Open Access Article
Open Access ArticlePreparation, characterization, antioxidant and antiglycation activities of selenized polysaccharides from blackcurrant
Changzong Wu†
,
Meimei Zhao†,
Xueying Bu,
Ziya Qing,
Libo Wang,
Yaqin Xu,
Yu Yang * and
Jingwen Bai
* and
Jingwen Bai *
*
College of Art and Science, Northeast Agricultural University, Harbin 150030, People's Republic of China. E-mail: yangyu@neau.edu.cn; baijingwen@neau.edu.cn; Fax: +86 451 55190243; Tel: +86 451 55191442 Tel: +86 451 55191810
First published on 2nd September 2020
Abstract
An ultrasound-assisted enzymatic method was used to extract the polysaccharides from blackcurrant fruits (BP), and then a nitric acid-sodium selenite method was employed to prepare twelve kinds of selenized blackcurrant polysaccharides (SBPs). Among them, SBP-1, SBP-2 and SBP-3 with different selenium contents of 250 ± 11, 312 ± 15 and 643 ± 24 μg g−1, displayed relatively higher 2,2-diphenyl-1-picrylhydrazyl radical (DPPH˙) scavenging activities than the others. After treating with a Sepharose-6B chromatography column, the purified blackcurrant polysaccharide (PBP) and three selenized polysaccharides (PSBP-1, PSBP-2, PSBP-3) with high purity were obtained. Compared with PBP, PSBPs possessed a larger absolute value of zeta potential (ZP) and smaller particle size, indicating the positive influence of selenized modification on physical stability of polysaccharides. Ultraviolet (UV), Fourier transform infrared (FT-IR) and circular dichroism (CD) spectra confirmed that selenium had been introduced onto the polysaccharide structure. X-ray diffraction (XRD) and I2–KI reaction results indicated that selenized modification did not cause an obvious change in crystal form and branch structure of blackcurrant polysaccharides. In addition, PSBPs were superior to PBP in antioxidant and antiglycation capacities, and the bioactivities of PSBPs were significantly improved in positive correlation with selenium content. This study suggested that PSBPs may be a potential selenium source and serve as functional food and medicine.
1. Introduction
Polysaccharides are a kind of important high-molecular carbohydrate, formed of multiple monosaccharide units joined together by glycosidic bonds.1,2 During recent years, natural polysaccharides from vegetables and fruits have attracted more and more attention for their multiple biological and potential pharmacological activities, such as free radical scavenging capacity, inhibition of oxidative damage and tumor-cell proliferation, hypoglycemic and radioprotective effect.2–5 Therefore, the polysaccharides extracted from natural resources have promising application in food, nutraceutical and pharmaceutical industries.Berry fruits, such as strawberry, blueberry, blackcurrant and bayberry, are delicious and powerful disease-fighting foods and are popularly consumed in the human diet.6 Among these berries, blackcurrant (Ribes nigrum L.) is recognized as one of the major commercial berries, and displayed diuretic, diaphoretic and febrifuge effects in traditional Chinese medicine.7,8 Recently, the polysaccharides from blackcurrant were demonstrated as being responsible for various health beneficial effects, including immunostimulatory, antimicrobial, antitumor, antioxidant and anti-inflammatory activities,2,9 and thereby it is an excellent natural product with broad development prospects.
In general, the polysaccharides contain hydroxyl, carboxyl and other groups that are easy to react with other compounds, providing a basis for the chemical modification.4,13 Meanwhile, chemical modification can improve the physicochemical properties, functional properties and biological activities of natural polysaccharides.5,13 As a result, more and more studies have focused on the chemical modification of the natural polysaccharides.4,5,14 Currently, selenized treatment is the most concerned method for polysaccharides modification. When selenium combines with polysaccharides, the selenized polysaccharides will possess the duple activities of selenium and polysaccharides.13 Moreover, the special selenium–oxygen bonds are formed in selenized polysaccharides, which makes it possible to be easily absorbed and utilized by human body as an organic selenium supplement.15,16 Many research findings have confirmed the positive effects of selenized modification on native polysaccharides from various resources, such as Radix hedysari,16 Momordica charantia L.4 and Cordyceps militaris,17 etc. Our previous studies found that several native polysaccharides extracted from blackcurrant displayed apparent antioxidant and glycation inhibitory activities.10–12 However, the relatively high molecular weight may affect the biological activities and applications of the polysaccharides.2 Some reports showed that selenization modification could not only cause the degradation of polysaccharides, but also improve the biological activities and functional properties of polysaccharides.4,14 Therefore, the investigation on the selenized modification of blackcurrant polysaccharides is needed.
In the present study, the selenized blackcurrant polysaccharides were prepared using nitric acid–sodium selenite (HNO3–Na2SeO3) method. The physicochemical, functional properties, structural characteristics and bioactivities in vitro of three selenized polysaccharides with different selenium contents were evaluated and compared with non-modified polysaccharides. The results of this study will provide valuable information for the selenized modification of natural polysaccharides from other berries.
