Open Access Article
Open Access ArticleRecent advances and prospects in the palladium-catalyzed cyanation of aryl halides
Mohan
Neetha
a,
C. M. A.
Afsina
a,
Thaipparambil
Aneeja
a and
Gopinathan
Anilkumar
 *abc
*abc
aSchool of Chemical Sciences, Mahatma Gandhi University, Priyadarsini Hills P. O., Kottayam, Kerala, India 686560. E-mail: anilgi1@yahoo.com; anil@mgu.ac.in
bAdvanced Molecular Materials Research Centre (AMMRC), Mahatma Gandhi University, Priyadarsini Hills P. O., Kottayam, Kerala, India 686560
cInstitute for Integrated Programs and Research in Basic Sciences (IIRBS), Mahatma Gandhi University, Priyadarsini Hills P. O., Kottayam, Kerala, India 686560
First published on 11th September 2020
Abstract
Aryl nitriles are compounds with wide significance. They have made their own space in various sectors including pharmaceuticals, industries, natural product chemistry, and so on. Furthermore, they are also key intermediates in various transformations in organic chemistry. Transition metal-catalyzed cyanation reactions have proved to be a better replacement for the existing traditional synthetic strategies for aryl nitriles. Palladium is one of the most studied transition metals other than copper and nickel owing to its wide functional group compatibility and catalytic efficacy. There have been drastic developments in the field of palladium-catalyzed cyanation since its discovery in the 1973. This review summarizes the recent developments in the palladium-catalyzed cyanation of aryl halides and covers literature from 2012–2020.
1 Introduction
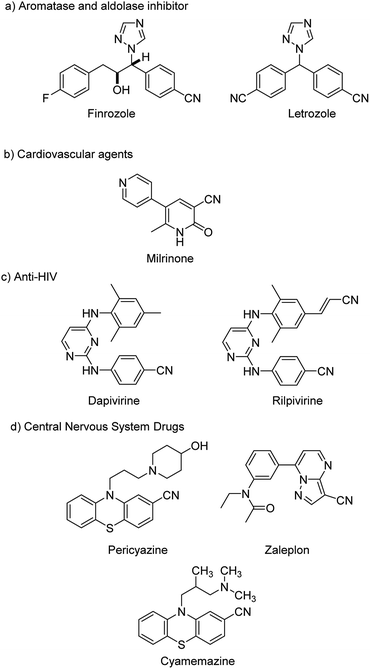
Cyanation in organic synthesis is the introduction of a cyanide group in to a reacting molecule resulting in the formation of diverse cyanides or otherwise called nitriles. Aryl nitriles are privileged compounds finding wide significance in various disciplines such as pharmaceuticals,1,2 industry,3 agrochemicals,4etc. They have been building blocks in various natural products, dyes and pigments. Some pharmaceutically relevant molecules containing nitrile moieties are portrayed in Fig. 1. The major highlight of nitriles lies in the fact that they can undergo smooth transformations into various other functional scaffolds like-aldehydes, amides, carboxyls, amidines, esters etc.5 This has been suitably exploited to synthesize and transform various molecules with structural complexity and diversity.Pongratz in 1927, was the first to develop a strategy towards cyanation reactions.6 From then, cyanation achieved significant relevance and gained much acceptance. Some of the conventional synthetic methodologies towards aryl nitriles involved, the Sandmeyer reaction7,8 from anilines, the Rosenmund–von Braun reaction9,10 from halides and industrial ammoxidation reactions.11 However, these reactions involved harsh conditions like high temperatures and utilized almost stoichiometric amounts of CuCN leading to the generation of heavy metal wastes in equimolar quantities. Hence, the situation demanded a much better alternative for the cyanide synthesis apart from these.
In this scenario, transition-metal catalyzed syntheses of aryl cyanides from aryl halides were explored and were found to be highly acceptable. Among them palladium,12–15 nickel16–18 and copper19–21 catalyzed cyanation have been well explored. Takagi et al. in 1973 introduced the first palladium-catalyzed cyanation from bromo and iodoarenes using potassium cyanide.22,23 They employed palladium(II) acetate/palladium(II) cyanide as the catalyst in DMF at 140–150 °C for 2–12 h. Later on, explorations on cyanations catalyzed by palladium were carried out extensively and above all, these investigations were the best in affording benzonitriles efficiently. Palladium has always intrigued scientists by virtue of its applications in the field of industry and laboratory.24 With this aspect, they have gained a command over other transition metals. Palladium enables an easy pathway for many transformations which are otherwise difficult to occur by the means of conventional strategies.25 It is a unique catalyst and is incorporated in homogeneous as well as heterogeneous catalysis. It catalyzes different organic reactions such as Heck,26 Stille,27 Tsuji–Trost,28 Suzuki,29 Wacker,30 Sonogashira31etc. Palladium is usually associated with mild reaction conditions and could tolerate a range of functional groups.32 Also they exhibit good endurance towards air and moisture. The mechanisms of palladium are well comprehended and hence are very convenient. Palladium as palladium(II) is mostly chosen as the substrate owing to its stability. These are then reduced to palladium(0) motifs to carry out the catalytic action. CuCN, Zn(CN)2, CUSCN, K4[Fe(CN)6], KCN, TMSCN and NaCN are the different metal and metalloid cyanation media, and cyanohydrin, N-cyano-N-phenyl-p-toluenesulfonamide (NCTS), acetone cyanohydrin, aminoacetonitrile, benzyl cyanide, α-iminonitrile, ethyl cyanoacetate, formamide, hexamethylenetetramine and cyanuric chloride formed the class of organo cyanation motifs.33
In 2003 and 2011, Beller et al. published two reviews solely on palladium catalyzed cyanation of aryl halides.34,35 Due to its wide applicability and importance, we intend to summarize the recent advances in palladium catalyzed cyanation of aryl halides using various cyanation strategies from 2012–2020. A model scheme for the palladium catalyzed cyanation of aryl halides is depicted in Scheme 1. For more clarity and simplicity, the review is classified based on the cyanating medium as reactions with metal-based cyanide sources and reactions with organic cyanide sources.
1.1. Reactions with metal-based cyanide sources
In 2013, palladium as a polymer aided macrocyclic palladium complex was utilized for the synthesis of various benzonitriles from a range of iodo and bromo arenes.36 The reaction was performed in water in the presence of Triton X-100 as non-ionic surfactant and avoided the use of any organic solvents (Scheme 2). The synthesis was compatible with the electronic effects and the steric effects exerted by the substituents on the aryl halides. Aryl chlorides were totally unreactive towards this reaction. Moreover, the catalyst was found to display exceptional recyclability, retaining its efficiency at the same time.
