62 × 10−34 joule s), c is the velocity of light in vacuum (3 × 108 meter per s), ε is the relative permittivity (i.e. dielectric constant) and n is the refractive index of the solvent.56,57 The Onsagar cavity radius (a) can be calculated using eqn (3) as described elsewhere.58where, M = molecular weight of molecule, N = Avogadro number = 6.022 × 1023, δ = molecular density of molecule.
2.5 First hyperpolarizability calculation
The first hyperpolarizability coefficient (β) is related to second order nonlinear optical (NLO) properties of molecule. The solvatochromism method is used to obtain β in methanol using Oudar formula59 as reported elsewhere38–44 using eqn (4) and eqn (5).| | |
(β) = (3/2h2c2) × {(vabs)2(rg)2(Δμ)}/{(vabs2 − v2)(vabs2 − 4v2)}
| (4) |
where, vabs: absorption maximum wave number and; v: incident reference wave number, 1064 nm of Nd:YAG laser source to which the β value is referred;
The transition dipole moment (rg) is calculated using eqn (5).
| | |
(rg)2 = [(3e2h)/(8π2mc)] × (f/vabs) = 2.13 × 10−30 × (f/vabs)
| (5) |
where,
f is the Oscillator strength,
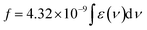
, which is obtained from the plot, molar absorption coefficient (
ε)
vs. wave number (
ν).
60
2.6 Computed parameters using time dependent density functional theory (TDDFT)
For computational calculation, the Orca quantum chemical software package61–63 with time dependent density functional theory (TDDFT)64–66 is used. The ground state dipole moment, absorption wavelength, the vertical excitation energy, oscillator strength of the optimized ethenyls were obtained using B3LYP functional with a def2 SVP basis set.67
3. Results and discussion
3.1 Synthesis
The trans-olefins of 1–9 were obtained through condensation reaction as shown in Scheme 2 with reasonable yield (31–83%). Overall the yield of reaction is obtained satisfactory. All the compounds were characterized through 1H NMR, 13C NMR, FTIR, MS (EI + method) and CHN analysis. In 1H NMR, the two doublet peaks correspond to trans olefin protons of compound 1–9 appear near δ 7.2 and δ 7.4 (each of 1H, J = 15.8–16.5 Hz). Similarly, multiplate peaks near δ 7.1–7.2 (2H, m –C5–H, C6–H), one singlet peak near δ 7.3–7.4 (1H, s, –C2–H), two doublet peaks near δ 7.9 (1H, d, J = 7.5–8.2 Hz, –C4–H) and δ 8.0 (1H, J = 6.2–7.5 Hz, –C7–H) correspond to the indole ring protons. In FTIR, the indole N–Hst is identified near 3370 cm−1. The C–Hst appear near 3040 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cst near 1620 cm−1. Similarly, four peaks correspond to the C
Cst near 1620 cm−1. Similarly, four peaks correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cst and C–Cst of phenyl ring are observed near 1520 cm−1, 1488 cm−1, 1455 cm−1, 1400 cm−1. For compound 1, the symmetrical and asymmetrical stretching frequency of nitro group is confirmed at 1330 cm−1 and 1520 cm−1. In compound 4, the methoxy, O–Cst is confirmed at 1244 cm−1. For compound 6, two sharp peaks at 3394 cm−1 and 3341 cm−1 are confirmed as primary amine NHst. For compound 7–9, the NHst peaks are disappeared upon N-substitution. For 8, the C
Cst and C–Cst of phenyl ring are observed near 1520 cm−1, 1488 cm−1, 1455 cm−1, 1400 cm−1. For compound 1, the symmetrical and asymmetrical stretching frequency of nitro group is confirmed at 1330 cm−1 and 1520 cm−1. In compound 4, the methoxy, O–Cst is confirmed at 1244 cm−1. For compound 6, two sharp peaks at 3394 cm−1 and 3341 cm−1 are confirmed as primary amine NHst. For compound 7–9, the NHst peaks are disappeared upon N-substitution. For 8, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peak at 1703 cm−1 correspond to N-acetyl group and for 9, the S
O stretching peak at 1703 cm−1 correspond to N-acetyl group and for 9, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching appears at 1180 cm−1. Thus, olefins bearing indole heterocyclic unit and indole N-substituted ethenyls were synthesized using mild reaction condition and characterized successfully. The detail of characterization data is shown in ESI.†
O stretching appears at 1180 cm−1. Thus, olefins bearing indole heterocyclic unit and indole N-substituted ethenyls were synthesized using mild reaction condition and characterized successfully. The detail of characterization data is shown in ESI.†
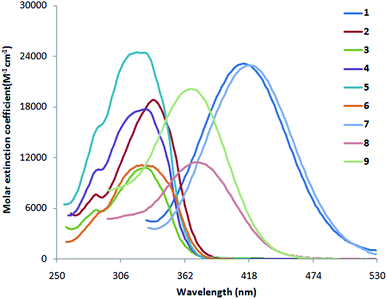
3.2 Absorption and fluorescence studies of indole compounds 1–9
The absorption and fluorescence data of compounds 1–9 in different solvents of varying polarity are summarized in Table 1. From the absorption (Fig. 1 and S1a-i†) and fluorescence spectra (Fig. 2 and S2a-i†), it is shown that the absorption coefficient of ethenyl indoles (1–9) is in between 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–23
800–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 (Table 2). On increasing the solvent polarity, the absorption and fluorescence wavelength maximum are red shifted. This suggest, a π–π* nature of electronic transition. On increasing the solvent polarity from n-hexane to dimethylformamide (DMF), the absorption maximum (λabs max) is moderately red shifted by 24 nm, 29 nm, 22 nm, 17 nm and 13 nm respectively for strong electron withdrawing nitro ethenyls (1, 7–9) and for strong electron donating amino compound 6. The λabs max of compound 5 with phenolic group is also moderately red shifted by 11 nm from n-hexane to polar solvent, DMF. On the other hand, the λabs max of other ethenyls 2–4 (Cl, H, OCH3) is not much sensitive to solvent polarity and a red shift of 0–5 nm is observed. In contrast to λabs max, the fluorescence maximum (λem max) is significantly red shifted by 151 nm, 133 nm, 172 nm, 202 nm for 1, 7–9 respectively from n-hexane to DMF. For 2–6, λem max is moderately red shifted by 14 nm, 20 nm, 22 nm, 09 nm and 11 nm respectively. This suggest the drastic stabilization of the excited state of 1, 7–9 due to coulomb interaction between the dipolar solute and solvent molecules.68,69 A red-shifted λem max in ethenyl indole (1, 7–9) is due to the electron delocalization from the indole moiety-to-the electron acceptor nitro group. As the transition is π → π* nature, the more stabilization of excited state with respect to the ground state leads to a red shifted in absorption and fluorescence wavelength. From the correlation of Hammett substituent constant,70 it is shown that strong electron acceptor –NO2 group (σp +0.81, for 1, 7–9) at the p-phenyl ring induces large electron delocalization in the excited state as compared to the weak electron acceptor chloro group –Cl (σp +0.24, in case of 2), –H (σp 0.0 in case of 3) and weak electron donor methoxy group –OCH3, (σp −0.28, in case of 4).
