Open Access Article
Open Access ArticleLithium bromide: an inexpensive and efficient catalyst for imine hydroboration with pinacolborane at room temperature†
Hanbi Kim,
Hyun Tae Kim,
Ji Hye Lee ,
Hyonseok Hwang
,
Hyonseok Hwang and
Duk Keun An
and
Duk Keun An *
*
Department of Chemistry, Kangwon National University, Institute for Molecular Science and Fusion Technology, Chunchon, 24341, Republic of Korea. E-mail: dkan@kangwon.ac.kr
First published on 17th September 2020
Abstract
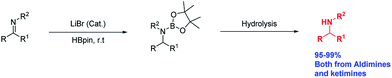
An efficient protocol for the hydroboration of imines is reported. Lithium halide salts are effective catalysts to convert aldimines and ketimines to their corresponding amines. Here, we report excellent isolated yield of secondary amines (>95%) using 3 mol% lithium bromide in THF at room temperature. In addition, DFT calculations for a plausible reaction pathway are reported.
Introduction
The hydroboration reaction was first reported in 1956 by H. C. Brown and co-workers.1 Thereafter, numerous alcohols and amines have been synthesized through hydroboration/borohydride reduction of carbonyls, imines and unsaturated hydrocarbons using boron hydride reagents such as NaBH4 and LiBH4.2Amines are ubiquitous functional groups; they are found in many natural products and are used as building blocks for the production of agrochemicals, pharmaceuticals, polymers, and dyes.3 Therefore, the synthesis of amines and their derivatives has gained significant attention in chemistry and biology.
Catalytic hydroboration of imines is a simple and straight-forward method for the preparation of amines, unlike conventional hydrogenation reactions and stoichiometric reductions, which are carried out at high pressure and temperature.4 Several catalytic systems for imine hydroboration have been reported, including the use of transition metals (e.g., Ti, Co, Ni, Ru),5 rare-earth metal complexes (Er, Y, Dy, Gd),6 an actinide group metal (Th),7 main group metals (Li, Na, Mg, Al, etc.),8 non-metals (P, B),9 and frustrated Lewis pairs.10
Recently, the hydroboration of imines has also been realized under catalyst-free conditions. In 2019, Pandey et al. reported the catalyst-free, solvent-free hydroboration of imines using pinacolborane (HBpin).11 However, although this method provided amines from aldimines in good to reasonable yields, the hydroboration of ketimines proceeded with lower yields, even under high temperatures and prolonged reaction times.
Speed et al. reported hydroboration of ketimines promoted by stoichiometric amounts of protic additives such as alcohols or water. The method provided good yields for aliphatic imines, while aniline derived imines (electron deficient substituents) were highly ineffective. The dehydrocoupling of pinacolborane with additives is the major issue in this protocol.12
Thus, methods that achieve efficient hydroboration using sustainable protocols are necessitated.
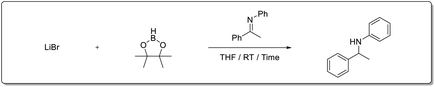
Recently, our group reported that Li+ salts13 are superior and effective catalysts for the hydroboration of carbonyl compounds.14 Prompted by those results, we decided to extend the methodology to the hydroboration of other functional groups. Herein, we report an economical method for the hydroboration of imines with pinacolborane and LiBr under mild conditions to afford excellent yields of secondary amines from both aldimines and ketimines (Scheme 1).
Results and discussion
First, we investigated the hydroboration of N-benzylideneaniline with pinacolborane using various Li, Na, and K metal halide salts in THF at room temperature (Table 1). The Li+ salts—LiCl, LiBr, and LiI showed superior catalytic activity after 30 min reaction time (entries 2–4). Among these salts, LiBr was chosen as the catalyst for further optimization because of its easy availability and cost-effectiveness.| Entity | Cat | Time | Yielda (%) (imine/amine) |
|---|---|---|---|
| a Yields were determined by GC. The values in parenthesis belong to those for aldehyde. | |||
| 1 | LiF | 1 h | 48/37 (15) |
| 2 | LiCl | 30 min | 0/99 |
| 3 | LiBr | 30 min | 0/99 |
| 4 | Lil | 30 min | 0/99 |
| 5 | NaF | 1 h | 51/45 (4) |
| 6 | Nacl | 1 h | 52/39 (7) |
| 7 | NaBr | 1 h | 66/30 (4) |
| 8 | Nal | 1 h | 4/96 |
| 9 | KF | 1 h | 47/38 (15) |
| 10 | KCl | 1 h | 55/28 (15) |
| 11 | KBr | 1 h | 77/18 (3) |
| 12 | Kl | 1 h | 33/50 (16) |
Moreover, further optimization of the reaction conditions was conducted by adjusting the catalyst loading, pinacolborane stoichiometry, type of solvent, and reaction time. The results are summarized in Table 2. When hydroboration was carried out in the absence of a catalyst, only 24% yield of the desired product (N-benzylaniline) was afforded after 1 h (entry 1, Table 2). As expected, a dramatic improvement was observed in the presence of LiBr (3.0 mol%), affording quantitative conversion to the product (entry 6). Only moderate conversions were achieved with a reduced catalyst loading (entry 2), lower stoichiometries of pinacolborane (entries 3 and 4) and a shorter reaction time (entry 5). Next, the reaction was optimized in various solvent systems including n-hexane, toluene, diethyl ether, and dichloromethane (entries 7–10). Both diethyl ether and dichloromethane afforded yield comparable to THF, whereas the reaction performed poorly in n-hexane and toluene. We also conducted hydroboration of imine in wet THF (trace amounts of water/tBuOH with THF respectively under catalyst-free condition). A similar result (lower conversions) was observed from aniline derived imines as suggested by Speed et al. Consequently, the optimal reaction conditions for the hydroboration of N-benzylideneaniline was determined to be 3.0 mol% of LiBr, 1.5 equiv. of HBpin in THF with a reaction time of 1 h (entry 6).
| Entry | LiBr (mol%) | Pinacolborane (equiv.) | Solvent (0.5 ml) | Time | Yielda (%) (S. M/product) |
|---|---|---|---|---|---|
| a Yields were determined by GC. The values in parenthesis belong to those for aldehyde. | |||||
| 1 | none | 1.5 | THF | 1 h | 71/24 (2) |
| 2 | 1.0 | 1.5 | THF | 1 h | 45/56 (2) |
| 3 | 3.0 | 1.2 | THF | 1 h | 16/76 (8) |
| 4 | 3.0 | 1.2 | THF | 3 h | 21/73 (4) |
| 5 | 3.0 | 1.5 | THF | 30 min | 13/82 (2) |
| 6 | 3.0 | 1.5 | THF | 1 h | 0/100 |
| 7 | 3.0 | 1.5 | Hexane | 1 h | 86/7 (4) |
| 8 | 3.0 | 1.5 | Toluene | 1 h | 52/40 (4) |
| 9 | 3.0 | 1.5 | Ether | 1 h | 1/95 |
| 10 | 3.0 | 1.5 | MC | 1 h | 6/87 (1) |
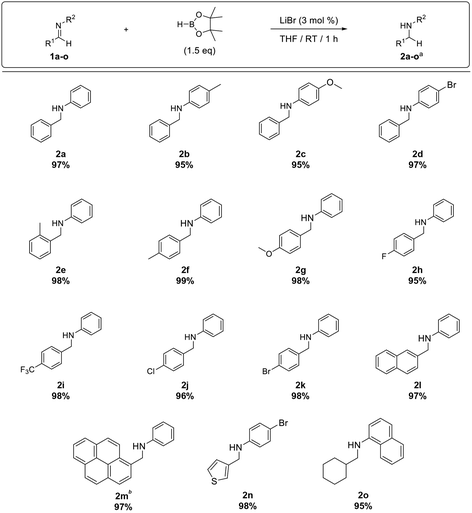
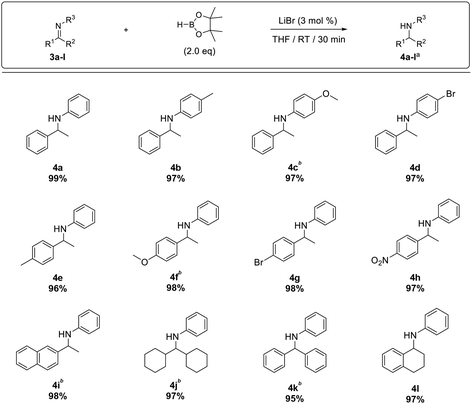
Furthermore, the substrate scope was extended to aldimines 1b–o (Table 3). We were pleased to observe that all aldimines tested underwent smooth hydroboration to afford the corresponding amines in excellent yield (2b–k). The reaction was equally efficient with aldimines bearing electron-withdrawing substituents (1d, and 1h–k) and electron-donating substituents (1b, 1c, and 1e–g). Furthermore, polyaromatic imines 1l and 1m underwent hydroboration to afford the respective amines 2l and 2m in excellent yield. Although pyrenyl imine 1m required a 3 h reaction time for complete conversion, it afforded amine 2m in 97% isolated yield. In addition, the hydroboration of heteroaromatic imine 1n and aliphatic imine 1o proceeded smoothly, affording the respective amines 2n and 2o in excellent yield.
Next, the hydroboration of ketimines was investigated using N-(1-phenylethylidene)aniline (Table 4). We observed that 20% of the starting imine remained unreacted (entry 1, Table 4), and increasing the reaction time from 1 to 3 h showed only a marginal improvement in the conversion with 1.5 equiv. of HBpin (entry 2). Finally, with 2.0 equiv. of HBpin and 3 mol% LiBr, the ketimine underwent hydroboration smoothly to afford the desired product in 99% yield over 30 min reaction time (entry 4, Table 4).
Next, the substrate scope was extended to a range of ketimines (3b–l). Substrates bearing electron-withdrawing groups, e.g. 4-bromo (3d and 3g) and 4-nitro (3h), and electron-donating substitutions, e.g. 4-methyl (3b and 3e) and 4-methoxy (3c and 3f), afforded their corresponding amines in excellent yield, although the ketimines bearing a 4-methoxy substituent required 3.0 equiv. of HBpin for complete conversion. Similarly, 3.0 equiv. of HBpin was required to convert polyaromatic (3i) and sterically hindered imines (3j–k) to the desired amines (4i–k) in high yield. However, dihydronaphthalene imine 3l underwent smooth hydroboration to afford the corresponding amine 4l in 97% yield with 2.0 equiv. of pinacolborane (Table 5). The use of excess HBpin in the reaction is mainly due to the decomposition of HBpin to B2(pin)3 during the reduction (11B NMR of crude reaction, refer S62 in ESI†) which was observed comparatively more in ketamine hydroboration.
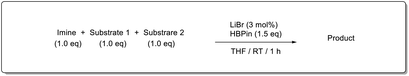
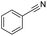
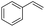
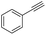
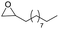
Finally, we investigated the chemoselectivity of our protocol. Accordingly, N-benzylideneaniline was treated with 3.0 equiv. of HBpin and 3 mol% LiBr in the presence of other reducible functional groups, such as esters and amides as well as nitrile, alkene, alkyne, alkyl halide, and epoxide moieties (Table 6). In all cases, our protocol exhibited high chemoselectivity toward imine hydroboration and excellent yield.
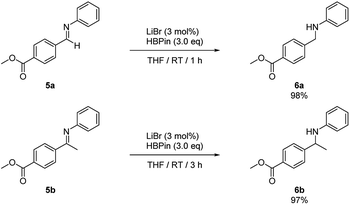
In addition, intramolecular hydroboration of imines was analyzed in the presence of an ester group. Both aldimine (5a) and ketamine (5b) proceeded selective hydroboration with 3 mol% LiBr to afford desired amines 6a and 6b in 98% and 97% yield, respectively, by leaving the ester group unreacted (Scheme 3).
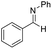
The reaction pathway for LiBr catalyzed hydroboration of aldimine (PhHCNPh) was explored using density functional theory (DFT) calculations at the M06-2X/6-31+G(d,p) level of theory. The electronic energy profile for the reaction pathway is illustrated in Scheme 2. The mechanism was predicted based on recent work done by Thomas15 et al., Clark16 et al., and Wuesthoff17 et al. Accordingly, HBpin undergoes reaction with LiBr catalyst initially to produce the hydridoborate intermediate INT1.15,16 The subsequent reaction of the INT1 with aldimine generates the INT2 which turns into the INT3 through a hexagonal ring transition state TS1. INT3 then undergoes a unimolecular transformation into the INT4 through a four-membered ring transition state TS2. Upon the reaction of INT4 with HBpin via ligand exchange, providing desired substrate dioxolan amine with regeneration of INT1 for the next catalytic cycle (Scheme 4).
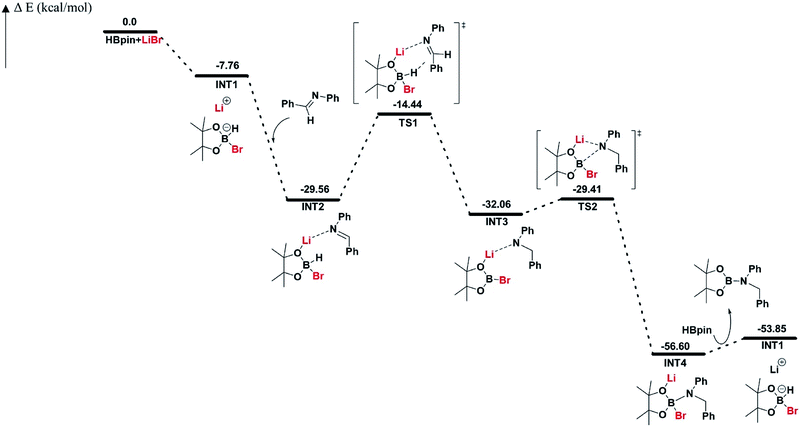 | ||
| Scheme 2 Free energy profile (in kcal mol−1) for LiBr catalyzed hydroboration of aldimine (PhHCNPh). | ||
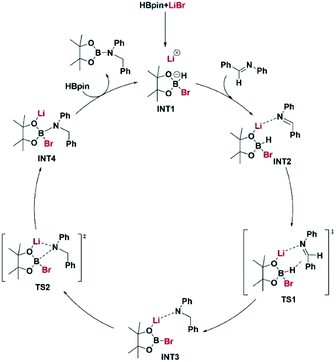 | ||
| Scheme 4 A plausible mechanism based on the energy profiles shown in Scheme 2. Coordinates for the intermediate and transition state structures are provided in ESI.† | ||
Conclusions
We demonstrated that LiBr, an inexpensive, mild, and stable reagent, efficiently catalyzes the hydroboration of imines at room temperature. Various imines (aldimines and ketimines) successfully underwent hydroboration with HBpin in the presence of 3 mol% LiBr to afford the desired amines in excellent yield. Furthermore, we demonstrated the high chemoselectivity of our protocol. DFT calculations for a plausible reaction pathway are reported. The proposed method involving the use of a very mild and easy-to-handle catalyst (LiBr) is a potential, industrially viable protocol for the preparation of secondary amines through the hydroboration of imines.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2017R1D1A1B03035412 for D. K. An and 2020R1I1A1A01073381 for J. H. Lee).Notes and references
- H. C. Brown and B. S. Rao, A new technique for the conversion of olefins into organoboranes and related alcohols, J. Am. Chem. Soc., 1956, 78, 5694 CrossRef CAS.
- (a) H. C. Brown, B. C. Subba Rao and I. Hydroboration, The reaction of olefins with sodium borohydride-aluminum chloride. a convenient route to organoboranes and the anti-Markownikoff hydration of olefins, J. Am. Chem. Soc., 1959, 81(24), 6423 CrossRef CAS; (b) H. C. Brown and S. Krishnamurthy, Forty years of hydride reductions, Tetrahedron, 1979, 35, 567 CrossRef CAS; (c) H. C. Brown, Y. M. Choi and S. Narasimhan, Convenient procedure for the conversion of sodium borohydride into lithium borohydride in simple ether solvents, Inorg. Chem., 1981, 20, 4454 CrossRef CAS; (d) N. Satyanarayana and M. Periyssamy, Hydroboration or hydrogenation of alkenes with CoCl2–NaBH4, Tetrahedron Lett., 1984, 25, 2501 CrossRef CAS; (e) J. H. Billman and A. C. Diesing, Reduction of schiff bases with sodium borohydride, J. Org. Chem., 1957, 22, 1068 CrossRef CAS.
- (a) T. C. Nugent and M. El-Shazly, Chiral amine synthesis–recent developments and trends for enamide reduction, reductive amination, and imine reduction, Adv. Synth. Catal., 2010, 352, 753 CrossRef CAS; (b) C. Pubill-Ulldemolins, A. Bonet, C. Bo, H. Gulyás and E. Fernández, A new context for palladium mediated B-addition reaction: an open door to consecutive functionalization, Org. Biomol. Chem., 2010, 8, 2667 RSC; (c) C. C. Chong and R. Kinjo, Catalytic hydroboration of carbonyl derivatives, imines, and carbon dioxide, ACS Catal., 2015, 5, 3238 CrossRef CAS.
- (a) K. Hayes, Industrial processes for manufacturing amines, Appl. Catal., A, 2001, 221, 187 CrossRef CAS; (b) T. E. Muller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Hydroamination: direct addition of amines to alkenes and alkynes, Chem. Rev., 2008, 108, 3795 CrossRef; (c) S. Nishimura, Handbook of heterogeneous catalytic hydrogenation for organic synthesis, Wiley New York etc, 2001 Search PubMed; (d) J. G. de Vries and C. J. Elsevier, Handbook of homogeneous hydrogenation, WeinheimWiley-VCH9783527311613, 2007 Search PubMed.
- (a) C. C. Chong and R. Kinjo, Catalytic hydroboration of carbonyl derivatives, imines, and carbon dioxide, ACS Catal., 2015, 5, 3238 CrossRef CAS; (b) A. Kaithal, B. Chatterjee and C. Gunanathan, Ruthenium-catalyzed selective hydroboration of nitriles and imines, J. Org. Chem., 2016, 81, 11153 CrossRef CAS; (c) J. Wu, H. Zeng, J. Cheng, S. Zheng, J. A. Golen, D. R. Manke and G. Zhang, Cobalt(II) coordination polymer as a precatalyst for selective hydroboration of aldehydes, ketones, and imines, J. Org. Chem., 2018, 83, 9442 CrossRef CAS.
- Z. Huang, S. Wang, X. Zhu, Q. Yuan, Y. Wei, S. Zhou and X. Mu, Well-Defined amidate-functionalized N-heterocyclic carbene-supported rare-earth metal complexes as catalysts for efficient hydroboration of unactivated imines and nitriles, Inorg. Chem., 2018, 57, 15069 CrossRef CAS.
- S. Saha and M. S. Eisen, Catalytic recycling of a Th–H bond via single or double hydroboration of inactivated imines or nitriles, ACS Catal., 2019, 9, 5947 CrossRef CAS.
- (a) K. Manna, P. Ji, F. X. Greene and W. Lin, Metal–organic framework nodes support single-site magnesium–alkyl catalysts for hydroboration and hydroamination reactions, J. Am. Chem. Soc., 2016, 138, 7488 CrossRef CAS; (b) Y. Wu, C. Shan, J. Ying, J. Su, J. Zhu, L. L. Liu and Y. Zhao, Catalytic hydroboration of aldehydes, ketones, alkynes and alkenes initiated by NaOH, Green Chem., 2017, 19, 4169 RSC; (c) V. A. Pollard, M. Á. Fuentes, A. R. Kennedy, R. McLellan and R. E. Mulvey, Comparing neutral (monometallic) and anionic (bimetallic) aluminum complexes in hydroboration catalysis: influences of lithium cooperation and ligand set, Angew. Chem., Int. Ed., 2018, 57, 10651 CrossRef CAS; (d) A. K. Jaladi, H. Kim, J. H. Lee, W. K. Shin, H. Hwang and D. K. An, Lithium diisobutyl-tert-butoxyaluminum hydride (LDBBA) catalyzed hydroboration of alkynes and imines with pinacolborane, New J. Chem., 2019, 43, 16524 RSC; (e) D. Yan, X. Wu, J. Xiao, Z. Zhu, X. Xu, X. Bao, Y. Yao, Q. Shen and M. Xue, n-Butyllithium catalyzed hydroboration of imines and alkynes, Org. Chem. Front., 2019, 6, 648 RSC.
- (a) Y. Lin, E. Hatzakis, S. M. McCarthy, K. D. Reichl, T. Lai, H. P. Yennawar and A. T. Radosevich, P–N Cooperative borane activation and catalytic hydroboration by a distorted phosphorous triamide platform, J. Am. Chem. Soc., 2017, 139, 6008 CrossRef CAS; (b) C. Tien, M. R. Adams, M. J. Ferguson, E. R. Johnson and A. W. Speed, Hydroboration catalyzed by 1,2,4,3-triazaphospholenes, Org. Lett., 2017, 19, 5565 CrossRef CAS; (c) T. Lundrigan, E. N. Welsh, T. Hynes, C. Tien, M. R. Adams, K. R. Roy, K. N. Robertson and A. W. Speed, Enantioselective imine reduction catalyzed by phosphenium ions, J. Am. Chem. Soc., 2019, 141, 14083 CrossRef CAS.
- P. Eisenberger, A. M. Bailey and C. M. Crudden, Taking the F out of FLP: simple Lewis acid–base pairs for mild reductions with neutral boranes via borenium ion catalysis, J. Am. Chem. Soc., 2012, 134, 17384 CrossRef CAS.
- V. K. Pandey, S. N. R. Donthireddy and A. Rit, Catalyst-free and solvent-free facile hydroboration of imines, Chem.–Asian J., 2019, 14, 3255 CrossRef CAS.
- B. S. N. Huchenski and A. W. H. Speed, Protic additives or impurities promote imine reduction with pinacolborane, Org. Biomol. Chem., 2019, 17, 1999 RSC.
- J. H. Kim, A. K. Jaladi, H. T. Kim and D. K. An, Lithium tert-butoxide-catalyzed hydroboration of carbonyl compounds, Bull. Korean Chem. Soc., 2019, 40, 971 CrossRef CAS.
- (a) M. M. Mojtahedi, E. Akbarzadeh, R. Sharifi and M. S. Abaee, Lithium bromide as a flexible, mild, and recyclable reagent for solvent-free Cannizzaro, Tishchenko, and Meerwein–Ponndorf–Verley reactions, Org. Lett., 2007, 9, 2791 CrossRef CAS; (b) R. Patel, V. P. Srivastava and L. D. S. Yadav, The first example of saccharin-lithium bromide catalysis: direct synthesis of N-tosylimines from alcohols, Adv. Synth. Catal., 2010, 352, 1610 CrossRef CAS; (c) C. N. Rao, C. Bentz and H. Reissig, Lithium bromide/water as additives in dearomatizing samarium-ketyl (hetero) arene cyclizations, Chem.–Eur. J., 2015, 21, 15951 CrossRef CAS.
- A. D. Bage, T. A. Hunt and S. P. Thomas, Hidden boron catalysis: nucleophile-promoted decomposition of HBpin, Org. Lett., 2020, 22(11), 4107 CrossRef CAS.
- I. P. Query, P. A. Squier, E. M. Larson, N. A. Isley and T. B. Clark, Alkoxide-catalyzed reduction of ketones with pinacolborane, J. Org. Chem., 2011, 76, 6453 CrossRef.
- B. Rickborn and M. T. Wuesthoff, Kinetics, stereochemistry, and mechanism of the sodium borohydride reduction of alkyl-substituted cyclohexanones, J. Am. Chem. Soc., 1970, 92, 6894 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06023b |
| This journal is © The Royal Society of Chemistry 2020 |