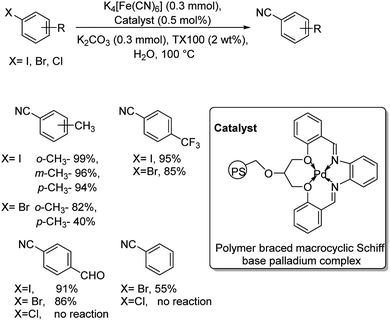 | ||
| Scheme 2 Water-mediated synthesis of benzonitriles using polymer braced macrocyclic palladium complex. | ||
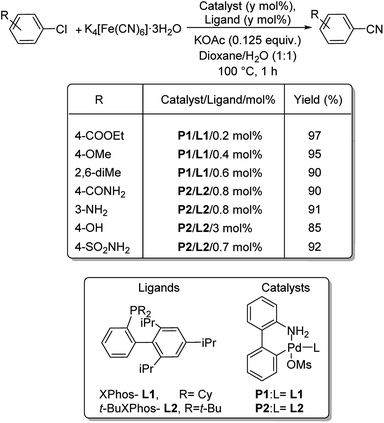
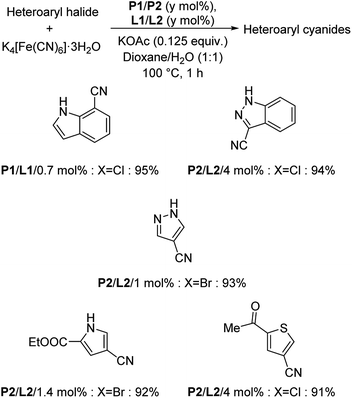
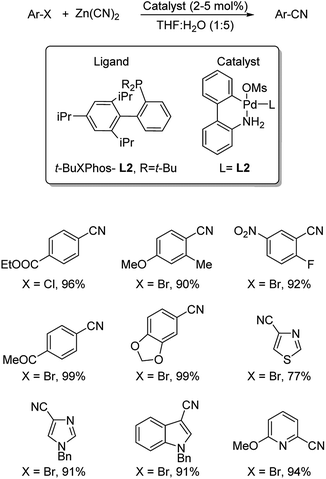
XPhos (L1) and t-BuXPhos (L2) were chosen as the best ligands forming two palladacycle precursor catalysts-P1 and P2 and cyanation of aryl and heteroaryl halides using K4[Fe(CN)6] in the presence of these palladacycle precatalysts was revealed by Buchwald and co-workers.37 Aryl chlorides with substitutions by COOEt, OMe, Me, COMe, NH2, SO2NH2, OH etc. underwent the reaction furnishing excellent yields (Scheme 3). In addition, synthesis of various heteroaryl cyanides with indole, pyrrole, thiophene, pyrazole, indazoles etc. backbones were also carried out easily (Scheme 4).
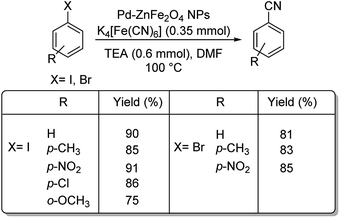
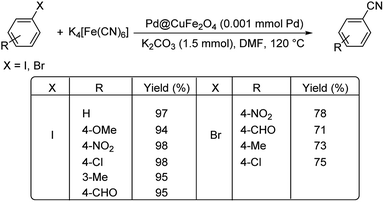
Interestingly, palladium reinforced on zinc ferrite nanoparticles (Pd–ZnFe2O4) was investigated for its ability to catalyse cyanation of diverse aryl iodides and bromides.38 The reaction was conducted using 10 mg catalyst, 0.35 mmol K4[Fe(CN)6] and 0.6 mmol TEA (triethylamine) in DMF at 100 °C (Scheme 5). It was observed that various aryl bromides and iodides with substitutions by H, NO2, OCH3, CH3 and Cl, could endure the reaction well furnishing a maximum yield of 91%.
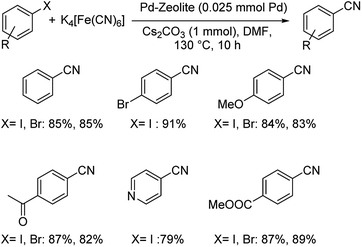
Pd-beta zeolite catalyzed synthesis of aryl cyanides using K4[Fe(CN)6] was developed by Sajadi and co-workers.39 Zeolites are crystalline, microporous, hydrated aluminosilicates finding wide applications in catalysis,40 surface chemistry41 and industry.42 Beta-zeolites are a class of zeolites acting as catalyst in various chemical transformations. The efficiency lies in the high Si/Al ratio, thermal stability, large pore size and higher acidity of the zeolites. Suitable tuning of these beta-zeolites with palladium generated a highly efficient catalyst. A range of aryl bromides and iodides with different functional groups reacted well yielding the desired benzonitriles effectively (Scheme 6). 79% to 91% of overall yield of the product was reported.
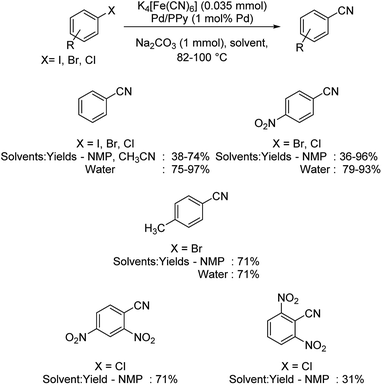
Magdesieva et al. in 2014 discussed the cyanation of haloarenes using a novel Pd/PPy (palladium–polypyrrole) nanocomposite.43 Benzonitriles were effectively synthesized using K4[Fe(CN)6] in different organic solvents (Scheme 7). The reaction was also carried out using water as the solvent. In either of the cases, the presence of electron-withdrawing moieties improved the yields more than that by those with electron-donating ones. Contradiction to this, nitro and dinitro substitutions on aryl chlorides imparted lower yields. The major highlight of the reaction is that even aryl chlorides, which are usually unreactive, also underwent the reaction, though the yield was only moderate.
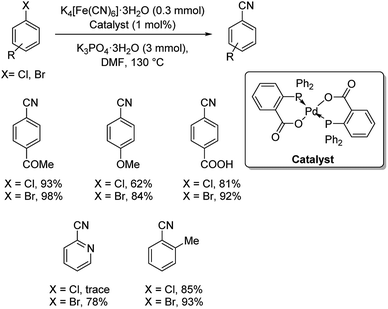
P–O bidentate chelate palladium complex catalyzed synthesis of benzonitriles using K4[Fe(CN)6]·3H2O was put forward.44 Cyanation of various aryl bromides and inert aryl chlorides were carried out efficiently by this strategy (Scheme 8). Presence of activating groups improved the yields of the reaction on comparison with electron-donating groups. Also, the reaction was extended to the cyanation of 2-bromopyridine and 2-chloropyridine, where 78% yield was obtained in the former case and traces in the latter one.
Palladium nanoparticles braced by zinc oxide as a potential catalyst for the cyanation of aryl bromides and chlorides was disclosed by Ranu et al.45 This reaction employed no additives, ligands or base and still could achieve benzonitriles in moderate to good yields. The cyanation was performed using K4[Fe(CN)6] in DMF solvent at 130 °C for 12–17 h (Scheme 9). Heteroaryl as well as styryl derived bromides could also undergo the reaction smoothly. High catalytic recyclability and low loading of catalyst are the highlights of the protocol.
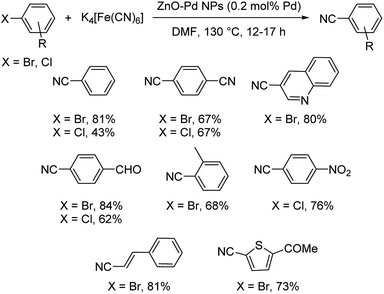 | ||
| Scheme 9 Palladium supported on zinc oxide nanoparticles-catalyzed cyanation of aryl bromides and chlorides. | ||
A microwave-assisted synthesis of aryl cyanides using a CN-dimeric ortho-palladated complex-[Pd{C6H2(CH2CH2NH2)–(OMe)2,2,3}(m-Br)]2 was also reported.46 The cyanation of diverse aryl halides were performed using 0.5 mol% of catalyst, 22 mol% of K4[Fe(CN)6], 1 equiv. of K2CO3, 1 equiv. TBAB in DMF at 130 °C (Scheme 10). Under the microwave condition (600 W), a maximum of 93% of overall yield was obtained with varying reaction times for various aryl halides. Aryl chlorides furnished lower yields in comparison to aryl iodides and bromides and at a slower pace. Moreover, the reaction was also carried out under conventional heating conditions, where the yields obtained were slightly lower and the duration of reaction was higher with respect to microwave heating.