900 M−1 cm−1 (Table 2). On increasing the solvent polarity, the absorption and fluorescence wavelength maximum are red shifted. This suggest, a π–π* nature of electronic transition. On increasing the solvent polarity from n-hexane to dimethylformamide (DMF), the absorption maximum (λabs max) is moderately red shifted by 24 nm, 29 nm, 22 nm, 17 nm and 13 nm respectively for strong electron withdrawing nitro ethenyls (1, 7–9) and for strong electron donating amino compound 6. The λabs max of compound 5 with phenolic group is also moderately red shifted by 11 nm from n-hexane to polar solvent, DMF. On the other hand, the λabs max of other ethenyls 2–4 (Cl, H, OCH3) is not much sensitive to solvent polarity and a red shift of 0–5 nm is observed. In contrast to λabs max, the fluorescence maximum (λem max) is significantly red shifted by 151 nm, 133 nm, 172 nm, 202 nm for 1, 7–9 respectively from n-hexane to DMF. For 2–6, λem max is moderately red shifted by 14 nm, 20 nm, 22 nm, 09 nm and 11 nm respectively. This suggest the drastic stabilization of the excited state of 1, 7–9 due to coulomb interaction between the dipolar solute and solvent molecules.68,69 A red-shifted λem max in ethenyl indole (1, 7–9) is due to the electron delocalization from the indole moiety-to-the electron acceptor nitro group. As the transition is π → π* nature, the more stabilization of excited state with respect to the ground state leads to a red shifted in absorption and fluorescence wavelength. From the correlation of Hammett substituent constant,70 it is shown that strong electron acceptor –NO2 group (σp +0.81, for 1, 7–9) at the p-phenyl ring induces large electron delocalization in the excited state as compared to the weak electron acceptor chloro group –Cl (σp +0.24, in case of 2), –H (σp 0.0 in case of 3) and weak electron donor methoxy group –OCH3, (σp −0.28, in case of 4).
Table 1 UV-Vis absorption and fluorescence data of 1–9 in different solvents
| |
Solvents |
λabs max (nm) |
ε (M−1 cm−1) |
λem max (nm) |
λex max (nm) |
Stokes' shift (va − vf) nm (cm−1) |
Quantum yield Φf |
| 1 |
n-Hexane |
403 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 423 |
497 |
408 |
94 (4693) |
0.0104 |
| 1,4-Dioxan |
407 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011 011 |
554 |
372 |
147 (6520) |
0.0216 |
| THF |
416 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
607 |
443 |
191 (7564) |
0.0418 |
| MeOH |
413 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
590 |
370 |
177 (7264) |
0.0013 |
| AcCN |
411 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 924 |
642 |
380 |
231 (8754) |
0.0118 |
| DMF |
427 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 068 |
648 |
446 |
221 (7987) |
0.0052 |
| 2 |
n-Hexane |
338 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
384 |
351 |
46 (3544) |
0.0026 |
| 1,4-Dioxan |
336 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
388 |
341 |
52 (3988) |
0.0037 |
| THF |
333 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
387 |
345 |
54 (4191) |
0.0020 |
| MeOH |
335 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
395 |
354 |
60 (4534) |
0.0016 |
| AcCN |
340 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475 475 |
396 |
355 |
56 (4159) |
0.0015 |
| DMF |
338 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 075 |
398 |
350 |
60 (4460) |
0.0024 |
| 3 |
n-Hexane |
328 |
4675 |
384 |
335 |
56 (4446) |
0.0066 |
| 1,4-Dioxan |
329 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 575 |
384 |
325 |
55 (4354) |
— |
| THF |
331 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
397 |
347 |
66 (5023) |
0.0083 |
| MeOH |
327 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
396 |
323 |
69 (5329) |
0.0072 |
| AcCN |
325 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925 925 |
407 |
333 |
82 (6199) |
— |
| DMF |
332 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
404 |
346 |
72 (5368) |
0.0110 |
| 4 |
n-Hexane |
328 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 496 496 |
389 |
340 |
61 (4781) |
0.0027 |
| 1,4-Dioxan |
330 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229 229 |
397 |
329 |
67 (5115) |
0.0209 |
| THF |
331 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 101 101 |
403 |
324 |
72 (5398) |
0.0163 |
| MeOH |
327 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
404 |
344 |
77 (5829) |
0.0137 |
| AcCN |
326 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
409 |
349 |
83 (6225) |
0.0176 |
| DMF |
333 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
411 |
349 |
78 (5700) |
0.0196 |
| 5 |
n-Hexane |
310 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987 987 |
389 |
308 |
79 (6552) |
0.0114 |
| 1,4-Dioxan |
318 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333 333 |
384 |
330 |
66 (5405) |
0.0035 |
| THF |
319 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754 754 |
385 |
332 |
66 (5373) |
0.0058 |
| MeOH |
322 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
390 |
330 |
68 (5414) |
0.0030 |
| AcCN |
323 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741 741 |
395 |
335 |
72 (5643) |
0.0042 |
| DMF |
331 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588 588 |
398 |
347 |
67 (5086) |
0.0081 |
| 6 |
n-Hexane |
320 |
8467 |
389 |
333 |
69 (5544) |
0.0361 |
| 1,4-Dioxan |
324 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522 522 |
394 |
354 |
70 (5484) |
0.0041 |
| THF |
325 |
2989 |
397 |
355 |
72 (5581) |
0.0334 |
| MeOH |
324 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
405 |
339 |
81 (6173) |
0.0301 |
| AcCN |
325 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 814 |
397 |
337 |
72 (5581) |
0.0745 |
| DMF |
333 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
400 |
365 |
67 (5030) |
0.0056 |
| 7 |
n-Hexane |
402 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026 026 |
508 |
395 |
106 (5190) |
0.0005 |
| 1,4-Dioxan |
415 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
566 |
406 |
151 (6429) |
0.0006 |
| THF |