2. Materials and methods
2.1. Materials
The fully mature blackcurrant fruits (Heifeng) were obtained from College of Horticulture, Northeast Agricultural University and kept frozen at −20 °C.D4006 macroporous resin and Sepharose-6B were purchased from Nankai University Chemical Plant (Tianjin, China). 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), phenanthroline, nicotinamide adenine dinucleotide (NADH), nitroblue tetrazolium (NBT), phenazine methosulfate (PMS), ascorbic acid (VC), albumin from bovine serum (BSA) and T-series dextrans were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All other reagents used were of analytical grade.
2.2. Extraction of the polysaccharides
Prior to extract, the blackcurrant fruits were defrosted at room temperature and then crushed by a homogenizer (JJ-2, Changzhou Guohua Electric Appliance Co., Ltd, Jiangsu, China). Afterwards, a certain amount of homogenate (50.0 g) and deionized water (1000.0 mL) were mixed in a beaker, and the cellulase (150 mg) and pectinase (75 mg) were added in sequence. Polysaccharides were then extracted at a temperature of 50 °C and a power of 640 W in an ultrasonic cleaning bath (SB-800 DTD, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China) for 60 min. After heating at 100 °C for 15 min to inactivate the enzymes, the extract was filtrated, concentrated, and precipitated with a four-fold volume of ethanol at 4 °C for 24 h. Subsequently, the precipitate collected by membrane filtration (0.45 μm, New Asia Pharmaceutical Co., Ltd., Shanghai, China) was dialyzed against distilled water (molecular weight cutoff 3500 Da) for 72 h and then the dialysate was concentrated and lyophilized to obtain the crude polysaccharides. Finally, D4006 macroporous resin was used for the preliminary purification of the crude polysaccharides based on previous reported method,2 and the main fraction was collected, concentrated, lyophilized, and designated “BP” for further preparation of the selenized polysaccharides.2.3. Preparation of the selenized polysaccharides
Twelve groups of modification conditions were designed to prepare the selenized polysaccharides (shown in Table 1). Under fully stirring, 500 mg of BP was mixed with 250 mL of HNO3 (0.5%, v/v) containing 2.5 g BaCl2. The selenized reactions were then started by adding 500 mg of Na2SeO3 (5 mg mL−1) under different ultrasonic powers (500 and 800 W) at various temperatures (50 and 80 °C) for various periods (3, 5 and 7 h). Afterwards, the mixtures were cooled to room temperature and the pH was adjusted to 5.0–6.0 with the saturated Na2CO3. Anhydrous Na2SO4 (1.6 g) was subsequently added and the supernatant without Ba2+ was gathered by centrifuging at 3500 rpm (SC-3610, Anhui USTZ Zookia Scientific Instruments Co., Ltd, Anhui, China) for 20 min. The resulting aqueous fraction was extensively dialyzed against distilled water (molecular weight cutoff 3500 Da) until no free sodium selenium was detected by ascorbic acid method.16 Finally, the dialysates were concentrated and lyophilized to obtain a series of selenized polysaccharides (SBPs).| No. | Time/power/temperature (h/W/°C) | Yield (%) | Se content (μg g−1) | DPPH˙ scavenging rate |
|---|---|---|---|---|
| a Means with different letters for the same species are significantly different (p < 0.05). | ||||
| 1 | 3 h/500 W/50 °C | 40.41 ± 0.98d | 171 ± 10a | 18.27 ± 0.33a |
| 2 | 5 h/500 W/50 °C | 43.19 ± 1.55h | 343 ± 15f | 27.79 ± 1.17c |
| 3 | 7 h/500 W/50 °C | 41.27 ± 2.07f | 424 ± 22g | 34.15 ± 1.09e |
| 4 | 3 h/800 W/50 °C | 43.47 ± 0.87i | 182 ± 10b | 40.71 ± 1.61h |
| 5 | 5 h/800 W/50 °C | 45.35 ± 2.41k | 250 ±11c | 50.90 ± 1.34j |
| 6 | 7 h/800 W/50 °C | 50.03 ± 0.99l | 312 ± 15d | 52.00 ± 1.21k |
| 7 | 3 h/500 W/80 °C | 43.98 ± 1.41j | 643 ± 24i | 55.55 ± 1.12l |
| 8 | 5 h/500 W/80 °C | 38.31 ± 1.39c | 541 ± 16h | 36.46 ± 1.13f |
| 9 | 7 h/500 W/80 °C | 35.25 ± 2.14a | 732 ± 21k | 46.00 ± 1.21i |
| 10 | 3 h/800 W/80 °C | 42.58 ± 1.68g | 709 ± 13j | 32.93 ± 1.32d |
| 11 | 5 h/800 W/80 °C | 40.82 ± 1.85e | 821 ± 16l | 37.57 ± 1.10g |
| 12 | 7 h/800 W/80 °C | 36.90 ± 1.22b | 318 ± 12e | 20.20 ± 0.86b |
2.4. Determination of selenium content
20.00 mg of SBPs were soaked in 4.0 mL of mixed acid (VHClO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2SO4
VH2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VHNO3 = 1
VHNO3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at room temperature overnight. The mixture was then heated at 95 °C for 1.5 h and diluted to 25.0 mL with deionized water after cooling. Following steps were performed in a separatory funnel (125.0 mL). Firstly, 30.0 mL of deionized water was mixed with the solution obtained above, and then the pH was adjusted to 2.0 with NaOH solution (10 mol L−1). Subsequently, 3.0 mL of o-phenylenediamine hydrochloride solution (1%) was added, and the mixture was incubated in the dark for 1 h. Finally, 7.0 mL of toluene each was used to extract selenium in the extract for five times. The sample in toluene layer was collected, concentrated, and the absorbance at 333 nm was measured using a UV spectrophotometer (T6 New Century, Beijing Purkinje General Instrument Co., Ltd, Beijing, China). Selenium content was calculated according to the standard curve: y = 0.0119x + 0.0184, R2 = 0.9968, where, y is the absorbance value at 333 nm, x is the corresponding selenium content (μg).
