Open Access Article
Open Access ArticleThree-dimensional hierarchical ZnCo2O4@C3N4-B nanoflowers as high-performance anode materials for lithium-ion batteries†
Haihong Xiao ,
Guoqing Ma,
Junyu Tan,
Shuai Ru
,
Guoqing Ma,
Junyu Tan,
Shuai Ru ,
Zhaoquan Ai* and
Caixia Wang*
,
Zhaoquan Ai* and
Caixia Wang*
Hubei Collaborative Innovation Center for Advanced Organic Chemical Materials, Ministry of Education Key Laboratory for the Synthesis and Application of Organic Functional Molecules, College of Chemistry and Chemical Engineering, Hubei University, Wuhan 430062, P. R. China. E-mail: 1045065959@qq.com; 2326978832@qq.com
First published on 2nd September 2020
Abstract
ZnCo2O4 has become one of the most widely used anode materials due to its good specific capacity, cost-efficiency, high thermal stability and environmental benignity. However, its poor conductivity and cycle stability have limited its practical application in lithium-ion batteries. To overcome these issues, we constructed a 3D nanoflower composite material (ZnCo2O4@C3N4-B) by combining ZnCo2O4 as a framework and B-doped g-C3N4 (g-C3N4-B) as a new carbon source material via a simple hydrothermal method. ZnCo2O4@C3N4-B exhibited exceptional specific capacitance of 919.76 mA h g−1 after 500 cycles at 0.2 A g−1 and a long-term capacity retention of 97.8% after 1000 cycles at 2 A g−1. The high reversible capacity, long cycling life and good rate performance could be attributed to the 3D interconnected architecture and doping of g-C3N4-B. This work provides a simple and general strategy to design high-performance anode materials for lithium-ion batteries to meet the needs of practical applications.
1. Introduction
Rechargeable lithium-ion batteries (LIBs) have been utilized widely as the main choice for mobile terminal equipment and electric vehicles.1–3 Transition metal oxides have become the preferred next-generation selectable materials for lithium-ion battery anodes due to their high specific capacity.4,5 Among these transition metal oxides, the binary transition metal oxides AB2O4 with a spinel structure can not only overcome the shortcomings of the large first-discharge capacity loss and poor cyclic stability of simple metal oxides by using the compounding principle and taking advantage of properties of multiple components, but also have attracted growing attention owing to their synergistic effect on various action mechanisms.6 Especially relative to other binary transition metal oxides, ZnCo2O4 with good specific capacity, cost-efficiency, high thermal stability and environmental benignity has become one of the most widely studied materials.7 Simultaneously, as a binary metal oxide, it has two reaction mechanisms of insertion and conversion in the electrode reaction, which improve and counteract the problem of electrochemical performance degradation common with single metal oxides.34–36 However, its poor conductivity and cycle stability have limited its practical application in LIBs.8,9In order to improve the electrochemical performance of the material, researchers have increased the specific surface area of the material by changing its morphology, such as nanospheres,10 nanotubes11 and nanosheets,12,13 but the requirement for precise control of morphology in the process of experimental synthesis can limit the wide application of the material. Therefore, it is important to develop an easy, general and environmentally benign process for the synthesis of 3D hierarchical ZnCo2O4 for electrode materials.
The other effective method is to synthesize transition metal oxide composites doped with a carbon material that can effectively alleviate the volume expansion by adjusting the 2D/3D network matrix.14,15 Graphene nanosheets with good conductivity and large specific surface area are often used as the carbon source, but their low theoretical specific capacity (372 mA h g−1) and high cost limit their pervasive application.16 In recent years, graphite-like materials have gradually replaced graphene materials because of their simple preparation processes and low cost.17 Among these, g-C3N4 has emerged as a promising carbon source of anode materials owing to its many advantages, such as excellent thermal and chemical stability, environmental friendliness, many preparation methods and easy access.18,19
Moreover, in the field of electrocatalysis and photocatalysis, doping non-metals and metals in g-C3N4 is an effective strategy to enhance the catalytic activity. Non-metal-doping materials, such as B-doped and S-doped g-C3N4, have been reported to enhance both the photocatalytic and electro-catalytic activity of g-C3N4.20 Moreover elemental doping can readily tune the band gap structure of g-C3N4, facilitating the separation and transfer of electron–hole pairs at the heterojunction interface.21 Therefore, B-doped and S-doped g-C3N4 may be used as a new carbon source material to improve the electrochemical performance of the electrode.
Inspired by the fascinating electrocatalytic performances of ion-doped g-C3N4 materials, B-doped or S-doped g-C3N4 as a new carbon source material was used to construct a modified ZnCo2O4@C3N4 hybrid anode material by a simple hydrothermal method. Interestingly, nanosphere, nanosheet and nanoflower composite materials of ZnCo2O4 and modified graphene-like materials were obtained by doping g-C3N4, g-C3N4-S and g-C3N4-B, respectively. Also, the morphology has a great effect on the electrochemical performance of the anode materials. The g-C3N4-B doped ZnCo2O4 nanoflower composite materials (ZCO@C3N4-B) showed a high reversible capacity, long cycling life and good rate performances due to large surface area and g-C3N4-B doping. Given these advantageous of this composite, it provides a simple and effectual method for manufacturing high-performance anodes for lithium-ion batteries, especially in practical use.
2. Experimental
2.1 Materials and instrumentations
Melamine, boric acid, thiourea, PVP, Zn(CH3COOH)2·2H2O and Co(CH3COOH)2·4H2O were obtained from Shanghai Chemical Co. The microstructures and morphologies of the samples were characterized by scanning electron microscopy (SEM, JSM-6510LV) and high-resolution transmission electron microscopy (HRTEM, Tecnai G20). The crystallographic structure was examined by X-ray diffraction (XRD, D8-ADVANCE) and Raman spectrometry (NEXUS670). Surface analysis of the samples was carried out by X-ray photoelectron spectroscopy (XPS, Escalab 250X, Thermo Fisher Scientific). The surface area measurements were performed according to the Brunauer–Emmett–Teller (BET, 3H-2000A) method.2.2 Electrochemical measurements
Electrochemical performances were measured using coin cells (LIR 2025) assembled in a glove box filled with argon. The electrode was prepared with 80 wt% of the composite materials (ZCO@C3N4, ZCO@C3N4-S or ZCO@C3N4-B), 10 wt% carbon black, and 10 wt% sodium carboxymethyl cellulose (CMC). The electrolyte consisted of a solution of 1 M LiPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Galvanostatic tests of the assembled cells were carried out using a Xinwei (BTS7.6.x 5 V/50 mA) system in the voltage range of 0.01–3.0 V (vs. Li+/Li). Cyclic voltammetry (CV) characteristics of the samples were analyzed within the potential range between 0.01–3.0 V at a scanning speed of 0.1 mV s−1 on a Shanghai Chenhua electrochemical workstation (CHI 600E). Also, electrochemical impedance spectroscopy (EIS) characterization was also performed on cells assembled with active materials at a testing frequency of 0.01 Hz to 100 kHz at room temperature.
1, v/v). Galvanostatic tests of the assembled cells were carried out using a Xinwei (BTS7.6.x 5 V/50 mA) system in the voltage range of 0.01–3.0 V (vs. Li+/Li). Cyclic voltammetry (CV) characteristics of the samples were analyzed within the potential range between 0.01–3.0 V at a scanning speed of 0.1 mV s−1 on a Shanghai Chenhua electrochemical workstation (CHI 600E). Also, electrochemical impedance spectroscopy (EIS) characterization was also performed on cells assembled with active materials at a testing frequency of 0.01 Hz to 100 kHz at room temperature.
2.3 Sample preparation
3. Results and discussion
3.1 Synthesis and characterization
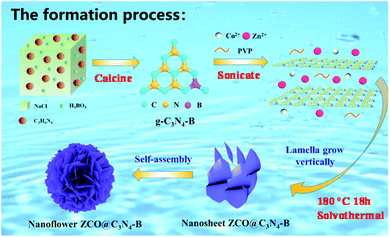
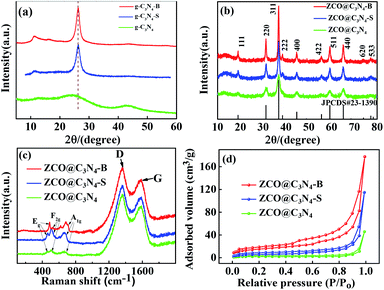
As schematically illustrated in Fig. 1, the ZCO@C3N4-B sample was synthesized via the following steps. First, the g-C3N4-B carbon source materials were synthesized by template calcination using NaCl, C3H6N6 and H3BO3. Afterwards, using PVP as the surface stabilizer, Zn(CH3COOH)2 and Co(CH3COOH)2 as the zinc source and cobalt source, respectively, g-C3N4-B was used as the carbon source for ultrasonic dispersion and the precursor was synthesized by the solvent heat method. Finally, the product ZCO@C3N4-B was obtained by calcining the precursor under the condition of 400 °C argon gas. Meanwhile, the sample ZCO@C3N4-S adopted the same operation process, except that boric acid was replaced by thiourea, while the ZCO@C3N4 sample only uses C3N4 as the carbon source for comparison. The morphologies of the composite materials strongly depended on the doping carbon materials, which had a great effect on the electrochemical performance of the