420 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141 141 |
590 |
420 |
170 (6860) |
0.0007 |
| MeOH |
415 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 823 |
556 |
417 |
141 (6111) |
0.0006 |
| AcCN |
419 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238 238 |
649 |
415 |
230 (8482) |
0.0008 |
| DMF |
430 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318 318 |
640 |
426 |
210 (7630) |
0.0007 |
| 8 |
n-Hexane |
362 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
423 |
363 |
61 (3984) |
0.0003 |
| 1,4-Dioxan |
378 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655 655 |
533 |
370 |
155 (7694) |
0.0007 |
| THF |
375 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
537 |
375 |
162 (8045) |
0.0008 |
| MeOH |
375 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464 464 |
564 |
374 |
189 (8936) |
0.0009 |
| AcCN |
376 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
602 |
374 |
226 (9984) |
0.0010 |
| DMF |
382 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
592 |
380 |
220 (9287) |
0.0009 |
| 9 |
n-Hexane |
360 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 722 |
403 |
356 |
43 (3197) |
0.0003 |
| 1,4-Dioxan |
369 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742 742 |
529 |
371 |
160 (8196) |
0.0008 |
| THF |
374 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
576 |
377 |
202 (9376) |
0.0008 |
| MeOH |
370 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
588 |
371 |
218 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020) 020) |
0.0009 |
| AcCN |
369 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065 065 |
593 |
370 |
224 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 236) 236) |
0.0010 |
| DMF |
377 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178 178 |
605 |
369 |
228 (9996) |
0.0009 |
 |
| | Fig. 1 Absorption spectra of 1–9 in methanol. | |
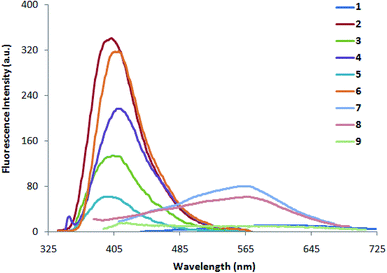 |
| | Fig. 2 Fluorescence spectra of 1–9 (0.5 × 10−5 M) in methanol. | |
Table 2 Comparison of absorption wavelength maximum (λabs max), fluorescence wavelength maximum (λem max), extinction coefficient, oscillator strength (f), S0–S1 transition state energy (ΔE, eV), transient dipole moment between ground and excited states (rg), change of excited state dipole moment (Δμ), optical band gap (ΔE), first hyperpolarizability (β) of ethenyl indoles 1–9 in methanola
| |
λabs max (nm) |
λem max (nm) |
(ε) (M−1 cm−1) |
f |
S0–S1 (ΔE) (eV) |
(rg) debye |
(Δμ) debye |
(μe) debye |
(β) (in 10−30) esu−1 cm5 |
| Onsagar cavity radius “a” (in Å); 1: 4.53; 2: 4.47; 3: 4.38; 4: 4.55; 5: 4.43; 6: 4.45; 7: 4.74; 8: 4.75; 9: 5.12; ground state dipole moment μg (in debye): 1: 8.39; 2: 3.99; 3: 2.57; 4: 1.05; 5: 1.37; 6: 2.44; 7: 8.94; 8: 4.34; 9: 9.32; excited state dipole moment μe = Δμ(McRay method) + μ(TDDFT)g. |
| 1 |
413 |
590 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.69 |
2.50 |
7.79 |
9.86 |
18.25 |
115 |
| 2 |
335 |
395 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.57 |
3.34 |
6.37 |
5.52 |
9.51 |
20 |
| 3 |
327 |
396 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.34 |
3.41 |
4.86 |
5.19 |
7.76 |
9 |
| 4 |
327 |
404 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.58 |
3.40 |
6.35 |
5.77 |
6.82 |
17 |
| 5 |
322 |
390 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.81 |
3.40 |
7.45 |
6.29 |
7.66 |
24 |
| 6 |
324 |
405 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.44 |
3.34 |
5.51 |
4.18 |
6.62 |
9 |
| 7 |
418 |
558 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 823 |
0.61 |
2.47 |
7.36 |
9.44 |
18.38 |
106 |
| 8 |
374 |
563 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464 464 |
0.32 |
2.83 |
5.03 |
14.21 |
18.55 |
43 |
| 9 |
370 |
588 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.56 |
2.87 |
6.64 |
17.78 |
27.10 |
90 |
The singlet state energy of 1–9 is calculated from their absorption and fluorescence wavelength maximum (Table 2). The first singlet excited state energy band gap for 1, 7–9 is 2.47–2.87 eV, whereas it is 3.34–3.40 eV for 2–6. As per Tauc plot and TDDFT calculation, the order is 3 > 2 > 9 > 8 > 1 > 2 and 3 > 4 = 5 > 6 (Fig. S3†). The optical band gap of 1 and 7–9 is 0.70–0.94 eV lower than compound 2–6. Interestingly, indole is acting as a strong electron donor in presence of an electron withdrawing p-phenyl nitro substituent (for 1, 7–9), whereas indole acts as a weak electron acceptor in presence of an electron donating p-phenyl methoxy and amine (OCH3, NH2) substituent. In order to understand the effect of substituent and solvent polarity on the excited state, McRay plot, the Stokes' shift (va − vf) vs. solvent polarity parameter, F(ε,n) is drawn, (Fig. 3) for 1–9 and the excited state dipole moment is calculated (Tables 2 and S1†). For all compounds, the Stokes' shift values are increased linearly with increasing the solvent polarity. This further improved by the deletion of two solvents, 1,4-dioxan and acetonitrile. In order to get a good correlation factor, these two solvents were excluded from our calculation and the following correlation are obtained (1: m1 = 4245, R = 0.91; 2: m1 = 1384, R = 0.99; 3: m1 = 1304, R = 0.99; 4: m1 = 1434, R = 0.99; 5: m1 = −1847, R = 0.92; 6: m1 = 805, R = 0.85; 7: m1 = 3397, R = 0.92, 8: m1 = 7645, R = 0.97, 9: m1 = 9563, R = 0.95). It is shown that a large change in the excited state dipole moment is observed for 1, 7, 8 and 9 (9.44–17.78 debye) as compared to 2, 3, 4, 5 and 6 (4.18–6.29 debye). Similarly, the ground state dipole moment (μg) is computed for 1–9 using TDDFT. The μg for 2–6 is in between 1.05–3.99 debye, whereas μg for 1, 7, 8, 9 is 4.34–9.32 debye (Table S1†). Thus, the large solvatochromic shift in 1 and 7–9 is due to charge transfer excited state. On the other hand, ethenyl indoles 2–6 with a weak electron acceptor or donor group (–Cl, –H, –OCH3, –OH, –NH2) show small change in excited state dipole moment (4.18–6.29 D) and exhibit non polar excited state as compared to 1 and 7–9.