4) at room temperature overnight. The mixture was then heated at 95 °C for 1.5 h and diluted to 25.0 mL with deionized water after cooling. Following steps were performed in a separatory funnel (125.0 mL). Firstly, 30.0 mL of deionized water was mixed with the solution obtained above, and then the pH was adjusted to 2.0 with NaOH solution (10 mol L−1). Subsequently, 3.0 mL of o-phenylenediamine hydrochloride solution (1%) was added, and the mixture was incubated in the dark for 1 h. Finally, 7.0 mL of toluene each was used to extract selenium in the extract for five times. The sample in toluene layer was collected, concentrated, and the absorbance at 333 nm was measured using a UV spectrophotometer (T6 New Century, Beijing Purkinje General Instrument Co., Ltd, Beijing, China). Selenium content was calculated according to the standard curve: y = 0.0119x + 0.0184, R2 = 0.9968, where, y is the absorbance value at 333 nm, x is the corresponding selenium content (μg).
2.5. Purification of the polysaccharides
BP and three selected selenized polysaccharides (1.0 mL, 10.0 mg mL−1) were loaded on a Sephrose-6B chromatography column (1.5 × 30 cm) and then eluted with deionized water at a flow rate of 0.8 mL min−1.12 The concentrations of polysaccharide in each eluted aliquot (1.0 mL per tube) were determined by using phenol-sulfuric acid method,10 and the elution curves of four polysaccharides were constructed. The main fractions of four polysaccharides were collected, concentrated, lyophilized and separately termed as “PBP”, “PSBP-1”, “PSBP-2” and “PSBP-3” for subsequent experiments.2.6. Physicochemical properties of PBP and PSBPs
2.7. Structural characterization of PBP and PSBPs
2.8. Functional properties of PBP and PSBPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min. The volume fraction of oil (%) and the mass of polysaccharide (g) were marked as φ and m. Subsequently, 100 μL of the emulsion was immediately blended with 5.0 mL of SDS (0.1%, w/v), and the absorbance A0 was measured at a wavelength of 500 nm. Ten minutes later, the absorbance A10 was also read by the same method. The emulsifying activity index (EAI) and emulsion stability (ES) of the polysaccharides were calculated as follows: EAI (m2 g−1) = (2 × 2.303 × A0)/(φ × m), ES = A10/A0 × 100%.
000 rpm for 2 min. The volume fraction of oil (%) and the mass of polysaccharide (g) were marked as φ and m. Subsequently, 100 μL of the emulsion was immediately blended with 5.0 mL of SDS (0.1%, w/v), and the absorbance A0 was measured at a wavelength of 500 nm. Ten minutes later, the absorbance A10 was also read by the same method. The emulsifying activity index (EAI) and emulsion stability (ES) of the polysaccharides were calculated as follows: EAI (m2 g−1) = (2 × 2.303 × A0)/(φ × m), ES = A10/A0 × 100%.2.9. Determination of antioxidant activities in vitro
DPPH˙ and ABTS+˙ scavenging rates were calculated based on the following equation:
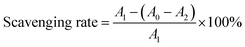 | (1) |
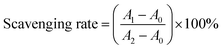 | (2) |
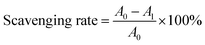 | (3) |
2.10. Determination of antiglycation activities
2.11. Statistical analysis
The results were represented as mean of three independent replicates ± standard deviation. Statistical analysis was analyzed using SPSS 18.0, and p < 0.05 was taken as statistically significant.3. Results and discussion
3.1. Preparation of the polysaccharides
Through ultrasound-assisted enzymatic extraction, ethanol precipitation, filtration, dialysis, concentration and lyophilization, the crude polysaccharide was obtained with a purity of 43.61% ± 0.33%. After one run treatment with D4006 macroporous resin, the eluted polysaccharide solution was gathered, concentrated, lyophilized, and the polysaccharide (BP) with a purity of 52.34% ± 0.26% and a recovery yield of 66.90% ± 0.32% was obtained.3.2. Screening of selenized polysaccharides
As listed in Table 1, twelve kinds of selenized polysaccharides with different yields, selenium contents and DPPH˙ scavenging activities were prepared by varying conditions. It could be found that the yield of selenized polysaccharide reached the peak value of 50.03% ± 0.99% at groups 6, followed by group 5 (45.35% ± 2.41%) and groups 7 (43.98% ± 1.41%). Concurrently, the selenized polysaccharides obtained at these three groups showed the relatively higher DPPH˙ scavenging activities (50.90–55.55%) than the other groups (18.27–46.00%). In addition, the selenium content determined by spectrophotometry revealed the significantly different presence at groups 5–7, which were 250 ± 11, 312 ± 15 and 643 ± 24 μg g−1, respectively. Generally, selenium can bind to oxygen of –OH groups on the polysaccharide in Se–O form, and the binding rate was related to the approachability and number of hydroxyl group of polysaccharide. Therefore, different monosaccharide units and their sequence in the polysaccharides from different plant sources result in different selenium binding rate.13 In addition, the prepared selenized polysaccharides in this study displayed higher selenium content (171–821 μg g−1) than the selenium modified polysaccharide from Cordyceps militaris (16.3–576.4 μg g−1) reported by Zhu et al.,17 which may be caused by the difference in the structure of polysaccharides extracted from different materials.5,13Based on the comprehensive consideration of yield, selenium content and DPPH˙ scavenging activity, the selenized polysaccharides obtained at groups 5–7 were selected for the subsequent study and were termed as “SBP-1”, “SBP-2” and “SBP-3”, respectively.
3.3. Purification of the polysaccharides
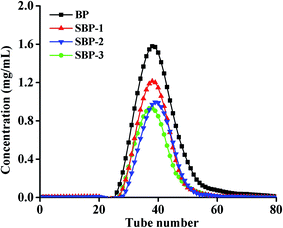
As shown in Fig. 1, the elution curves of the four polysaccharide samples (BP, SBP-1, SBP-2 and SBP-3) displayed sharp and symmetric chromatography peaks. The main fractions were pooled, concentrated, and lyophilized, the homogeneous native (PBP) and selenized polysaccharides (PSBP-1, PSBP-2, PSBP-3) were acquired, whose purity reached to 83.10% ± 0.45%, 82.12% ± 0.13%, 83.58% ± 0.44% and 85.50% ± 0.62%, and the recovery yields were 75.60% ± 0.14%, 72.14% ± 0.19%, 71.31% ± 0.50% and 73.24% ± 0.37%, respectively.3.4. Effect of selenized modification on the physicochemical properties
| Sample | Polyphenol (%) | Protein (%) | Reducing sugar (%) | Uronic acid (%) | Purity (%) | Recovery (%) | Mw (kDa) |
|---|---|---|---|---|---|---|---|
| a Means with different letters for the same species are significantly different (p < 0.05). | |||||||
| PBP | 0.22 ± 0.05a | 1.85 ± 0.02a | 0.74 ± 0.15a | 26.67 ± 0.13a | 83.10 ± 0.45 | 75.60 ± 0.14 | 2.04 × 104 |
| PSBP-1 | 0.17 ± 0.01b | 0.98 ± 0.03b | 4.40 ± 0.11b | 27.66 ± 0.18b | 82.12 ± 0.13 | 72.14 ± 0.19 | 1.55 × 104 |
| PSBP-2 | 0.16 ± 0.04b | 0.87 ± 0.03c | 5 ± 0.26c | 29.26 ± 0.11c | 83.58 ± 0.44 | 71.31 ± 0.50 | 1.29 × 104 |
| PSBP-3 | 0.15 ± 0.07b | 0.91 ± 0.02b | 6.50 ± 0.19d | 31.85 ± 0.16d | 85.50 ± 0.62 | 73.24 ± 0.37 | 9.09 × 103 |
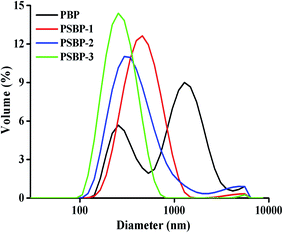
| Sample | Average particle size (nm) | PDI | Zeta potential (mV) |
|---|---|---|---|
| PBP | 632.9 | 0.50 | −12.9 |
| PSBP-1 | 406.3 | 0.26 | −14.6 |
| PSBP-2 | 342.9 | 0.21 | −16.3 |
| PSBP-3 | 250.4 | 0.18 | −18.6 |
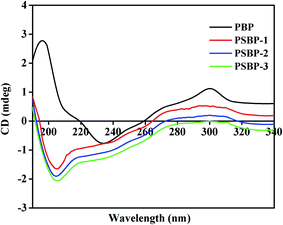
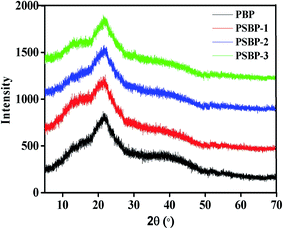
3.5. Effect of selenized modification on the structural characteristics
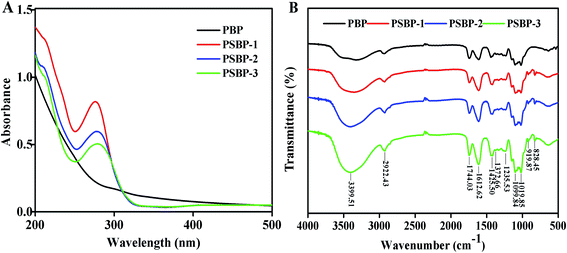
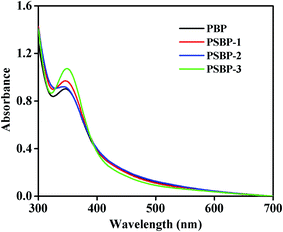
As exhibited in Fig. 3B, the FT-IR spectra of PBP and PSBPs displayed the typical absorption peaks of polysaccharides. Among them, the strong band around 3399.51 cm−1 was attributed to the stretching vibration of O–H, which illustrated the presence of intermolecular hydrogen bonding.19,28 The signal at 1744.03 cm−1 represented the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The obvious peaks toward about 1612.62 cm−1 and 1425.50 cm−1 were assigned to the absorption of deprotonated carboxylic groups, indicating the existence of uronic acid.2 In addition, the specific sharp peaks distributed in the region of 1200–1000 cm−1 were inferred to be the pyranose form of sugar residues.2,12 Compared with PBP, two new absorption peaks appeared at 919.87 cm−1 and 828.45 cm−1 in the spectra of three selenized polysaccharides, which were caused by the stretching vibrations of C–Se–O and Se
O. The obvious peaks toward about 1612.62 cm−1 and 1425.50 cm−1 were assigned to the absorption of deprotonated carboxylic groups, indicating the existence of uronic acid.2 In addition, the specific sharp peaks distributed in the region of 1200–1000 cm−1 were inferred to be the pyranose form of sugar residues.2,12 Compared with PBP, two new absorption peaks appeared at 919.87 cm−1 and 828.45 cm−1 in the spectra of three selenized polysaccharides, which were caused by the stretching vibrations of C–Se–O and Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. Similarly, Gao et al.14 and Wei et al.15 also reported the bands around these wavelength for selenized polysaccharides from alfalfa roots and Radix hedysari. These results signified that selenium was successfully conjunct with the polysaccharides from blackcurrant fruits by O–Se–O or Se
O, respectively. Similarly, Gao et al.14 and Wei et al.15 also reported the bands around these wavelength for selenized polysaccharides from alfalfa roots and Radix hedysari. These results signified that selenium was successfully conjunct with the polysaccharides from blackcurrant fruits by O–Se–O or Se![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond.
O bond.
3.6. Effect of selenized modification on the functional properties
Previous studies suggested that hydration property, oil retention capacity and emulsifying ability of polysaccharides in functional foods were greatly associated with the rheological properties of the final products and the mouth-feel experienced by consumers.19,33 Therefore, it is necessary to evaluate these functional properties of polysaccharides.| Sample | Functional properties | |||
|---|---|---|---|---|
| WHC (g g−1) | OHC (g g−1) | EAI (m2 g−1) | ES (%) | |
| a Means with different letters for the same species are significantly different (p < 0.05). | ||||
| PBP | 0.42 ± 0.03c | 1.15 ± 0.08a | 121.35 ± 1.32d | 87.75 ± 1.27d |
| PSBP-1 | 0.40 ± 0.05b | 1.18 ± 0.04b | 92.98 ± 1.43c | 79.70 ± 1.39c |
| PSBP-2 | 0.39 ± 0.02a,b | 1.19 ± 0.05b,c | 88.93 ± 1.88b | 67.64 ± 1.67b |
| PSBP-3 | 0.38 ± 0.02a | 1.20 ± 0.10c | 69.77 ± 1.23e | 60.39 ± 1.12e |
| Soy lecithin | — | — | 143.27 ± 1.49e | 94.85 ± 1.33e |
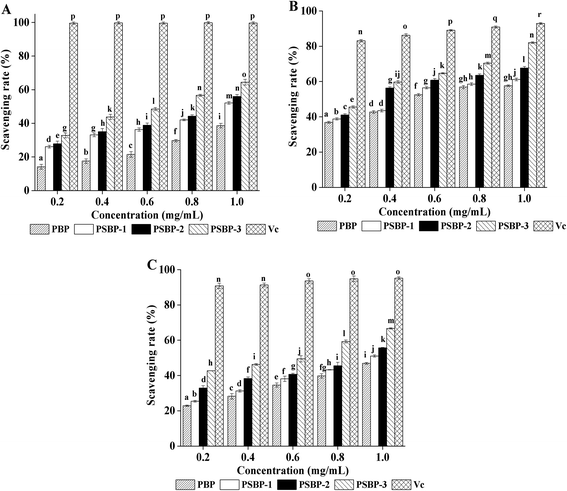
3.7. Effect of selenized modification on the antioxidant activities
In summary, the results of ABTS+, hydroxyl and superoxide anion radical scavenging assays above demonstrated that selenized modification is an effective method to enhance the antioxidant activity of blackcurrant polysaccharides. Moreover, the higher the selenium content was, the better the scavenging effect was. The selenium groups in the obtained selenized polysaccharide can activate the hydrogen atom of the anomeric carbon, and thereby contributing to the abilities scavenging free radicals.14,17,40 In addition, it has been reported that selenium as the cofactor of selenium dependent antioxidant enzymes can be transformed into the active center of these enzymes in vivo, such GSH-Px, TRx and SOD.40 Therefore, PSBPs in this study may increase the selenium-dependent antioxidant enzymes activities, and further display potent antioxidant activity in vivo.