anode material.X-ray diffraction (XRD) analysis was used to determine the crystalline phase of the sample. As shown in the Fig. 2(a), strong characteristic diffraction peaks of the graphene-like materials (g-C3N4, g-C3N4-S and g-C3N4-B) were observed at 24.23° and 43.51°, which were attributed to the (002) and (100) crystal planes of graphite.22 The graphitization degree of the g-C3N4 graphene materials was low. After doping the S and B elements, the corresponding peak diffraction angle of the (002) crystal plane was closer to 26° of the pure graphite crystal and the diffraction intensity was increased remarkably.23 These results demonstrated that the degree of graphitization of the composite materials was enhanced after doping with the S or B element. Also, Fig. 2(b) displays the diffraction patterns of the ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B composites materials. The diffraction peaks of those materials were located at 18.96°, 31.22°, 36.81°, 38.49°, 44.74°, 55.57°, 59.28°, 65.15°, 74.00° and 77.22°, corresponding to the crystal faces (111), (220), (311), (222), (400), (422), (511), (440), (620) and (533) of cubic ZnCo2O4 with a typical spinel structure (JCPDS Card No. 23-1390).24 No other impurity peaks were observed. This indicated that all of the composite materials had an excellent crystallization degree. Also, doping with B and S elements increased the peak strength, suggesting the formation of more stable crystal structures of the ZCO@C3N4-B and ZCO@C3N4-S. The absence of carbon peaks showed that graphene-like materials had been graphitized.25
 | ||
| Fig. 2 (a) XRD patterns of the C3N4, C3N4-S and C3N4-B; (b–d) XRD patterns, Raman spectra and nitrogen adsorption–desorption of ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B. | ||
Raman spectroscopy was performed to study the structural properties of the composite materials. As shown in Fig. 2(c), all the samples had three peaks at 473, 512 and 654 cm−1, respectively. The peaks at 473 cm−1 from the stretching vibration of Zn–O and Co–O bond were attributed to the active mode Eg. The characteristic peak at 512 cm−1 was from the vibration of the Co–O bond, corresponding to a symmetric mode of F2g. Also, the expansion vibration of the Co–O bond at 654 cm−1 was ascribed to the vibration mode of A1g.26 The D peak (1357 cm−1) was the disordered vibration peak of graphitic carbon, and the G peak (1584 cm−1) was the in-plane vibration peak of the carbon atom sp2. The relative dimensional intensity ID/IG (R = ID/IG) reflected the degree of carbonization of the material. The R values of ZCO@C3N4, ZCO@C3N4-S, and ZCO@C3N4-B were 0.87, 0.84, and 0.82, respectively. After doping with B and S elements, the R values decreased. Also, the R value of ZCO@C3N4-B was smaller than that of ZCO@C3N4-S, indicating the higher degree of carbonization of ZCO@C3N4-B.
In order to further analyze the specific surface area and pore size of the sample, the adsorption and desorption isotherms of the ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B samples were tested by N2. As shown in Fig. 2(d), all three materials conformed to type IV and the mesoporous hysteresis loop belonged to type H3, which may lead to an inconspicuous saturated adsorption platform due to the irregular mesoporous structure of the material.28 The formula of the multi-molecule layer adsorption was derived from BET theory:29 the specific surface area of the ZCO@C3N4-B sample was calculated to be 137.02 cm3 g−1 (ZCO@C3N4-S and ZCO@C3N4 were 63.27 and 27.86 cm3 g−1, respectively), and the pore size (shown in the ESI Fig. S5†) was mainly distributed between 9–12 nm (ZCO@C3N4-S and ZCO@C3N4 were between 15–18 nm and 42–50 nm, respectively). It could be clearly seen that the specific surface area of the ZCO@C3N4-B sample was greater than that others. However, this was mainly attributed to the layer with the nanoflower structure being beneficial to increase the specific surface area and its aperture size was also relatively small, prompting the material surface to form a large number of free channels, which makes it more conducive to lithium ions embedded in the charge and discharge process, and this unique advantages is more suitable for some of the applications related to the surface structure.