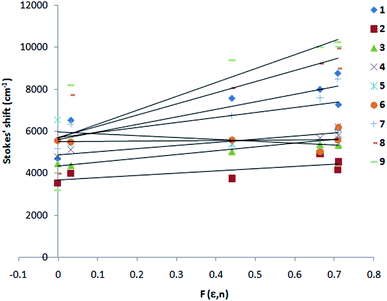 |
| | Fig. 3 McRay plot, Stokes' shift vs. F(ε,n) of 1–9. Solvents used are n-hexane, 1,4-dioxane, THF, MeOH, AcCN and DMF. | |
From Kamlet–Taft plot71 (Fig. 4 and Table S1†), it is shown that nitro compounds (1, 7–9) are highly polarized in the excited state. A large slope is observed for 1 and 7–9 as compared to 2–6 (slope: −5.14 × 10−3, R = 0.98 for 1; −0.93 × 10−3, R = 0.85 for 2; −1.32 × 10−3, R = 0.99 for 3; slope −1.43 × 10−3, R = 0.99 for 4; slope −2.69 × 10−3, R = 0.91 for 5; slope −0.71 × 10−3, R = 0.96 for 6; −4.03 × 10−3, R = 0.94 for 7; −7.38 × 10−3, R = 0.98 for 8; −9.22 × 10−3, R = 0.97 for 9). The formation of charge transfer excited state in 1 and 7–9 could be due to twist over the single bond attached to the p-phenyl ring and such phenomena is also suggested in other donor–acceptor substituted ethenyl systems.45,46,72–79
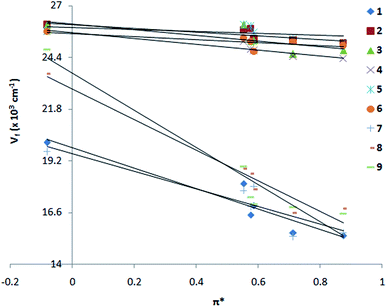 |
| | Fig. 4 Kamlet–Taft plot, emission wave number vs. solvent polarizability parameter π* of 1–9 in solvents, n-hexane, 1,4-dioxane, THF, MeOH, AcCN and DMF. | |
3.3 Time dependent density functional theory (TDDFT) studies
The geometry of the molecules are optimized and the parameters such as absorption, oscillator strength, HOMO–LUMO energy, optical band gap is computed for 1–9 using TDDFT method (Table S2†). The parameters obtained through computation methods are also followed the similar trend as compared to the experimental results. Compound with strong electron withdrawing p-phenyl substituent, the ground and excited states are stabilized, whereas, the ground and excited state are destabilized in presence of electron donating substituent compared to the unsubstituted ethenyl indole 3. This is due to the pushing or pulling of π electrons from indole to the p-phenyl substituted ring, which leads to the stabilization or destabilization of the ground or excited state.
All these compounds show one intense band (λabs max 300–440 nm). Compound 1 exhibits longest λabs max of 434 nm and 3 has the lowest λabs max of 341 nm. This absorption is due to the HOMO → LUMO (S0 → S1) transition (Fig. S4†). From solvatochromism data, these absorption is due to the π → π* transition. As compared to, ethenyl indole (3), the HOMO–LUMO optical band gap is decreased upon increasing the electron withdrawing or electron donating p-phenyl substitution. The energy band gap is decreased by 0.81 eV from phenyl ethenyl indole (3) to p-nitro phenyl ethenyl indole (1) [3.85 eV (for 3) to 3.05 eV (for 1)] (Fig. 5 and S5†). Similarly, the energy band gap is slightly decreased by 0.07 eV from phenyl ethenyl indole (3) to p-hydroxy phenyl ethenyl indole (5) [3.86 eV for 3 to 3.79 eV for 5], whereas the band gap is comparable for amino and methoxy substituent [3.86 eV for 3 to 3.84 eV for 4, 3.85 eV for 6]. Interestingly, N-ethyl substitution destabilized the ground and excited state of 1, whereas the ground and excited state is stabilized upon N-acetyl and N-sulfonyl substitution. Overall, the HOMO and LUMO energy of ethenyl indole, is progressively stabilized for electron withdrawing group due to the delocalization of π electron from indole to the p-phenyl ring (for NO2, Cl) (Fig. S6†). In case of electron donating substituents (methoxy, hydroxy and amino) (4–6), the destabilization of HOMO and LUMO energy level could be due to the hindrance in effective π conjugation.
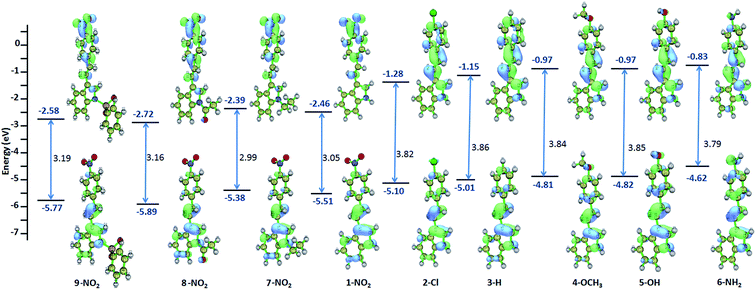 |
| | Fig. 5 TDDFT computed molecular orbitals, optical band gap and HOMO–LUMO energy of 1–9. | |
3.4 Second order non-linear optical properties
The first hyperpolarizability coefficient (β) is related to the second order non-linear optical properties of the molecule. Thus, β of the compounds 1–9 is calculated in solvent, methanol using solvatochromism method. In general, non linear optical properties of the molecule is influenced by the delocalization of π electron. Thus, the effect of p-phenyl and N-substitution on the NLO response of the ethenyl indole 3 is studied. For this purpose, the absorption, oscillator strength (f), dipole moment (Δμ) and transition dipole moment (rg) of the molecules were calculated. It is shown that as compared to the reference compound 3, the β increases for strong electron withdrawing p-nitro phenyl substituent (Table 2, Fig. 6 and S7†). Compound 1 and 7–9, which have strong electron withdrawing p-nitro phenyl substituent, exhibit large β value. Similarly, as compared to 3, the β of other ethenyl increases slightly with increasing the electron donating nature of p-phenyl substitution. From previously report on 6-substituted indole based NLO materials, NLO response is also increased with increasing the donor ability of indole moiety through pyrrolidine ring.47,48 Compounds with weak electron donating or weak electron withdrawing substituent (Cl, OCH3, OH) (2, 4–5), however, exhibit a low β value as compared to nitro compounds, 1 and 7–9. The order of β obtained in ethenyl indoles with electron withdrawing group is NO2 (1, 7–9) > Cl (2) > H (3) and electron donating OH (5) > OCH3 (4) > NH2 (6) (β; 1: 115, 2: 20, 3: 9, 4: 17, 5: 24, 6: 9, 7: 106, 8: 43, 9: 87) (in 10−30 esu−1 cm5).
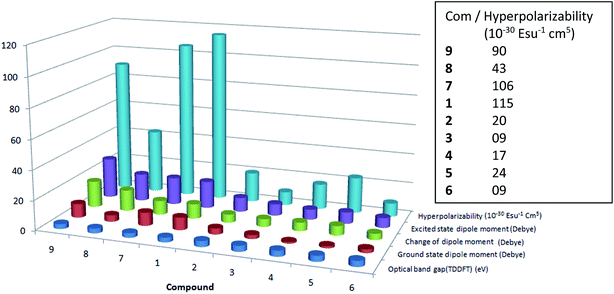 |
| | Fig. 6 Comparative chart of first hyperpolarizability (βCT), excited state dipole moment, change of excited state dipole moment, ground state dipole moment and optical band gap of 1–9. | |
The β value of some of the donor–acceptor nitro compounds, such as p-nitro aniline, 4-amino-4′-nitro stilbene, it is shown that the β value is increased from 20 to 100 (in 10−30 esu−1, cm5) with increasing the conjugation length and charge transfer nature of the molecule.28 From our previous report on thiophene and furan based conjugated compounds, similar results are also found.45,46 On the other hand, molecule with moderate or weak electron donor/acceptor substituent (cyano, chloro, methoxy, hydroxy), the effect on the β is very small as compared to the nitro substitution.45,46 For compound 7–9 with N-ethyl, N-acetyl and N-sulfonyl substituent, there is a hindrance in effective π conjugation, which leads to lower β value as compared to 1. The μg for 2–6 is in between 1.05–3.99 debye, whereas μg for 1, 7–9 is 4.34–9.32 debye (Tables S1–S3†). Thus, molecule with charge transfer behavior exhibits large ground state dipole moment, lower optical band gap and larger β value.