3.8. Effects of selenized modification on inhibition of non-enzymatic glycation
Diabetes mellitus (DM) is a chronic metabolic disease that threatens the health of the global population. Many complications related to DM such as hyperlipidemia, atherosclerosis, cardiovascular diseases and renal failure prompt that is very urgent for its control.40 Non-enzymatic glycation generally includes three reaction phases. In the early phase, Amadori products are formed by the reaction between the reduced amino groups at the end of proteins and aldehyde groups on the reducing sugars. They are further oxidized, degraded, and rearranged, and generated the dicarbonyl compounds in the intermediate phase. In the late phase, dicarbonyl compounds react with free amino groups on proteins to form AGEs. Reducing the formation of AGEs can help prevent the occurrence and development of DM.10 Therefore, antiglycation assay can be used to evaluate the anti-diabetic potential of the polysaccharide.As shown in Fig. 8A–C, all three kinds of selenized polysaccharide samples (PSBP-1, PSBP-2 and PSBP-3) displayed the stronger inhibitory activities against the non-enzymatic glycated products formation than PBP and AG at two test concentrations, and the inhibition rate at 0.5 mg mL−1 was higher than that at 0.1 mg mL−1. At the early phase, the inhibitory activities of PSBPs and PBP on Amadori products obviously increased from 1 to 13 days, and reached maximum values at the 15th day (Fig. 8A). The maximum inhibitory rates of PSBP-1, PSBP-2 and PSBP-3 at 0.5 mg mL−1 were 60.11 ± 1.72%, 66.12 ± 1.50% and 69.95 ± 0.38%, which increased 7.65%, 13.66% and 17.49% over that of PBP. As presented in Fig. 8B and C, with the increase of culture time, the inhibitory effect of PSBPs and PBP at two concentrations on the formation of dicarbonyl compounds and AGEs increased significantly. At the 15th day, PSBP-1, PSBP-2 and PSBP-3 at 0.5 mg mL−1 showed the maximum inhibitory rates of 57.54 ± 0.72%, 65.23 ± 1.17% and 67.08 ± 0.55% for dicarbonyl compounds and 64.58 ± 1.56%, 71.88 ± 0.46%, 73.29 ± 1.53% for AGEs, which were 1.09-, 1.24-, 1.27-fold and 1.03-, 1.15-, 1.17-fold higher than that of PBP (52.62 ± 0.49%) and (62.58 ± 1.15%), respectively. Obviously, the inhibitory effects of PSBPs at the late phase were more obvious than that at the early and intermediate phases, which was related to the inhibition of PSBPs on free radicals generation derived from the glycation process.10,23 In addition, at the same concentration, the inhibitory activities increased in the order: PSBP-3 > PSBP-2 > PSBP-1, indicating a positive effect of selenium content. Similarly, in comparison with native polysaccharides, the selenized polysaccharides from Momordica charantia L.4 and sweet potato40 can significantly reduce fasting blood glucose, MDA and LPO levels and increase glycogen and insulin contents to exert therapeutic effect for DM.
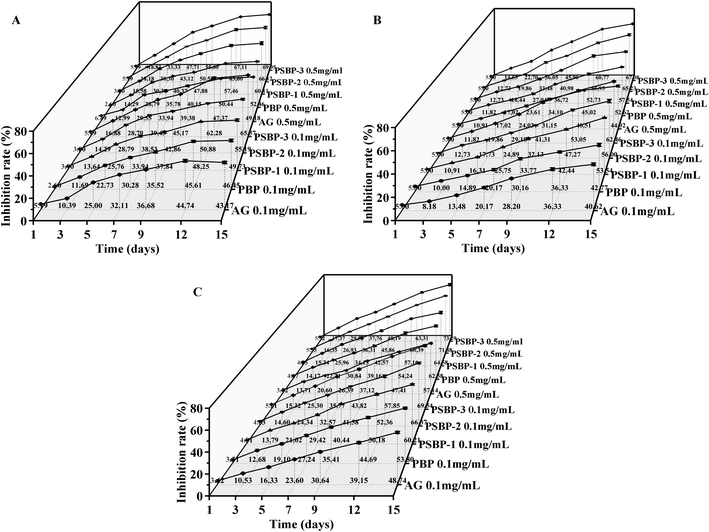 | ||
| Fig. 8 Inhibitory effects of PBP and PSBPs on the formation of Amadori product (A), dicarbonyl compound (B) and AGEs (C). | ||
In this study, PSBPs have been confirmed to possess stronger free radical scavenging activities than PBP in vitro. Therefore, the better inhibitory effects of PSBPs on non-enzymatic glycation may be associated with the excellent radical scavenging activities. After selenized modification, the presence of selenium in PSBPs not only functions as an antioxidant agent but also prevents the progress of diabetes.