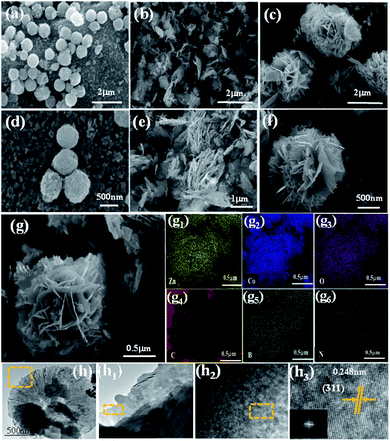
The morphologies and structural features of the samples were observed by SEM. It can be seen from Fig. S3(a–c)† that the morphology of the material C3N4, C3N4-S mainly presented a block structure, but the sample C3N4-B had a lamellar structure, and it can also be clearly seen that C, N, B were evenly distributed on the C3N4-B surface from Fig. S3(c1–c4).† However, after coating the composite material, ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B could be observed as mainly nanospheres, nanopieces and nanoflowers, as shown in Fig. 3(a–f). The nanoflower structure was composed of many loosely packed nanosheets and had many channel pores and a large surface area, which was also consistent with the test results from the nitrogen adsorption–desorption. At the same time, the interlaced nanosheets inside the electrodes were tightly combined, facilitating sufficient contact between the electrode/electrolyte.
The SEM-EDS mapping images of Zn, Co, O, C, B and N for ZCO@C3N4-B are shown in Fig. 3(g–g6). The Zn, Co, O, C, B and N elements were uniformly distributed in the composite material. As shown in Fig. 3(h), the transmission electron microscopy (TEM) images of the nanometer flower ZCO@C3N4-B showed a lamellar structure (the h1 diagram is the local enlarged diagram of Fig. 3(h)), and in the high-resolution transmission electron microscopy (HRTEM) image shown in Fig. 3(h2), the ZCO@C3N4-B nanoflower visualized well-defined lattice fringes with a spacing of ∼0.248 nm (shown in Fig. 3(h3)), corresponding to the (311) crystal plane of the ZnCo2O4 phase.24 Therefore, it can be concluded that the nanoflower composite material was mainly composed of (+2) Zn and (+3) Co, which strictly agrees with the above XRD results.
To further investigate the elemental composition and valence state of the ZCO@C3N4-B nanoflower, X-ray photoelectron spectroscopy (XPS) was performed and the results shown in Fig. 4(a) (ZCO@C3N4 and ZCO@C3N4-S shown in Fig. S1 and S2†). The strong characteristic peaks at 1086, 1021, 781, 521, 290, 502 and 714 eV correspond to Zn 2p1/2, Zn 2p3/2, Co 2p, B 1s, O 1s, C 1s and N 1s, respectively. This indicated that ZCO@C3N4-B was mainly composed of Zn, Co, O, C, N and B elements. There were two characteristic peaks at 1044.3 and 1020.8 eV in Fig. 4(b), corresponding to Zn 2p1/2 and Zn 2p3/2 orbitals of the Zn(II) oxidation state. The peaks of Co 2p at 795.0 and 779.7 eV were attributed to Co 2p1/2 and Co 2p3/2 orbitals in Fig. 4(c). Also, the two weaker peak at 804.3 and 789.3 eV indicated the Co(III) oxidation state of ZCO@C3N4-B.7 As shown in Fig. 4(d), the peaks at 531.1 and 529.4 eV of O 1s were attributed to chemisorbed oxygen (C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and metal–oxygen bond (Co–O),27 respectively. The Zn and Co elements in the calcined product ZCO@C3N4-B were in the form of divalent and trivalent, which were consistent with the XRD and Raman results. The large characteristic peak of C 1s at 284.3 eV represented the C–C peak of a graphene-like material, while the peak at 288.3 eV was attributed to the sp2-bonded carbon (N–C
O) and metal–oxygen bond (Co–O),27 respectively. The Zn and Co elements in the calcined product ZCO@C3N4-B were in the form of divalent and trivalent, which were consistent with the XRD and Raman results. The large characteristic peak of C 1s at 284.3 eV represented the C–C peak of a graphene-like material, while the peak at 288.3 eV was attributed to the sp2-bonded carbon (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) in Fig. 4(e). The peaks of the graphite N and pyridine N were at 400.4 and 398.8 eV, as shown in Fig. 4(f),18,30 respectively. As shown in Fig. 4(g), the B 1s peak at 191.6 eV was typical for C–B coordination, suggesting that B was more likely to replace N to form the C–B bond in the carbon nitride framework. This was consistent with the electronegativity principle. The binding energy of C–B should be 1–2 eV lower than N–B according to the literature.19 Therefore, the XPS results demonstrated that B atoms successfully entered the framework of C3N4 and maintained the stability of the skeleton.
N) in Fig. 4(e). The peaks of the graphite N and pyridine N were at 400.4 and 398.8 eV, as shown in Fig. 4(f),18,30 respectively. As shown in Fig. 4(g), the B 1s peak at 191.6 eV was typical for C–B coordination, suggesting that B was more likely to replace N to form the C–B bond in the carbon nitride framework. This was consistent with the electronegativity principle. The binding energy of C–B should be 1–2 eV lower than N–B according to the literature.19 Therefore, the XPS results demonstrated that B atoms successfully entered the framework of C3N4 and maintained the stability of the skeleton.
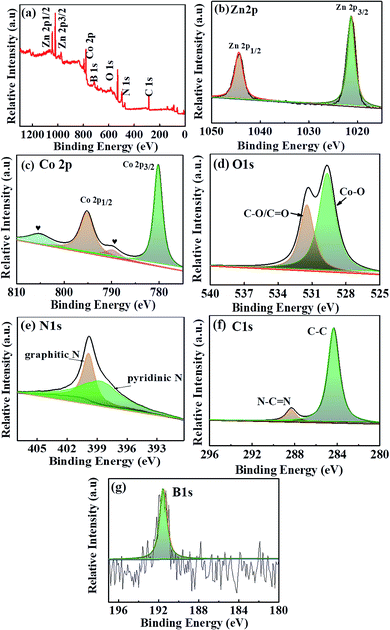 | ||
| Fig. 4 (a) XPS full spectrum for the ZCO@C3N4-B nanoflower. (b–g) XPS high-resolution spectra of Zn 2p, Co 2p, O 1s, C 1s, N 1s and B 1s for the ZCO@C3N4-B. | ||
The high-resolution Zn 2p and Co 2p spectra of ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B are compared in Fig. 5(a and b). As shown in Fig. 5(a), compared with ZCO@C3N4-B, two characteristic peaks in the Zn 2p spectrum of ZCO@C3N4 or ZCO@C3N4-S showed a negative shift, indicating that the Zn(II) valence state in ZCO@C3N4-B was more stable. Similarly, compared to ZCO@C3N4-B, a slight negative shift occurred in the Co 2p peak (Fig. 5(b)) of ZCO@C3N4 or ZCO@C3N4-S. These results suggested that ZCO@C3N4-B was more electropositive because of the redistribution of the electron density.
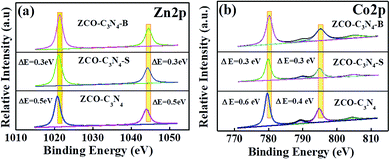 | ||
| Fig. 5 (a and b) XPS spectra comparison of the Zn 2p and Co 2p spectra of ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B. | ||
3.2 Electrochemical properties
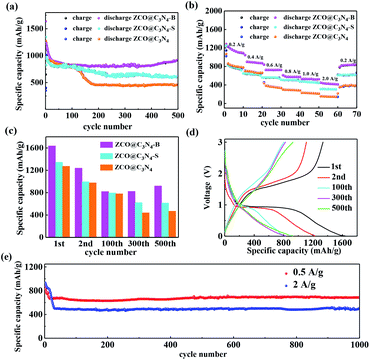
The lithium storage properties of the as-prepared ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B were investigated as the anode in LIBs. Fig. 6(a) displays the cycling behaviour for different electrodes at 0.2 A g−1. The reversible capacities of the ZCO@C3N4, ZCO@C3N4-S and ZCO@C3N4-B electrodes were 468.23, 616.07 and 919.76 mA h g−1 after 500 cycles, respectively. Comparing the three materials, the specific discharge capacity of the ZCO@C3N4-B electrodes increased slowly after 450 cycles, which could be the result of the reversible formation/dissolution of polymer-like gels during the transition of the metal oxide-activated electrode material.33 While the specific capability of the other two electrodes obviously decreased gradually, which was mainly attributed to the stability of the 3D nanoflower structure of ZCO@C3N4-B. Meanwhile, Fig. 6(a) exhibited the almost 100% coulombic efficiency of the ZCO@C3N4-B electrode at a current density of 0.2 A g−1. The charge–discharge properties of the electrodes at different current densities were tested and are shown in Fig. 6(b). The capacitance of the ZCO@C3N4-B electrode was much better than that of the other two electrodes at different current densities (0.2, 0.4, 0.6, 0.8, 1 and 2 A g−1), especially at a high current density (2 A g−1). Compared with ZCO@C3N4 and ZCO@C3N4-S electrodes, ZCO@C3N4-B retained a higher retention capacity (Fig. 6(c)). This could be because the integrity of the 3D nanoflower structure provided a shorter diffusion distance for lithium ions and enhanced the contact area between the electrolyte and graphene-like materials.31Galvanostatic discharging and charging measurements were conducted to study the electrochemical performance of the ZCO@C3N4-B electrode. Fig. 6(d) shows the 1st, 2nd, 100th, 300th, and 500th discharge (Li+ insertion) and charge (Li+ extraction) profiles at a current density of 0.2 A g−1 in the voltage window of 0.01–3 V. The initial discharge capacity of the ZCO@C3N4-B electrode was 1636.34 mA h g−1, which far exceeded the theoretical specific capacity of ZnCo2O4 (975.2 mA h g−1). It decreased to 1238 mA h g−1 in the second cycle and the irreversible capacity loss was nearly 25%, which may be due to the formation of a solid electrolyte interface (SEI) and the reduction of the metal oxide to metal and Li2O.32 Also, the circulation capacity remained above 919 mA h g−1 after 500 cycles, mainly owing to the stability of the 3D flower-like structure. However, the discharge capacities of the ZCO@C3N4-S and ZCO@C3N4 electrodes were 1345.56 and 1272.26 mA h g−1 for the 1st cycle, respectively, and remained only at 616.53 and 468 mA h g−1 after 500 cycles (shown in Fig. S4†). The ZCO@C3N4-B electrode showed better cycle capacity than the other two electrodes. This was because the structure of the electrode was well adjusted after doping with the B element; thereby improving the electrical properties of the electrode. The voltage platform was maintained at about 0.9–1.1 V during 500 cycles discharge, suggesting that the ZCO@C3N4-B electrode had excellent electrochemical reversibility in lithium insertion and extraction reactions. A long cycle test of the ZCO@C3N4-B electrode at a high current density was performed and the result is shown in Fig. 6(e). ZCO@C3N4-B delivered a high reversible capacity of 686.78 mA h g−1 after 1000 cycles at 0.5 A g−1, and maintained 95.3% capacity from the tenth cycles. In addition, a stable reversible capacity of 497.76 mA h g−1 at 2 A g−1 was obtained after 1000 cycles. These results showed the excellent cycling stability of the 3D nanoflower ZCO@C3N4-B. Furthermore, as demonstrated in Table S1,† in respect to the current rate and the cycling life, the ZCO@C3N4-B composite demonstrated outstanding electrochemical properties compared to the other ZnCo2O4-based anodes for LIBs (Table 1).
| Electrode material | Voltage range (V) | Current density (mA g−1) | Cycle number | Capacity (mA h g−1) | Reference |
|---|---|---|---|---|---|
| ZnCo2O4 nanocluster particles | 0.005–3.0 | 100 | 100 | 700 | 5 |
| Porous Zn–Co–O | 0.01–3.0 | 100 | 200 | 997 | 6 |
| Nanophase ZnCo2O4 | 0.005–3.0 | 60 | 30 | 900 | 8 |
| ZnO/ZnCo2O4 nanocomposites | 0.01–3.0 | 500 | 200 | 847 | 14 |
| ZnCo2O4@TiO2 nano-composite | 0.01–3.0 | 100 | 90 | 827 | 15 |
| ZnCo2O4@C3N4-B nanoflower | 0.01–3.0 | 200 | 500 | 919 | This work |
| 500 | 1000 | 686 |
To examine the electrochemical characteristics of the ZCO@C3N4-B electrodes, cyclic voltammetry profiles of the initial three cycles were performed and the results are displayed in Fig. 7(a). There was an intense peak at 0.31 V and a minor peak at 0.97 V in the first discharge process, which could result from the reduction of ZnCo2O4 to metallic Zn and Co and the decomposition of the electrolyte to form the SEI films, respectively. In the 2nd to 5th discharge processes, the reduction peaks were shifted to 1.52 and 1.01 V, indicating the structural modification and different lithium reaction after the first cycle. During the charge process, two oxidation peaks located at 1.82 and 2.24 V were seen, attributed to the oxidation of Zn to Zn2+ and CoO to Co3+, respectively. The electrochemical reactions involved in the discharge and charging process could be clarified as follows:22
| ZnCo2O4 + 8Li+ +8e− → Zn + 2Co + 4Li2O | (1) |
| Zn + Li+ + e− ↔ LiZn | (2) |
| Zn + Li2O ↔ ZnO + 2Li+ + 2e− | (3) |
| 2Co + 2Li2O ↔ 2CoO + 4Li+ + 4e− | (4) |
| 2CoO + 2/3Li2O ↔ 2/3Co3O4 + 4/3Li+ + 4/3e− | (5) |
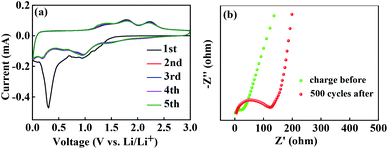 | ||
| Fig. 7 (a) Cyclic voltammetry curves during the initial five cycles for the ZCO@C3N4-B electrode; (b) electrochemical impedance of the ZCO@C3N4-B electrodes after 500 cycles and the charge before. | ||
Electrochemical impedance spectroscopy (EIS) of the ZCO@C3N4-B electrodes before cycling and after the 500th cycle was measured and the corresponding Nyquist plots were obtained and are shown in Fig. 7(b). The Nyquist plots constituted a semicircle in the high-to-medium frequency from the charge transfer and a sloping long line in the low-frequency from the mass transfer of lithium ions. The surface layer resistance after 500 cycles (≈122 Ω) was very low, indicating that the ZCO@C3N4-B electrodes with the special flower-like structure could produce an interlaced conductive network and continuous Li+ and e− pathways, thus significantly enhancing the battery performance.
In order to further investigate the reason for the excellent electrochemical performance of the ZCO@C3N4-B electrode, the surface property of ZCO@C3N4-B after 500 cycles was observed by SEM (Fig. 8). After 500 cycles, the 3D nanoflower ZCO@C3N4-B maintained its structural integrity without cracking, leading to the excellent cycling stability. It is well known that nanostructures can provide large electrolyte contact areas and large specific surface areas, which can greatly increase the battery capacity. Also, the nanoflower structure can form a staggered conductive network and provide continuous Li+ and e− pathways to ensure the transfer of ions and electrons, thereby improving the long-term conductivity.
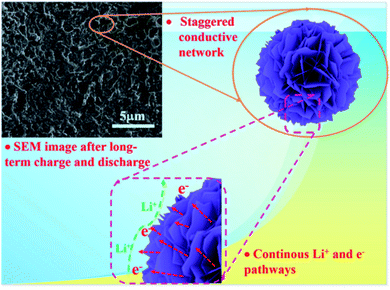 | ||
| Fig. 8 Mechanism explanations of the excellent electrochemical performance of the nanoflower ZCO@C3N4-B electrode. | ||
4. Conclusion
In summary, a special three-dimensional (3D) nanoflower ZCO@C3N4-B was successfully synthesized using a simple hydrothermal method. At a current density of 0.2 A g−1, the nanoflower electrode capacity remained above 919 mA h g−1 after 500 cycles. Also, it displayed excellent cycling stability at 0.5 A g−1 and 2 A g−1 after 1000 cycles. The excellent electrochemical performance of the ZCO@C3N4-B electrode was attributed to its steady special secondary structure, integrated staggered conductive network, large electrolyte contact area and continuous lithium ion and electron pathways. The nanoflower ZCO@C3N4-B has potential to serve as an anode material for lithium-ion batteries and is expected to be used in industrial applications.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the high-tech research and development project fund of Wuhan Science and Technology Bureau (2017010201010107).Notes and references
- Z. Yang, J. Ren, Z. Zhang, X. Chen, G. Guan, L. Qiu, Y. Zhang and H. Peng, Chem. Rev., 2015, 115, 5159–5223 CrossRef CAS.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS.
- Z. Zhao-Karger, P. Gao, T. Ebert, S. Klyatskaya, Z. Chen, M. Ruben and M. Fichtner, Adv. Mater., 2019, 31, 1806599 CrossRef.
- S. Zheng, X. Li, B. Yan, Q. Hu, Y. Xu, X. Xiao, H. Xue and H. Pang, Adv. Energy Mater., 2017, 7, 1602733 CrossRef.
- Y. Pan, W. Zeng, L. Li, Y. Zhang, Y. Dong, D. Cao, G. Wang, B. L. Lucht, K. Ye and K. Cheng, Nano-Micro Lett., 2016, 9, 3–9 Search PubMed.
- M. H. Soo, Y. K. So, J.-G. Kim, Ki J. Kim, J.-W. Lee, M.-S. Park, Y.-J. Kim, M. Shahabuddin, Y. Yamauchi and J. Ho Kim, Nanoscale, 2015, 7, 8351–8355 RSC.
- K. N. Jung, S. M. Hwang, M. S. Park, Ki J. Kim, J. Kim, S. X. Dou, J. Ho Kim and J. Lee, Sci. Rep., 2015, 5, 7665 CrossRef CAS.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Adv. Energy Mater., 2007, 17, 2855–2861 CAS.
- R. Zhao, Q. Li, C. Wang and L. Yin, Electrochim. Acta, 2016, 197, 58–67 CrossRef CAS.
- Y. Wang, W. Guo, Y. Yang, Y. Yu, Q. Li, D. Wang and F. Zhang, Electrochim. Acta, 2018, 262, 1–8 CrossRef CAS.
- A. W. Nemaga, J. Mallet, J. Michel, C. Guery, M. Molinari and M. Morcrette, J. Power Sources, 2018, 393, 43–53 CrossRef CAS.
- Y. Ouyang, H. Ye, X. Xia, X. Jiao, G. Li, S. Mutahir, L. Wang, D. Mandler, W. Lei and Q. Hao, J. Mater. Chem. A, 2019, 7, 3228–3237 RSC.
- J. B. Boland, A. Harvey, R. Tian, D. Hanlon, V. Vega-Mayoral, B. Szydlowska, A. Griffin, T. Stimpel-Lindner, S. Jaskaniec, V. Nicolosi, G. Duesberg and J. N. Coleman, Nanoscale Adv., 2019, 1, 1560–1570 RSC.
- J. Xu, L. He, Y. Wang, C. Zhang and Y. Zhang, Electrochim. Acta, 2016, 191, 417–425 CrossRef CAS.
- W. Shi, H. Zhao and B. Lu, Nanotechnology, 2017, 28, 165403 CrossRef.
- X. Cheng, D. Li, Y. Wu, R. Xu and Y. Yu, J. Mater. Chem. A, 2019, 7, 4913–4921 RSC.
- G. Wang, Z. Wen, Y.-E. Yang, J. Yin, W. Kong, S. Li, J. Sun and S. Ji, J. Mater. Chem. A, 2018, 6, 7557–7565 RSC.
- H. Yang, Y. Zhou, Y. Wang, S. Hu, B. Wang, Q. Liao, H. Li, J. Bao, G. Ge and S. Jia, J. Mater. Chem. A, 2018, 6, 16485–16494 RSC.
- H. Yu, X. Jiang, Z. Shao, J. Feng, X. Yang and Y. Liu, Nanoscale Res. Lett., 2018, 13, 52–57 CrossRef.
- Q. Yan, G.-F. Huang, D.-F. Li, M. Zhang, A.-L. Pan and W.-Q. Huang, J. Mater. Sci. Technol., 2018, 34, 2515–2520 CrossRef.
- Y. Wang, Y. Li, J. Zhao, J. Wang and Z. Li, Int. J. Hydrogen Energy, 2019, 44, 618–628 CrossRef CAS.
- Y. V. Kaneti, N. L. Wulan Septiani, I. Saptiama, X. Jiang, B. Yuliarto, M. J. A. Shiddiky, N. Fukumitsu, Y.-M. Kang, D. Golberg and Y. Yamauchi, J. Mater. Chem. A, 2019, 7, 3415–3425 RSC.
- R. Ding, J. Zhang, J. Qi, Z. Li, C. Wang and M. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 13470–13478 CrossRef CAS.
- M. Liu, Z. Zhang, M. Dou, Z. Li and F. Wang, Carbon, 2019, 151, 28–35 CrossRef CAS.
- Y. Xiao, G. Tian, W. Li, Y. Xie, B. Jiang, C. Tian, D. Zhao and H. Fu, J. Am. Chem. Soc., 2019, 141, 2508–2515 CrossRef CAS.
- Y. Wang, M. Wang, G. Chen, C. Dong, Y. Wang and L.-Z. Fan, Ionics, 2014, 21, 623–628 CrossRef.
- Q. Li, Y. Feng, P. Wang and R. Che, Nanoscale, 2019, 11, 5080–5093 RSC.
- J. Li, X. Li, L. Zeng, S. Fan, M. Zhang, W. Sun, X. Chen, M. O. Tade and S. Liu, Nanoscale, 2019, 11, 3877–3887 RSC.
- Z. Chen, C. Wang, M. Chen, C. Ye, Z. Lin, L. Xing, Y. Liao, M. Xu, G. Cao and W. Li, J. Mater. Chem. A, 2019, 7, 3924–3932 RSC.
- H. Che, A. Liu, X. Zhang, J. Mu, Y. Bai and J. Hou, Ceram. Int., 2015, 41, 7556–7564 CrossRef CAS.
- R. Fang, K. Chen, L. Yin, Z. Sun, F. Li and H. M. Cheng, Adv. Mater., 2019, 31, 1800863 CrossRef.
- J. Zhou, B. Zhao and J. Bai, Scr. Mater., 2019, 166, 87–91 CrossRef CAS.
- Y. R. Zhang, Z. L. Wang, F. uz Zaman, Z. W. Zhao, X. Sun, J. Y. Zhang, L. R. Hou and C. Z. Yuan, J. Mater. Chem. A, 2019, 7, 3264–3277 RSC.
- A. P. Wang, S. Kadam, H. Li, S. Q. Shi and Y. Qi, npj Comput. Mater., 2018, 4, 34–39 CrossRef.
- J. J. Zhang and A. S. Yu, Sci. Bull., 2015, 60, 823–838 CrossRef CAS.
- N. Vicente and G. Garcia-Belmonte, J. Phys. Chem. Lett., 2017, 8, 1371–1374 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary figures of electrochemical performances. See DOI: 10.1039/d0ra05203e |
| This journal is © The Royal Society of Chemistry 2020 |