Overall, the β value is increased with (i) increasing the dipole moment, (ii) increasing the % of charge transfer behavior (iii) increasing the polarizability and (iv) with increasing the change of excited state dipole moment of 3-substituted indole compounds, whereas β value is decreased with (v) increasing the optical band gap of the molecule. It is the combination of all five factors involved in deciding the NLO response of the molecule. Mostly, for withdrawing substituent, the order of μg, μe, Δμ, π* and charge separation in 3-substituted indoles is NO2 (1, 7–9) > Cl (2) > H (3); and the order of β: NO2 (1, 7–9) > Cl (2) > H (3), where as for electron donating substituent, the order of μe, Δμ, π* and charge separation is OH (5) > OCH3 (4) > NH2 (6); and the order of β: OH (5) > OCH3 (4) > NH2 (6). On the other hand higher optical band gap reduced the β value. The order of optical band gap (ΔE) for electron withdrawing substituent: H (3) > Cl (2) > NO2 (1, 7–9) and the order of β is NO2 (1, 7–9) > Cl (2) > H (3). Similarly, the order ΔE for donating substituent: NH2 (6) > OCH3 (4) > OH (5) and the order of β: OH (5) > OCH3 (4) > NH2 (6). The optical band gap of N-substituted nitro compound (7–9) is little larger as compared to 1 and thus, NLO response of 7–9 is slightly lower as compared to compound 1.
4. Conclusion
In summary, it is shown that the excited state of ethenyl indole is highly sensitive to the solvent and the substituent present on it. Compound 1 and 7–9 with strong electron-attracting substituent exhibits charge transfer and highly dipolar excited state as compared to other ethenyls. Compound 2–6 with a moderate electron donating substituent or weak electron withdrawing or weak electron donating substituent exhibit non polar excited state and insensitive to solvent polarity. The energy band gap of 3 (phenyl ethenyl indole) is decreased by substituting either with an electron withdrawing (Cl, NO2) or an electron donating (OCH3, OH, NH2) substituent at the p-phenyl position. The compound with a strong electron accepting, p-nitrophenyl ethenyl indole shows 12 times better NLO response as compared to the reference ethenyl indole 3, whereas, for ethenyls 2–6 bearing a weak or moderately electron withdrawing or electron accepting substituent, exhibit lower NLO response. The β of ethenyl is increased with increasing the order of electron withdrawing nature of phenyl ring. On the other hand, in case of compounds bearing electron donating substituent shows comparable β value. The NLO response is also proportional to the ground state dipole moment, polarizability, dipolar nature and ionic character of the molecule, whereas it is inversely proportional to the optical band gap of the molecule. Overall, the optical properties of indole compound is highly dependent upon the substituent present in phenyl ring and N-substitution. In addition, studies on the macroscopic NLO properties of indole compound is an interesting aspect and a future prospective to look into. Thus, the above studies will help in designing and developing optical material for various electronic applications.
Conflicts of interest
There is no conflicts of interest to declare.
Acknowledgements
PKH, JK, NK are thankful to University Grants Commission, New Delhi for research grant (No. F.30-72/2014-BSR) and research fellowship. Authors acknowledged AMRC, IIT Mandi for 1H and 13C NMR facility.
References
- K. Palczewski, Chemistry and biology of vision, J. Biol. Chem., 2012, 287, 1612–1619 CrossRef CAS PubMed.
- C. Dugave and L. Demange, Cis-trans isomerisation of organic molecules and biomolecules: implications and applications, Chem. Rev., 2003, 103, 2475–2532 CrossRef CAS PubMed.
- A. Wand, I. Gdor, J. Zhu, M. Sheves and S. Ruhman, Shedding new light on retinal protein photochemistry, Annu. Rev. Phys. Chem., 2013, 64, 437–458 CrossRef CAS PubMed.
- A. K. Singh and P. K. Hota, Development of bacteriorhodopsin analogues and studies of charge separated excited states in the photoprocesses of linear polyenes, Photochem. Photobiol., 2007, 83, 50–62 CrossRef CAS PubMed.
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, New strategies for fluorescent probe design in medical diagnostic imaging, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- A. K. Singh and P. K. Hota, Ethenyl indoles as neutral hydrophobic fluorescence probes, J. Phys. Org. Chem., 2007, 20, 624–629 CrossRef CAS.
- B.-K. An, J. Gierschner and S. Y. Park, π-Conjugated cyanostilbene derivatives: a unique self-assembly motif for molecular nanostructures with enhanced emission and transport, Acc. Chem. Res., 2012, 45, 544–554 CrossRef CAS PubMed.
- H. Meir, The photochemistry of stilbenoid compounds and their role in materials technology, Angew. Chem., Int. Ed. Engl., 1992, 31, 1399–1420 CrossRef.
- M. Irie, Diarylethenes for memories and switches, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- R. Klajn, Spiropyran-based dynamic materials, Chem. Soc. Rev., 2014, 43, 148–184 RSC.
- M. Dudek, Z. Pokladek, M. Deiana and K. Matczyszyn, Molecular design and structural characterization of photoresponsive azobenzene-based polyamide units, Dyes Pigm., 2020, 180, 108501 CrossRef CAS.
- A. Georgiev, D. Yordanov, D. Dimov, I. Zhivkov, D. Nazarova and M. Weiter, Azomethine phthalimides fluorescent E-Z photoswitches, J. Photochem. Photobiol., A, 2020, 393, 112443 CrossRef CAS.
- L. Beverina and G. A. Pagani, π-Conjugated zwitterions paradigm of donor-acceptor building blocks in organic based materials, Acc. Chem. Res., 2014, 47, 319–329 CrossRef CAS PubMed.
- B. Wex and B. R. Kaafarani, Perspective on carbazole-based organic compounds as emitters and hosts in TADF applications, J. Mater. Chem. C, 2017, 5, 8622–8653 RSC.
- H. Hug, M. Bader, P. Mair and T. Glatzel, Biophotovoltaics: natural pigments in dye- sensitized solar cells, Appl. Energy, 2014, 115, 216–225 CrossRef CAS.
- J. A. Mikroyannidis, D. V. Tsagkournos, S. S. Sharma, Y. K. Vijay and G. D. Sharma, Conjugated small molecules with broad absorption containing pyridine and pyran units: synthesis and application for bulk heterojunction solar cells, Org. Electron., 2010, 11, 2045–2054 CrossRef CAS.
- T. Wang, Y. Chen, X. Bao, Z. Du, J. Guo, N. Wang, M. Sun and R. Yang, A new isoindigo-based molecule with ideal energy levels for solution-processable organic solar cells, Dyes Pigm., 2013, 98, 11–16 CrossRef CAS.
- S. Paek, J. K. Lee and J. Ko, Synthesis and photovoltaic characteristics of push–pull organic semiconductors containing an electron-rich dithienosilole bridge for solution-processed small-molecule organic solar cells, Sol. Energy Mater. Sol. Cells, 2014, 120, 209–217 CrossRef CAS.
- L. Beverina and G. A. Pagani, π-Conjugated zwitterions as paradigm of donor-acceptor building blocks in organic based material, Acc. Chem. Res., 2014, 47, 319–329 CrossRef CAS PubMed.
- I. Benesperi, H. Michaels and M. Freitag, The researcher's guide to solid-state dye-sensitized solar cells, J. Mater. Chem. C, 2018, 6, 11903–11942 RSC.
- T. Wang, Y. Chen, X. Bao, Z. Du, J. Guo, N. Wang, M. Sun and R. Yang, A new isoindigo based molecule with ideal energy levels for solution processable organic solar cells, Dyes Pigm., 2013, 98, 11–16 CrossRef CAS.
- J. K. Lee, B.-S. Jeong, J. Kim, C. Kim and J. Ko, Synthesis and photochemical characterization of fumaronitrile based organic semiconductor and its use in solution processed small molecule organic solar cells, J. Photochem. Photobiol., A, 2013, 251, 25–32 CrossRef CAS.
- G. D. Sharma, J. A. Mikroyannidis, S. S. Sharma and T. K. R. Justin Thomas, Bulk heterojunction organic photovoltaic devices based on small molecules featuring pyrrole and carbazole and 2-(4-nitrophenyl)acrylonitrile acceptor segments as donor and fullerene derivatives as acceptor, Dyes Pigm., 2012, 94, 320–329 CrossRef CAS.
- J. A. Mikroyannidis, D. V. Tsagkournos, S. S. Sharma, Y. K. Vijay and G. D. Sharma, Conjugated small molecules with broad absorption containing pyridine and pyran units: synthesis and application for bulk heterojunction solar cells, Org. Electron., 2010, 11, 2045–2054 CrossRef CAS.
- J. Solovjova, T. Malinauskas, M. Daskeviciene, E. Kasparavicius, A. Ilciukaite, A. Sackus, V. Paulauskas and V. Getautis, Triphenylamine-based phenylhydrazone-indolium cationic dyes for solid state DSSC applications, Mater. Lett., 2020, 274, 128001 CrossRef CAS.
- Nonlinear Optical Properties of Organic Molecules and Crystals, ed. D. S. Chemla and J. Zyss, Academic Press, New York, 1987, vol. 1–2 Search PubMed.
- P. N. Prasad and D. J. Williams, Introduction to Nonlinear Optical Effects in Molecules and Polymers, Wiley, New York, 1991 Search PubMed.
- D. R. Kanis, M. A. Ratner and T. J. Marks, Design and construction of molecular assemblies with large second order optical nonlinearities. Quantum chemical aspects, Chem. Rev., 1994, 94, 195–242 CrossRef CAS.
- J. Zyss and I. Ledoux, Nonlinear optics in multipolar media: theory and experiments, Chem. Rev., 1994, 94, 77–105 CrossRef CAS.
- E. Goovaerts, W. Wenseleers, M. H. Garcia and G. H. Cross, Design and characterization of organic and organometallic molecules for second order nonlinear optics, in Nonlinear optical materials, ed. H. S. Nalwa, Academic press, San Diego, CA, 2001, vol. 9, pp. 127–191 Search PubMed.
- S. Boomadevi, H. P. Mittal and R. Dhansekaran, Synthesis, crystal growth and characterization of 3-methyl 4-nitropyridine 1-oxide (POM) single crystal, J. Cryst. Growth, 2004, 261, 55–62 CrossRef CAS.
- S. R. Forrest and M. E. Thompson, Introduction: organic electronics and optoelectronics, Chem. Rev., 2007, 107, 923–925 CrossRef CAS.
- G. S. He, L.-S. Tan, Q. Zheng and P. N. Prasad, Multiphoton absorbing materials: molecular designs, characterizations and applications, Chem. Rev., 2008, 108, 1245–1330 CrossRef CAS PubMed.
- J. Campo, A. Painelli, F. Terenziani, T. V. Regemorter, D. Beljonne, E. Goovaerts and W. Wenseleers, First hyperpoarizability dispersion of the octupolar molecule crystal violet: multiple resonances and vibrational and solvent effects, J. Am. Chem. Soc., 2010, 132, 16467–16478 CrossRef CAS PubMed.
- D. Dini, M. J. F. Calvete and M. Hanack, Nonlinear optical materials for the smart filtering of optical radiation, Chem. Rev., 2016, 116, 13043–13233 CrossRef CAS PubMed.
- L. R. Mingabudinova, V. V. Vinogradov, V. A. Milichko, E. Hey-Hawkins and A. V. Vinogradov, Metal-organic frameworks as competitive materials for non-linear optics, Chem. Soc. Rev., 2016, 45, 5408–5431 RSC.
- Z. Liu, T. Lu and Q. Chen, An sp-hybridized all-carboatomic ring, cyclo[18]carbon: electronic structure, electronic spectrum and optical nonlinearity, Carbon, 2020, 165, 461–467 CrossRef CAS.
- M. S. Paley, J. M. Harris, H. Looser, J. C. Baumert, G. C. Bjorklund, D. Jundt and R. J. Twieg, A solvatochromic method for determining second order polarizabilities of organic molecules, J. Org. Chem., 1989, 54, 3774–3778 CrossRef CAS.
- C. Bosshard, G. Knopfle, P. Pretre and P. Gunter, Second order polarizabilities of nitropyridine derivatives determined with electric field induced second harmonic generation and a solvatochromic method: a comparative study, J. Appl. Phys., 1992, 71, 1594–1605 CrossRef CAS.
- S.-S. P. Chou, G.-T. Hsu and H.-C. Lin, Synthesis and second order nonlinearities of sulfonyl substituted pyrrole imino dyes, Tetrahedron Lett., 1999, 40, 2157–2160 CrossRef CAS.
- S. Bruni, E. Cariati, F. Cariati, F. A. Porta, S. Quici and D. Roberto, Determination of the quadratic hyperpolarizability of trans-4-[4-(dimethylamino)styryl]pyridine and 5- dimethylamino-1,10-phenanthroline from solvatochromism of absorption and fluorescence spectra: a comparison with electric-field-induced second harmonic generation technique, Spectrochim. Acta, Part A, 2001, 57, 1417–1426 CrossRef CAS.
- M. E. Reish, A. J. Kay, A. Teshome, I. Asselberghs, K. Clays and K. C. Gordon, Testing computational models of hyperpolarizability in a merocyanine dye using spectroscopic and DFT methods, J. Phys. Chem. A, 2012, 116, 5453–5463 CrossRef CAS PubMed.
- R. V. Solomon, P. Veerapandian, S. A. Vedha and P. Venuvanalingam, Tuning nonlinear optical and optoelectronic properties of vinyl coupled triazene chromophores: a density functional theory and time-dependent density functional theory investigation, J. Phys. Chem. A, 2012, 116, 4667–4677 CrossRef CAS PubMed.
- S. B. Chemate and N. Sekar, Novel iminocoumarin derivatives: synthesis, spectroscopic and computational studies, J. Fluoresc., 2015, 25, 1615–1628 CrossRef CAS PubMed.
- N. Kumar, M. Paramasivam, J. Kumar, A. Gusain and P. K. Hota, Substituent dependent optical properties of p-phenyl substituted ethenyl-E-thiophene, J. Fluoresc., 2018, 28, 1207–1216 CrossRef CAS PubMed.
- N. Kumar, M. Paramasivam, J. Kumar, A. Gusain and P. K. Hota, Tuning of optical properties of p-phenyl ethenyl-E-furans: a solvatochromism and density functional theory, Spectrochim. Acta, Part A, 2019, 206, 396–404 CrossRef CAS PubMed.
- J. Liu, M. Zhang, W. Gao, A. A. Fedorchuk and I. V. Kityk, Synthesis and non-linear optical properties of novel conjugated small molecules based on indole donor, J. Mol. Struct., 2018, 1165, 223–227 CrossRef CAS.
- M. Zhang, G. Qin, J. Liu, Z. Zhen, A. A. Fedorchuk, G. Lakshminarayana, A. A. Albassam, A. M. El-Naggar, K. Ozga and I. V. Kityk, Modification of indole by electron-rich atoms and their application in novel electron donor materials, Chem. Phys. Lett., 2017, 681, 105–109 CrossRef CAS.
- C. Ouyang, J. Liu, Q. Liu, Y. Li, D. Yan, Q. Wang, M. Guo and A. Cao, Preparation of main- chain polymers based on novel monomers with D-pi-a structure for application in organic second-order nonlinear optical materials with good long-term stability, ACS Appl. Mater. Interfaces, 2017, 9, 10366–10370 CrossRef CAS PubMed.
- J. Kumar, N. Kumar, N. Sati and P. K. Hota, Antioxidant properties of ethenyl indole: DPPH assay and TDDFT studies, New J. Chem., 2020, 44, 8960–8970 RSC.
- A. K. Singh and P. K. Hota, Fluorescence and photoisomerization studies of p-nitrophenyl substituted indolic ethenes, J. Phys. Org. Chem., 2006, 19, 43–52 CrossRef CAS.
- E. Wenkert, J. H. Udelhofen and N. K. Bhattacharyya, 3-Hydroxy methyleneoxindole and its derivatives, J. Am. Chem. Soc., 1959, 81, 3763–3768 CrossRef CAS.
- G. F. Smith, Indoles. Part I. The formylation of indole and some reactions of 3-formyl indole, J. Chem. Soc. Part IV, 1954, 3842–3846 RSC.
- D. Nagarathnam, A facile synthesis of 3-substituted indoles, J. Heterocycl. Chem., 1992, 29, 953–958 CrossRef CAS.
- E. G. McRae, Theory of solvent effects on molecular electronic spectra, frequency shifts, J. Phys. Chem., 1957, 61, 562–572 CrossRef CAS.
- C. Reichardt, Solvatochromic dyes as solvent polarity indicators, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- C. Reichardt, Solvents
and solvent effects in organic chemistry, VCH, Weinheim, Basel, Germany, 2nd edn, 1990, ch. 6, pp. 285–338 Search PubMed.
- N. Kumar, J. Kumar and P. K. Hota, Substituent dependence charge transfer and photochemical properties of donor-acceptor substituted ethenyl thiophenes, J. Fluoresc., 2017, 27, 1729–1738 CrossRef CAS PubMed.
- J. L. Oudar and D. S. Chemla, Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment, J. Chem. Phys., 1977, 66, 2664 CrossRef CAS.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley-Interscience, London, 1970 Search PubMed.
- F. Neese, The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- T. Petrenko and F. Neese, A general efficient quantum chemical method for predicting absorption bandshapes, resonance raman spectra and excitation profiles for larger molecules, J. Chem. Phys., 2007, 127, 164319 CrossRef PubMed.
- T. Petrenko, O. Krylova, F. Neese and M. Sokolowski, Optical absorption and emission properties of rubrene: insight by a combined experimental and theoretical study, New J. Phys., 2009, 11, 015001 CrossRef.
- A. Dreuw and M. Head-Gordon, Single reference ab initio methods for the calculation of excited states of large molecules, Chem. Rev., 2005, 105, 4009–4037 CrossRef CAS PubMed.
- J. Kulhánek, F. Bureš, A. Wojciechowski, M. Makowska-Janusik, E. Gondek and I. V. Kityk, Optical operation by chromophores featuring 4,5-dicyanoimidazole embedded within poly(methyl methacrylate) matrices, J. Phys. Chem. A, 2010, 114, 9440–9446 CrossRef PubMed.
- T. Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the coulomb-attenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balance basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- B. S. Neporent and N. G. Bakhshiev, On the role of universal and specific intermolecular interactions in the influence of the solvent on the electronic spectra of molecules, Opt. Spectrosc., 1960, 8, 408–413 CAS.
- C. Reichardt, S. Lobbecke, A. M. Mehranpour and G. Schafer, Pyridinium N-Phenoxide betaines and their application to the determination of solvent polarities synthesis and Uv-visible spectroscopic properties of new lipophillic tert-butyl- and 1-adamantyl substituted, negatively solvatochromic pyridinium N-Phenolate betaine dyes, Can. J. Chem., 1998, 76, 686–694 CrossRef CAS.
- M. B. Smith and J. March, in March's Advanced organic chemistry, reactions, mechanism, and structure, ed. M. B. Smith, A wiley-interscience publication, New York, 6th edn, 2007 Search PubMed.
- M. J. Kamlet, J.-L. M. Abboud and R. W. Taft, The solvatochromic comparison method. 6. The π* scale of solvent polarities, J. Am. Chem. Soc., 1977, 99, 6027–6038 CrossRef CAS.
- A. K. Singh and P. K. Hota, Photoreactivity of donor-acceptor ethenes, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2003, 42, 2048–2053 Search PubMed.
- A. K. Singh and P. K. Hota, Absorption and fluorescence spectral properties of donor acceptor ethenes bearing indole and p-nitrophenyl substituents, Res. Chem. Intermed., 2005, 31, 85–101 CrossRef CAS.
- Y. Sonoda, Y. Shimoi, M. Goto, N. Tohnai and M. Kanesato, Fluorescence properties of (E,E,E)-1,6-di(n-naphthyl)-1,3,5-hexatriene (n = 1, 2): effects of internal rotation, J. Phys. Chem. A, 2013, 117, 566–578 CrossRef CAS PubMed.
- C. Singh, R. Ghosh, J. A. Mondal and D. K. Palit, Excited state dynamics of a push-pull stilbene: a femtosecond transient absorption spectroscopic study, J. Photochem. Photobiol., A, 2013, 263, 50–60 CrossRef CAS.
- C.-K. Lin, Y.-F. Wang, Y.-C. Cheng and J.-S. Yang, Multisite constrained model of trans-4-(N,N-dimethylamino)-4-nitrostilbene for structural elucidation of radiative and nonradiative excited states, J. Phys. Chem. A, 2013, 117, 3158–3164 CrossRef CAS PubMed.
- N. Kumar, J. Kumar and P. K. Hota, Substituent dependent photoreactivity of donor-acceptor phenyl ethene, Lett. Org. Chem., 2018, 15, 479–484 CrossRef CAS.
- J. Kumar, N. Kumar and P. K. Hota, Excited state and fluorescence probe properties of donor-acceptor substituted ethenes: a plausible photochromic material for organic electronics, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2018, 57, 301–307 Search PubMed.
- P. K. Hota and A. K. Singh, Donor-acceptor conjugated linear polyenes: a study of excited state intramolecular charge transfer, photoisomerisation and fluorescence probe properties, J. Fluoresc., 2018, 28, 21–28 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra05405d |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
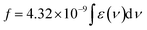 , which is obtained from the plot, molar absorption coefficient (ε) vs. wave number (ν).60
, which is obtained from the plot, molar absorption coefficient (ε) vs. wave number (ν).60
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cst near 1620 cm−1. Similarly, four peaks correspond to the C
Cst near 1620 cm−1. Similarly, four peaks correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cst and C–Cst of phenyl ring are observed near 1520 cm−1, 1488 cm−1, 1455 cm−1, 1400 cm−1. For compound 1, the symmetrical and asymmetrical stretching frequency of nitro group is confirmed at 1330 cm−1 and 1520 cm−1. In compound 4, the methoxy, O–Cst is confirmed at 1244 cm−1. For compound 6, two sharp peaks at 3394 cm−1 and 3341 cm−1 are confirmed as primary amine NHst. For compound 7–9, the NHst peaks are disappeared upon N-substitution. For 8, the C
Cst and C–Cst of phenyl ring are observed near 1520 cm−1, 1488 cm−1, 1455 cm−1, 1400 cm−1. For compound 1, the symmetrical and asymmetrical stretching frequency of nitro group is confirmed at 1330 cm−1 and 1520 cm−1. In compound 4, the methoxy, O–Cst is confirmed at 1244 cm−1. For compound 6, two sharp peaks at 3394 cm−1 and 3341 cm−1 are confirmed as primary amine NHst. For compound 7–9, the NHst peaks are disappeared upon N-substitution. For 8, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching peak at 1703 cm−1 correspond to N-acetyl group and for 9, the S
O stretching peak at 1703 cm−1 correspond to N-acetyl group and for 9, the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching appears at 1180 cm−1. Thus, olefins bearing indole heterocyclic unit and indole N-substituted ethenyls were synthesized using mild reaction condition and characterized successfully. The detail of characterization data is shown in ESI.†
O stretching appears at 1180 cm−1. Thus, olefins bearing indole heterocyclic unit and indole N-substituted ethenyls were synthesized using mild reaction condition and characterized successfully. The detail of characterization data is shown in ESI.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–23
800–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1 (Table 2). On increasing the solvent polarity, the absorption and fluorescence wavelength maximum are red shifted. This suggest, a π–π* nature of electronic transition. On increasing the solvent polarity from n-hexane to dimethylformamide (DMF), the absorption maximum (λabs max) is moderately red shifted by 24 nm, 29 nm, 22 nm, 17 nm and 13 nm respectively for strong electron withdrawing nitro ethenyls (1, 7–9) and for strong electron donating amino compound 6. The λabs max of compound 5 with phenolic group is also moderately red shifted by 11 nm from n-hexane to polar solvent, DMF. On the other hand, the λabs max of other ethenyls 2–4 (Cl, H, OCH3) is not much sensitive to solvent polarity and a red shift of 0–5 nm is observed. In contrast to λabs max, the fluorescence maximum (λem max) is significantly red shifted by 151 nm, 133 nm, 172 nm, 202 nm for 1, 7–9 respectively from n-hexane to DMF. For 2–6, λem max is moderately red shifted by 14 nm, 20 nm, 22 nm, 09 nm and 11 nm respectively. This suggest the drastic stabilization of the excited state of 1, 7–9 due to coulomb interaction between the dipolar solute and solvent molecules.68,69 A red-shifted λem max in ethenyl indole (1, 7–9) is due to the electron delocalization from the indole moiety-to-the electron acceptor nitro group. As the transition is π → π* nature, the more stabilization of excited state with respect to the ground state leads to a red shifted in absorption and fluorescence wavelength. From the correlation of Hammett substituent constant,70 it is shown that strong electron acceptor –NO2 group (σp +0.81, for 1, 7–9) at the p-phenyl ring induces large electron delocalization in the excited state as compared to the weak electron acceptor chloro group –Cl (σp +0.24, in case of 2), –H (σp 0.0 in case of 3) and weak electron donor methoxy group –OCH3, (σp −0.28, in case of 4).
900 M−1 cm−1 (Table 2). On increasing the solvent polarity, the absorption and fluorescence wavelength maximum are red shifted. This suggest, a π–π* nature of electronic transition. On increasing the solvent polarity from n-hexane to dimethylformamide (DMF), the absorption maximum (λabs max) is moderately red shifted by 24 nm, 29 nm, 22 nm, 17 nm and 13 nm respectively for strong electron withdrawing nitro ethenyls (1, 7–9) and for strong electron donating amino compound 6. The λabs max of compound 5 with phenolic group is also moderately red shifted by 11 nm from n-hexane to polar solvent, DMF. On the other hand, the λabs max of other ethenyls 2–4 (Cl, H, OCH3) is not much sensitive to solvent polarity and a red shift of 0–5 nm is observed. In contrast to λabs max, the fluorescence maximum (λem max) is significantly red shifted by 151 nm, 133 nm, 172 nm, 202 nm for 1, 7–9 respectively from n-hexane to DMF. For 2–6, λem max is moderately red shifted by 14 nm, 20 nm, 22 nm, 09 nm and 11 nm respectively. This suggest the drastic stabilization of the excited state of 1, 7–9 due to coulomb interaction between the dipolar solute and solvent molecules.68,69 A red-shifted λem max in ethenyl indole (1, 7–9) is due to the electron delocalization from the indole moiety-to-the electron acceptor nitro group. As the transition is π → π* nature, the more stabilization of excited state with respect to the ground state leads to a red shifted in absorption and fluorescence wavelength. From the correlation of Hammett substituent constant,70 it is shown that strong electron acceptor –NO2 group (σp +0.81, for 1, 7–9) at the p-phenyl ring induces large electron delocalization in the excited state as compared to the weak electron acceptor chloro group –Cl (σp +0.24, in case of 2), –H (σp 0.0 in case of 3) and weak electron donor methoxy group –OCH3, (σp −0.28, in case of 4).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423
423![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 011
011![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603
603![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924
924![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068
068![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750
750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600
600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475
475![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075
075![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575
575![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 925
925![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975
975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 496
496![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229
229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 101
101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020
020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530
530![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 987
987![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 333
333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 754
754![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 741
741![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 588
588![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 522
522![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814
814![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185
185![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026
026![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 141
141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823
823![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238
238![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 318
318![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975
975![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655
655![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464
464![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722
722![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 742
742![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773
773![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020)
020)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 065
065![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 236)
236)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 178
178![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900
900![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800
800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400
400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823
823![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 464
464![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000