4. Conclusion
In the present study, the selenized blackcurrant polysaccharides (PSBPs) with different selenium contents were successfully prepared. Both PBP and PSBPs were non-crystalline substances, and PSBPs displayed the larger zeta potential and smaller particle size compared to PBP. In addition, PSBPs displayed better OHC than PBP, while selenized modification did not improve the WHC and emulsifying property of blackcurrant polysaccharides. Meanwhile, PSBPs exhibited potent radicals scavenging capacities and antiglycation activities. Therefore, selenized modification was an efficient method to improve the biological activities of blackcurrant polysaccharides. However, the precise chemical structures and the structure–effect relationship of PSBPs have to be further investigated in later study.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 31600276), Natural Science Foundation of Heilongjiang Province of China (No. C2018013), Postdoctoral Scientific Research Start-up Fund Projects of Heilongjiang Province of China (LBH-Q19010, LBH-Q17030) and Student's Innovation Training Program of Northeast Agricultural University (201910224076).References
- Q. W. Liu, X. D. Ge, L. G. Chen, D. L. Cheng, Z. Yun, W. Xu and R. Shao, Int. J. Biol. Macromol., 2018, 107, 2262–2268 CrossRef CAS.
- Y. Q. Xu, X. J. Niu, N. Y. Liu, Y. K. Gao, L. B. Wang, G. Xu, X. G. Li and Y. Yang, Food Chem., 2018, 243, 26–35 CrossRef CAS.
- X. Y. Li, L. Wang and B. B. Wang, Food Chem., 2017, 229, 479–486 CrossRef CAS.
- Y. Ru, K. X. Liu, X. Y. Kong, X. Y. Li, X. R. Shi and H. M. Chen, Int. J. Biol. Macromol., 2020, 152, 295–304 CrossRef CAS.
- L. Wang, X. Y. Li and B. B. Wang, Int. J. Biol. Macromol., 2018, 120, 1362–1368 CrossRef CAS.
- S. H. Nile and S. W. Park, Nutrition, 2014, 30(2), 134–144 CrossRef CAS.
- M. M. Zhao, J. W. Bai, X. Y. Bu, Y. Tang, W. Q. Han, D. L. Li, L. B. Wang, Y. Yang and Y. Q. Xu, Food Control, 2020, 107449 Search PubMed.
- Y. Q. Xu, L. Zhang, Y. Yang, X. M. Song and Z. Y. Yu, Carbohydr. Polym., 2015, 117, 895–902 CrossRef CAS.
- R. Takata, T. Yanai, R. Yamamoto and T. Konno, Biosci., Biotechnol., Biochem., 2007, 71, 1342–1344 CrossRef CAS.
- Y. Q. Xu, Y. Y. Guo, Y. K. Gao, X. J. Niu, L. B. Wang, X. G. Li, H. C. Chen, Z. Y. Yu and Y. Yang, LWT--Food Sci. Technol., 2018, 93, 16–23 CrossRef CAS.
- Y. Q. Xu, F. Cai, Z. Y. Yu, L. Zhang, X. G. Li, Y. Yang and G. J. Liu, Food Chem., 2016, 194, 650–658 CrossRef CAS.
- Y. Yang, Z. X. Lei, M. M. Zhao, C. Z. Wu, L. B. Wang and Y. Q. Xu, Ind. Crops Prod., 2020, 147, 112249 CrossRef CAS.
- D. J. Chen, H. Y. Sun, Y. X. Shen, M. Luo, X. Xin and Z. M. Xu, J. Funct. Foods, 2019, 61, 103492 CrossRef CAS.
- P. Y. Gao, J. Bian, S. S. Xu, C. F. Liu, Y. Q. Sun, G. L. Zhang, D. Q. Li and X. G. Liu, Int. J. Biol. Macromol., 2020, 149, 207–214 CrossRef CAS.
- D. F. Wei, T. Chen, M. F. Yan, W. H. Zhao, F. Li, W. D. Cheng and L. X. Yuan, Carbohydr. Polym., 2015, 125, 161–168 CrossRef CAS.
- S. L. Qiu, J. Chen, X. Chen, Q. Fan, C. S. Zhang, D. Y. Wang, X. P. Li, X. Y. Chen, X. L. Chen and C. Liu, Carbohydr. Polym., 2014, 103, 148–153 CrossRef CAS.
- Z. Y. Zhu, F. Liu, H. Gao, H. Q. Sun, M. Meng and Y. M. Zhang, Int. J. Biol. Macromol., 2016, 93, 1090–1099 CrossRef CAS.