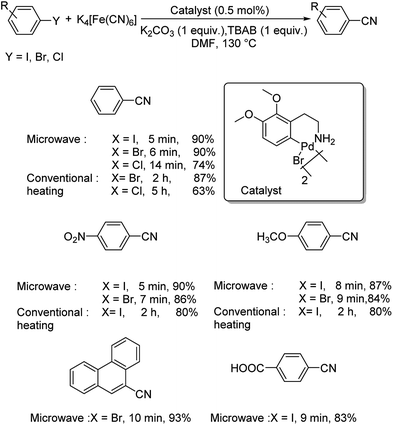 | ||
| Scheme 10 Investigations on cyanation of aryl halides catalyzed by CN-dimeric ortho-palladated complex via microwave and conventional heating. | ||
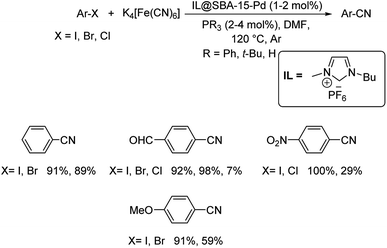
Ionic liquids (ILs) have always gained keen attention due to its green character. This insight led scientists to employ them in various chemical transformations. Ionic liquid assisted cyanation of diverse aryl halides was effected by Karimi et al.47 Here, palladium was functionalized by SBA-15 (mesoporous silica) and was associated with ionic liquid to develop the catalyst for the cyanation reaction. The ionic liquid utilised for the reaction was 1-butyl-3-methylimidazolium hexafluorophosphate (Scheme 11). The IL stabilizes the Pd moiety and at the same time it also prevents their agglomeration and hence, improving the catalytic recyclability. Aryl bromides with a range of substitutions by electron-donating and electron-withdrawing groups underwent the reaction to furnish aryl cyanides in good to excellent yields. Cyanation of aryl chlorides was also carried out, but the yield reported was very low. The studies on the activity of the catalyst revealed that, the superior catalytic efficiency was due to the ionic liquid which was present in the passages of mesoporous silica. The imidazolium ionic liquids could undergo phase transfer, which was exploited for faster penetration of Fe(CN)6 into the pores of the system. Thus, in the vicinity of Pd NPs, a highly concentrated site was accomplished which resulted in the higher activity of the catalyst.
Aryl chlorides have been mostly inert and unreactive towards cyanation reactions. So an approach for synthesis of aryl cyanides from unreactive chloroarenes via catalysis by palladium acetate in the presence of 2-di-tert-butylphosphino-2′-isopropoxy-1,1′-binaphthyl (L3) was disclosed by Xie and co-workers.48 The cyanation medium employed was potassium hexacyanoferrate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 t-BuOH–H2O (Scheme 12). Electron-donating groups on the aryl chloride were well tolerated by the reaction. Heterocyclic substrates also underwent the reaction giving good results. The activated palladium centre was stabilised by the hemi-labile coordination of oxygen atom on the ligand with palladium. This assisted the oxidative addition of aryl chloride. At a later stage, the bulkiness of the ligand eased the reductive elimination to form the aryl cyanides.
1 t-BuOH–H2O (Scheme 12). Electron-donating groups on the aryl chloride were well tolerated by the reaction. Heterocyclic substrates also underwent the reaction giving good results. The activated palladium centre was stabilised by the hemi-labile coordination of oxygen atom on the ligand with palladium. This assisted the oxidative addition of aryl chloride. At a later stage, the bulkiness of the ligand eased the reductive elimination to form the aryl cyanides.
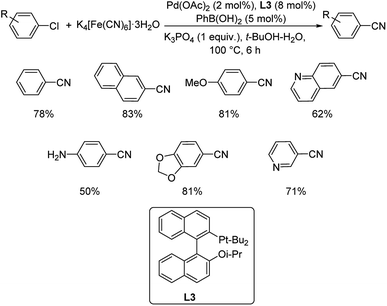 | ||
| Scheme 12 Optimized reaction condition for the synthesis of various aryl cyanides catalyzed by Pd(OAc)2. | ||
Palladium nanoparticles anchored on (3-aminopropyl)triethoxysilane-modified copper ferrite as an efficient catalyst for the cyanation of bromo and iodo arenes was disclosed in 2015.49 Diverse aryl iodides and bromides were analysed to provide very good results (Scheme 13). Moreover, due to the higher reactivity of Ar–I over Ar–Br, excellent yields were obtained in the former case than in the latter. The yields reported were more when the para-position of the aryl halides was substituted with electron-withdrawing groups than when it was substituted with electron-donating ones.
A combination of palladium and N-heterocyclic carbene (NHC) was employed as catalyst for the mono and dicyanation of various haloarenes by Shi et al.50 The optimised condition was 1 mol% Pd(OAc)2, 1 mol% ligand L4 (NHC), 0.25 equiv. K4[Fe(CN)6]·3H2O, 1 equiv. Na2CO3 in DMAc (N,N-dimethylacetamide) at 120 °C (Scheme 14). Smooth conduct of the reaction was observed with electron-deficient and electron-rich aryl iodides. The reaction was tolerant to the steric effects offered by the substituents. The reaction was also amenable to aryl bromides as well as aryl chlorides, though the latter afforded only moderate yields. Double cyanation of different aryl halides was also analysed by the group and they noted that aryl bromides with iodide substitution required lesser time for the conversion to dicyano products than aryl dibromides and also provided slightly higher yields (Scheme 15).
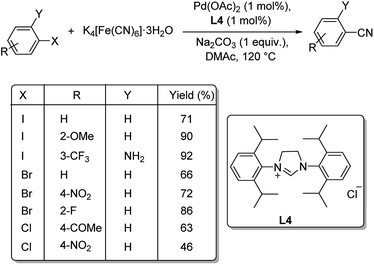 | ||
| Scheme 14 One pot mono-cyanation of various aryl halides using N-heterocyclic carbene and palladium. | ||
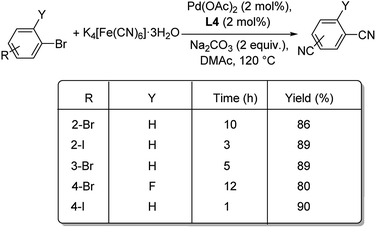 | ||
| Scheme 15 Double cyanation of substituted aryl bromides catalyzed by palladium acetate and N-heterocyclic carbene. | ||
Pitchumani et al. disclosed a strategy towards the synthesis of aryl cyanides using core–shell hybrid nano spheres (Pd NPs on C@Fe3O4) as the novel catalyst.51 The reaction could investigate a range of substrates and it was noted that the presence of electron-deficient substituents on iodo and bromoarenes gave slightly higher yields in comparison with electron-rich ones (Scheme 16). Chloroarenes underwent the reaction, but exhibited lower reactivity. The group could also carry out the cyanation of hetero aryl halides with good results. The catalyst displayed superlative properties such as-good efficiency, exceptional stability and recyclability. A possible mechanistic trajectory was proposed where, initially, the haloarene undergoes oxidative addition to Pd(0). This generates a Pd(II) moiety. Subsequently, from the inner coordination sphere of K4[Fe(CN)6], a ligand exchange takes place to the Pd(II) species forming a complex. A reductive elimination from the complex finally renders the aryl cyanides (Scheme 17).
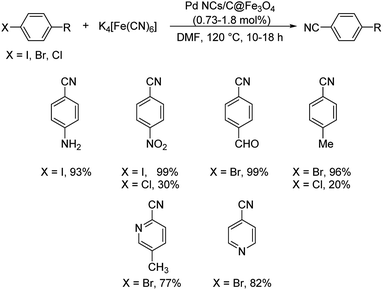 | ||
| Scheme 16 Pd NPs on C@Fe3O4 catalyzed synthesis of diversely substituted haloarenes and heterohaloarenes. | ||
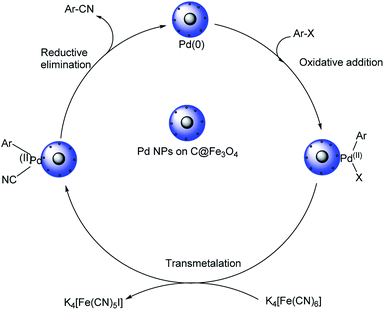 | ||
| Scheme 17 Possible mechanistic trajectory for the cyanation reaction. [This figure has been reproduced from ref. 51 with permission from AMERICAN CHEMICAL SOCIETY, copyright 2015]. | ||
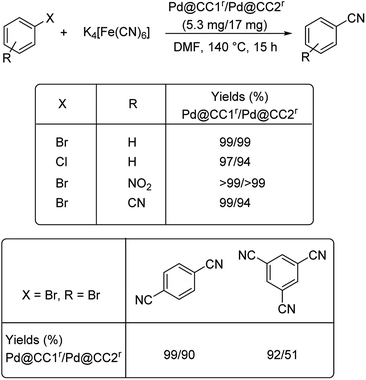
Delightfully in 2016, covalent cages loaded with palladium nanoparticles were developed and found to catalyse the cyanation of various aryl halides effectively.52 The cages were designed from triphenylamine based trialdehydes and cyclohexane diamine. Two cages CC1r and CC2r are the ones that impregnate palladium. The optimised reaction condition was 5.3 mg/17 mg Pd@CC1r/Pd@CC2r, 0.17 mmol K4[Fe(CN)6]·3H2O, in DMF, 140 °C, 15 h (Scheme 18). As is evident from the reaction condition, the reaction does not employ any additives. Various aryl halides underwent the reaction rendering excellent results. It can also be noted that, dibromo and tribromobenzenes were transformed to dicyano and tricyanobenzenes respectively in very high yields. Here, the normally least reactive aryl chloride could also afford the desired cyanobenzene very efficiently.
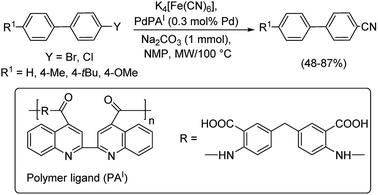
A polymer based catalyst having palladium(II) ions coordinated to biquinolyl fragments in the polyamic type polymer chain was introduced by Magdesieva et al. in 2016.53 The source of cyanide was K4[Fe(CN)6] (Scheme 19). Here, the catalyst was capable of bringing about the cyanation of aryl halides in good yields. The catalyst was found to be stable under microwave as well as under conventional heating conditions.
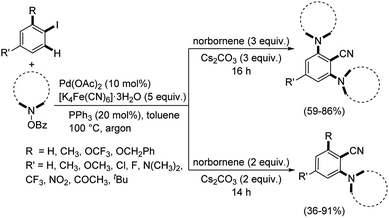
Ranu and Majhi designed a strategy towards ortho-C–H-amination/ipso-C–I-cyanation of aryl iodides using palladium catalyst.54 The cyanation media utilized was K4[Fe(CN)6]·3H2O and the protocol was assisted by norbornene (Scheme 20). A range of 2-aminobenzonitriles were effectively synthesized with good results. Here, they could carry out an ortho-mono-C–H-amination and ortho-bis-C–H-amination along with the ipso-C–I-cyanation.
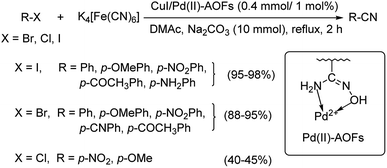
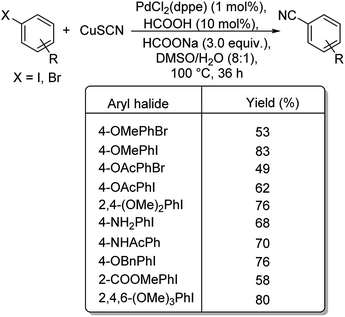
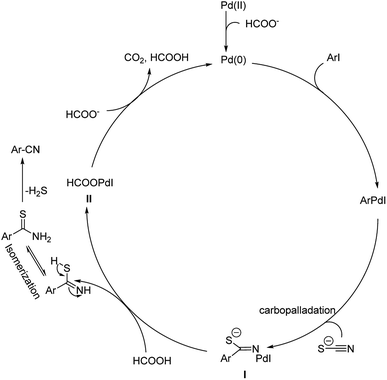
In 2017, Wu and co-workers have designed a facile protocol towards the synthesis of aryl cyanides from haloarenes using Cu(I)Pd(II)-AOFs (Pd–Cu bimetallic catalyst anchored on amidoxime fibres).55 The synthesis of amidoxime fibres was carried out by the reaction between NH2OH and polyacrylonitrile. The cyanation was brought about by K4[Fe(CN)6] and the reaction exhibited high endurance towards various substitutions on the haloarene. Excellent yields were reported with iodo and bromoarenes whereas, chloroarenes could impart only moderate yields (Scheme 21). Here, the coordination between Pd(II) ions and –OH/–NH2 in AOFs result in Pd(II)-AOFs. CuI reduces Pd(II)-AOFs to Pd(0)-AOFs. CuI also enhances the dissociation of the nitrile ion from K4[Fe(CN)6]. The haloarene is then subjected to an oxidative-addition to the Pd(0)-AOFs forming Ar–Pd–X intermediate. Finally, a reductive elimination establishes the aryl nitriles and also the Pd(0)-AOFs is regenerated for the next catalytic cycle.
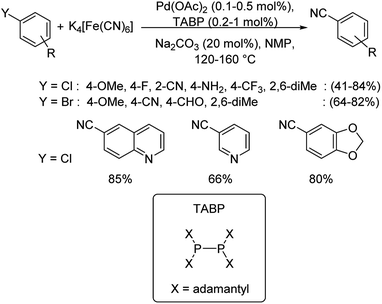
In most of the above reactions, the reactivity of aryl chlorides was found to be least in comparison to aryl bromides and iodides. Hence, Beller and co-workers in 2018 investigated the cyanation of aryl halides especially, aryl chlorides using TABP (tetraadamantylbiphosphine) as the efficient ligand along with palladium acetate as the catalyst.56 This was the first report on the utilization of biphosphine ligands in coupling reactions catalyzed by palladium (Scheme 22). Substitutions by electron-rich, electron-deficient and sterically hindered moieties were well explored. Furthermore, the cyanation of heteroaryl halides were also analysed which afforded the products satisfactorily.
A straightforward approach using palladium nanoparticles as the catalyst for the synthesis of aryl nitriles was disclosed by Kandathil and co-workers.57 Here, the aqueous ethanolic extract of Piper nigrum (black pepper) biogenetically converted palladium acetate into palladium NPs. The synthesized palladium NPs and K4[Fe(CN)6] were employed for the transformation of haloarenes to aryl nitriles (Scheme 23). The conversion towards various cyanides were more effective in the case of bromo and iodoarenes, but least for chloroarenes.
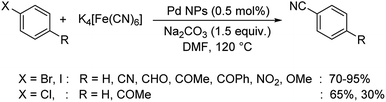 | ||
| Scheme 23 Biogenetically synthesized palladium nanoparticles catalyzed formation of various benzonitriles. | ||
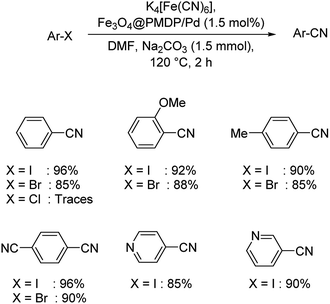
A palladium based core–shell catalyst using poly-methyldopa(PMDP)-coated Fe3O4 NPs and palladium NPs was designed by Veisi and co-workers.58 Adsorption of the palladium ions on the Fe3O4@PMDP resulted in the generation of the catalyst (Scheme 24). The cyanation of diverse iodo and bromoarenes was carried out using K4[Fe(CN)6] and manifested excellent yields. However, it was noticed that chloroarenes were unreactive substrates in the reaction. Nitrogen heterocylces which are electron-deficient like 3-iodopyridine and 4-iodopyridine underwent the cyanation reaction exhibiting good results.
Palladium nanoparticles anchored by polydopamine (PDA) as catalyst for the synthesis of aryl nitriles was disclosed by Gazdag et al.59 Initially, it was noted that under the optimised condition (10 mg Pd/PDA, 0.125 mmol K4[Fe(CN)6], 0.5 mmol Na2CO3 in NMP at 140 °C) the yields were favourable only for electron-deficient aryl halides, other substrates reacted less effectively (Scheme 25). But, the addition of tetrabutyl ammonium bromide (TBAB), improved the efficiency of the reaction and rendered moderate to good yields. This may be due to the potential of TBAB in stabilising the dissolved Pd(0) motifs in solution and thus displayed greater catalytic ability.
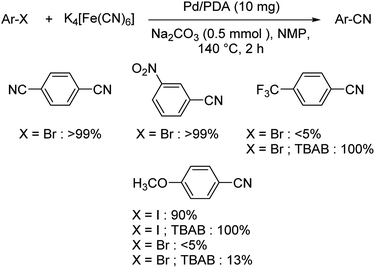 | ||
| Scheme 25 Substrate scope investigations of cyanation of aryl halides using Pd/PDA with and without TBAB. | ||
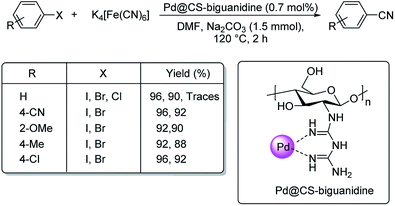
Haloarene cyanation using core–shell nanomaterial catalyst was established by Veisi in 2018.60 Here, a matrix of biguanidine-functionalized chitosan was impregnated with palladium NPs to form the core–shell-Pd@CS-biguanidine. The medium for cyanation was K4[Fe(CN)6] in solvent DMF at a temperature of 120 °C (Scheme 26). Excellent yields of the aryl nitriles were observed with high catalytic recyclability. A mechanistic route towards the products is also depicted (Scheme 27) where an oxidative addition of the haloarene takes place on the catalyst, followed by the transfer of cyanide ion from K4[Fe(CN)6]. In the end, a reductive elimination completes the reaction, rendering the cyanobenzenes.
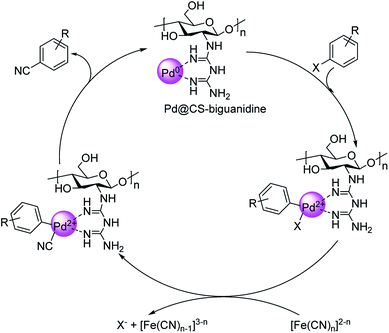 | ||
| Scheme 27 Plausible mechanism for the formation of aryl cyanides. [This figure has been reproduced from ref. 60 with permission from ELSEVIER, copyright 2020]. | ||
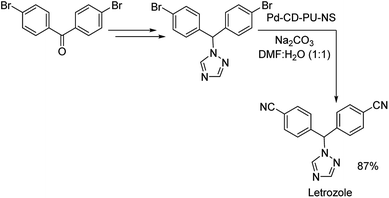
Nanosponge is one of the diverse forms of the available nanomaterials. Recently in 2019, a cyclodextrin-polyurethane based nanosponge was developed by Panahi and co-workers, where they immobilized palladium NPs to form Pd-CD-PU-NS.61 The catalyst was investigated for its efficiency in bringing about the cyanation of various aryl halides. Diverse functional groups were well tolerated and rendered good results (Scheme 28). Bis-halogenated substrates were also capable of affording the desired mono as well as bis-substituted compounds in good yields. Using the same catalyst, they could also synthesize a non-steroidal aromatase inhibitor letrozole in 87% overall yield (Scheme 29).
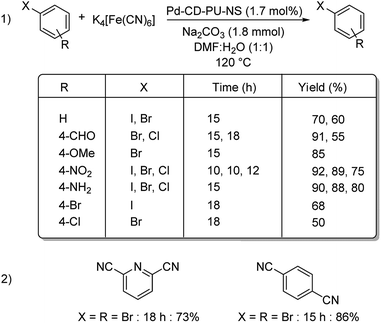 | ||
| Scheme 28 (1) Mono-cyanation reactions catalyzed by Pd-CD-PU-NS. (2) Synthesized bis-cyanated products by the same protocol. | ||
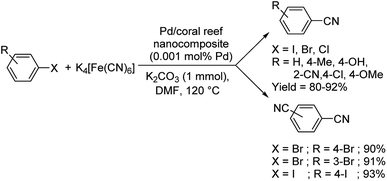
Nasrollahzadeh and co-workers established a lucid methodology for the formation of benzonitriles from haloarenes using Pd/coral reef nanocomposite and K4[Fe(CN)6].62 In this approach, the extract from Cucurbita pepo leaves was employed as a reducing agent for palladium, converting it from Pd(II) to Pd(0) and also as a stabilising medium (Scheme 30). Diverse haloarenes reacted to render the benzonitriles in excellent yields. Also, a double-addition was observed when there were two bromo or iodo groups in the aryl halides i.e., 1,4-dibromobenzene, 1,3-dibromobenzene and 1,4-diiodobenzene.
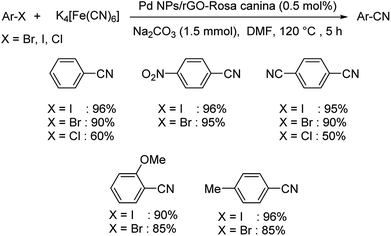
Rosa canina fruit extract modified graphene oxide decorated with palladium nanoparticles was showcased as a novel green catalyst for haloarene cyanation.63 Here also K4[Fe(CN)6] was the suitable cyanating medium. The optimised condition for the reaction was 0.5 mol% Pd nanoparticles (NPs)/reduced graphene oxide(rGO)-Rosa canina, 1.5 mmol Na2CO3 in DMF at 120 °C for 5 h (Scheme 31). It was observed that electronic factors were not much pronounced in this reaction. Iodo and bromoarenes displayed high reactivity providing excellent results while, chloroarenes reduced the yields of the products significantly. The catalyst was stable through seven catalytic cycles and thus displayed high efficiency. The proposed mechanism discusses an oxidative addition of the haloarene over the catalyst followed by the movement of the cyanide anion from the cyanating agent and lastly, a reductive elimination led to the formation of the desired product. This is similar to the mechanism discussed in Scheme 27, but there Pd@CS-biguanidine was used as the catalyst instead of PdNPs/rGO.
Veisi et al. discovered a hybrid material as catalyst like in the previous report with the only change in the plant extract used.64 Now they incorporated the extract of Thymbra spicata and developed Pd NPs/rGO-T. spicata. K4[Fe(CN)6] was again used as the cyanating agent. The catalyst was then evaluated for its cyanation capability and revealed that cyanation of a range of haloarenes was possible with similar substrate scope as above. Electronic as well as steric effects were tolerated imparting good yields. Here also it was noticed that bromo and iodoarenes required only lower reaction times than chloroarenes in affording the expected products.
Lately in 2020, palladium as Pd NPs@Fe3O4/chitosan/pumice hybrid beads for the effective cyanation of haloarenes for the formation of various benzonitriles was investigated by Baran.65 The Fe3O4/chitosan/pumice hybrid beads acted as the stabilising agent and was suitably decorated with the synthesized palladium nanoparticles. The cyanation was carried out in the presence of K4[Fe(CN)6] as the cyanating agent (Scheme 32). The efficiency of the catalyst was obvious from the yields of the benzonitriles formed. A maximum yield of 98% was reported and the catalyst displayed good recyclability.
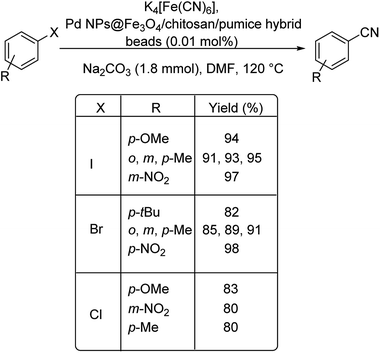 | ||
| Scheme 32 Synthesis of different aryl cyanide motifs from various aryl halides using Fe3O4/chitosan/pumice hybrid beads. | ||
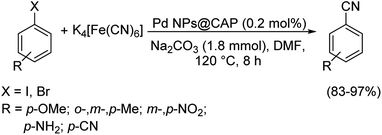
Baran and co-workers also prepared palladium nanoparticles on microbeads possessing chitosan, β-cyclodextrin and agarose forming the catalytic system-PdNPs@microcapsules (CAP).66 This was as before investigated for cyanation of haloarenes using K4[Fe(CN)6] as the cyanating agent (Scheme 33). Bromo and iodoarenes were successfully converted into different benzonitriles with good yields. The formation of no by-products, high catalytic efficiency and easy purification are the most acceptable traits of the reaction.
 | ||
| Scheme 35 Suggested mechanistic route towards the product synthesis. [This figure has been reproduced from ref. 67 with permission from AMERICAN CHEMICAL SOCIETY, copyright 2013]. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 THF
5 THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
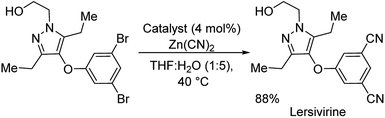
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solvent (Scheme 36). Electron-deficient and electron-rich substituents on aryl bromides were well tolerated. Aryl chloride underwent the reaction providing excellent yield. Various five and six-membered heterocyclic cyanides were also synthesized effectively. As a further application, the protocol was extended towards the synthesis of a reverse transcriptase inhibitor-lersivirine (Scheme 37). About 88% overall yield of lersivirine was reported.
H2O solvent (Scheme 36). Electron-deficient and electron-rich substituents on aryl bromides were well tolerated. Aryl chloride underwent the reaction providing excellent yield. Various five and six-membered heterocyclic cyanides were also synthesized effectively. As a further application, the protocol was extended towards the synthesis of a reverse transcriptase inhibitor-lersivirine (Scheme 37). About 88% overall yield of lersivirine was reported.
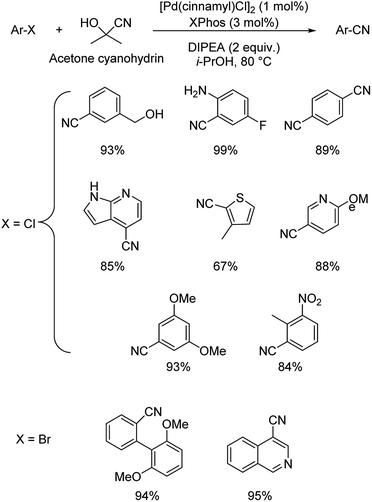
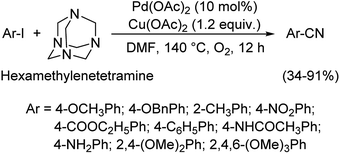
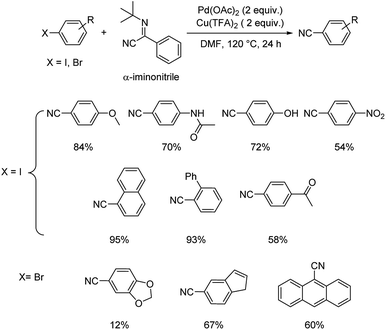
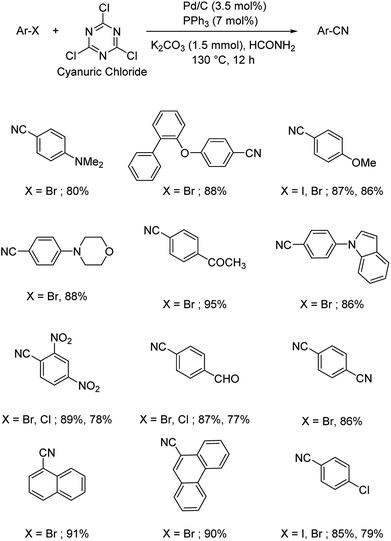
1.2. Reactions with organic cyanide sources
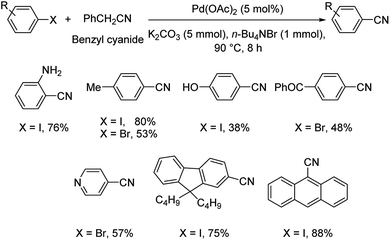
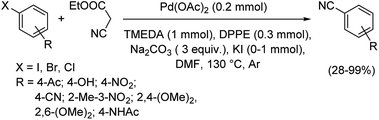
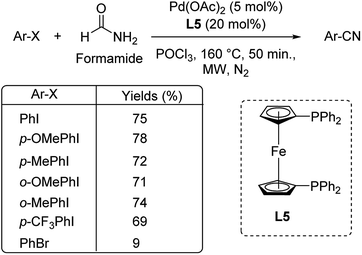
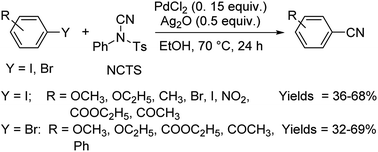
Different protocols utilizing various organic cyanide sources such as benzyl cyanide, ethyl cyanoacetate, acetone cyanohydrin, formamide, N-cyano-N-phenyl-p-toluenesulfonamide, α-iminonitrile, hexamethylenetetramine and cyanuric chloride, during the period 2012–2020 are discussed below.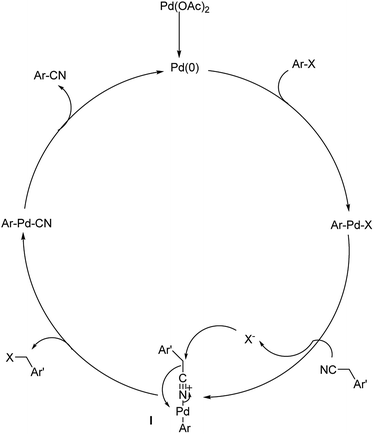 | ||
| Scheme 39 The plausible trajectory through which the reaction proceeds. [This figure has been reproduced from ref. 69 with permission from ROYAL SOCIETY OF CHEMISTRY, copyright 2020]. | ||
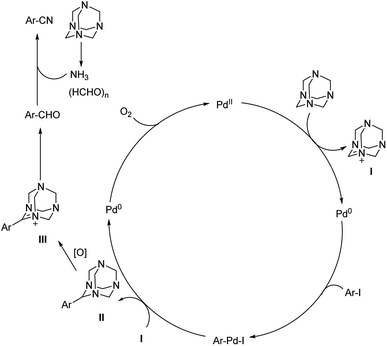 | ||
| Scheme 45 Synthetic pathway for the cyanation reaction carried out by HMTA. [This figure has been reproduced from ref. 75 with permission from AMERICAN CHEMICAL SOCIETY, copyright 2017]. | ||
2 Conclusions
Cyanations reactions have a pivotal role in synthetic organic chemistry, as it synthesizes aryl cyanides finding superior relevance in pharmaceuticals, natural products, industries etc. Recently, there have been many developments in the cyanation chemistry particularly, the ones catalyzed by transition metals. Palladium, copper and nickel are the major transition metals acting as catalysts for cyanation reactions. Palladium catalyzed cyanation has always gained much importance due to its high catalytic efficiency. Various palladium sources such as-palladium acetate, palladium chloride, ligand–palladium complexes, palladium nanoparticles, nanoshells, nanospheres, nanobeads, core–shells and so on have been widely employed. K4Fe(CN)6, Zn(CN)2, CuSCN, NCTS, α-iminonitrile, ethyl cyanoacetate etc. are the different cyanating media utilized. Among these, the studies have revealed that K4Fe(CN)6 is the one majorly used. This may be due to its innocuous nature, low cost and easy handling properties. Various investigations on the aryl halide cyanation using these catalysts and cyanation media are discussed briefly in the present review focussing mainly on the functional group compatibility, synthetic yields of the aryl cyanides and the mechanisms involved. Being a flexible functional group, the nitriles can be efficiently transformed into various other functionalities. Diverse approaches on their synthesis as well as transformations are under study. Aryl nitriles hence form a field with high applicability. Cyanation catalyzed by palladium, as summarized in this review, is a well emerging field having its very roots from the mid 1970's and it inspires scientists to prove their potential to finally realize some major outputs that impart high relevance to the scientific world. This may be projected as a practical and functional approach for the synthesis of aryl nitriles for diversely oriented disciplines.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MN and GA thank the Kerala State Council for Science, Technology and Environment (KSCSTE, Trivandrum) for the award of a research fellowship and a research grant (Order No. 341/2013/KSCSTE dated 15.03.2013) respectively. CMA and TA thank the Council of Scientific and Industrial Research (CSIR, New Delhi) for junior research fellowships.References
- F. F. Fleming, L. Yao, P. C. Ravikumar, L. Funk and B. C. Shook, J. Med. Chem., 2010, 53, 7902 CrossRef CAS.
- (a) S. I. Murahashi, Science of Synthesis, Georg Thieme, Stuttgart, 2004, vol. 19, p. 345 Search PubMed; (b) A. Kleemann, J. Engel, B. Kutscher and D. Reichert, Pharmaceutical Substances: Syntheses, Patents, Applications, Georg Thieme Verlag, Stuttgart, 4th edn, 2001, p. 241 Search PubMed.
- Industrial Biotransformations, ed. A. Liese, K. Seelbach and C. Wandrey, Wiley-VCH, Weinheim, Germany, 2nd edn, 2006 Search PubMed.
- G. Yana, Y. Zhang and J. Wang, Adv. Synth. Catal., 2017, 359, 4068 CrossRef.
- (a) J. Fatiadi, in Preparation and Synthetic Applications of Cyano Compounds, ed. S. Patai and Z. Rappoport, Wiley-VCH, New York, NY, 1983 Search PubMed; (b) Z. Rappoport, in Chemistry of the Cyano Group, John Wiley & Sons, London, UK, 1970, p. 121 Search PubMed.
- A. Pongratz, Monatsh. Chem., 1927, 48, 585 CrossRef CAS.
- T. Sandmeyer, Ber. Dtsch. Chem. Ges., 1884, 17, 1633 CrossRef.
- H. H. Hodgson, Chem. Rev., 1947, 40, 251 CrossRef CAS PubMed.
- K. W. Rosenmund and E. Struck, Ber. Dtsch. Chem. Ges., 1919, 52, 1749 CrossRef.
- C. F. Koelsch and G. G. Whitney, J. Org. Chem., 1941, 06, 795 CrossRef CAS.
- A. C. Stevenson, Ind. Eng. Chem., 1949, 41, 1846 CrossRef CAS.
- D. M. Tschaen, R. Desmond, A. O. King, M. C. Fortin, B. Pipik, S. King and T. R. Verhoeven, Synth. Commun., 1994, 24, 887 CrossRef CAS.
- R. Chidambaram, Tetrahedron Lett., 2004, 45, 1441 CrossRef CAS.
- C. Yang and J. M. Williams, Org. Lett., 2004, 6, 2837 CrossRef CAS.
- R. S. Jensen, A. S. Gajare, K. Toyota, M. Yoshifuji and F. Ozawa, Tetrahedron Lett., 2005, 46, 8645 CrossRef CAS.
- R. K. Arvela and N. E. Leadbeater, J. Org. Chem., 2003, 68, 9122 CrossRef CAS PubMed.
- U. S. Kanchana, T. V. Mathew and G. Anilkumar, J. Organomet. Chem., 2020, 920, 121337 CrossRef CAS.
- A. Xia, X. Xie, H. Chen, J. Zhao, C. Zhang and Y. Liu, Org. Lett., 2018, 20, 7735 CrossRef CAS PubMed.
- J. Zanon, A. Klapars and S. L. Buchwald, J. Am. Chem. Soc., 2003, 125, 2890 CrossRef CAS PubMed.
- Y. Ren, W. Wang, S. Zhao, X. Tian, J. Wang, W. Yin and L. Cheng, Tetrahedron Lett., 2009, 50, 4595 CrossRef CAS.
- M. Jiang, T. Yuan, F. Yi and J. Chen, ARKIVOC, 2016, (v), 13 Search PubMed.
- K. Takagi, T. Okamoto, Y. Sakakibara and S. Oka, Chem. Lett., 1973, 471 CrossRef CAS.
- K. Takagi, T. Okamoto, Y. Sakakibara, A. Ohno, S. Oka and N. Hayama, Bull. Chem. Soc. Jpn., 1975, 48, 3298 CrossRef CAS.
- A. Biffis, P. Centomo, A. D. Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249 CrossRef CAS.
- J. D. Wolfe and J. J. Li, in Chapter 1-An Introduction to Palladium Catalysis, ed. J. J. Li and G. W. Gribble, Elsevier, Amsterdam, Netherlands, 2007, vol. 26, p. 1 Search PubMed.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508 CrossRef.
- B. M. Trost and D. L. V. Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437 CrossRef.
- B. W. Michel, L. D. Steffens and M. S. Sigman, Org. React., 2014, 84, 2 Search PubMed.
- K. Sonogashira, J. Organomet. Chem., 2002, 653, 46 CrossRef CAS.
- V. L. M. Silva and A. M. S. Silva, Molecules, 2019, 24, 228 CrossRef.
- For more information on cyanating agents see- A. M. Nauth and T. Opatz, Org. Biomol. Chem., 2019, 17, 11 RSC.
- M. Sundermeier, A. Zapf and M. Beller, Eur. J. Inorg. Chem., 2003, 3513 CrossRef CAS.
- P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049 RSC.
- H. Jiang, J. Jiang, H. Wei and C. Cai, Catal. Lett., 2013, 143, 1195 CrossRef CAS.
- T. D. Senecal, W. Shu and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 1 CrossRef PubMed.
- A. S. Singh, S. S. Shendage and J. M. Nagarkar, Tetrahedron Lett., 2013, 54, 6319 CrossRef CAS.
- S. M. Sajadi, M. Maham and S. A. Mahmoud, J. Chem. Res., 2013, 37, 620 CrossRef CAS.
- R. Gläser and J. Weitkamp, The Application of Zeolites in Catalysis, in Basic Principles in Applied Catalysis; Springer Series in Chemical Physics, ed. M. Baerns, Springer, Berlin, Heidelberg, 2004, vol. 75 Search PubMed.
- T. L. Barr and M. A. Lishka, J. Am. Chem. Soc., 1986, 108, 3178 CrossRef CAS.
- B. Yilmaz and U. Müller, Top. Catal., 2009, 52, 888 CrossRef CAS.
- T. V. Magdesieva, O. M. Nikitin, E. V. Zolotukhina and M. A. Vorotyntsev, Electrochim. Acta, 2014, 122, 289 CrossRef CAS.
- L. Fu, X. Li, Q. Zhu, S. Chen, Y. Kang and M. Guo, Appl. Organomet. Chem., 2014, 28, 699 CrossRef CAS.
- T. Chatterjee, R. Dey and B. C. Ranu, J. Org. Chem., 2014, 79, 5875 CrossRef CAS.
- A. R. Hajipour and F. Rafiee, J. Iran. Chem. Soc., 2014, 11, 1391 CrossRef CAS.
- B. Karimi, A. Zamani and F. Mansouri, RSC Adv., 2014, 4, 57639 RSC.
- Y. Tu, Y. Zhang, S. Xu, Z. Zhang and X. Xie, Synlett, 2014, 25, 2938 CrossRef CAS.
- M. Gholinejad and A. Aminianfar, J. Mol. Catal. A: Chem., 2015, 397, 106 CrossRef CAS.
- Z. Xu, Y. Xiao, H. Ding, C. Cao, H. Li, G. Pang and Y. Shi, Synthesis, 2015, 47, 1560 CrossRef CAS.
- B. S. Kumar, A. J. Amali and K. Pitchumani, ACS Appl. Mater. Interfaces, 2015, 7, 22907 CrossRef PubMed.
- B. Mondal, K. Acharyya, P. Howlader and P. S. Mukherjee, J. Am. Chem. Soc., 2016, 138, 1709 CrossRef CAS PubMed.
- O. M. Nikitin, O. V. Polyakova, P. K. Sazonov, A. V. Yakimansky, M. Y. Goikhman, I. V. Podeshvo and T. V. Magdesieva, New J. Chem., 2016, 40, 10465 RSC.
- B. Majhi and B. C. Ranu, Org. Lett., 2016, 18, 4162 CrossRef CAS.
- Z.-C. Wu, Q. Yang, X. Ge, Y.-M. Ren, R.-C. Yang and T.-X. Tao, Catal. Lett., 2017, 147, 1333 CrossRef.
- S. Zhang, H. Neumann and M. Beller, Chem.–Eur. J., 2018, 24, 67 CrossRef CAS PubMed.
- V. Kandathil, R. B. Dateer, B. S. Sasidhar, S. A. Patil and S. A. Patil, Catal. Lett., 2018, 148, 1562 CrossRef CAS.
- H. Veisi, S. Hemmati and P. Safarimehr, J. Catal., 2018, 365, 204 CrossRef CAS.
- T. Gazdag, A. Kunfi and G. London, React. Kinet., Mech. Catal., 2018, 125, 567 CrossRef CAS.
- H. Veisi, Polyhedron, 2018, 159, 212 CrossRef.
- S. K. Dangolani, S. Sharifat, F. Panahi and A. Khalafi-Nezhad, Inorg. Chim. Acta, 2019, 494, 256 CrossRef.
- M. Nasrollahzadeh, F. Ghorbannezhad, S. M. Sajadi and R. S. Varma, Nanomaterials, 2019, 9, 565 CrossRef CAS PubMed.
- S. Hemmati, A. Sedrpoushan, N. Alizadeh, K. Khosravi and M. Hekmati, Appl. Organomet. Chem., 2019, 33, e5103 CrossRef.
- H. Veisi, T. Tamoradi, B. Karmakar, P. Mohammadi and S. Hemmati, Mater. Sci. Eng., C, 2019, 104, 109919 CrossRef CAS PubMed.
- T. Baran, Carbohydr. Polym., 2020, 237, 116105 CrossRef CAS PubMed.
- T. Baran and M. Nasrollahzadeh, Int. J. Biol. Macromol., 2020, 148, 565 CrossRef CAS PubMed.
- G.-Y. Zhang, J.-T. Yu, M.-L. Hu and J. Cheng, J. Org. Chem., 2013, 78, 2710 CrossRef CAS PubMed.
- D. T. Cohen and S. L. Buchwald, Org. Lett., 2015, 17, 202 CrossRef CAS PubMed.
- Q. Wen, J. Jin, B. Hu, P. Lu and Y. Wang, RSC Adv., 2012, 2, 6167 RSC.
- S. Zheng, C. Yu and Z. Shen, J. Org. Chem., 2013, 78, 2710 CrossRef.
- D. N. Sawant and B. M. Bhanage, J. Chem. Sci., 2014, 126, 319 CrossRef CAS.
- S. Mo, Synlett, 2014, 25, 1337 CrossRef CAS.
- J. Li, W. Xu, J. Ding and K.-H. Lee, Tetrahedron Lett., 2016, 57, 1205 CrossRef CAS.
- F. Burg, J. Egger, J. Deutsch and N. Guimond, Org. Process Res. Dev., 2016, 20, 1540 CrossRef CAS.
- Y. Yan, S. Sun and J. Cheng, J. Org. Chem., 2017, 82, 12888 CrossRef CAS PubMed.
- Y.-L. Shi, Q. Yuan, Z.-B. Chen, F.-L. Zhang, K. Liu and Y.-M. Zhu, Synlett, 2018, 29, 359 CrossRef CAS.
- E. Niknam, F. Panahi and A. Khalafi-Nezhad, Eur. J. Org. Chem., 2020, 2020, 2699 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |