Open Access Article
Open Access ArticleHKUST-1 derived Cu@CuOx/carbon catalyst for base-free aerobic oxidative coupling of benzophenone imine: high catalytic efficiency and excellent regeneration performance†
Huang Kaimenga,
Chen Siyuanb,
Xia Changjiua,
Li Chenhaoa,
Zhu Bina,
Gao Hongyi b,
Peng Xinxina,
Lin Mina,
Luo Yibin
b,
Peng Xinxina,
Lin Mina,
Luo Yibin *a,
Wang Ge
*a,
Wang Ge *b and
Shu Xingtiana
*b and
Shu Xingtiana
aState Key Laboratory of Catalytic Materials and Reaction Engineering, Research Institute of Petroleum Processing, SINOPEC, 100083, Beijing, The People's Republic of China. E-mail: luoyibin.ripp@sinopec.com
bBeijing Advanced Innovation Center for Materials Genome Engineering, School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, The People's Republic of China. E-mail: gewang33@126.com
First published on 1st October 2020
Abstract
The oxidative coupling of imines to ketazine with molecular oxygen is a green process towards the synthesis of hydrazine or hydrazine hydrate, which could efficiently address the economic and environmental issues of the traditional Raschig or peroxide-ketazine process. Herein, we developed an efficient heterogeneous base-free benzophenone imine oxidative coupling route with O2 catalyzed by Cu/CuOx/carbon materials derived from MOFs under mild conditions. Under optimized conditions, the conversion of BI is up to 98.2% and the selectivity of ketamine is 94.9%. This catalyst has excellent structure stability, recycling, and regeneration performance, owing to the carbonization of organic ligands of MOF at high temperature. More importantly, it is confirmed that the metallic Cu core is essential to improve the catalytic performance of the CuO shell in the BI oxidative coupling reaction, due to the promotion of electron transfer in the CuO surface, making dissolved O2 molecules more easily insert oxygen vacancies. This strategy might open an avenue to the sustainable catalytic synthesis of hydrazine or hydrazine hydrate.
1 Introduction
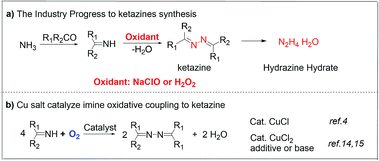
The oxidative coupling of imines (especially for benzophenone imine, abbreviated as BI) provides a remarkable novel and green approach to prepare ketazines, which are the key intermediates for the synthesis of hydrazine (N2H4) or hydrazine hydrate (N2H4·H2O).1–4 N2H4 and N2H4·H2O are widely used as polymer blowing agents,5,6 reducing reagents,7,8 precursors of pharmaceuticals,9,10 and high-energy fuels.11 Traditionally, N2H4·H2O is majorly manufactured by the highly polluting Raschig or urea-based Raschig process (Scheme 1a).1,12 Although H2O2 solution has been used as a clean oxidant to produce ketazines,13 the peroxide-ketazine process still suffers severe economic and environmental issues, due to the high price of H2O2 and the generation of N-containing wastewater.13 A fascinating alternative catalytic imine oxidative coupling route with O2 as sole oxidant, based on Hayashi's CuCl/ZnCl2 or CuCl2/organic base catalytic systems, have been developed (Scheme 1b). But it is restricted by catalyst separation/recycling and alkaline waste issues.4,14Recently, metal–organic frameworks (MOFs) as precursors provide a promising platform for preparing efficient carbon supported metal or metal oxide catalysts due to their high surface area, highly ordered characteristics of metal ions, tunable porosity, and multi-functionality.15,16 Due to their advantages of excellent electron conductivity, high porosity of carbon-supported metal materials derived from MOFs, they are introduced with promising applications in batteries,17 supercapacitors,18,19 electrocatalytic14 and other reactions.20 Among of them, carbon-supported Cu materials derived from Cu-MOFs have been widely concerned, attribute to the valence changing ability between copper and its oxides, as well as the absorption and activation ability of Cu and CuOx to H2, O2, CO and other small molecules. Recently, Wu group obtained a new Cu-MOF derived carbon-supported Cu/Cu2O materials by tuning copper ligands and pyrolytic conditions, which demonstrating excellent catalytic properties in liquid-phase hydrogenation of furfural into furfuryl alcohol.21 Lu group22 and Chen group23 independently found that Cu/CuOx/C materials derived from Cu–BTC can also be applied to catalyze CO oxidation reaction. Cu/CuOx/C materials with various morphologies or sizes have important influence on catalytic activity. The significant increase in electron density on the Cu/Cu2O and Cu/CuO interface also play a pivotal role in the enhancement of CO oxidation. Although progress have been made in the field of Cu-MOFs derivatives catalysis, the types of catalytic reactions are extremely limited. In particular, there are few reports on the synthesis of organic chemicals by MOFs derivatives catalytic oxidation in liquid phase.24,25
Herein, to address the drawbacks in the synthesis of hydrazine hydrate, inspired by homogeneous Cu catalytic systems,14,26 a heterogeneous Cu-MOF derived Cu@CuOx nanoparticles supported on carbon matrix (Cu@CuOx/carbon) catalyst prepared via the direct anaerobic pyrolysis of HKUST-1 precursor in N2 or H2/Ar atmosphere (see Fig. 1a),20,27–30 which exhibits excellent catalytic performance in the aerobic oxidative coupling of benzophenone imine without adding any organic base.24,31–33 Under optimized conditions, the conversion of benzophenone imine was up to 98.2% and the selectivity of ketazine was 94.9%. More importantly, this catalyst has excellent structure stability, recycling and regeneration performance, owing to the carbonization of organic ligands of MOF at high temperature.29,34 Consequently, this catalytic route unfolds a novel viewpoint on efficient heterogeneous preparation of ketazines, which has great industrial application potential and academic meaning.
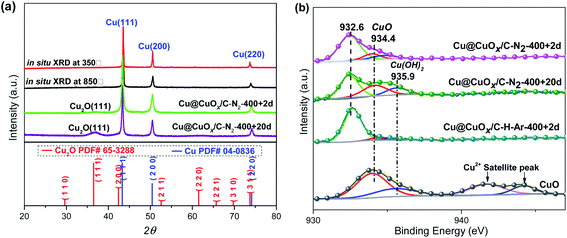 | ||
| Fig. 1 (a) XRD and in situ XRD patterns of HKUST-1 derivatives; (b) XPS spectrums of HKUST-1 derivatives and CuO. | ||
2 Experimental
2.1 Materials
All the reagents and solvents were commercially available and used for the synthesis without further purification. Copper nitrate trihydrate (Cu(NO3)2·3H2O, AR, Sinopharm Chemical Reagent Co., Ltd.), 1,3,5-benzenetricarboxylic acid (BTC, 98%, Aladdin), ethanol (EtOH, AR, Sinopharm Chemical Reagent Co., Ltd.), and N,N-dimethylformamide (DMF, AR, Sinopharm Chemical Reagent Co., Ltd.) were used as received.2.2 Catalyst preparation
The HKUST precursor was prepared based on the reported literature with some modifications.28 First, 0.48 g Cu(NO3)2·3H2O was added into 24 mL H2O to prepare solution A. And 0.36 g benzene-1,3,5-tricarboxylic acid (BTC) was added into the mixture of 12 mL ethanol and 12 mL N,N-dimethylformamide to prepare solution B. Then solution A was added into solution B with stir constantly for 30 min. The mixed solution was transferred into 100 mL Teflon-lined stainless steel autoclave and heated at 85 °C for 24 h. The obtained precipitate was washed with DMF and methanol several times, then dried at 80 °C for 12 h. Then the blue HKUST-1 (Cu3BTC2) product was obtained.35 1 g HKUST was added into a crucible with a cover. The HKUST-1 was heated to the specific temperatures and kept for 2 h under N2 or H2/Ar atmosphere. Both the heat and cool rate was set as 5 °C min−1. For example, when the catalyst is calcined in nitrogen at 400 °C for 2 hours and exposed to air at room temperature for 2 days, the obtained catalyst was labeled as Cu@CuOx/C–N2-400 + 2d.2.3 Characterization of catalysts
X-ray photoelectron spectroscopic (XPS) experiments were carried out on a spectrometer (AXIS Supra, Kratos Analytical Ltd) with Al Kα radiation, the C 1s peak (284.6 eV) was used for the calibration of binding energy values. Scanning electron microscopy (SEM) images were taken from a Hitachi 4800 microscope (20 kV). Scanning-transmission electron microscopy (STEM) images and energy dispersive X-ray spectroscopy (EDX) of catalysts were recorded on a JEOL JEM-2100 microscope (200 kV). The samples were dispersed in anhydrous alcohol using the ultrasonic technique, the suspension obtained was added dropwise to a micro-grid membrane and dried in air. 1H, 13C-NMR spectra were recorded with a Varian 700 M spectrometer. Chemical shifts (in ppm) were referenced to CDCl3 (δ = 7.26 ppm) or TMS (δ = 0.00 ppm) as an internal standard.2.4 Catalytic performance tests
In a typical reaction, to a 25 mL reaction vessel, were added 50 mg catalyst and 1.0 mmol benzophenone imine in 2 mL of solvent. The heterogeneous solution was stirred at 80 °C for 24 h under 1 O2 balloon. Imine conversion and product selectivity were analyzed by gas chromatography (GC). The product and side products were determined by GC-MS and 700 MHz 1H NMR spectroscopy analysis.36 The performance of the catalyst was evaluated by imine conversion (x), selectivity (s) and ketazine formation rate r (mmol g−1 h−1).Conversion (%)
| x = (1 − n1) × 100% |
| s = (n2/(n2 + n3 + n4)) × 100% |
| r = 0.833sx |
2.5 Recycled experiments
Catalytic stability and regeneration method: add 220 mg of Cu@CuOx/C–N2-400-20d catalyst, 6 mL of dichloromethane, and 800 mg (5 mmol) of diphenylketimine to the reaction flask and keep the reaction temperature to 80 °C under the oxygen balloon. After 72 h, dichloromethane adds into the reaction to quench. After the catalyst was centrifuged twice, it was separated and dried at room temperature for 2 hours, followed by the next cycle experiment.3 Results and discussion
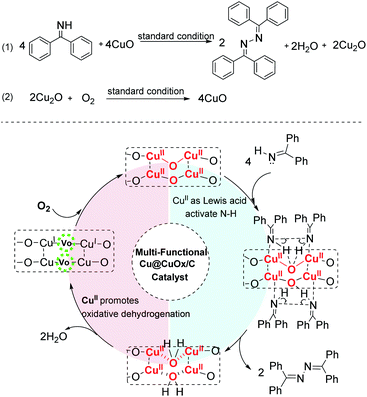
As illustrated in Fig. 1a, three sharp diffraction peaks with 2θ peaks located at 43°, 50° and 74° in X-ray powder diffraction (XRD) spectra are observed, which are indexed to the Cu (111), (200) and (220) crystalline planes of metallic Cu (JCPDS # 04-0836), respectively.37 It is inferred that under the effects of high temperature and reductants (such as ligand derived carbon and H2), the aggregated Cu particles are transformed from the framework Cu sites of HKUST-1 during pyrolysis processing. Moreover, by employing Scherrer's formula, it is confirmed that the calculated aggregation degree of Cu particles is along with the increase of pyrolysis temperature, as shown in Fig. S2.† When the as-made samples exposed to air for a specific duration (the corresponding samples are referred to as Cu@CuOx/C–400-N2 + 2d and Cu@CuOx/C–400-N2 + 20d, with the exposure time of 2 days and 20 days, respectively), a significant 2θ peak at around 36.5° ascribing to Cu2O (111) plane (JCPDS # 78-2076) formed. And the peak strength is correlated to the exposure time, suggesting the slow aerobic oxidation of metallic Cu particles by O2 in the air.38–40 Meanwhile, this oxidation process was directly verified by X-ray photoelectron spectroscopy (XPS). As shown in Fig. 1b, the peak intensity of CuO increases with the extension of exposure time, indicating that the surface Cu2O (binding energy of 932.6 eV) is transformed into CuO (binding energy of 934.4 eV), which can be powerfully supported by Cu Auger spectroscopy (Cu LMM). It is demonstrated that the oxidation Cu-containing particles occur from external surface to inner core, resulting in the formation of core–shell Cu@CuOx composite material.37–41After calcination and carbonization of trimethylbenzoic acid ligand, copper aggregates to form octahedral and irregular derivative particles (Fig. 2a). Due to the large volume of octahedron, it is hard to well characterized by TEM analysis. However, the irregular shape particles were partially dissociated after the sample was pretreated with ethanol and ultrasonic treatment before TEM experiments. We were surprised to find that the irregular shaped particles were composed of copper carbon nanospheres with the size of about 40–50 nm, as shown in Fig. 2c and S3a.† The high-resolution TEM image demonstrates that Cu2O (111) and Cu (111) located in the shell and core of Cu@CuOx/C–400-N2 + 20d (Fig. 2d), which is consistent with the analysis results of XRD and XPS. According to STEM-EDS mapping data, the atomic ratio of Cu, C, and O elements in the Cu@CuOx/C–N2-400 + 20d is 69%, 25%, and 6% (Fig. 2e and S4†). The N2 adsorption–desorption curves (see Fig. S5†) show that the specific surface and the external surface area of the sample is 24.2 m2 g−1 and 11.9 m2 g−1, respectively. The average pore width of Cu@CuOx/C–400-N2 + 20d is up to 71.6 nm, and therefore the Cu-based active sites have good substrate accessibility.
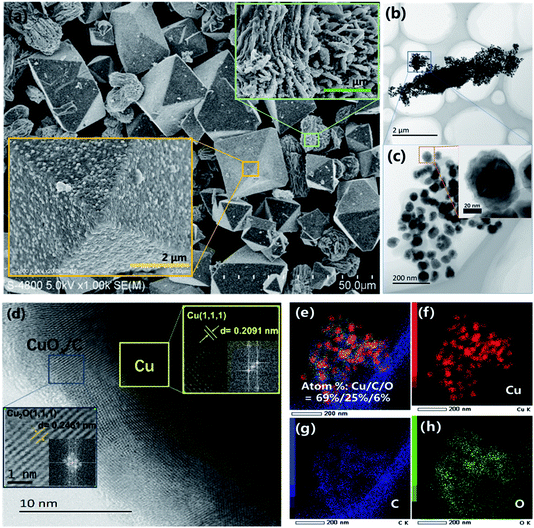 | ||
| Fig. 2 (a) SEM analysis of Cu@CuOx/C–N2-400 + 20d material; (b–d) HR-TEM spectrum of Cu@CuOx/C–N2-400 + 20d; (e–h) STEM-EDS mapping images of Cu@CuOx/C–N2-400 + 20d. | ||
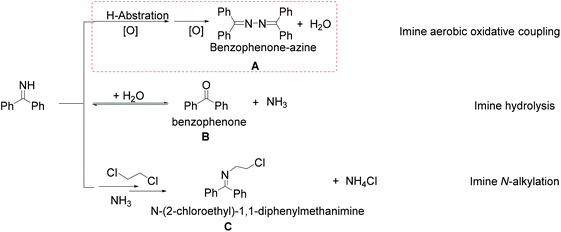
In Table 1, benzophenone-azine A is the target product of the BI oxidative coupling, while two side products, benzophenone B and N-(2-chloroethyl)-1,1-diphenylmethanimine C are caused by the hydrolysis of BI and proposed imine N-alkylation with dichloroethane, respectively. Worthy to note that all the products were determined by GC-MS and 1H, 13C NMR analysis in Fig. S6.†36 It is confirmed that B and C are two competitive products in benzophenone-azine A synthesis process, as shown in Table 1. It should be noted that BI is easy to hydrolyze to produce ammonia and benzophenone.42 To restrict the hydrolysis of BI, the pH value of the mixture of BI and various solvents is set at 8.0 in our experiments (Fig. S7†). When different homogeneous Cu salt catalysts are used, the generation of Cu-imine complexes as Lewis acid may facilitate the hydrolysis of BI, with side product B selectivity up to 47.6% and 58.4%, respectively, as entries 1 and 2 in Table 1. In the contrary, various kinds of heterogeneous Cu@CuOx/carbon catalysts possess more excellent benzophenone-azine A selectivity, higher than 85%, and up to 94.2% in maximum, as entries 3–6 in Table 1.
| Entry | Catalyst | Solvente | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| a Reaction conditions: 2 mL solvent, 50 mg HKUST-1 or HKUST-1 derived catalyst, 1 mmol BI, 80 °C, 24 h, 1 O2 balloon. Yields are determined by GC spectroscopy. All the product and by-products were determined by 1H, 13C NMR and GC-MS, see ESI for more information.b 20% mmol metal salts as the catalyst.c 1 mmol Cu catalyst.d No corresponding imine alkylation product was detected.e No corresponding imine alkylation products were detected. | ||||||
| 1b | Cu(OAc)2·H2O | 1,2-Dichloroethane | 97.5 | 51.2 | 47.6 | 1.2 |
| 2b | CuCl2·2H2O | 1,2-Dichloroethane | 96.4 | 40.7 | 58.4 | 0.8 |
| 3 | Cu@CuOx/C–N2-400 + 2d | 1,2-Dichloroethane | 83.5 | 94.0 | 4.9 | 1.1 |
| 4 | Cu@CuOx/C–N2-400 + 20d | 1,2-Dichloroethane | 77.7 | 94.1 | 4.6 | 1.3 |
| 5 | Cu@CuOx/C–N2-850 + 20d | 1,2-Dichloroethane | 75.2 | 94.2 | 4.6 | 1.2 |
| 6 | Cu@CuOx/C–H–Ar-400 + 2d | 1,2-Dichloroethane | 87.7 | 91.4 | 6.3 | 2.3 |
| 7 | HKUST-1 | 1,2-Dichloroethane | 30.1 | 3.4 | 75.4 | 21.2 |
| 8c | CuO | 1,2-Dichloroethane | 38.1 | 86.3 | 7.5 | 6.2 |
| 9c | Cu2O | 1,2-Dichloroethane | 46.2 | 90.5 | 4.8 | 4.7 |
| 10d | Cu@CuOx/C–N2-400 + 20d | Trichloromethane | 95.0 | 86.0 | 14.0 | 0 |
| 11e | Cu@CuOx/C–N2-400 + 20d | 1-Chloropropane | 91.0 | 94.8 | 5.2 | 0 |
| 12 | Cu@CuOx/C–N2-400 + 20d | Acetonitrile | 27.5 | 42.3 | 57.7 | 0 |
| 13 | Cu@CuOx/C–N2-400 + 20d | 1,4-Dioxane | 42.9 | 30.7 | 69.3 | 0 |
While under the same conditions, the selectivity of product A is only 3.4% for the HKUST-1 catalyst,43 see entry 7 in Table 1. As a result, it is suggested that Cu oxides are promising active sites for the oxidative coupling reaction of BI with O2, rather than CuII in alkaline solution or organic solvent.
Moreover, core–shell Cu@CuOx/carbon catalysts generally perform higher BI conversion and better selectivity than commercial CuO and Cu2O powders. More in detail, Cu@CuOx/C–N2-400 + 2d as a catalyst, the 83.5% BI conversion, and the 94.0% product A selectivity, which is much higher than that of CuO powder (38.1% BI conversion, 86.3% product A selectivity), as shown in entries 8 and 9, Table 1. It is reasonably attributed to the smaller particle size and unique induced effect of Cu core on CuOx shell,44 which is conducive to the adsorption of BI and the H abstraction by Lewis acid site to promote the imine dehydration oxidative coupling reaction. Moreover, it is found that increasing the exposure time and pyrolysis temperature (corresponding to Cu@CuOx/C–N2-400 + 20d and Cu@CuOx/C–N2-850 + 20d, respectively) have a slight negative impact on BI conversion. While the selectivity was maintained at 94%, the BI conversion decreased by 5.8% and 8.3%, respectively. When using H2 as reductant, the Cu@CuOx/C–H–Ar-400 + 2d shows even higher BI conversion (reaching 87.7%), but the selectivity of product A drops to 91.4%. Consequently, by combining with characterization results, it confirms that higher Cu and Cu2O content is in favor of more promising catalytic performance in oxidative coupling reaction, which may be accelerated by oxygen radical species and lattice oxygen species in principle. Therefore, in order to ruling out possible oxygen radical species, several free radical scavengers were added to the general reaction process, and without significant impact on the catalytic performance Cu@CuOx/C–N2-400 + 20d, as shown in Table S1,† indicating that lattice oxygen species involved in the oxidative reaction.45
Interestingly, owing to the difference of solubility between organic solvents and water, chlorinated solvents, e.g., dichloroethane, trichloromethane and 1-chloropropane, perform remarkably enhanced both BI conversion and A selectivity than those of other chloride-free solvents, e.g., acetonitrile and dioxane, as shown in entries 10–13 in Table 1. In more detail, BI and product A completely dissolve in hydrophobic chlorinated solvents to preventing BI hydrolysis, while acetonitrile and dioxane can make organic subtracts/products and water homogeneously dissolved. More than that, probably due to the number and position of chlorine atoms in these two solvents decrease the activity of alkylation reaction, when chloroform or 1-chloropropane was used as solvents, no corresponding N-alkylation of imines was detected by GC or GC-MS (Fig. S8†). The influence of halogen-containing solvents on this oxidative coupling reaction needs to be further explored and summarized, which will be further explained in the follow-up research.
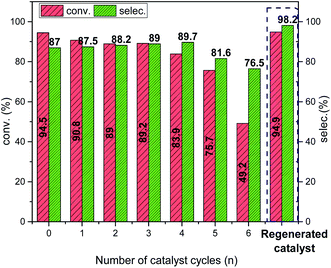
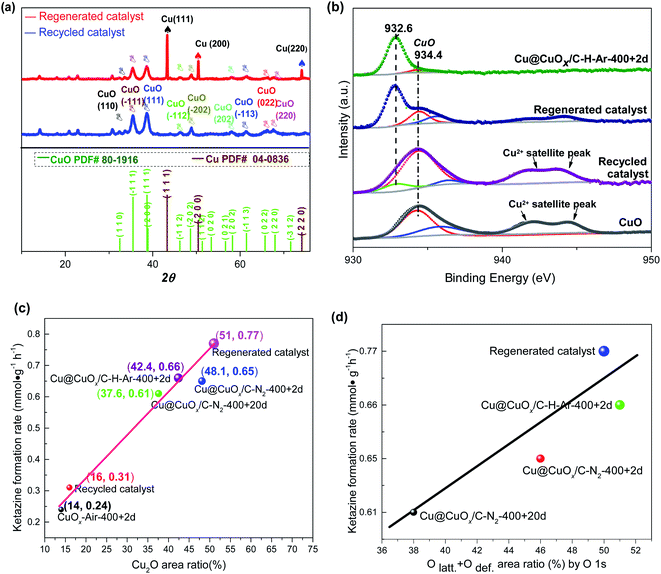
The catalytic performance of Cu@CuOx/C–N2-400 + 20d becomes gradually decreased along with the increasing of recycle times, with BI conversion and ketazine selectivity reducing from 94.5% and 87% at initial stage to 46.2% and 76.5% after six recycles, respectively (Fig. 3). Corresponding, in accordance with XRD analysis, deactivated catalyst possesses crystalline CuO phases with (110), (−111), (111) and (−202) planes (JCPDS # 80-1916), corresponding to 2θ of 32.5°, 35.6°, 38.8°, and 48.9° (blue line in Fig. 4a). Meanwhile, it can be seen from the Cu 2p3/2 XPS spectrum that the surface Cu species of the deactivated catalyst mainly exists in the form of CuO. The peak with binding energy of 934.4 eV and satellite peak are almost the same as that of commercial CuO powder, indicating that Cu and Cu2O of Cu@CuOx/C–N2-400 + 20d are completely oxidized to CuO (Fig. 4b).46 To recover the initial catalytic activity, the deactivated catalyst was regenerated by H2/Ar (5%/95%) gas mixtures at 400 °C for 1 hour. Interestingly, the BI conversion and ketazine selectivity of regenerated catalyst is up to 98.2% and 94.9%, respectively, even higher than those of fresh Cu@CuOx/C–N2-400 + 2d catalyst. In Fig. 4a, three sharp diffraction peaks of metallic Cu (JCPDS # 04-0836) were detected in the XRD patterns of regenerated catalyst (red line), suggesting that CuO can be quickly reduced to Cu2O and metallic Cu by H2/Ar atmosphere at high temperature through a facial reduction method.47,48 This transformation was further confirmed by the appearance of the peak at 932.6 eV in Cu 2p3/2 XPS (Fig. 4b). In addition, from SEM images of deactivated catalyst (Fig. S9†), the morphology and size of nanoparticles are well maintained. However, the original octahedral carbon matrix becomes irregular. Nevertheless, ICP-OES analysis demonstrates that the Cu leaching is negligible after several recycles, therefore showing its excellent application potential (Fig. S10†). Totally, it strongly infers that significant increase in the density of low-valence Cu species (including metallic Cu and Cu2O) favors the enhanced catalytic performance for BI oxidative coupling reaction, as shown in Fig. 2d and S11.† Specifically, when the Cu2O area ratio rises from 14% to 50%, the ketazine formation rate increased linearly from 0.24 mmol g−1 h−1 to 0.77 mmol g−1 h−1, which suggests that Cu2O and metallic Cu species have induction effects on CuO and then facilitate the generation and transformation of active oxygen species.49–51 Furthermore, the O 1s XPS spectra directly proved that the high content of lattice oxygen and defect oxygen in the catalyst was beneficial to the high yield of ketazine (see Fig. 4d and S12†). Owing to the enhancement of electron transfer on CuO surface induced by low valence Cu and Cu2O core, which makes the formation and donation of lattice oxygen much more accessible.52 Once the metal Cu is finally oxidized to CuO, the promoting electron effect would be weakened, and its catalytic performance is similar to that of commercial CuO powder.
On the basis of the study of surface composition and the corresponding catalytic activities, a synergetic catalytic mechanism was proposed where O2 molecules adsorbed on Cu2O to form Cu(II)–O2–Cu(II) intermediate, and then the oxygen activated by copper split into oxygen adatoms and transfer to fill the oxygen vacancies on CuO.53 Imine adsorbed on CuO is oxidatized dehydrogenation into Cu-imine species by the lattice oxygen of CuO. It is suggested that two redox semi-reactions involved in the heterogeneous BI oxidative coupling: (1) oxidative BI coupling by the active oxygen species of CuO, with the formation of ketazine (containing N–N bonds), water and Cu2O; (2) the aerobic oxidation of Cu2O to CuO, making oxygen adatoms insert the oxygen vacancy sites, as illustrated in Scheme 2.26 In a general procedure, the surface CuII species acted as Lewis acid sites make the H–N bonds of BI more active, through the charge transfer between Cu sites and N atoms in imine groups. Then, the activated H atom is oxidized by the surface lattice oxygen species to produce water and oxygen vacancies, and the divalent copper is reduced to monovalent copper; in the meantime, the N–N bond is oxidized to ketazine. After that, Cu2O is re-oxidized by O2 to CuO, with the finishing of one catalytic recycle. The Lewis acidity and redox capacity of Cu-containing sites are apparently synergistical to boost both BI activation and active oxygen species transfer, thus exhibiting excellent BI conversion and ketazine selectivity.
4 Conclusions
In summary, a novel and efficient heterogeneous oxidative BI coupling route catalyzed by Cu@CuOx/carbon composites without the assistance of organic base, has been developed. Under the optimized conditions, the Cu@CuOx/carbon catalyst not only offers 98.2% BI conversion and 94.9% ketazine selectivity, but also possess exciting regeneration ability. More importantly, it is suggested that the metallic Cu core is essential to improve the catalytic performance of CuO shell in BI oxidative coupling reaction, because it promotes the electron transfer on CuO surface and makes the dissolved O2 molecules more easily inserted into the oxygen vacancies. The enhanced redox cycle between CuI and CuII plays an essential role in BI activation, the dehydrated coupling of BI and the regeneration of active sites. Therefore, the low-valence Cu content is closely relevant with BI conversion and ketazine selectivity. With the increase of catalyst recycling times, Cu@CuOx/carbon catalyst was slowly deactivated but could be entirely regenerated by a facial hydrogenation process. Hence, this heterogeneous oxidative coupling method has ultra-important potential for sustainable catalytic synthesis of hydrazine hydrate.Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Hayashi, Catal. Rev., 1990, 32, 229–277 CrossRef CAS.
- E. W. Schmidt, in Hydrazine and Its Derivatives: Preparation, Properties, Applications, Wiley-Blackwell, New York, 2001, p. 2122 Search PubMed.
- E. Rothgery, in Hydrazine and Its Derivatives, 2004, vol. 13 Search PubMed.
- H. Hayashi, A. Kainoh, M. Katayama, K. Kawasaki and T. Okazaki, Prod. R&D, 1976, 15, 299–303 Search PubMed.
- A. S. Dutta, in Recycling of Polyurethane Foams, ed. S. Thomas, A. V. Rane, K. Kanny, V. K. Abitha and M. G. Thomas, William Andrew Publishing, 2018, pp. 17–27 Search PubMed.
- G. Wypych, in Handbook of Foaming and Blowing Agents, ed. G. Wypych, ChemTec Publishing, 2017, pp. 123–177 Search PubMed.
- A. Furst, R. C. Berlo and S. Hooton, Chem. Rev., 1965, 65, 51–68 CrossRef CAS.
- D. Formenti, F. Ferretti, F. K. Scharnagl and M. Beller, Chem. Rev., 2019, 119, 2611–2680 CrossRef CAS.
- C. H. Zhou and Y. Wang, Curr. Med. Chem., 2012, 19, 239–280 CrossRef CAS.
- J. P. Schirmann and P. Bourdauducq, Hydrazine, Wiley-VCH Verlag GmbH & Co. KGaA, 2001 Search PubMed.
- G. D. Byrkit and G. A. Michalek, Ind. Eng. Chem. Res., 1950, 42, 1862–1875 CrossRef CAS.
- F. Raschig, Ber. Dtsch. Chem. Ges., 1907, 40, 4580–4588 CrossRef CAS.
- P. Nikhitha and K. B. S. Saibabu, Chem. Eng. Technol., 2010, 33, 1543–1551 CrossRef CAS.
- A. Laouiti, M. M. Rammah, M. B. Rammah, J. Marrot, F. Couty and G. Evano, Org. Lett., 2012, 14, 6–9 CrossRef CAS.
- W. Yang, X. Li, Y. Li, R. Zhu and H. Pang, Adv. Mater., 2019, 31, 1804740 Search PubMed.
- W. Chaikittisilp, K. Ariga and Y. Yamauchi, J. Mater. Chem. A, 2013, 1, 14–19 RSC.
- X. Zhang, A. Chen, M. Zhong, Z. Zhang and X. H. Bu, Electrochem. Energy Rev., 2019, 2, 29–104 CrossRef CAS.
- R. R. Salunkhe, Y. V. Kaneti, K. Jeonghun, J. Ho and Y. Yamauchi, Acc. Chem. Res., 2016, 49, 2796–2806 CrossRef CAS.
- J. Wang, M. Rao, C. Ye, Y. Qiu, W. Su, S. R. Zheng, J. Fan, S. L. Cai and W. G. Zhang, RSC Adv., 2020, 10, 4621–4629 RSC.
- K. Shen, X. Chen, J. Chen and Y. Li, ACS Catal., 2016, 6, 5887–5903 CrossRef CAS.
- K. Chen, J. L. Ling and C. D. Wu, Angew. Chem., 2020, 59, 1925–1931 CrossRef CAS.
- S. Zhang, H. Liu, C. Sun, P. Liu, L. Li, Z. Yang, X. Feng, F. Huo and X. Lu, J. Mater. Chem. A, 2015, 3, 5294–5298 RSC.
- R. Zhang, L. Hu, S. Bao, R. Li, L. Gao, R. Li and Q. Chen, J. Mater. Chem. A, 2016, 4, 8412–8420 RSC.
- C. Bai, A. Li, X. Yao, H. Liu and Y. Li, Green Chem., 2016, 18, 1061–1069 RSC.
- R. Fang, R. Luque and Y. Li, Green Chem., 2016, 18, 3152–3157 RSC.
- M. C. Ryan, Y. J. Kim, J. B. Gerken, F. Wang, M. M. Aristov, J. R. Martinelli and S. S. Stahl, Chem. Sci., 2020, 11, 1170–1175 RSC.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS.
- H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415–5418 RSC.
- A. Corma, H. Garcia and F. X. Llabres i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS.
- P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC.
- X. Zhang, W. Dong, Y. Luan, M. Yang, L. Tan, Y. Guo, H. Gao, Y. Tang, R. Dang, J. Li and G. Wang, J. Mater. Chem. A, 2015, 3, 4266–4273 RSC.
- Y. Luan, Y. Qi, H. Gao, R. S. Andriamitantsoa, N. Zheng and G. Wang, J. Mater. Chem. A, 2015, 3, 17320–17331 RSC.
- W. Zhong, H. Liu, C. Bai, S. Liao and Y. Li, ACS Catal., 2015, 5, 1850–1856 CrossRef CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Commun., 2014, 50, 12800–12814 RSC.
- K. Schlichte, T. Kratzke and S. Kaskel, Microporous Mesoporous Mater., 2004, 73, 81–88 CrossRef CAS.
- K. Li, X. Shao, L. Tseng and S. J. Malcolmson, J. Am. Chem. Soc., 2018, 140, 598–601 CrossRef CAS.
- Y. J. Chen, M. H. Li, J. C. Huang and P. Chen, Sci. Rep., 2018, 8, 7646 CrossRef.
- Q. Zhu, L. Zou, G. Zhou, W. A. Saidi and J. C. Yang, Surf. Sci., 2016, 652, 98–113 CrossRef CAS.
- I. Platzman, R. Brener, H. Haick and R. Tannenbaum, J. Phys. Chem. C, 2008, 112, 1101–1108 CrossRef CAS.
- Z.-J. Zuo, J. Li, P.-D. Han and W. Huang, J. Phys. Chem. C, 2014, 118, 20332–20345 CrossRef CAS.
- M. C. Biesinger, Surf. Interface Anal., 2017, 49, 1325–1334 CrossRef CAS.
- R. W. Layer, Chem. Rev., 1963, 63, 489–510 CrossRef CAS.
- L. Mitchell, B. Gonzalez-Santiago, J. P. S. Mowat, M. E. Gunn, P. Williamson, N. Acerbi, M. L. Clarke and P. A. Wright, Catal. Sci. Technol., 2013, 3, 606–617 RSC.
- C. Sarkar, S. Pendem, A. Shrotri, D. Q. Dao, P. Pham Thi Mai, T. Nguyen Ngoc, D. R. Chandaka, T. V. Rao, Q. T. Trinh, M. P. Sherburne and J. Mondal, ACS Appl. Mater. Interfaces, 2019, 11, 11722–11735 CrossRef CAS.
- J. J. Varghese, Q. T. Trinh and S. H. Mushrif, Catal. Sci. Technol., 2016, 6, 3984–3996 RSC.
- R. P. Vasquez, Surf. Sci. Spectra, 1998, 5, 262–266 CrossRef CAS.
- S. D. Pike, E. R. White, A. Regoutz, N. Sammy, D. J. Payne, C. K. Williams and M. S. Shaffer, ACS Nano, 2017, 11, 2714–2723 CrossRef CAS.
- A. P. LaGrow, M. R. Ward, D. C. Lloyd, P. L. Gai and E. D. Boyes, J. Am. Chem. Soc., 2017, 139, 179–185 CrossRef CAS.
- S. Yadav, A. Jain and P. Malhotra, Green Chem., 2019, 21, 937–955 RSC.
- T.-J. Huang, Catal. Lett., 2003, 87, 173–178 CrossRef CAS.
- L. Wan, Q. Zhou, X. Wang, T. E. Wood, L. Wang, P. N. Duchesne, J. Guo, X. Yan, M. Xia, Y. F. Li, A. A. Jelle, U. Ulmer, J. Jia, T. Li, W. Sun and G. A. Ozin, Nat. Catal., 2019, 2, 889–898 CrossRef CAS.
- T. Kangas, K. Laasonen, A. Puisto, H. Pitkänen and M. Alatalo, Surf. Sci., 2005, 584, 62–69 CrossRef CAS.
- B. Wei, N. Yang, F. Pang and J. Ge, J. Phys. Chem. C, 2018, 122, 19524–19531 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06367c |
| This journal is © The Royal Society of Chemistry 2020 |