- L. Wang, Q. H. Huang, Y. F. Huang, J. H. Xie, C. Qu, J. P. Chen, L. Zheng, T. G. Yi, H. F. Zeng and H. L. Li, J. Funct. Foods, 2018, 46, 365–377 CrossRef CAS.
- Q. Q. Song, L. Jiang, X. Q. Yang, L. X. Huang, Y. Yu, Q. Yu, Y. Chen and J. H. Xie, Int. J. Biol. Macromol., 2019, 138, 874–880 CrossRef CAS.
- P. Muniandy, A. B. Shori and A. S. Baba, Food Packag. Shelf Life, 2016, 8, 1–8 CrossRef.
- K. B. Jeddou, F. Chaari, S. Maktouf, O. Nouri-Ellouz, C. B. Helbert and R. E. Ghorbel, Food Chem., 2016, 205, 97–105 CrossRef.
- M. B. Romdhane, A. Haddar, I. Ghazala, K. B. Jeddou, C. B. Helbert and S. Ellouz-Chaabouni, Food Chem., 2017, 216, 355–364 CrossRef.
- Y. Q. Xu, G. J. Liu, Z. Y. Yu, X. M. Song, X. G. Li and Y. Yang, Food Chem., 2016, 199, 694–701 CrossRef CAS.
- Y. B. Meng, Y. Y. Zhang, N. Jia, H. R. Qiao, M. H. Zhu, Q. H. Meng, Q. Lu and Y. G. Zu, Int. J. Biol. Macromol., 2018, 118, 1438–1448 CrossRef CAS.
- F. J. Mao, P. J. Shi, H. Chen, L. Song, Z. Y. Wang, C. Wu and M. Du, Food Chem., 2018, 254, 103–108 CrossRef CAS.
- M. Marchuk, M. J. Selig, G. B. Celli, P. Lawrence, D. M. Smilgies and A. Abbaspourrad, Food Hydrocolloids, 2019, 93, 226–234 CrossRef CAS.
- Q. Li, W. Wang, Y. Zhu, Y. Chen, W. J. Zhang, P. Yu, G. H. Mao, T. Zhao, W. W. Feng and L. Q. Yang, Carbohydr. Polym., 2017, 161, 42–52 CrossRef CAS.
- E. Malinowska, M. Klimaszewska, T. Strączek, K. Schneider, C. Kapusta, P. Podsadni, G. Łapienis, M. Dawidowski, J. Kleps and S. Górska, Carbohydr. Polym., 2018, 198, 407–417 CrossRef CAS.
- Q. Y. Song, Z. Y. Zhu, X. T. Wang, L. T. Chen and D. Y. Wang, J. Mol. Struct., 2019, 1178, 630–638 CrossRef CAS.
- F. Y. Kagimura, M. A. A. D. Cunha, T. V. Theis, C. R. M. Malfatti, R. F. H. Dekker, A. M. Barbosa, S. D. Teixeira and K. Salomé, Carbohydr. Polym., 2015, 127, 390–399 CrossRef CAS.
- R. B. A. Kolsi, H. B. Salah, N. Jardak, R. Chaaben, I. Jribi, A. E. Feki, T. Rebai, K. Jamoussi, N. Allouche and C. Blecker, Carbohydr. Polym., 2017, 170, 148–159 CrossRef.
- M. Z. Sun, Y. M. Li, T. X. Wang, Y. W. Sun, X. Y. Xu and Z. S. Zhang, Carbohydr. Polym., 2018, 184, 127–134 CrossRef CAS.
- L. Wang, B. Zhang, J. Xiao, Q. Huang, C. Li and X. Fu, Food Chem., 2018, 249, 127–135 CrossRef CAS.
- E. Alpizar-Reyes, H. Carrillo-Navas, R. Gallardo-Rivera, V. Varela-Guerrero, J. Alvarez-Ramirez and C. Pérez-Alonso, J. Food Eng., 2017, 209, 68–75 CrossRef CAS.
- M. Elleuch, D. Bedigian, O. Roiseux, S. Besbes, C. Blecker and H. Attia, Food Chem., 2010, 124(2), 411–421 CrossRef.
- I. Mateos-Aparicio, A. Redondo-Cuenca and M. J. Villanueva-Suárez, Food Chem., 2010, 122(1), 339–345 CrossRef CAS.
- A. Shah, F. A. Masoodi, A. Gani and B. A. Ashwar, LWT--Food Sci. Technol., 2017, 80, 19–26 CrossRef CAS.
- X. X. Liu, Y. Y. Yan, H. M. Liu, X. D. Wang and G. Y. Qin, LWT--Food Sci. Technol., 2019, 111, 242–251 CrossRef CAS.
- C. Ai, H. C. Meng, J. W. Lin, T. Zhang and X. M. Guo, Food Hydrocolloids, 2020, 109, 106049 CrossRef CAS.
- L. Z. Cheng, Y. F. Wang, X. X. He and X. L. Wei, Int. J. Biol. Macromol., 2018, 120, 82–92 CrossRef CAS.
Footnote |
| † Two authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |