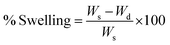Open Access Article
Open Access ArticleAdvances in chemistry and composition of soft materials for drug releasing contact lenses
Subir Chatterjee ,
Prashant Upadhyay,
Manjul Mishra,
Srividya M.,
M. R. Akshara,
Kamali N.,
Zahra Sifat Zaidi,
Sayeda F. Iqbal and
Santosh K. Misra
,
Prashant Upadhyay,
Manjul Mishra,
Srividya M.,
M. R. Akshara,
Kamali N.,
Zahra Sifat Zaidi,
Sayeda F. Iqbal and
Santosh K. Misra *
*
Department of Biological Sciences & Bioengineering, Indian Institute of Technology Kanpur, Kalyanpur, Uttar Pradesh, India-208016. E-mail: skmisra@iitk.ac.in; Tel: +91-512-259-4013
First published on 6th October 2020
Abstract
Ocular drug delivery has always been a challenging feat to achieve in the field of medical sciences. One of the existing methods of non-invasive ocular drug delivery is the use of eye drops. However, drugs administered through these formulations have low bioavailability in the ocular system. This limitation can been overcome by using contact lenses as drug delivery vehicles. According to USA FDA definitions they can be categorized into two main categories-hard and soft contact lenses. Based on the material properties, hard contact lenses are mostly produced from polymers of acrylate monomers such as MMA (methyl methacrylate). These have the least water retention capacity, thereby, having minimal ability to diffuse oxygen into the corneal layer and are not ideal for long term use. Soft material contact lenses are flexible and are mainly hydrogel based. They have higher water retention capacities as compared to rigid contact lenses, which gives them the ability to transmit oxygen to the corneal layer. These hydrogel based soft materials are mainly produced from polymers of acrylate monomers such as HEMA (hydroxyethyl methacrylate) and found to be better for drug delivery contact lenses. These polymer-based soft materials have been efficiently modified in terms of their chemistry to achieve diverse physicochemical properties to produce efficient ocular drug delivery systems. However, complications such as drug leaching during storage and distribution, sterilisation, preservation of integrity of the lens and the possibility of surface roughness due to the incorporated drug molecules still need to be optimised. This review highlights the chemistries of various polymeric molecules through which physicochemical properties can be modified to achieve optimum drug loading and sustained release of the drug for application in the ocular system.
1. Introduction
Eyes are one of the most intricate organs of the human body. They have tissues that are found to be arranged in layers.1,2 There are many ocular diseases that impede the normal functioning of the eye. Conjunctivitis is one of the most common ocular infections that occur in the conjunctiva of the eye. It is highly contagious and is characterised by excessive tear production. Ocular inflammation and dry eye are some of the most prevalent ocular disorders. Dry eye is caused by inadequate production of tears which in turn causes irritation in the eye. This can also lead to impaired vision. Of the most severe categories of ocular disease, glaucoma, is one of the disorders which causes damage to the optic nerve. This nerve damage can eventually lead to blindness.3,4 Age related Macular Degeneration (ADM), Diabetic Macular Edema (DME), Uveitis and CMV retinitis are some of the other ocular diseases affecting a big population across the world.5 A variety of therapeutic methods have been introduced to take care of such ocular problems but drug delivery to the eye has always been one of the most challenging tasks. As eyes have both static and dynamic barriers including static barriers of corneal layers, blood aqueous barrier and dynamic barriers of retinal blood and conjunctival barrier, it causes inhibition to the uptake of drug and thereby reducing the bioavailability.6,7 Different routes of administration exist to treat ocular diseases (Fig. 1). Each of the methods is associated with some drawbacks. Topical administration of drugs for ocular therapy is the most commonly used drug delivery method. It is non-invasive and can be self-administered by the patient. It has several disadvantages associated as the corneal barrier is difficult to penetrate and the drug is washed off by the continuous presence of tears. On the other side, oral administration of drug is generally coupled with topical application to increase the efficacy of the treatment whereas systemic route is the parenteral administration of drug and it leads to systemic toxicity. Other routes of administration include intravitreal, intracameral, subconjunctival and retro-lobular. All these routes also suffer from the similar obstacles.6 There are some transporters present in the eye which are known to efflux drug molecules outside and reduce bioavailability of the drug.8,9 Due to difference in morphologies and barrier properties of the anterior and posterior segments in eye, different therapeutic measures have to be devised to ensure successful therapy. The anterior ophthalmic disorders are mostly treated with topical eye drops, whereas posterior segment disorders require many of the new methods including dendrimers, microneedles,10 nanocrystals and lipid based nanosystems.11 Nanotechnology can be used into various drug delivery systems to increase specificity and decrease off target toxicity. Their compatibility and stability of such systems could be increased by coating them with naturally available polymers like chitosan, alginate, hyaluronan, dextran and lecithin.12 Ocular implants are good options for long term and controlled drug delivery but being an invasive process is generally associated with a lot of complications. Iontophoresis is another method of the delivery of drug using voltage gradient and can be used to deliver a wide variety of drugs to both the anterior and posterior segments of the eye. It is a non-invasive method of delivery and associated with less toxicity. But this process requires frequent administrations while sustained release of drug is not possible using this method.13 These mentioned methods have significant therapeutic abilities but many serious drawbacks exist, which can be overcome by using therapeutic contact lenses.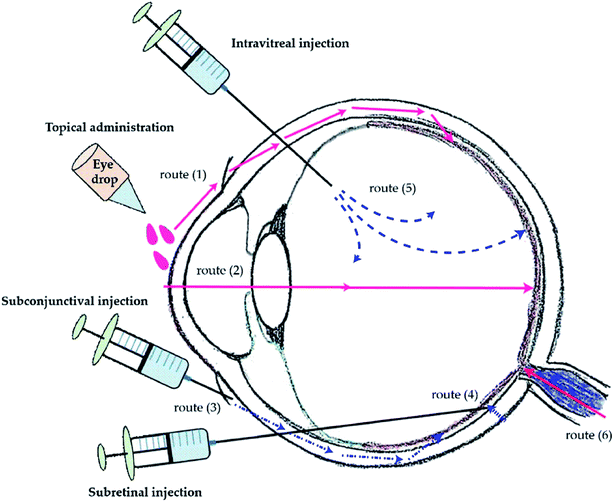 | ||
| Fig. 1 Methods of ocular drug administration and its delivery routes to the posterior segment. Routes of drug transportation to the back of the eye via topical administration (1) and (2), subconjunctival injection (3), subretinal injection (4), and intravitreal injection (5). The drug transportation from the systemic circulation via oral medication (6). Reprinted from ref. 134. | ||
Contact lens can be classified into hard or soft type based on the material used in preparation. Generally hard lenses are made up of rigid gas permeable material whereas soft contact lenses possess soft and flexible polymer which allows oxygen to pass through to the cornea. FDA further classifies soft material contact lenses into four groups as mentioned in Table 1. To consider the system to be ionic or non-ionic the pH 7.2 is used as the standard pH.
| Group | Water content | Percentage | Ionic/non-ionic |
|---|---|---|---|
| I | Low water content | (<50%) | Nonionic |
| II | High water content | (>50%) | Nonionic |
| III | Low water content | (<50%) | Ionic |
| IV | High water content | (>50%) | Ionic |
The cornea, an avascular tissue, depends on the atmosphere for oxygen requirement.14 Insufficient oxygen permeability (Dk) may lead to severities such as ocular and limbal redness neovascularization, corneal swelling, and endothelial pH changes.15,16,18 Therefore, it is important to ensure sufficient oxygen transmissibility of the contact lens. Holden et al., estimated the critical oxygen transmissibility level of hydrogel contact lens under daily wear conditions to avoid corneal edema. Many studies have reported that the high Dk silicone hydrogel contact lens with its sufficient oxygen flux had resolved the hypoxia-induced complications to a greater extent.17–20 Hydrogel based contact lenses are gaining popularity because of their capacity to hold water and allow oxygen diffusion. Hydrogels can be improved by adding silicone monomers to improve durability and other physical properties.21,22 FDA also categorises contact lens into two categories based on their chemical properties as hydrophilic and hydrophobic contact lenses. Hydrophilic lens can be used for a longer time and can absorb water. Oxygen present in water can diffuse into the desired location. Hydrophobic contact lenses are often combined with hydrophilic substances to increase biocompatibility.23 Few disadvantages associated with contact lens include discomfort, irritation, dry eye and red eye. These also contribute to the discontinuation of lens by people. If these properties can be improved in a lens, usage of contact lens can be increased.24,25 Lenses made up of hydrogel have become popular due to the numerous advantages like easy preparation, flexibility, transparency, high water content, drug loading possibilities and high biocompatibility.26,27 Silicone based contact lenses are the most recent addition to the market of soft material contact lens. FDA approved polymers which can be used in the manufacturing of contact lens is mentioned in Table 2 whereas the IUPAC name of the monomers used is mentioned in the Table 3.
| Sl no | Group | Polymer used for the fabrication of contact lens | Monomer composition | Water content (%) | Oxygen transmissibility (Dk/t)b | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| a HEMA (hydroxy ethylmethacrylate); MAA (methacrylic acid); MMA (methyl methacrylate); NVP (N-vinyl pyrrolidone); GMA (glycerol methacrylate); DMA (N,N-dimethylacryamide); EGDMA (ethylene glycol dimethacrylate); TEGDMA (tri ethylene glycol dimethacrylate); PDMS (polydimethylsiloxane); TPVC (tris(trimethylsioxy)silyl)propyl vinyl carbamate); TRIS (3-[tris(trimethylsiloxy)silyl]propyl methacrylate); DAA (diacetone acrylamide); IBMA (isobutyl methacrylate); PVP (poly(vinyl pyrrolidone); DEG (diethylene glycol); AMA (allyl methacrylate); MPC (methacryloyloxyethyl phosphorylcholine).b Higher the value of Dk/t, higher is the oxygen transmissibility. | |||||||
| 1 | I | Polymacon | HEMA | 38.3 | 8.5–24.3 | The lower water content makes them more rigid and easier to handle. Due to the non ionic nature of the lens material , these are less prone to deposition of proteins. | These are less wettable by the tear film. Due to this, a sense of dryness in the eye is felt by the patient, thus, making it uncomfortable to use |
| 2 | Crofilcon | GMA, MMA | 38 | 13 | The incorporation of siloxane into the fabrication process enhances the oxygen transmissibility of the lens | ||
| 3 | Lotrafilcon A | DMA, TRIS, siloxane | 24 | 140 | |||
| 4 | Lotrafilcon B | DMA, TRIS, siloxane | 33 | 110 | |||
| 5 | SenofilconA | HEMA, DMA, mPDMS, siloxane macromer, TEGDMA, PVP | 38 | 103–147 | |||
| 6 | Galyfilcon A | HEMA, DMA, mPDMS, siloxane macromer, EGDMA, PVP | 47 | 86 | |||
| 7 | II | Nelfilcon A | Modified PVA | 69 | 26 | The decreased rigidity of the lens increases patient compliance, as it decreases the sensation of irritation in the eye and also reduces chance of corneal abrasion | Due to increased water content, the mechanical property of the lens is altered which often makes the handling of the lens difficult |
| 8 | Omafilcon A | HEMA, MPC | 58–60 | 28–36.7 | |||
| 9 | Omafilcon B | HEMA, MPC | 62 | 21.3–52.3 | |||
| 10 | Netrafilcon A | EGDMA, MMA, DMA | 65 | 34.5 | |||
| 11 | Hilaficon A | HEMA, AMA, NVP | 70 | 35 | |||
| 12 | Hilaficon B | HEMA, AMA, NVP | 59 | 22 | |||
| 13 | Lidofilcon A | EGDMA, AMA, NVP, MMA | 70 | 31 | |||
| 14 | Lidofilcon B | EGDMA, AMA, NVP, MMA | 79 | 38 | |||
| 15 | Hioxifilcon A | HEMA, EGDMA, GMA | 59 | 28 | |||
| 16 | Hioxifilcon D | HEMA, EGDMA, GMA | 54 | 21 | |||
| 17 | Hilafilcon A | HEMA, EGDMA, AMA, NVP | 70 | 35 | |||
| 18 | Hilafilcon B | HEMA, EGDMA, AMA, NVP | 59 | 22 | |||
| 19 | III | Bufilcon A | HEMA, DAA, TMPT | 45 | 16 | Easier to handle due to the increased rigidity caused by the decrease in the water content | The ionic nature renders the surface of the contact lens reactive, thereby causing deposition of proteins present in the tear fluid |
| 20 | Deltafilcon A | HEMA, MAA, IBMA, TMPT | 43 | 10 | |||
| 21 | Phemficon | HEMA, β-ethoxyethyl methacrylate | 38 | 9 | |||
| 22 | IV | Ocufilcon B | HEMA, MAA | 52–53 | 16–24 | Flexible nature of the lens makes it comfortable to wear thereby curbing the feeling of irritation often felt in the eye | Some of them are difficult to handle due to the decreased rigidity caused by the increased water content. Due to ionic nature, they are more susceptible to deposition of proteins from the tear fluid |
| 23 | Ocufilcon C | HEMA, MAA | 55 | 16 | |||
| 24 | Ocufilcon D | HEMA, MAA | 55 | 17.8–28.1 | |||
| 25 | Ocufilcon E | HEMA, MAA | 65 | 22 | |||
| 26 | Ocufilcon F | HEMA, MAA | 60 | 24.3 | |||
| 27 | Etafilcon A | HEMA, MAA, PVP | 58 | 23.8–28 | |||
| 28 | Methafilcon A | HEMA, DEG, MAA, EGDMA | 55 | 14.5–31.3 | |||
| 29 | Methafilcon B | HEMA, DEG, MAA, EGDMA | 55 | 16 | |||
| 30 | Perfilcon A | HEMA, NVP, MAA | 71 | 34 | |||
| 31 | Etafilcon A | HEMA, MAA, PVP | 58 | 23.8–28 | |||
| Monomer used | IUPAC name |
|---|---|
| HEMA (hydroxy ethylmethacrylate) | 2-Hydroxyethyl-2-methylprop-2-enoate |
| MAA (methacrylic acid) | 2-Methylprop-2-enoic acid |
| MMA (methyl methacrylate) | 2-Methylprop-2-enoate |
| NVP (N-vinyl pyrrolidone) | 1-Ethenylpyrrolidin-2-one |
| GMA (glycerol methacrylate) | 2,3-Dihydroxypropyl-2-methylprop-2-enoate |
| DMA (N,N-dimethylacryamide) | N,N-Dimethylprop-2-enamide |
| EGDMA (ethylene glycol dimethacrylate) | 2-(2-Methylprop-2-enoyloxy)ethyl-2-methylprop-2-enoate |
| TEGDMA (triethylene glycol dimethacrylate) | 2-[2-[2-(2-Methylprop-2-enoyloxy)ethoxy]ethoxy]ethyl-2-methylprop-2-enoate |
| PDMS (polydimethylsiloxane) | Poly(dimethylsiloxane) |
| TPVC ((tris(trimethylsiloxy)silyl)propyl vinyl carbamate) | Ethenyl-N-[3-tris(trimethylsilyloxy)silylpropyl]carbamate |
| TRIS (3-[tris(trimethylsiloxy)silyl]propyl methacrylate) | 3-Tris(trimethylsiloxy)silylpropyl-2-methylprop-2-enoate |
| DAA (diacetone acrylamide) | N-(2-Methyl-4-oxopentan-2-yl)prop-2-enamide |
| IBMA (isobutyl methacrylate) | 2-Methylpropyl-2-methylprop-2-enoate |
| TMPT (trimethylolpropane trimethacrylate) | 2,2-Bis(2-methylprop-2-enoyloxymethyl)butyl-2-methylprop-2-enoate |
| PVP (poly(vinyl pyrrolidone)) | 1-Ethenylpyrrolidin-2-one |
| DEG (diethylene glycol) | 2,2′-Oxydi(ethan-1-ol) |
| DAA (diacetone acrylamide) | N-((2-Methyl-4-oxopentan-2-yl)prop-2-enamide) |
To develop an efficient ophthalmic drug delivery system one of the most important step is the proper use of the chemical compounds for the fabrication of the contact lens and the drug molecule to be used. The drug loading and delivery depends mainly on the interactions between the drug molecule and polymeric network of the contact lens. Other factors such as pH of the drug loading solution and temperature during the drug loading process also play significant roles. One of the most important characteristics of a formulation is the patient compliance. The prolonged use of contact lens have been reported to cause some adverse effects in the ocular system. Some of the most widely reported adverse effects of contact lens are:
• Corneal abrasion: scratches on the surface of the cornea, caused by the edges of the contact lens. This is mostly caused due to the prolonged use of rigid contact lens.
• Hypoxia: use of contact lenses having lower oxygen transmissibility deprives the corneal surface of oxygen supply, thereby causing hypoxia. The cornea needs 3–10 μl of oxygen per cm2 per h. Due to hypoxic condition, the corneal cells shift to anaerobic respiration leading to formation of lactic acid. The accumulation of lactic acid causes osmotic load, thereby, leading to accumulation of water which eventually causes edema.
• Neovascularization: the extended wear contact lens has been reported to induce corneal neovascularization, which is the pathological development of vascular capillaries from limbus to the corneal apex by 1 mm or less.29–31 Several factors like lack of oxygen, lactic acid, stromal softening, and vasogenic stimulations are known to induce corneal neovascularization.28 Kymionis et al., by slit-lamp inspection demonstrated that corneal neovascularization is the side effect of Intacs implantation and RGP contact lens fitting in patients ten years after implantation. Dumbleton et al., using randomized prospective clinical trials observed the development of moderate neovascularization in low Dk (oxygen permeability) lens wearers whereas high Dk hydrogel lens caused no neovascularization.32 Corneal neovascularization, which also leads to loss of immune privilege in the anterior segment has been seen as a serious complication of contact lens wear.33
• Microbial infection: the prolonged use of contact lens have often been the cause of several microbial infections. Repeated replacement of contact lenses makes the ocular system vulnerable to microbial growth. One of the most prevalent adverse effect is the development of fungal keratitis in the ocular system. The microbial growth accompanied with corneal abrasion may lead to corneal ulcer which may lead to loss of vision.
• Reduced corneal reflex: corneal reflex or blink reflex is an involuntary activity of the eyelids. It is caused by the stimulation of the cornea due to exposure to trauma. Prolonged use of contact lens often leads to reduced to corneal reflex.
• Formation of microcysts: prolonged hypoxia or mechanical injury to the corneal surface caused by the lens often leads to formation of cysts around the corneal layer.
• Meibomitis: meibomium glands are present in the upper and lower eyelids. Oil excreted by these glands is an important constituent of the tear film, which remains on the corneal surface after each blink. It helps in hindering the evaporation of tear film, thereby curbing dryness of the eye. Lens care solutions contain preservatives, which may plug the meibomium glands, thereby disrupting their functionality. This condition is known as meibomitis and it leads to dryness of the eye and raises a feeling of discomfort in the patient.
• Contact lens-induced papillary conjunctivitis(CLPC): a chronic inflammatory process that includes the development of giant papillae in the upper tarsal conjunctiva.34–39 The symptoms include excessive mucus production, reduction in lens tolerance, increased lens awareness, excessive lens movement, ocular redness, itching burning sensation, and irritation. Allansmith et al., reported the development of this syndrome in both soft and hard contact lens wearers.38 Chang et al., 2001 proposed the association of delayed tear clearance in contact lens with the pathogenesis of CLPC.40 Sorbara et al., 2009, in their study, found that the fitting of high Dk silicone hydrogel lenses has resulted in an increase in the prevalence of CLPC despite their efficiency in resolving corneal neovascularization. This clinically significant CLPC condition is one of the major reasons for contact lens dropout.37
• Dryness and irritation: use of contact lenses fabricated from non ionic monomers often causes dryness and sensation of irritation in the eye. Contact lenses having lower wettability hinder the spreading of the tear film over the lens surface, thereby causing a sensation of dryness and irritation in the eye. Due to this condition, often redness of the eye is observed.
• Biofouling: biomolecular interaction between the contact lens surface and the proteins present in the tear fluid leads to undesired deposition of protein molecules on the lens surface. This affects the visual clarity of the lens.
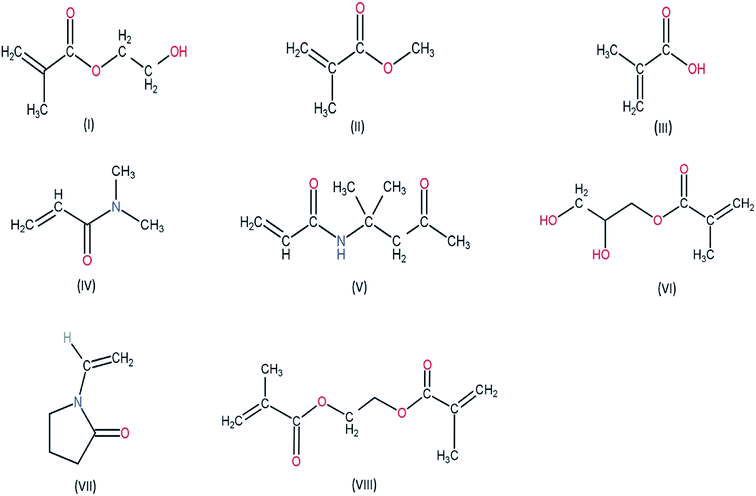
Utmost care must be taken while handling the contact lens. The patients must follow the instructions given by the manufacturers regarding the handling of the lens. Lens cases and lens storage solutions are often the cause of microbial contamination. Proper lens disinfection techniques must be followed. However, certain modifications during in the fabrication of the lens can also mitigate the adverse effects caused by the use of contact lens. Proper choice of monomers in the fabrication of the contact lens is a major step towards enhancing the patient compliance of the formulation. Various groups have tried to incorporate antibiotics into the polymeric matrix of the contact lens, to curb the microbial contamination in the ocular system. Incorporation of anti-inflammatory drugs such as dexamethasone into the polymeric matrix can be a potential solution to the occurrence of persistent inflammation caused by the prolonged use of contact lens. The thickness and mechanical property of the contact lens shall not interfere with the oxygen transmissibility and optical activity of the lens. The thickness of a contact lens is mainly governed by the purpose of its use. Lenses differ in their central and peripheral thickness according to the purpose of use. The power of the lens often influences the central and peripheral thickness of the lens. Lens used to treat myopia (lens having negative power) have higher peripheral thickness whereas those which are used to treat hypermetropia (lens having positive power) have higher central thickness. The thickness of the lens often influences the mechanical property and oxygen transmissibility of the lens. Lens having higher thickness have lower oxygen transmissibility. However, as the physicochemical property of the polymer influences the oxygen transmissibility, the choice of monomers used during fabrication has varying effect on the swelling capacity and other parameters such as oxygen transmissibility and mechanical property of the lens. The nature of crosslinking agent used during polymerisation plays a crucial role as well. Higher concentration of crosslinking agent inhibits the swelling capacity. Thus, the parameters – oxygen transmissibility, mechanical properties and swelling capacity of the lens has cumulative effect on the efficacy of the contact lens drug delivery system. Chemical structures of the monomers used in the fabrication of soft material contact lens are given in (Fig. 2 and 3).
The soft contact lenses are basically prepared from copolymers. Acrylic polymers such as HEMA (2-hydroxyethyl methacrylate) are used for the fabrication of the lens. The chemical structure of HEMA shows the presence of hydrophilic groups (hydroxyl group and carboxyl group) which make them soluble in water. However, the incorporation of a cross linking agent such as TEGDMA (triethylene glycol dimethacrylate) or EGDMA (ethylene glycol dimethacrylate) leads to copolymerization reaction forming the polymeric structure of pHEMA (poly hydroxyethyl methacrylate). pHEMA is a transparent hydrogel and has unique physicochemical property. The pendant hydroxyl groups in the polymeric structure of the pHEMA is responsible for the swelling property of hydrogels on coming in contact with aqueous environment. The water molecules diffuse into the polymeric structure and are retained due to the hydrophilic nature of the pendant hydroxyl groups. Another vital characteristic is that the hydrophilic nature causes the tear film to spread over the surface of the contact lens, which eventually helps in ocular drug delivery. However, various chemical modifications can be done to optimize the physicochemical properties of these hydrogel based contact lenses to ensure efficient drug loading and drug delivery.
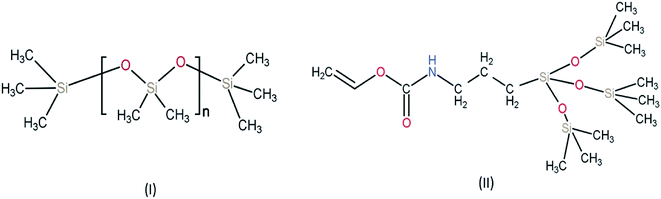 | ||
| Fig. 3 Chemical structure of monomers used for silicone-based hydrogel contact lens (I) PDMS (polydimethylsiloxane) (II) TPVC (tris(trimethylsiloxy)silyl)propyl vinyl carbamate. | ||
Drug polymer interactions influence the process of drug loading and drug delivery. The mesh size in the polymeric structure of hydrogel depends on the crosslinking density. Higher crosslinking density decreases the mesh size due to increased polymeric network which eventually decreases the ability of water molecules to diffuse into the polymeric structure of the hydrogel. Thus, for optimized drug loading of hydrophilic drugs the mesh size must be higher to help in increased water retention capacity of the hydrogel, thereby enhancing the drug loading capacity. In case of hydrophobic drugs, drug polymer interaction is enhanced by the incorporation of hydrophobic groups into the polymeric network of the hydrogel. The hydrophobic drug molecule has enhanced ability to interact with the polymeric network, thus increasing the drug loading capacity.
Incorporation of NVP (N-vinyl pyrrolidone), MMA (methyl methacrylate), MAA (methacrylic acid), styrene into the polymeric network of pHEMA with varying ratios influences the physicochemical properties of the contact lens.41–45 Among the four co-monomers used, styrene is most hydrophobic in nature. The presence of aromatic rings makes styrene hydrophobic as compared to the other monomers used in the study.41 Thus, when incorporated into pHEMA, it leads to loss of water retention capacity. With increasing concentration of styrene, the refractive index of the lens increases due to the high molar refraction of the phenyl ring present in styrene. Moreover, with concentrations beyond 60% wt of styrene, the lens turns opaque thereby leading to loss of visibility of the lens. MMA has a lesser hydrophobicity as compared to styrene but more than that of MAA. The chemical structure of MAA and MMA reveals that MAA has a carboxylic acid group which has the ability to form hydrogen bonds in the presence of water molecules. However, MMA is the methyl ester of MAA, the presence of methyl group leads to increased hydrophobicity as compared to MAA. The hydrophobicity of HEMA is more than MAA but less than that of MMA. Thus, the copolymer of HEMA and MMA had decreased water retention ability as compared to the copolymer of HEMA and MAA and also to that of pHEMA. The copolymer of HEMA and MAA has higher water retention capacity as that of pHEMA and it increases with increasing concentration of the comonomer MAA. Due to low molar refractions of MAA and MMA the refractive index of the contact lens decreases with their increasing concentrations in their respective copolymers.41 NVP has the highest hydrophilicity among the four comonomers used. The presence of the bipolar lactam group makes it highly polar thereby inducing hydrophilicity. With increasing concentration of NVP the refractive index of the contact lens increases due to the high molar refraction of the pyrrolidone ring of NVP.41 The copolymer of HEMA and NVP also showed no variation in the mechanical properties and the visibility of the lens.
Biomolecular level of interaction with biomaterials is a major factor which determines their biocompatibility. Acrylic biomaterials interact with proteins, lipids and other biomolecules. The tear fluid has proteins which on coming in contact with the surface of the contact lens causes adverse reactions to occur. The adsorption on the surface of the lens may cause changes leading to the polymeric structure to be collapsed. The deposition of proteins on the surface of the contact lens depends on the protein–polymer interactions. To minimize these biomaterial interactions on the surface of the contact lens modifications in the polymeric structure of the pHEMA hydrogel contact lens must be done. The copolymerization of HEMA with comonomers such as MAA, NVP and their effect on protein deposition on the surface of the contact lens have been studied.42–45 MAA, due to the presence of the carboxylic acid functional group is negatively charged at physiological pH 7.4, thus, acting as an ionic monomer. NVP on the other hand is a non-ionic comonomer. The difference in the chemistry of these two monomers influence the process of protein deposition on the surface of the contact lens. Charge density in the hydrogel affects the porosity of the polymer matrix of the hydrogel. With increasing charge density, the repulsive forces lead to increase in effective pore size of the polymeric matrix. The increase in pore size increases the hydration of the matrix thereby helping in better diffusion of biomolecules from the surrounding medium into the polymeric matrix.
The composition of tear fluid has proteins such as HSA (Human Serum Albumin) and lysozyme. At physiological pH (pH 7.4), lysozyme (pI 10.7), a globular protein having net positive charge and HSA (pI 4.9), a globular protein having net negative charge. The presence of negatively charged ionic comonomer, MAA increases the surface adsorption of lysozyme due to electrostatic attraction. Moreover, the increase in pore size due to increasing concentration of MAA would also eventually lead to increased diffusion of protein molecules into the polymeric matrix. In case of HSA, surface adsorption of the protein is inhibited due to the electrostatic repulsion caused by the presence of negatively charged ionic comonomer, MAA. Thus, pHEMA hydrogels have higher affinity towards the surface adsorption of HSA as compared to that of a copolymer of HEMA and MAA. Moreover, HSA has a larger molecular size as compared to lysozyme which hinders the diffusion of HSA into the polymeric matrix.42,43
2. Platforms of ocular drug delivery
2.1. Conventional hydrogel-based contact lens
Hydrogels have been extensively used for the past few decades as a mode of drug delivery due to their unique physicochemical properties. It enables them to be superabsorbent in nature allowing them to absorb and retain a significant amount of water. The water retention capacity depends on the cross linking of the polymer. A vital aspect is the nature in which the water interacts within the hydrogel. The water in the hydrogel can be of three types: bound water; intermediate water and free water.46 Free water has the ability to move within the polymeric network without getting hindered by any attractive or repulsive interaction. Bound water remains attached to the hydrophilic groups in the polymeric network via hydrogen bonding. Intermediate water is considered to be exchanged with free and bound water. Thus, the hydrophilicity and the polymer cross linking plays a critical role in transport of water within the hydrogel and also the swelling up of hydrogel. They are recently being used to produce soft material contact lenses. Ocular delivery of some drugs has been achieved with the help of soft material contact lenses made from hydrogels which are polymer of hydroxymethyl acrylate (pHEMA).47,48 To optimize the performance of the contact lens, hydrophobic monomer 4-vinylpyridine (VP) and ionic monomer, N-(3-aminopropyl) methacrylamide (APMA) was incorporated into the polymeric network of pHEMA.44 This strategy of polymerization was used successfully for the delivery of NSAIDs (nonsteroidal anti-inflammatory drugs) – diclofenac and ibuprofen. The incorporated monomers kept the viscoelastic properties and the water retention ability of pHEMA hydrogels unaltered. It was observed that the drug uptake capacity of the lenses was enhanced for ibuprofen (upto 10-fold) and diclofenac (upto 20-fold). However, for drug release the pH of the environment plays a vital role. Studies showed that dried loaded pHEMA – APA and pHEMA – VP effectively swelled up on exposure to water but due to the presence of ionic and hydrophobic interactions in the polymeric system the drug release was less than 10%. When the contact lens was kept in a solution of pH range of 5–8 they showed significant amount of drug release. The high polymeric density of the pHEMA hydrogel helped to achieve sustained release of the drugs. Ibuprofen was released for a period of 24 hours and diclofenac showed a prolonged release of about 1 week.These conventional contact lenses can be produced by soaking them in the drug solution. The drug uptake and release depend on the affinity with the drug. However, this can be manipulated by altering the loading temperature and the loading time of the drug Fig. 4.49 Silicone based hydrogel contact lenses have been fabricated and have been found to be feasible for ocular drug delivery.45 These soft material contact lenses have higher ability of oxygen transmission on the corneal surface as compared to conventional hydrogel-based contact lens. These were usually made of polysiloxanes. The flexibility of these lenses along with the ability of oxygen transmission paved the way for a new material for the fabrication of soft material contact lens. The first US FDA approved silicone based contact lens was under the brand name Silsoft manufactured by Bausch and Lomb in 1984. It was patented as 30 day wearable contact lens for aphakia.
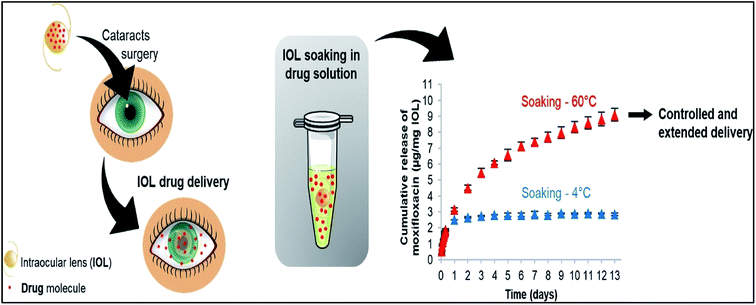 | ||
| Fig. 4 Effect of loading temperature on the release of dug. Reprinted with permission from ref. 49 copyright (2018), Elsevier. | ||
García-Millán E. et al. studied the effect of chemical and structural modifications of pHEMA (contact lens on the loading and release of corticosteroidal drug, triamcinolone acetonide).50 Two different types of contact lens was studied – (a) pHEMA with NVP ((N-vinyl pyrrolidone) as comonomer; (b) pHEMA with MAA (methacrylic acid) as comonomer. EGDMA was used as a cross linker to fabricate the lens. Varying concentrations of the comonomers were used to check for optimized loading conditions and release kinetics of the drug. Saline solution (0.9% NaCl solution) and PBS buffer solution of pH = 7.4 were used for drug loading. It was observed that the lenses show similar swelling property in saline solution but in case of contact lens with MAA as comonomer, swelling was enhanced in PBS buffer as compared to the swelling in saline solution. MAA, due to the presence of carboxylic groups has pKa = 4.661. The pH of 7.4 ionises the carboxyl groups in the polymeric structure, thereby increasing the electrostatic repulsion between the carboxyl groups, which causes the hydrogel to swell. The use of comonomers increased the amount of loaded drug in the polymeric network of the lens. The swelling property also influenced the phenomenon of drug loading in PBS buffer. Contact lens having 40% MAA (200 mM) showed the most efficient drug loading in when PBS buffer was used as the drug loading solution. Drug release profiles revealed that all the contact lenses fabricated with varying concentrations of comonomers – NVP and MAA showed similar release kinetics, when they were placed in artificial lachrymal fluid. No lag phase in the release of drug was observed and after 5 hours the release profile showed significant increase in drug release. More than 80% of drug release was achieved in the first 24 hours. So, this specific study showed that 40% MAA (200 mM) when used as a comonomer with pHEMA could be a potential formulation for the anti-inflammatory drug, triamcinolone acetonide. PBS buffer at pH = 7.4 would be the ideal drug loading solution.
The effect of salt induced modulation on the process of drug loading was studied by Zhu et al.51 The loading of three drugs – betaxolol hydrochloride, betaxolol base and diclofenac sodium into the polymeric matrix of pHEMA contact lens was studied. It was found that use of different types of salts influenced the ionic strength of the drug loading solution and also the solubility of drug, eventually, influencing the process of drug loading. The effect of three salts – NaCl, Na2SO4, NaSCN was studied. Drug loading was performed by immersing the dry contact lens in 1 mL aqueous drug solutions. In case of diclofenac sodium, use of NaCl and Na2SO4 increased the ionic strength of the drug loading solution leading to enhanced drug loading into the contact lens. However, use of NaSCN significantly reduced the loading of diclofenac sodium. In case of betaxolol hydrochloride, highest drug loading was achieved when NaSCN was used. At physiological ionic strength of 0.15 mol l−1 the amount of drug loading achieved is in the order of NaSCN > NaCl > Na2SO4. However, beyond the physiological ionic strength, increasing ionic strength of NaSCN lead to reduced drug loading. In case of betaxolol base, no significant change in drug loading was observed below ionic strength of 0.15 mol l−1. However, on increasing the ionic strength beyond 1.0 mol l−1, an increase in amount of drug loaded was observed during the usage of NaCl and Na2SO4. However, use of NaSCN decreased the amount of drug loading. Thus, both the nature of the drug and the salt system used, has an influence in the drug loading (Fig. 5).
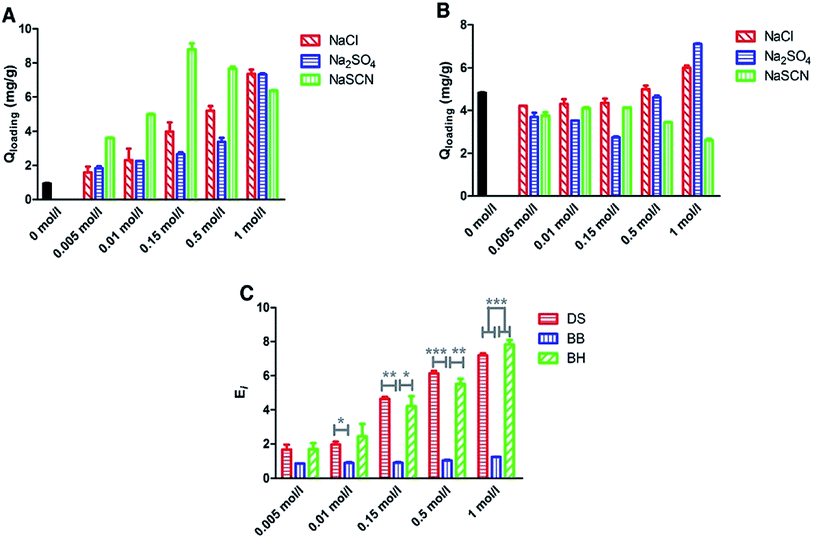 | ||
| Fig. 5 Loading results of betaxolol hydrochloride (BH) (A) and betaxolol base (BB) (B) in NaCl,Na2SO4 and NaSCN solutions with the ionic strength ranging from 0 to 1 mol l−1 and comparison of enhancement factor for diclofenac sodium (DS), betaxolol base and betaxolol hydrochloride in NaCl solutions (C). Each point represents the average of three measurements. (*P < 0.05, **P < 0.01, ***P < 0.001). Reprinted with permission from ref. 51 copyright (2019) Elsevier. | ||
Paradiso et al. studied the drug release in two different types of contact lenses – (a) pHEMA based hydrogel (HEMA and PVP-polyvinylpyrrolidone); (b) silicone hydrogel produced by using a hydrophobic comonomer, TRIS (3-[tris(trimethylsiloxy)silyl]propyl methacrylate) and NVP (N-vinyl pyrrolidone).52 An antibiotic, levofloxacin and an antiseptic, chlorhexidine were used as a model drug in which the contact lenses were soaked for drug loading. The swelling capacity of the hydrogel was dependent on temperature. Both the hydrogels showed decreased swelling on increasing the temperature. However, the pHEMA based hydrogel showed significant changes as compared to the silicone-based hydrogel. The silicone-based hydrogel had lower cross linking than the pHEMA hydrogel due to which the silicone based hydrogel had higher swelling capacity. The swelling capacity was highest at 4 °C, thus, drug loading was performed at this temperature for both the contact lenses. The drug release profile was studied after the contact lenses were soaked for 14 and 36 hours, respectively. The loading time of levofloxacin did not affect it's release profile for the silicone based contact lens and the release kinetics shows that there was an initial burst release. In case of the pHEMA based contact lens, higher loading time increased the duration of release of levofloxacin. The release kinetics also showed that the pHEMA based contact lens shows a controlled release of the drug. Release profiles of chlorhexidine showed that the loading time did not affect the pHEMA based contact lens. The release kinetics also showed that both the pHEMA and silicone based contact lens showed controlled release of the drug, though the silicone based contact lens showed an initial burst.
Minami et al. studied the loading and release of the selective histamine H1 receptor antagonist, epinastine hydrochloride (EH) from soft material contact lens having anionic, cationic and non-ionic co-monomers incorporated during the fabrication of the contact lens.53 The ionic nature of the comonomer used, influenced the and drug release kinetics. HPMA (N-(2-hydroxypropyl)methacrylamide), a non-ionic monomer was used of the fabrication of all the contact lenses. The other non-ionic monomers used were CHDMMA (1,4-cyclohexanedimethanol monoacrylate) and NVP (N-vinyl pyrrolidone). HO-MS (mono-2-(methacryloyloxy)ethyl succinate) was used as anionic comonomer. MAPTAC ([3-(methacrylamido)propyl]trimethylammonium chloride) was used as the cationic comonomer. Varying ratios of different ionic and non-ionic comonomers were cross linked with HPMA to fabricate five types of contact lenses: (a) anionic lens produced from non ionic monomers, HPMA and CHDMMA incorporated with anionic monomer, HO-MS; (b) cationic lens produced from non ionic monomers, HPMA and CHDMMA incorporated with cationic monomer, MAPTAC; (c) bi-ionic lens produced from non ionic monomers, HPMA and CHDMMA incorporated with anionic monomer, HO-MS and cationic monomer, MAPTAC (d) non ionic lens produced from non ionic monomers, HPMA, CHDMMA and NVP; (e) non ionic lens produced from non ionic monomers, HPMA and NVP. The contact lenses were soaked in 0.05% (w/v) EH solution in PBS buffer for 24 hours. In vitro release profile showed that the anionic monomer released the highest amount of EH and the release kinetics showed a linear release profile, releasing 69% of the drug in 12 h. The non-ionic, bi-ionic and cationic showed significantly lower amount of drug release, the cationic lens showing the least amount of dug release. The non-ionic, bi-ionic and cationic lenses also showed initial high burst release. The amount of drug released was dependent on the concentration of drug used in the loading solution. EH, with amine group, imparts cationic nature to the molecule. The anionic monomer, thus, achieves the highest drug loading due to the electrostatic attraction between the opposite charges. On the other hand, the cationic monomer had the least affinity for the cationic drug molecule, thereby curbing the drug loading, which in turn leads to least amount of drug release. In vivo studies showed that controlled release of drug was achieved and the amount of drug released was higher than that of conventional eye drops over a period of 12 hours.
In another important study, Yang et al. studied the drug loading and release profile by using eleven types of soft material contact lenses, which are commercially available and are produced form polymers approved by the US FDA.54 Pirfinedone (PFD), a drug used for treating scarring in the eyes was used as the model drug. The polymers used in the contact lenses were – Polymacon, Nelfilcon A, Omafilcon A, Hilaficon B, Ocufilcon D, Etafilcon A, Balafilcon A, Lotrafilcon A, Lotrafilcon B, Senofilcon A, Galyfilcon A. In vitro studies showed that polymacon and etafilcon lenses showed highest drug uptake. However, the amount of drug released was higher for polymacon and thus polymacon lens was chosen for the in vivo studies. In vivo studies showed that as compared to eye drops, the drug loaded contact lens showed higher drug release in different anatomical parts of the ocular system – cornea, sclera and aqueous humour. The in vivo release kinetics revealed that there was initial burst release and the drug was released for a period of 150 minutes. The retention time of the drug was 240 minutes in the ocular system, which was higher as compared to pirfinedone eye drops.
In some of the cases an innovative approach to extend duration for drug release was achieved by using Vitamin E as the diffusion barrier over the lens.55 Vitamin E is hydrophobic and a potent antioxidant and has low water solubility. It has been found useful in treatment of various eye diseases such as cataract and apoptosis of corneal epithelium. Additionally, Vitamin E serves as protective agent against UV radiation and prevent corneal damage. Considering the biocompatible nature and therapeutic properties, Vitamin E can be utilised in creating diffusion barriers for various hydrophilic drugs, which diffuse faster in the aqueous environment of tear film. Most of the hydrophilic drugs are charged at physiological pH, hence, a hydrophobic environment created by Vitamin E will serve as an effective drug barrier. Three hydrophilic ophthalmic drugs, timolol (a beta blocker, used for treatment of glaucoma), fluconazole and dexamethasone 21-disodium phosphate (an anti-inflammatory corticosteroid) have been tested for extended release using Vitamin E as a diffusion barrier. Increased lens thickness, reduced ion permeability and slight reduction in oxygen permeability have been observed with negligible toxicity. Although transparency of the Vitamin E coated lenses was maintained, the drugs were observed to have an extended release profile.
Molecular imprinting is an efficient technique to ensure high amount of drug loading into the contact lenses Fig. 6. During polymerization reactions, high affinity cavities are created using the same drug molecules. These drug molecules then serve as memory sites for the drugs. Initially, monomers undergo polymerization whereas drug molecules are mixed during polymerization and released after a network is formed. This creates drug sized pockets of very high affinity for the drug molecules. The properties of the functional monomer, its functionality, composition of the lens, drug release kinetics together comprehend for loading and release of the drug template into the contact lens. However, backbone monomers and other functional monomers decides the layout of structure of the polymeric network and size and shape of high affinity cavities.56 Adequate amounts of functional monomers and cross-linking agent should be provided as excessive quantity of them binds the drug molecules so tightly that they are retained in the polymeric matrix for a very long duration, which renders them ineffective.57,58
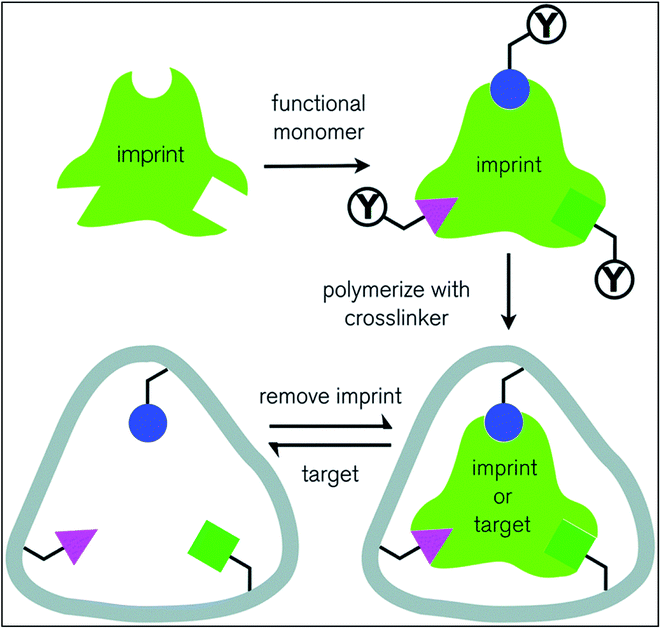 | ||
| Fig. 6 Schematic representation of molecular imprinting technique. Reprinted from ref. 63 with permission from the Royal Society of Chemistry. | ||
In 2011, White et al., reported that the concentration of cross-linking agent should be less than 5% to get better contact lenses.59 Higher concentrations were reported to increase the network stiffness and reduces drug release rate. However, Hiratani et al., showed that when using DMA (N,N-diethyl acrylamide) and EGDMA (ethylene glycol dimethacrylate) as cross-linking agent, the effective concentration was found to be 80 mM. In their study, the authors demonstrated the use of weakly cross-linked hydrogels for the loading of timolol using MAA (methacrylic acid) as functional monomer. The study demonstrates that timolol loading increased effectively. The imprinted adsorption sites enhance the drug loading efficiency of the contact lens.60 Different drugs have different capability of release with a range of concentration of MAA monomers. Contact lenses imprinted with dorzolamide show greatest capability to release at MAA with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar ratio.61 However, low molecular weight drugs were usually exploited for these studies, another approach using high molecular weight drugs came up in 2009, when Ali et al., demonstrated control of drug release from contact lenses using hyaluronic acid. In their study, the authors observed that hyaluronic acid can be delivered with the release rate of 6 μg h−1 for 24 hours.62
4 molar ratio.61 However, low molecular weight drugs were usually exploited for these studies, another approach using high molecular weight drugs came up in 2009, when Ali et al., demonstrated control of drug release from contact lenses using hyaluronic acid. In their study, the authors observed that hyaluronic acid can be delivered with the release rate of 6 μg h−1 for 24 hours.62
2.2. Use of nanoparticles for ocular drug delivery by contact lens
Researchers have been trying to use drug loaded nanoparticles conjugated on the surface of the contact lens for improved drug delivery.64 The nanoparticle-laden contact lens can be produced mainly by four methods – (a) dispersion of drug encapsulated nanoparticles in the hydrogel monomer (b) formation of micelles by addition of surfactant and drugs to the pre monomer of the mixture.65,66 (c) Soaking the contact lens in suspension of the nanoparticles42 and (d) attachment of nanoparticles on the surface of the contact lenses by chemical modifications.67,68In some of the cases, drugs are encapsulated in nanoparticles and loaded in therapeutic contact lenses by dispersing them in HEMA monomers followed by polymerisation of the monomer by using ethylene glycol-dimethacrylate (EGDMA) as a cross linking agent. Process was performed in presence of photo initiator Darocur. Colloidal nanoparticles used for the purpose of ocular drug delivery encapsulate the drug to prevent them from getting degraded by the enzymes such as lysozymes which are present in tear fluid. This increases the residence time in the ocular system. Further studies also showed that a drug delivery system comprising of both the nanoparticles and the contact lens shows a better sustained drug release profile as compared to either of them when used alone. The drug is released from the nanoparticle into the hydrogel matrix of the contact lens and then it diffuses into the tissues. The use of nanoparticles laden contact lens curbs the leaching of drugs during the process of storage and sterilization. However, a major concern of using nanoparticle-laden contact lens is the aggregation of nanoparticles which would eventually hamper the transparency of the contact lens thus leading to improper vision.
One of the most recent work in this field has been a study of the effect of gold nanoparticles (AuNPs) on the uptake and release of a drug, timolol from contact lens which is a potent non-selective beta blocker.69 The release profile of the drug was analyzed under both in vitro and in vivo conditions. The main objective of this study was to find novel treatment methodologies for glaucoma by the use of therapeutic contact lens. Timolol was used as a model drug as being a beta blocker shows fall in intraocular pressure in the aqueous humour fluid of the eye. The drug loading of the AuNPs was done by two methods – (a) the AuNPs were soaked in a solution of the drug timolol (b) the AuNPs were incorporated into the contact lens during the process of fabrication and then the contact lenses were soaked in two different concentrations of the drug solution. The release kinetics of both the cases were analysed. Both the cases showed a significant uptake of the drug timolol. However, release kinetics under in vitro conditions did not show any significant result. Under in vivo conditions, when applied on the eye of rabbit, the tear fluid showed significant concentration of timolol in case of the AuNPs laden contact lens. Moreover, the release kinetics showed that there was no case of initial high burst release while a prolonged decrease in the intraocular pressure as compared to that of conventional eye drops was also noticed. The swelling property of the lens remained unaltered and the optical activity of the contact lens was unhindered. So, this methodology was a novel approach to the treatment of glaucoma with the help of therapeutic contact lens.
A major concern of developing therapeutic contact lens loaded with drug is the leaching of drug during storage. The drug should only diffuse out of the contact lens on being placed in the eye. An attempt to curb the drug leaching has been made to develop timolol containing contact lenses which do not show any leaching.70 Timolol was encapsulated in crosslinked particles formed by the cross linking of monomers of EGDMA (ethylene glycol dimethacrylate) and PGT (propoxylated glyceryl triacylate). Then, these crosslinked polymeric nanoparticles were incorporated in the monomers of HEMA before performing polymerization. This work also gave an insight to the drug release mechanism from the polymeric matrix, which could later be manipulated to obtain a drug delivery system of better efficacy. The drug release kinetics was studied by putting the polymeric nanoparticles encapsulating the drug in PBS (phosphate buffer saline). It was observed that there was prolonged release of drug timolol and when packed in storage conditions, the drug did not show any leaching. However, on exposing the drug to higher temperatures of greater than 40 °C showed rapid disintegration. Another concern of this drug delivery system was that oxygen permeability of the lens was decreased due to the thick layer of nanoparticles. The optical activity was also hampered as there was a decrease in light transmission. However, this study gave an insight to the mechanism of drug diffusion from the polymeric matrix. Analysis of the drug release profile showed that timolol was conjugated to the polymeric matrix with the help of ester linkage. The aqueous environment of the interior of the polymeric matrix caused the hydrolysis of the ester linkage, thereby causing a release of the drug from the polymeric matrix.
Another approach of development of ocular drug delivery system was the use of a pH sensitive drug releasing system.71 This work helped in developing a system which would also prevent drug leaching when the contact lens would be stored in a specific pH range. Drug release kinetics of two cases was studied – (a) normal contact lenses without nanoparticles in which the drug was loaded directly by the conventional soaking method; (b) nanoparticles of Eudragit S 100, a copolymer of methacrylic acid and methyl methacrylate, was incorporated in the contact lens. Cyclosporine was used as the model drug. This study showed that there was release of drug only above pH of 7.2. So, the therapeutic contact lens could be easily stored at a pH of 6.5 without any drug leaching. The nanoparticle-laden contact lens showed a higher drug release in vitro and it also showed a sustained release of the drug cyclosporine. The optical activity of the lens also remained unaltered. Contact lens loaded with drug by direct soaking showed decrease in the optical activity. Under in vivo conditions the nanoparticle-laden contact lens showed higher bioavailability of the drug as compared to in vitro conditions and the bioavailability was increased by a significant amount as compared to the conventional eye drops. However, these drug delivery systems show decrease in the oxygen permeability which is a major drawback of this ocular drug delivery system.
Recently researchers have been working in the field of developing nanodiamonds (NDs) for the purpose of ocular drug delivery.72 NDs have huge potential to be used as a vehicle for drug delivery as many in vivo studies found them to be biocompatible. The unique structural property of NDs allow them to bind reversibly to the drug, thus, influencing the drug uptake and release kinetics. However, ocular compatibility of NDs have not yet been studied but nanohorns, a structure which shows resemblance to that of NDs have been found to be compatible to the eye. The aim of using nanodiamond was to improve the mechanical property of soft material contact lens. Moreover, studies showed that nanodiamond arrays do not interfere with the optical activity of the lens. NDs coated with polyethyleneimine (PEI) were crosslinked with biodegradable polymer chitosan forming a ND nanogel based ocular drug delivery system. Chitosan, when degraded by the enzyme lysozyme present in the tear fluid, caused the release of the drug timolol. Thus, this ocular drug delivery system is an enzyme sensitive system (Fig. 7).
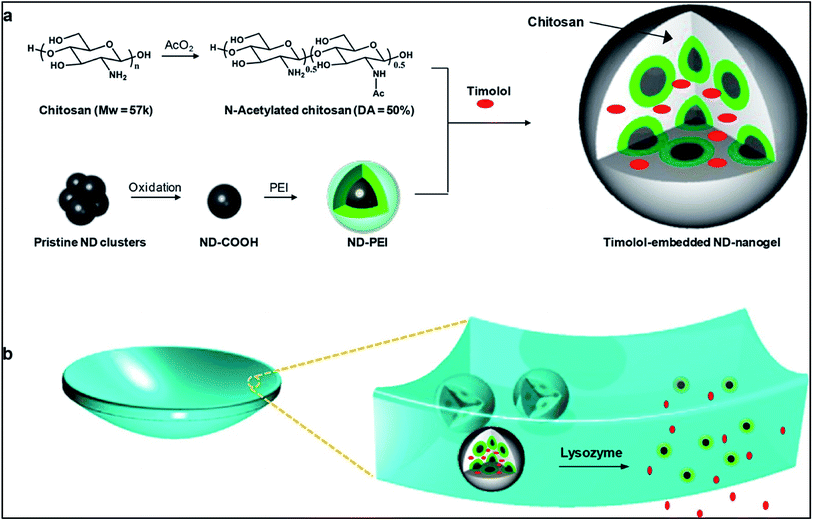 | ||
| Fig. 7 Schematic illustration of lysozyme-activated drug eluting contact lens. (a) Drug loaded ND-nanogels are synthesized by cross-linking PEI-coated NDs and partially N-acetylated chitosan (MW = 57 kDa; degree of N-acetylation = 50%) in the presence of timolol maleate. The ND-nanogels are then embedded in a hydrogel and cast into enzyme-responsive contact lenses. (b) Exposure to lacrimal fluid lysozyme cleaves the N-acetylated chitosan, degrading the ND-nanogels and releasing the entrapped timolol maleate while leaving the lens intact. Reprinted with permission from ref. 72. Copyright 2014, American Chemical Society. | ||
Drug release kinetics study of this system showed that the sustained release of timolol was under the control of the degradation of the polysaccharide chitosan by the enzyme lysozyme. The optical activity and the oxygen permeability of the lens was also within the desired limits. However, this drug delivery system was studied under in vitro conditions (Fig. 8 and 9). Studies under in vivo conditions would confirm the possibility of clinical application of this drug delivery system paving way for a novel therapeutic approach to the treatment of glaucoma.
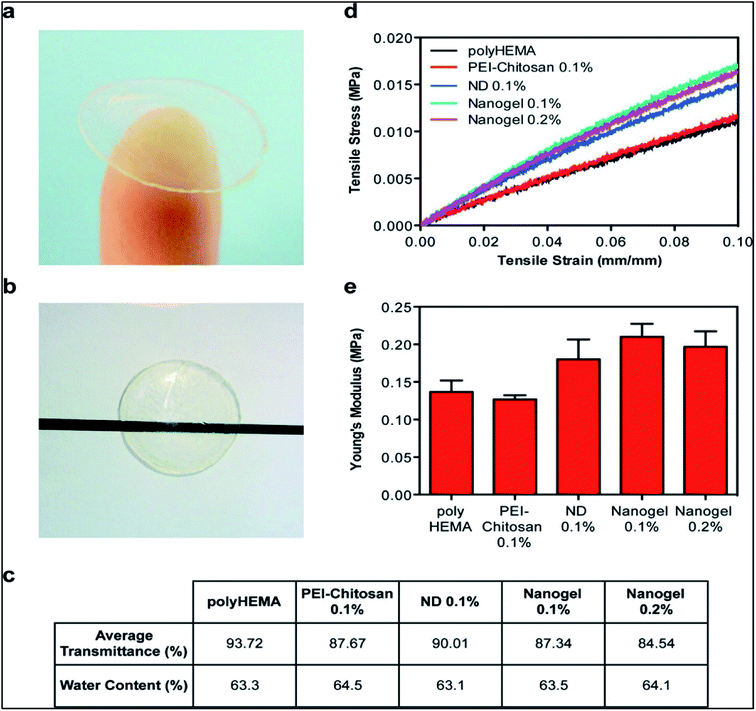 | ||
| Fig. 8 Characterization of physical properties of contact lenses. (a) ND-nanogels can be embedded into polyHEMA gels and cast into contact lenses. (b) ND-nanogel embedded lenses maintain optical transparency. (c) Comparison of average visible light transmittance (400–700 nm) and water content of polyHEMA lenses without additives, with 0.1% (w/w) PEI-chitosan, with 0.1% (w/w) pristine ND, with 0.1% (w/w) ND-nanogel or 0.2% (w/w) ND-nanogel. (d) Tensile stress–strain curves comparing polyHEMA lenses without additives, with 0.1% (w/w) PEI-chitosan, with 0.1% (w/w) pristine ND, with 0.1% (w/w) ND-nanogel or 0.2% (w/w) ND-nanogel. (e) Young's modulus of the polyHEMA lenses without additives, with 0.1% (w/w) PEI-chitosan, with 0.1% (w/w) pristine ND, with 0.1% (w/w) ND-nanogel or 0.2% (w/w) ND-nanogel, as determined by the first 5% of the stress–strain curve slope. Reprinted with permission from ref. 72. Copyright 2014, American Chemical Society. | ||
Chitosan nanoparticles have also been studied for the ocular delivery of anti-inflammatory drug, dexamethasone sodium phosphate (DXP).73 Chitosan nanoparticles were prepared by the crosslinking mediated by sodium tripolyphosphate (TPP). These nanoparticles encapsulated the anti-inflammatory drug DXP. The nanoparticles were incorporated into monomers of HEMA while fabrication of the polymeric soft material contact lens. In vitro studies of this ocular drug delivery system showed enhanced bioavailability as compared to the conventional eye drops. Sustained release of the drug was also achieved. Although there was no significant loss in the optical activity but the oxygen permeability of the lens was affected. So, these contact lenses must only be used for shorter duration of time. Cytotoxicity studies of this drug delivery system under in vivo conditions have not yet been done. If this system is found biocompatible then this can be used as therapeutic approach to the treatment of eye injuries. The use of silver nanoparticles incorporated in the contact lens74–76 were found biocompatible with the human tissues and had shown antimicrobial effect due to its ability to interrupt the cellular metabolic processes of the microbes. It also interacts with the microbial enzymes, often, causing conformational changes, leading to loss of activity. These silver nanoparticle laden contact lenses can also be used to treat microbial infections in the eyes which are often caused by the prolonged use of contact lens. They were found effective against P. aeruginosa and S. aureus.75
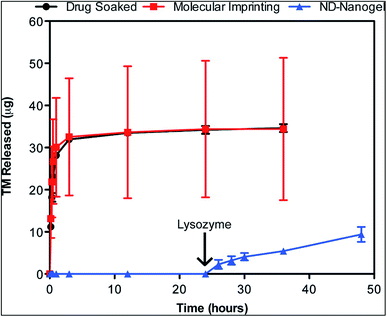 | ||
| Fig. 9 Enzyme-triggered drug release. Drug-eluting profiles from drug-soaked (black line), molecularly imprinted (red line) and ND nanogel-embedded contact lenses (blue line) in saline solution at 37 °C as determined by HPLC analysis of TM. Lysozyme (2.7 mg mL−1) in PBS was added after 24 h of incubation (N = 3 for each type of lens). Reprinted with permission from ref. 72. Copyright 2014, American Chemical Society. | ||
Polymeric micelles based core–shell nanocarriers are widely used as a vehicle for drug delivery (Fig. 10). Micelles help in achieving sustained release of a drug. Polymeric micelles loaded with drug are incorporated into HEMA hydrogels to develop therapeutic contact lens.77,78 The use of surfactant Brij98 along with the drug loaded micelle laden contact lens showed a controlled and extended drug release. There was no initial burst release of the drug. The use of surfactant played a critical role in achieving the controlled release of the drug. Drug release profile showed that the release of the drug was affected significantly by the partitioning of the drug in the surfactant aggregates. Moreover, further analysis showed that pHEMA contact lenses with micelles and surfactant laden in them showed better optical activity than the normal pure pHEMA contact lenses. The oxygen permeability of the lens was also not hampered. Thus, these may be used as an effective ocular drug delivery system.
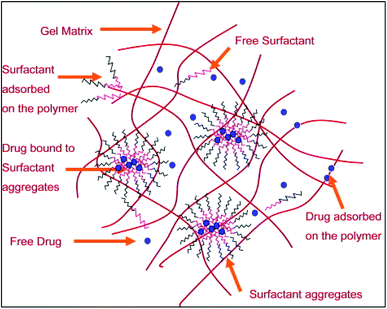 | ||
| Fig. 10 A schematic representation of the microstructure of the surfactant laden gels. Reprinted with permission from ref. 66. Copyright (2009), Elsevier. | ||
2.3. Liposomal drug delivery-based contact lens
Ocular drug delivery has also been studied by the incorporation of liposomes in the contact lenses (Fig. 11). In one of the cases, dimyristoyl phosphatidylcholine (DMPC) liposomes were incorporated in poly-2-hydroxyethyl methacrylate (p-HEMA) hydrogels.79 DMPC, a lipid was converted to liposomes and loaded with drug, lidocaine. Drug release kinetic studies showed that there was an initial burst in the release of the drug, however, the drug release was decreased significantly after a certain duration of time. This effect was due to the diffusion of drug which slowed down the release process after a certain time. However, this is a major drawback of this drug delivery system. The liposomes on getting aggregated also hampers the optical activity of the lens, but this can be optimised by manipulating the size of the liposomes to prevent aggregation. The initial burst of the drug can be altered by conjugating the liposomes with polyethylene glycol (PEG).Therapeutic contact lens can also be produced by surface modification with drug loaded liposomes.80 Polyethylene amine was conjugated to the hydroxyl group while surface amine group was conjugated with NHS–PEG–Biotin molecules to prepare contact lenses. Simultaneously, the biotin molecules were bound to neutravidin and further surface immobilized the liposomes containing the PEG–biotin layer possessing neutravidin. The liposomal layer can be of single to multiple layers. Drug release profile of double layer of liposome loaded with levofloxacin showed a release for 30 hours and about ten layers of liposome showed drug release of upto 120 hours.81 However, multiple layers of liposome affect the oxygen permeability of the contact lens.
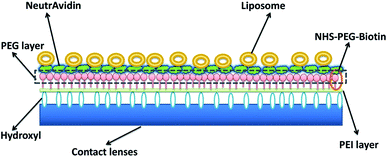 | ||
| Fig. 11 Schematic diagram of liposomes immobilized on the surface of contact lens. Reprinted with permission from ref. 82. Copyright (2018), Elsevier. | ||
2.4. Microemulsion based contact lenses
Microemulsions, integrated with contact lenses, are another set of interesting ocular drug delivery vehicles. They are simple to prepare and sterilise which give them advantage over other drug delivery means. The drug release kinetics depends on microemulsion stability.83 A study shows that oil-in-water microemulsions of the drug timolol were incorporated in the contact lenses that were made up of pHEMA. High drug loading was achieved. However, the drawback in this experiment was a rapid release of the drug. This posed a problem as the drug could not have a long-lasting effect. This drawback was explained by the assumption that pluronic surfactant used in microemulsions doesn't provide with a barrier strong enough to impede drug release.84 This problem was overcome by using Brij 97 as a surfactant. It was seen that this surfactant enabled slow release of drug, cyclosporine A, from HEMA lenses that were incorporated with microemulsions. It was observed that the release of cyclosporine A from HEMA lenses in in vitro conditions was maintained for around 20 days when Brij97 was used.85 Another method devised to extend the release of drugs from the lenses was adding a silica shell around microemulsions. Octadecyltrimethoxysilane was used as a silica source. It was noticed that due to the silica shell the release of lidocaine extended upto 8 days, though 50% of the drug got released within the first few hours.86 When silica shell reinforced-microemulsions were made using Brij 97 or Tween 20, the HEMA lenses showed lidocaine release over 4–8 days. However, initial burst release was also observed. Moreover, the lenses with microemulsions prepared using Brij 97 showed more transparency than those without use of it.872.5. Cyclodextrin based contact lenses for ocular drug delivery
Cyclodextrin are cyclic oligosaccharides which contain α-1,4-linkages in α-D-glucopyranose unit. Cyclodextrin's have truncated cone like structure because of glucopyranose having chair conformation. Cyclodextrin are widely used to form inclusion complexes. While forming inclusion complexes the inner naturally occurring water molecule of cyclodextrin are replaced by appropriate guest moieties (Fig. 12).88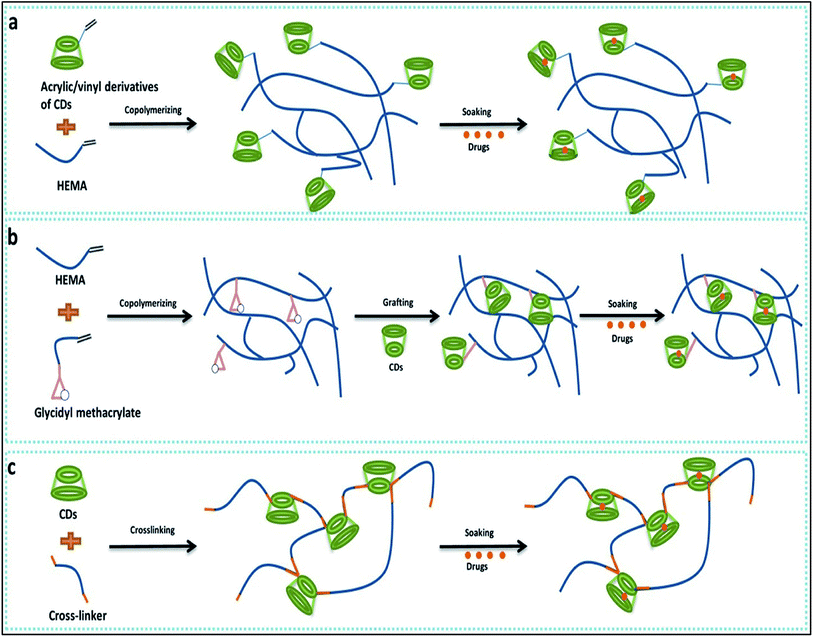 | ||
| Fig. 12 Cyclodextrin-based contact lens used for drug delivery. (a) Copolymerization of acrylic/vinyl CDs derivatives; (b) grafting of CDs to preformed polymer networks; (c) directing cross-linking of CDs. Reprinted with permission from ref. 82. Copyright (2018), Elsevier. | ||
Primarily hydrogels were used for designing of drug delivery due to high degree of comfort, significant release of the drug into target and bioavailability. Recent studies have demonstrated that cyclodextrin based hydrogel contact lens that can be a useful addition to ophthalmic drug delivery.89,90 Efficient drug loading was reported with CD/Hydrogel based contact lenses as well as the sustained drug release for a prolonged period was also reported.91 The methods to develop cyclodextrin loaded into contact lenses are classified in three major groups. The co-polymerisation of various monomers used to design soft contact lens with the glycidyl methacrylate provides a polymeric network with binding points for cyclodextrin.92 Vivid application of such contact lenses can be envisioned like various hydrophobic drugs used to tackle anti-fungal and antiviral activity in ophthalmology, for example, TSC (thiosemicarbazone), a hydrophobic drug which display a higher tendency to self-aggregate in water, hampers the reproducibility of these drug in vitro. This can be improved with cyclodextrin mediated protected delivery of these drugs.93 Contact lens formed after the co-polymerisation of HEMA and GMA, are grafted with the beta-cyclodextrin using the reaction of the glycidyl group of contact lens and the hydroxyl group of cyclodextrin under mild condition. By this strategy, soft contact lens with pendent beta-cyclodextrin which is not part of the contact lens structure are made.92 As medicated soft contact lenses have various strict requirements like oxygen permeability, device compatibility91 a CD grafted contact lens can overcome such problems. This soft contact lens with pendent beta-cyclodextrin showed reduction in frictional coefficient by 50% without hampering the useful property of contact lens like optical transparency, swelling degree, glass transition and oxygen permeability. This modified soft contact lens were also checked for their ability like drug loading capacity, drug affinity and sustained drug delivery and reported with approximately 1300% increase in loading capacity, 15 fold in increase in drug affinity and sustained drug delivery for 2 weeks.67 Different types of cyclodextrin (alpha, beta, gamma) were also used to check their influence in soft contact lenses, out of which gamma cyclodextrin were noted to have high affinity for contact lens structural network and remarkable decrease in protein deposition.94
Contact lens fabricated with crosslinked cyclodextrin use method of hydrogel/CD contact lens preparation, obtained by direct cross-linking of hydroxypropyl-β-CD (HPβ-CD) and HPβ-CD/hydroxypropylmethyl cellulose (HPMC) (1.0%, w/v) with ethylene glycol diglycidyl ether (EGDE) (Fig. 13).95 After preparation, these contact lenses are directly soaked with drug. In vitro release assay was performed with 1-indanone thiosemicarbazones drug (TCSs) and sustained release of drugs for 2 weeks was reported (Fig. 14). Finally, antibacterial activity of these drugs was also checked against pathogen associated with eye infection and localized release of therapeutic drug in to the eye was reported. These contact lenses were 90% transparent as well.95 Various entity are essential and should be consider while developing contact lenses based on CD and hydrogel cross-linking like mechanical property, oxygen permeability, and transmittance. Such properties can conquer the suitability of hydrogel/CD based contact lenses. Other novel approaches are also prominent where poly CDs were designed by directly cross-linking CDs with citric acid. Then these poly CDs were loaded with a drug ethoxzolamide and finally incorporated into HEMA. It was reported that sustained drug release occurred for six days and were having extremely high loading capacity.96
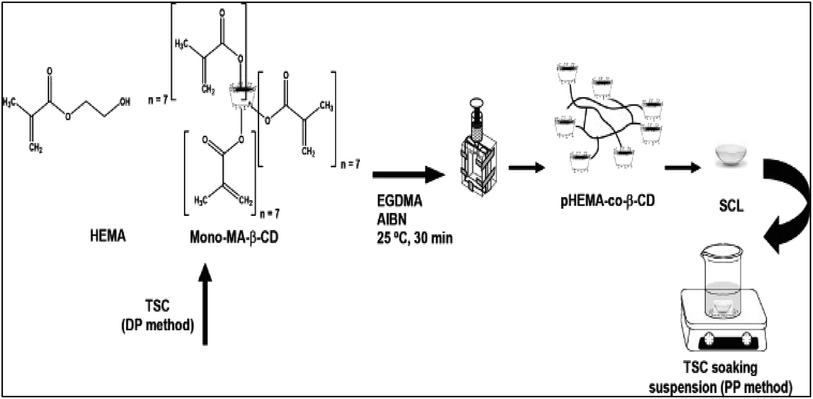 | ||
| Fig. 13 Schematic representation of the synthesis of TSC (thiosemicarbazone) loaded beta CD conjugated soft contact lenses by DP (during polymerization) method and PP (post polymerization) method. Reprinted with permission from ref. 95. Copyright (2013), Elsevier. | ||
Copolymerization of acrylic/vinyl CDs derivates can be performed for preparation of contact lenses. In this method of medicated contact lens preparation, hydroxyethyl methacrylate (HEMA) are copolymerized with methacrylate-derivative of β-cyclodextrin (β-CD) to produce hydrogel having adjustable drug loading and drug release property.96,97 With the content of methacrylate β-CD in contact lenses, properties like glass transition temperature and degree of swelling were also affected. Further study were carried out by loading of hydrocortisone and acetazolamide.97 It was reported that hydrocortisone loading was decreased while loading of acetazolamide was found to be maximum with decrease in beta-CDs content. The increase in the beta-CDs content increased the cross linking in the polymeric network, thereby, decreasing the pore size. The decreased pore size reduces the water retention capacity of the polymeric network. Drugs such as, hydrocortisone and acetazolamide are loaded into the hydrogel as they had the ability to form complexes with CDs with water molecules of the hydrogel matrix. Thus, increase in the beta-CDs content reduces the drug loading capacity of the hydrogel. Another study was carried out for investigating sustainable ophthalmic drug release by poly(2-hydroxyethyl methacrylate) hydrogel containing β-cyclodextrin (pHEMA/β-CD).98 Here contact lenses were prepared by photopolymerization of HEMA, mono-methacrylate β-CD (mono-MA-β-CD) and trimethylolpropane trimethacrylate using a cast molding process. It was reported that such mixtures increase in release duration of puerarin, as well as increase in tensile strength and hydrogel swelling.
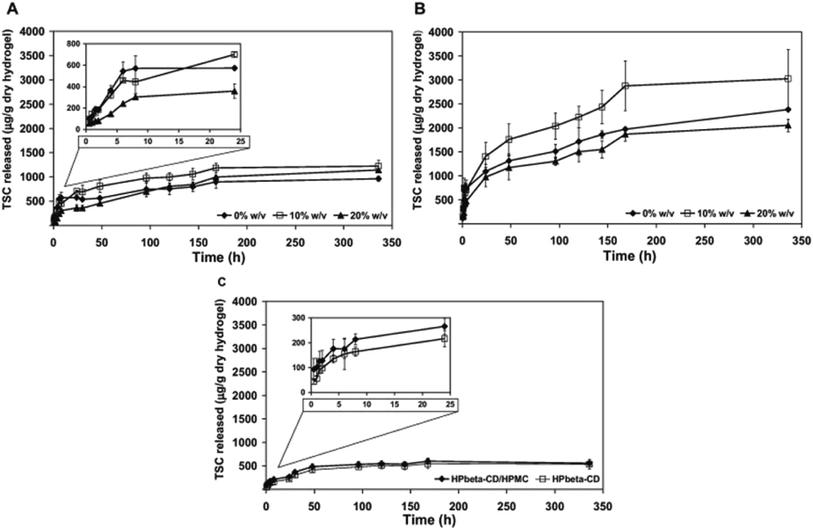 | ||
| Fig. 14 Thiosemicarbazone release kinetics from different hydrogels over two weeks, at 25 °C. (A) pHEMA-co-β-CD produced by the DP method, (B) pHEMA-co-β-CD produced by the PP method and (C) HPβ-CD and HPβ-CD/HPMC SHHs. The artificial lacrimal fluid medium was replaced to maintain sink conditions every (A) 30 h, (B) 4 h and (C) 8 h. Figure insets show the release during the first 24 h. Reprinted with permission from ref. 95. Copyright (2013), Elsevier. | ||
2.6. Use of drug-polymer film embedding to contact lens
Sustained release of drugs can also be achieved by encapsulation of drug molecules in polymer films. Ciolino et al., developed various types of prototypes that employed this technique. PLGA (poly[lactic-co glycolic acid]) films that were impregnated with an antifungal drug (econazole) in pHEMA were developed as therapeutic contact lenses. These lenses showed antifungal activity against Candida albicans.99 Ciprofloxacin, a well-known antimicrobial drug for Staphylococcus aureus, showed sustained release when encapsulated in PLGA films with pHEMA lenses. Altering the ratio of ciprofloxacin to PLGA can affect the rate of release. pHEMA can also affect the kinetics of drug release.100 Similar lenses have been developed for treatment of glaucoma. UV polymerisation was used to embed Latanoprost in poly(lactic-co-glycolic acid) films with methafilcon lenses. These lenses showed prolonged release of drug for 30 days and the results were similar to topical application of the same drug. The thickness of the film affects the rate of release for loaded drug.101Zhu et al., designed two different types of such lenses where drug delivery is controlled by pH or by presence of various ionic species. Diclofenac sodium was embedded in a film made using a blend of ethyl cellulose and Eudragit S100 with pHEMA lenses. When the lenses are stored in a phosphate buffer (pH 6.8), no leaking of drug was observed. When the contact lenses are applied on rabbit eyes, drug release occurs upon contact with tear fluid (pH 8.2) (Fig. 15).102 A similar prototype that used a mixture of cellulose acetate and Eudragit S100 for the polymer film with a silicone hydrogel base also showed sustained drug release under both in vivo and in vitro conditions upon pH change in tear fluid.103 Ion-triggered drug release was achieved by a silicone-based contact lens with a cellulose acetate film embedded with betaxolol hydrochloride104 and the drug was conjugated to poly(styrene-divinyl benzene) sulfonic acid resin. The lens could be stored in distilled water with negligible drug loss and allowed controlled release of drug for up to 1 week in rabbit model. The electrolytes present in tear fluid triggers sustained drug release. Carreira et al., developed drug eluting lenses for use after keratoprosthesis as a bandage. Vancomycin chlorhydrate was embedded in a PVA and chitosan blend film that used glyoxal for crosslinking. These lenses showed promising biocompatibility under in vitro conditions and sustained drug release for over 8 hours.105
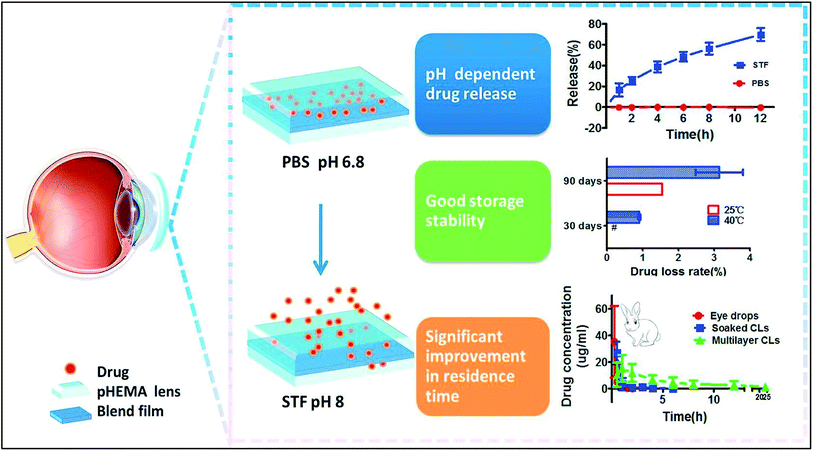 | ||
| Fig. 15 Schematic representation of the pH dependent release of drug from pHEMA lens embedded with drug. Reprinted with permission from ref. 102. Copyright (2018), Elsevier. | ||
3. Optimization of contact lenses for clinical application
Infections, allergies, immune reactions, accidental damage to cornea and vascular degeneration are the generally reported drawbacks of the ophthalmic implants. Thus it can be emphasized that biocompatibility is one of the most important issues which raises concern about the extended wear life of the soft contact lenses (SCLs). Spoilage of contact lenses by the proteins and lipid adsorption is the major factor compromising the biocompatibility of contact lenses.106 Silicone hydrogels were found to adsorb less amount of protein than the conventional hydrogels. This behavior can be attributed to the hydrophilic properties of the silicone hydrogels which increases the wettability of the surface, and hence less protein adsorption. Protein adsorption are often accompanied by the denaturation and structural changes which accounts for irreversibility of these interactions. Some of the consequences are inflamed cornea, hazy vision, foreign body reactions and disrupted tear film. Protein adsorption from tear fluid to the lens surface can be managed using different measures. Hence, there has been an increasing demand of biocompatible contact lenses, which can provide comfort to the user and can be used as a potential therapeutic drug delivery device. Hydrophobic interactions and static electric field are considered two most important factors which cause protein adsorption.107 To achieve reduced protein and lipid adsorption, there was a need to increase hydrophilicity of the material used. PEG (polyethylene glycol) is a condensation polymer of ethylene glycol and it possess some unique properties such as strong polarity, high water solubility and hydrophilicity. PEG has been widely used to modify the surfaces of materials. Being long chain polymer and electrically neutral in wide range of pH, it can resist electrostatic interactions between protein and lens surface. Polymerization, grafting and immobilization on the lens surface are some of the common ways to introduce PEG and to decrease the surface roughness.108The siloxane based lens are relatively more hydrophobic as compared to that of pHEMA lens. Lipids present in the tear fluid are deposited on the surface of the contact lens, thereby decreasing the wettability of the contact lens. Hydrophilic copolymers carry functional groups which make them soluble in water, thus, they have the potential to increase the wettability of the contact lens on being incorporated into the polymeric network of the contact lens. PVP (polyvinylpyrrolidone), polyethylene glycol have been used for this purpose.109,110 Additionally, to improve the wettability of surface of soft contact lenses, the presence of hydrophilic moieties is essential, and protein and lipid deposition must be minimal as much as possible. To tackle this problem, Valint and colleagues developed and patented this method in 2001 called plasma surface treatment to the soft contact lenses.111 The principle of this method lies in the oxidation of the silicone, which due to the addition of oxygen shows increased hydrophilicity and more surface wettability. Combination of hydrophilic monomeric hydrocarbon, oxygen and several oxidizing agents were used for plasma treatment to modify the surface of lens material. The use of pendant β cyclodextrins grafted to the copolymer network of pHEMA (polyhydroxyethylmethacrylate) and glycidyl methacrylate were found to decrease friction coefficient by 50%. These pendant cyclodextrins did not affect viscoelasticity, tensile strength, light transmittance, water content, glass transition (Tg), melting temperature (Tm), contact angle with the surface and oxygen permeability. Presence of phosphate group in the side chains of the polymer forming hydrogels for SCLs, is also appreciated to enhance biocompatibility and sustained drug delivery.112 The drug release profile was tested for naphazoline, a decongestant used for eyes. It contains a cationic group and is introduced in equimolar amount which provides sufficient polymer–drug interactions. With this study, it was observed that the hydrogel polymer with the inclusion of amide and phosphate groups imparted high transparency and proper shape (Fig. 16).
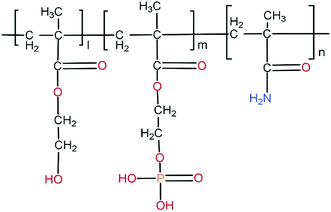 | ||
| Fig. 16 Chemical structure of poly(HEMA-co-MOEP-co-Mam). The presence of phosphate groups in the side chain of the polymer forming hydrogel is seen. | ||
Smart hydrogels which are responsive towards some stimuli such as light intensity, change in pH, hydrophilicity were also used for controlled and sustained drug delivery. One such example is the use of grafted PNIPAAm/PAAc (poly-N-isopropyl acrylamide/poly acrylic acid) on polypropylene surface to study the release of vancomycin.113 Vancomycin helps to reduce the chance of biofilm formation which are formed by harmful microorganisms such as Staphylococcus aureus which enters the blood circulation during implantation of a prosthetic device. A remarkable enhancement in vancomycin loading (2.5 mg cm−2) was achieved by grafting AAc to the polypropylene surface and reduced the friction coefficient from 0.28 ± 0.03 to 0.05 ± 0.02 with pH-dependent release. It also increased the retention time of vancomycin for several hours.113
A major aspect of ocular drug delivery deals with the microbial interactions on the interface of contact lens and surface of the eyes. However, various attempts have been made to provide antimicrobial activity to the soft contact lenses to deal with such issues. Incorporation of selenium,114 silver,115 cationic peptide,116 NSAIDs (Non-Steroidal Anti-Inflammatory Drugs)117,118 phosphoryl choline,119 fimbrolide-coated,120 PEO coated121 were the strategies analysed for the effective anti-microbial behaviour of soft contact lenses. These modifications proved to be effective to some extent, however, they were also associated with certain limitations. Surface coatings such as Mel4 peptide coatings,122 covalently attached melamine123,124 and immobilized nanoparticle coatings like Zn-doped CuO125 have been observed to show stable and efficient anti-microbial activity against Staphylococcus aureus and Pseudomonas aeruginosa.
4. Sterilization of contact lenses
After manufacturing the lenses, they must be sterilised against contamination and preserved until they are used. Some of the major methods for the sterilisation and packaging of contact lenses are(i) The lens is washed manually using various cleaning agents and packed in a single-use plastic case. Alternatively, glass vials can be used for packing since they are more stable when placed in high temperatures.
(ii) The lens remains immersed in a saline solution within the case, which is then closed by heat-sealing with aluminium foil to prevent entry of microbes.
(iii) The packed lenses are sterilised using pressurised steam at 121 °C in an autoclave. An alternative to this method is the use of UV irradiation.
(iv) Appropriate measures should be taken to keep the packaging intact during sterilisation process.
The properties of the biomaterial must be preserved after sterilisation and the agents used for sterilisation should not alter the composition of the drug. Depending on the material and drug used, suitable sterilisation techniques must be optimised.
5. Evaluation of drug laden contact lens
The swelling behaviour of the contact lens is a significant study to understand the optimization of drug loading and delivery. It gives the effect of any incorporated material on the structural integrity of the contact lens. Maulvi, F. A. et al., studied the swelling behaviour of gold nanoparticles and timolol incorporated contact lenses. They observed an insignificant increase in % swelling of the drug-laden contact lens compared to the blank contact lens.69 This increase may be due to the effect of incorporated nanoparticle and drug on the crosslinking capacity of the fabricated lens. The dry weights of the blank contact lens (lens without soaking) and nanoparticles-drug soaked contact lens were measured and the swelling capacity measured using the formulaWater content and optical transparency is an important parameter to compare between a blank contact and drug-laden contact lens. Optical transparency is measured by calculating the transmittance percentage using an UV spectrophotometer in the range of 200–800 nm.69,71
Depending on the chemical nature of the drug, different methods are adapted to measure the amount of drug uptake by the lens. Solvent extraction and colorimetric analysis are two of the important methods to study drug uptake and release. During the solvent extraction method, a solvent in which drug can be solubilized was used to extract the drug from the drug-laden contact lens and the amount of drug present in the lens was estimated using the HPLC column.69 The in vivo drug release study was carried out in rabbit models where the amount of drug released in the tear fluid was calculated by LCMS studies. Draize technique scoring system was used to check the ocular tolerance of the drug-laden contact lens. White rabbits were used for in vivo pharmacodynamic study to measure the efficiency of drug release in vivo systems. To measure the drug release, sterilized drug-laden contact lens was placed in the rabbit eyes and tear fluid was collected at regular intervals for LC-MS analysis. Maulvi et al., compared the kinetic release of timolol drug for glaucoma by drug-laden nanoparticles contact lens and conventional eye drop using in vivo pharmacokinetic studies in rabbit eyes.126 They achieved the sustained release of drug by nanoparticle laden ring hydrogel contact lens and overcame the burst release limitation. In vivo pharmacodynamic study is used to measure the efficacy of the drug. Intraocular pressure of the rabbit eye was measured and reduced for a prolonged time in a modified contact lens than the normal lens, comparatively.126 Additionally, ex vivo experiments were carried out to measure the release of the drug. The penetration effect of the drug was studied in isolated rabbit cornea using the perfusion apparatus where drug penetration were measured in osmometer quantitatively.126 When particles incorporated contact lenses were used for drug release, it was found that swelling property is not affected and intraocular pressure of the eyes was decreased for a prolonged time. Drug residence time was improved along with sustained release of the drug.126
Various assays such as comet assay, elution assay, and agar overlay/diffusion assay have been generally performed to check the possibility of toxicity from drug-releasing contact lens. One of the most commonly used assays for measuring the cytotoxic effect of drug-releasing contact lens is colorimetric MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay.127 The metabolically viable cells convert the substrate MTT into formazan, which is then dissolved for quantitative colorimetric measurement. This assay is performed in cell lines ahead of animal studies to deduce the possibility of toxicity. Maulvi et al., observed significantly lower cell viability in microemulsion and silica shell nanoparticle-laden hydrogel extract when compared with the control using MTT assay.126 Ciolino et al., developed a drug-eluting contact lens for delivering a glaucoma drug.128 They assessed the cytotoxicity of latanoprost laden contact lens on human corneal limbal epithelial (HCLE) cell lines and found no cytotoxic effect of contact lens and its eluted products.
The elution assay is an indirect assay that is used to measure the cytotoxicity effect of extract solution from the test material. It does not measure the physical or mechanical impact because the test material is not in direct contact with the cells.130 Danion et al., investigated the potential toxicity of the extracted solution from a soft contact lens coat with liposomes on the epithelial cells.129 They incubated the lens on the culture media, which was then used for the growth of the cells without involving direct contact of the lens and calculated the cell viability by XTT assay, which suggested that no cytotoxic compounds were present in the extract solution.
Agar overlay/diffusion assay, an indirect assay in which the cells are covered with an agar layer, and the test material is placed on top of the layer. After 24–72 hours of the incubation period, the cytotoxicity effect can be evaluated.130,131 The free material from the contact lens has the potential to penetrate the cell and interact with DNA. Comet assay is a microgel electrophoresis technique for measuring DNA damage and DNA repair. In this assay, the cells embedded in the agarose are lysed with detergents or high salts, which then subjected to electrophoresis.132 The principle is that cells with increased DNA damage possess increased migration towards the anode. Coelho et al., used this assay to evaluate the genotoxicity of the nine types of therapeutic contact lenses developed from bacterial cellulose and observed genotoxicity caused by two types of lenses.133
6. Future perspective
With recent advancements in the field of material science there are ample opportunities to develop drug releasing soft material contact lenses. Hydrogel based contact lenses would be one of the most effective ocular drug delivery systems as oxygen permeability is not hindered in such materials. However, modification to optimize the mechanical and optical properties must be done. Combinations of a variety of monomers and the crosslinkers must be studied in order to develop the contact lens with the most optimised properties. The choice of copolymers for the fabrication of the contact lens is one of the most vital parameters. For efficient loading and release of the drug it must be ensured that proper drug–polymer interaction is established. Hydrophilic and hydrophobic drugs differ in their interaction with the polymeric network and should be kept in consideration while co-polymerising two different type of polymers for preparing contact lenses. Additionally, use of smart polymers can be explored for the fabrication of contact lenses. As these polymers respond to any significant changes to the environment such as change in pH, temperature, etc. these can be used to deliver the required drug at different physiological conditions in the eye. Different combinations of monomers and crosslinkers must be studied to get the most optimised network of polymer which would be able to release the drug in the required rate. PLGA (poly(lactic-co-glycolic acid)) is a biodegradable and biocompatible copolymer which has been used for various therapeutic applications. PLGA has been observed to be degraded in the vitreous humour of the eye. Drug molecules can be entrapped in the polymeric matrix of PLGA and can be incorporated into the hydrogel polymeric network. The encapsulation makes the drug to be released over a prolonged period of time, thereby increasing the residence time of the drug in the eye. Moreover, the problems – such as initial burst release, excretion of major amount of the drug leading to decreased bioavailability, which are associated with the use of eye drops, can be solved, thereby, making ocular drug delivery to the posterior portion of the eye more feasible. The concept of prodrugs can also be utilised. The drug can be modified chemically in such a way that the drug can increase the permeability to the posterior part of the eye and on reaching the site of action it gets converted to the pharmacologically active form. This would increase the bioavailability of the drug. Moreover, the proper encapsulation of the drug can be utilised to increase the shelf life of the drug as the drug is masked from the external environment. However, a major drawback of all the modifications is that the optical clarity, oxygen permeability and the mechanical properties of the lens is often compromised which makes the lens unfit for use. Thus, an approach to develop drug releasing contact lens should be such that these essential properties of the lens are not compromised.7. Summary
The increasing incidence of ocular diseases have made it necessary for the development of ocular drug delivery platforms. At present topical administration of drugs with the help of eye drops is the most widely used method of ocular drug delivery. The various anatomical barriers of the eye lead to decreased bioavailability of the drug. The use conventional hydrogel based contact lens soaked in drug have also been studied, but a major problem associated with this technique is the initial burst release of the drug which may lead to systemic toxicity. Thus, an ocular drug delivery platform needs to be developed which would give control over the process of drug loading and drug release. The ongoing research in the field of developing soft material contact lenses as ocular drug delivery vehicle aims at overcoming the challenges of the existing drug delivery techniques. The recent advancements in the field of material science has paved the way for a plethora of techniques which can be utilised to develop the drug releasing soft material contact lens. Combinations of different monomers and cross linkers have produced hydrogel based soft material contact lens with optimised mechanical properties. Copolymerisation of HEMA (hydroxyethyl methacrylate) with monomers such as NVP (N-vinyl pyrrolidone), MMA (methyl methacrylate), MAA (methacrylic acid) has optimised the mechanical properties of the contact lens leading to enhanced mechanical strength of the contact lens making them fit for use. The use of various ionic and non ionic monomers have made it possible for the incorporation of various categories of drugs into the polymeric network. The use of nanotechnology has also made a significant impact in this field. Drug molecules encapsulated by nanoparticles helped in enhanced drug loading. Contact lens loaded with drug by conventional soaking method lead to leaching of drug from the contact lens during storage causing decrease in the concentration of the drug. Encapsulation of drug molecules hindered the process of drug leaching. As an improvement the encapsulation of drugs by nanoparticles produced from various polymers leads to release of drug on being exposed to the enzyme like lysozyme, thereby, decreasing the occurrence of burst release. For the fabrication of an ideal contact lens it must be ensured that some of the adverse effects caused by the prolonged use of contact lens are being mitigated. Thus, proper use of monomers and crosslinkers for the fabrication of the contact lens would produce the contact lens with the most optimised properties. However, it must be ensured that properties such as oxygen transmission and optical clarity should never be compromised during the production of drug delivering soft material contact lens.Conflicts of interest
There are no conflicts to declare.References
- C. L. Rowe-Rendleman, S. A. Durazo, U. B. Kompella, K. D. Rittenhouse, A. Di Polo, A. L. Weiner, H. E. Grossniklaus, M. I. Naash, A. S. Lewin, A. Horsager and H. F. Edelhauser, Drug and gene delivery to the back of the eye: from bench to bedside, Investig. Ophthalmol. Vis. Sci., 2014, 55(4), 2714–2730 CrossRef CAS
.
- C. E. Willoughby, D. Ponzin, S. Ferrari, A. Lobo, K. Landau and Y. Omidi, Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function–a review, Clin. Exp. Ophthalmol., 2010, 38, 2–11 CrossRef
.
- S. Reimondez-Troitiño, N. Csaba, M. J. Alonso and M. De La Fuente, Nanotherapies for the treatment of ocular diseases, Eur. J. Pharm. Biopharm., 2015, 95, 279–293 CrossRef
.
- H. A. Quigley, Neuronal death in glaucoma, Prog. Retin. Eye Res., 1999, 18(1), 39–57 CrossRef CAS
.
- K. G. Janoria, S. Gunda, S. H. Boddu and A. K. Mitra, Novel approaches to retinal drug delivery, Expet Opin. Drug Deliv., 2007, 4(4), 371–388 CrossRef CAS
.
- V. Gote, S. Sikder, J. Sicotte and D. Pal, Ocular drug delivery: present innovations and future challenges, J. Pharmacol. Exp. Ther., 2019, 370(3), 602–624 CrossRef CAS
.
- J. Barar, A. Aghanejad, M. Fathi and Y. Omidi, Advanced drug delivery and targeting technologies for the ocular diseases, Bioimpacts, 2016, 6(1), 49 CrossRef CAS
.
- R. Gaudana, H. K. Ananthula, A. Parenky and A. K. Mitra, Ocular drug delivery, AAPS J., 2010, 12(3), 348–360 CrossRef CAS
.
- M. R. Jain, Drug delivery through soft contact lenses, Br. J. Ophthalmol., 1988, 72(2), 150–154 CrossRef CAS
.
- E. B. Souto, J. Dias-Ferreira, A. López-Machado, M. Ettcheto, A. Cano, A. Camins Espuny, M. Espina, M. L. Garcia and E. Sánchez-López, Advanced formulation approaches for ocular drug delivery: state-of-the-art and recent patents, Pharmaceutics, 2019, 11(9), 460 CrossRef CAS
.
- M. C. Peters, E. D. Santos Neto, L. M. Monteiro, M. N. Yukuyama, M. G. Machado, I. F. de Oliveira, M. H. Zanin, R. Löbenberg and N. Bou-Chacra, Advances in ophthalmic preparation: The role of drug nanocrystals and lipid-based nanosystems, J. Drug Targeting, 2020, 28(3), 259–270 CrossRef CAS
.
- A. R. Pontillo and A. Detsi, Nanoparticles for ocular drug delivery: modified and non-modified chitosan as a promising biocompatible carrier, Nanomedicine, 2019, 14(14), 1889–1909 CrossRef CAS
.
- E. M. Del Amo and A. Urtti, Current and future ophthalmic drug delivery systems: a shift to the posterior segment, Drug Discov. Today, 2008, 13(3–4), 135–143 CrossRef CAS
.
- G. K. Smelser and V. Ozanics, Importance of atmospheric oxygen for maintenance of the optical properties of the human cornea, Science, 1952, 115(2980), 140 CrossRef CAS
.
- J. A. Bonanno, Effects of contact lens-induced hypoxia on the physiology of the corneal endothelium, Optom. Vis. Sci., 2001, 78(11), 783–790 CrossRef CAS
.
- B. A. Holden and G. W. Mertz, Critical oxygen levels to avoid corneal edema for daily and extended wear contact lenses, Investig. Ophthalmol. Vis. Sci., 1984, 25(10), 1161–1167 CAS
.
- S. M. Dillehay and M. B. Miller, Performance of Lotrafilcon B silicone hydrogel contact lenses in experienced low-Dk/t daily lens wearers, Eye Contact Lens, 2007, 33(6), 272–277 CrossRef
.
- D. F. Sweeney, Have silicone hydrogel lenses eliminated hypoxia?, Eye Contact Lens, 2013, 39(1), 53–60 CrossRef
.
- J. Pozuelo, V. Compañ, J. M. González-Méijome, M. González and S. Mollá, Oxygen and ionic transport in hydrogel and silicone-hydrogel contact lens materials: An experimental and theoretical study, J. Membr. Sci., 2014, 452, 62–72 CrossRef CAS
.
- L. Alvord, T. O. Davis, C. F. Morgan, K. L. Schindhelm, J. Ü. Vogt and L. Y. Winterton, Oxygen permeability of a new type of high Dk soft contact lens material, Optom. Vis. Sci., 1998, 75(1), 30–36 CrossRef CAS
.
- C. S. Musgrave and F. Fang, Contact lens materials: a materials science perspective, Materials, 2019, 12(2), 261 CrossRef CAS
.
- G. Foulks, R. Chalmers, N. Keir, C. A. Woods, T. Simpson, R. Lippman, W. Gleason, D. A. Schaumberg, M. D. Willcox and I. Jalbert, The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on clinical trial design and outcomes, Investig. Ophthalmol. Vis. Sci., 2013, 54(11), TFOS157–TFOS182 CrossRef
.
- C. González-Chomón, A. Concheiro and C. Alvarez-Lorenzo, Soft contact lenses for controlled ocular delivery: 50 years in the making, Ther. Deliv., 2013, 4(9), 1141–1161 CrossRef
.
- N. Pritchard, D. Fonn and D. Brazeau, Discontinuation of contact lens wear: a survey, Int. Contact Lens Clin., 1999, 26(6), 157–162 CrossRef CAS
.
- H. Chen, Y. Jin, L. Sun, X. Li, K. Nan, H. Liu, Q. Zheng and B. Wang, Recent developments in ophthalmic drug delivery systems for therapy of both anterior and posterior segment diseases, Colloid Interface Sci., 2018, 24, 54–61 CrossRef CAS
.
- X. Hu, L. Hao, H. Wang, X. Yang, G. Zhang, G. Wang and X. Zhang, Hydrogel contact lens for extended delivery of ophthalmic drugs, Int. J. Polym. Sci., 2011, 2011, 814163 Search PubMed
.
- G. Jha and A. Kumar, Drug delivery through soft contact lenses: An introduction, Chronicles Young Sci., 2011, 2(1), 3 CrossRef CAS
.
- N. Efron Contact lens complications, Elsevier, 2019 Search PubMed
.
- J. M. Dixon and E. Lawaczeck, Corneal vascularization due to contact lenses, Arch. Ophthalmol., 1963, 69(1), 72–75 CrossRef CAS
.
- J. M. Dixon, Corneal vascularization due to corneal contact lenses: the clinical picture, Trans. Am. Ophthalmol. Soc., 1967, 65, 333 CAS
.
- G. D. Kymionis, G. A. Kontadakis. Severe corneal vascularization after intacs implantation and rigid contact lens use for the treatment of keratoconus, in Seminars in ophthalmology, Taylor & Francis, 2012, vol. 27, no. 1–2, pp. 19–21 Search PubMed
.
- K. A. Dumbleton, R. L. Chalmers, D. B. Richter and D. Fonn, Vascular response to extended wear of hydrogel lenses with high and low oxygen permeability, Optom. Vis. Sci., 2001, 78(3), 147–151 CrossRef CAS
.
- M. R. Dana and J. W. Streilein, Loss and restoration of immune privilege in eyes with corneal neovascularization, Investig. Ophthalmol. Vis. Sci., 1996, 37(12), 2485–2494 CAS
.
- C. C. Skotnitsky, T. J. Naduvilath, D. F. Sweeney and P. R. Sankaridurg, Two presentations of contact lens-induced papillary conjunctivitis (CLPC) in hydrogel lens wear: local and general, Optom. Vis. Sci., 2006, 83(1), E27–E36 CrossRef
.
- W. H. Elhers and P. C. Donshik, Giant papillary conjunctivitis, Curr. Opin. Allergy Clin. Immunol., 2008, 8(5), 445–449 CrossRef CAS
.
- D. J. Fuerst, J. Sugar and S. Worobec, Superior limbic keratoconjunctivitis associated with cosmetic soft contact lens wear, Arch. Ophthalmol., 1983, 101(8), 1214–1216 CrossRef CAS
.
- L. Sorbara, L. Jones and D. Williams-Lyn, Contact lens induced papillary conjunctivitis with silicone hydrogel lenses, Contact Lens Anterior Eye, 2009, 32(2), 93–96 CrossRef CAS
.
- M. R. Allansmith, D. R. Korb, J. V. Greiner, A. S. Henriquez, M. A. Simon and V. M. Finnemore, Giant papillary conjunctivitis in contact lens wearers, Am. J. Ophthalmol., 1977, 83(5), 697–708 CrossRef CAS
.
- F. Stapleton, S. Stretton, E. Papas, C. Skotnitsky and D. F. Sweeney, Silicone hydrogel contact lenses and the ocular surface, Ocul. Surf., 2006, 4(1), 24–43 CrossRef
.
- S. W. Chang and C. J. Chang, Delayed tear clearance in contact lens associated papillary conjunctivitis, Curr. Eye Res., 2001, 22(4), 253–257 CrossRef CAS
.
- P. Chhabra, R. Gupta, G. Suri, M. Tyagi, G. Seshadri, S. Sabharwal, U. K. Niyogi and R. K. Khandal, Studies on Development of Polymeric Materials Using Gamma Irradiation for Contact and Intraocular Lenses, Int. J. Polym. Sci., 2009, 2009, 906904 Search PubMed
.
- M. S. Lord, M. H. Stenzel, A. Simmons and B. K. Milthorpe, The effect of charged groups on protein interactions with poly (HEMA) hydrogels, Biomaterials, 2006, 27(4), 567–575 CrossRef CAS
.
- Q. Garrett, B. Laycock and R. W. Garrett, Hydrogel lens monomer constituents modulate protein sorption, Investig. Ophthalmol. Vis. Sci., 2000, 41(7), 1687–1695 CAS
.
- P. Andrade-Vivero, E. Fernandez-Gabriel, C. Alvarez-Lorenzo and A. Concheiro, Improving the loading and release of NSAIDs from pHEMA hydrogels by copolymerization with functionalized monomers, J. Pharmaceut. Sci., 2007, 96(4), 802–813 CrossRef CAS
.
- P. C. Nicolson and J. Vogt, Soft contact lens polymers: an evolution, Biomaterials, 2001, 22(24), 3273–3283 CrossRef CAS
.
- A. Yamada-Nosaka, K. Ishikiriyama, M. Todoki and H. Tanzawa, 1H-NMR studies on water in methacrylate hydrogels. I., J. Appl. Polym. Sci., 1990, 39(11-12), 2443–2452 CrossRef CAS
.
- C. C. Li and A. Chauhan, Modeling ophthalmic drug delivery by soaked contact lenses, Ind. Eng. Chem. Res., 2006, 45(10), 3718–3734 CrossRef CAS
.
- C. C. Li and A. Chauhan, Ocular transport model for ophthalmic delivery of timolol through p-HEMA contact lenses, J. Drug Deliv. Sci. Technol., 2007, 17(1), 69–79 CrossRef CAS
.
- A. Topete, A. S. Oliveira, A. Fernandes, T. G. Nunes, A. P. Serro and B. Saramago, Improving sustained drug delivery from ophthalmic lens materials through the control of temperature and time of loading, Eur. J. Pharm. Sci., 2018, 117, 107–117 CrossRef CAS
.
- E. García-Millán, S. Koprivnik and F. J. Otero-Espinar, Drug loading optimization and extended drug delivery of corticoids from pHEMA based soft contact lenses hydrogels via chemical and microstructural modifications, Int. J. Pharm., 2015, 487(1–2), 260–269 CrossRef
.
- Q. Zhu and S. Mao, Enhanced drug loading efficiency of contact lenses via salt-induced modulation, Asian J. Pharm. Sci., 2019, 14(2), 204–215 CrossRef
.
- P. Paradiso, R. Galante, L. Santos, A. P. Alves de Matos, R. Colaço, A. P. Serro and B. Saramago, Comparison of two hydrogel formulations for drug release in ophthalmic lenses, J. Biomed. Mater. Res. B Appl. Biomater., 2014, 102(6), 1170–1180 CrossRef CAS
.
- T. Minami, W. Ishida, T. Kishimoto, I. Nakajima, S. Hino, R. Arai, T. Matsunaga, A. Fukushima and S. Yamagami, In vitro and in vivo performance of epinastine hydrochloride-releasing contact lenses, PloS One, 2019, 14(1), e0210362 CrossRef CAS
.
- M. Yang, Y. Yang, M. Lei, C. Ye, C. Zhao, J. Xu, K. Wu and M. Yu, Experimental studies on soft contact lenses for controlled ocular delivery of pirfinedone: in vitro and in vivo, Drug Deliv., 2016, 23(9), 3538–3543 CrossRef CAS
.
- C. C. Peng, J. Kim and A. Chauhan, Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing vitamin E diffusion
barriers, Biomaterials, 2010, 31(14), 4032–4047 CrossRef CAS
.
- S. Venkatesh, J. Saha, S. Pass and M. E. Byrne, Transport and structural analysis of molecular imprinted hydrogels for controlled drug delivery, Eur. J. Pharm. Biopharm., 2008, 69(3), 852–860 CrossRef CAS
.
- H. Hiratani, Y. Mizutani and C. Alvarez-Lorenzo, Controlling drug release from imprinted hydrogels by modifying the characteristics of the imprinted cavities, Macromol. Biosci., 2005, 5(8), 728–733 CrossRef CAS
.
- C. J. White, A. Tieppo and M. E. Byrne, Controlled drug release from contact lenses: a comprehensive review from 1965-present, J. Drug Deliv. Sci. Technol., 2011, 21(5), 369–384 CrossRef CAS
.
- C. J. White, M. K. McBride, K. M. Pate, A. Tieppo and M. E. Byrne, Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses, Biomaterials, 2011, 32(24), 5698–5705 CrossRef CAS
.
- H. Hiratani and C. Alvarez-Lorenzo, Timolol uptake and release by imprinted soft contact lenses made of N,N-diethylacrylamide and methacrylic acid, J. Controlled Release, 2002, 83(2), 223–230 CrossRef CAS
.
- B. M. Nikouei, S. A. Vahabzadeh and S. A. Mohajeri, Preparation of a molecularly imprinted soft contact lens as a new ocular drug delivery system for dorzolamide, Curr. Drug Deliv., 2013, 10(3), 279–285 CrossRef
.
- M. Ali and M. E. Byrne, Controlled release of high molecular weight hyaluronic acid from molecularly imprinted hydrogel contact lenses, Pharmaceut. Res., 2009, 26(3), 714–726 CrossRef CAS
.
- J. E. Lofgreen and G. A. Ozin, Controlling morphology and porosity to improve performance of molecularly imprinted sol–gel silica, Chem. Soc. Rev., 2014, 43(3), 911–933 RSC
.
- N. Gupta, S. Goel and H. Gupta, Patent review on nanotechnology in ocular drug delivery, Recent Pat. Nanomed., 2013, 3(1), 37–46 CrossRef CAS
.
- D. R. Janagam, L. Wu and T. L. Lowe, Nanoparticles for drug delivery to the anterior segment of the eye, Adv. Drug Deliv. Rev., 2017, 122, 31–64 CrossRef CAS
.
- Y. Kapoor, J. C. Thomas, G. Tan, V. T. John and A. Chauhan, Surfactant-laden soft contact lenses for extended delivery of ophthalmic drugs, Biomaterials, 2009, 30(5), 867–878 CrossRef CAS
.
- Y. Kapoor and A. Chauhan, Drug and surfactant transport in Cyclosporine A and Brij 98 laden p-HEMA hydrogels, J. Colloid Interface Sci., 2008, 322(2), 624–633 CrossRef CAS
.
- H. J. Jung, M. Abou-Jaoude, B. E. Carbia, C. Plummer and A. Chauhan, Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses, J. Contr. Release, 2013, 165(1), 82–89 CrossRef CAS
.
- F. A. Maulvi, R. J. Patil, A. R. Desai, M. R. Shukla, R. J. Vaidya, K. M. Ranch, B. A. Vyas, S. A. Shah and D. O. Shah, Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: in vitro and in vivo evaluation, Acta Biomater., 2019, 86, 350–362 CrossRef CAS
.
- H. J. Jung and A. Chauhan, Temperature sensitive contact lenses for triggered ophthalmic drug delivery, Biomaterials, 2012, 33(7), 2289–2300 CrossRef CAS
.
- F. A. Maulvi, H. H. Choksi, A. R. Desai, A. S. Patel, K. M. Ranch, B. A. Vyas and D. O. Shah, pH triggered controlled drug delivery from contact lenses: Addressing the challenges of drug leaching during sterilization and storage, Colloids Surf. B Biointerfaces, 2017, 157, 72–82 CrossRef CAS
.
- H. J. Kim, K. Zhang, L. Moore and D. Ho, Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release, ACS Nano, 2014, 8(3), 2998–3005 CrossRef CAS
.
- G. Behl, J. Iqbal, N. J. O'Reilly, P. McLoughlin and L. Fitzhenry, Synthesis and characterization of poly(2-hydroxyethylmethacrylate)
contact lenses containing chitosan nanoparticles as an ocular delivery system for dexamethasone sodium phosphate, Pharmaceut. Res., 2016, 33(7), 1638–1648 CrossRef CAS
.
- B. S. Bazzaz, B. Khameneh, M. M. Jalili-Behabadi, B. Malaekeh-Nikouei and S. A. Mohajeri, Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles, Contact Lens Anterior Eye, 2014, 37(3), 149–152 CrossRef
.
- K. Kalishwaralal, S. BarathManiKanth, S. R. Pandian, V. Deepak and S. Gurunathan, Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis, Colloids Surf. B Biointerfaces, 2010, 79(2), 340–344 CrossRef CAS
.
- J. F. Huang, J. Zhong, G. P. Chen, Z. T. Lin, Y. Deng, Y. L. Liu, P. Y. Cao, B. Wang, Y. Wei, T. Wu and J. Yuan, A hydrogel-based hybrid theranostic contact lens for fungal keratitis, ACS Nano, 2016, 10(7), 6464–6473 CrossRef CAS
.
- J. Xu, Y. Ge, R. Bu, A. Zhang, S. Feng, J. Wang, J. Gou, T. Yin, H. He, Y. Zhang and X. Tang, Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma, J. Controlled Release, 2019, 305, 18–28 CrossRef CAS
.
- C. Lu, A. S. Mikhail, X. Wang, M. A. Brook and C. Allen, Hydrogels containing core cross-linked block co-polymer micelles, J. Biomater. Sci., Polym. Ed., 2012, 23(8), 1069–1090 CrossRef CAS
.
- D. Gulsen, C. C. Li and A. Chauhan, Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery, Curr. Eye Res., 2005, 30(12), 1071–1080 CrossRef CAS
.
- A. Danion, H. Brochu, Y. Martin and P. Vermette, Fabrication and characterization of contact lenses bearing surface-immobilized layers of intact liposomes, J. Biomed. Mater. Res., 2007, 82(1), 41–51 CrossRef
.
- A. Danion, I. Arsenault and P. Vermette, Antibacterial activity of contact lenses bearing surface-immobilized layers of intact liposomes loaded with levofloxacin, J. Pharmaceut. Sci., 2007, 96(9), 2350–2363 CrossRef CAS
.
- J. Xu, Y. Xue, G. Hu, T. Lin, J. Gou, T. Yin, H. He, Y. Zhang and X. Tang, A comprehensive review on contact lens for ophthalmic drug delivery, J. Controlled Release, 2018, 281, 97–118 CrossRef CAS
.
- F. A. Maulvi, A. R. Desai, H. H. Choksi, R. J. Patil, K. M. Ranch, B. A. Vyas and D. O. Shah, Effect of surfactant chain length on drug release kinetics from microemulsion-laden contact lenses, Int. J. Pharm., 2017, 524(1–2), 193–204 CrossRef CAS
.
- C. C. Li, M. Abrahamson, Y. Kapoor and A. Chauhan, Timolol transport from microemulsions trapped in HEMA gels, J. Colloid Interface Sci., 2007, 315(1), 297–306 CrossRef CAS
.
- Y. Kapoor and A. Chauhan, Ophthalmic delivery of cyclosporine A from Brij-97 microemulsion and surfactant-laden p-HEMA hydrogels, Int. J. Pharm., 2008, 361(1–2), 222–229 CrossRef CAS
.
- D. Gulsen and A. Chauhan, Ophthalmic drug delivery through contact lenses, Investig. Ophthalmol. Vis. Sci., 2004, 45(7), 2342–2347 CrossRef
.
- D. Gulsen and A. Chauhan, Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle, Int. J. Pharm., 2005, 292(1–2), 95–117 CrossRef CAS
.
- G. Crini, A history of cyclodextrins, Chem. Rev., 2014, 114(21), 10940–10975 CrossRef CAS
.
- J. Xu, X. Li and F. Sun, Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release, Acta Biomater., 2010, 6(2), 486–493 CrossRef CAS
.
- M. J. Garcia-Fernandez, N. Tabary, B. Martel, F. Cazaux, A. Oliva, P. Taboada, A. Concheiro and C. Alvarez-Lorenzo, Poly-(cyclo) dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses, Carbohydr. Polym., 2013, 98(2), 1343–1352 CrossRef CAS
.
- N. A. Brennan, M. L. Coles, A. Jaworski and D. Rosario, Proposed practice guidelines for continuous contact lens wear, Clin. Exp. Optom., 2001, 84(2), 71–77 CrossRef
.
- J. F. dos Santos, C. Alvarez-Lorenzo, M. Silva, L. Balsa, J. Couceiro, J. J. Torres-Labandeira and A. Concheiro, Soft contact lenses functionalized with pendant cyclodextrins for controlled drug delivery, Biomaterials, 2009, 30(7), 1348–1355 CrossRef
.
- A. Rasheed, Cyclodextrins as drug carrier molecule: a review, Sci. Pharm., 2008, 76(4), 567–598 CrossRef CAS
.
- J. F. Dos Santos, J. J. Torres-Labandeira, N. Matthijs, T. Coenye, A. Concheiro and C. Alvarez-Lorenzo, Functionalization of acrylic hydrogels with α-, β-or γ-cyclodextrin modulates protein adsorption and antifungal delivery, Acta Biomater., 2010, 6(10), 3919–3926 CrossRef CAS
.
- R. J. Glisoni, M. J. García-Fernández, M. Pino, G. Gutkind, A. G. Moglioni, C. Alvarez-Lorenzo, A. Concheiro and A. Sosnik, β-Cyclodextrin hydrogels for the ocular release of antibacterial thiosemicarbazones, Carbohydr. Polym., 2013, 93(2), 449–457 CrossRef CAS
.
- M. J. Garcia-Fernandez, N. Tabary, B. Martel, F. Cazaux, A. Oliva, P. Taboada, A. Concheiro and C. Alvarez-Lorenzo, Poly-(cyclo)dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses, Carbohydr. Polym., 2013, 98(2), 1343–1352 CrossRef CAS
.
- J. F. dos Santos, R. Couceiro, A. Concheiro, J. J. Torres-Labandeira and C. Alvarez-Lorenzo, Poly(hydroxyethyl methacrylate-co-methacrylated-β-cyclodextrin) hydrogels: synthesis, cytocompatibility, mechanical properties and drug loading/release properties, Acta Biomater., 2008, 4(3), 745–755 CrossRef CAS
.
- J. Xu, X. Li and F. Sun, Cyclodextrin-containing hydrogels for contact lenses as a platform for drug incorporation and release, Acta Biomater., 2010, 6(2), 486–493 CrossRef CAS
.
- J. B. Ciolino, S. P. Hudson, A. N. Mobbs, T. R. Hoare, N. G. Iwata, G. R. Fink and D. S. Kohane, A prototype antifungal contact lens, Investig. Ophthalmol. Vis. Sci., 2011, 52(9), 6286–6291 CrossRef CAS
.
- J. B. Ciolino, T. R. Hoare, N. G. Iwata, I. Behlau, C. H. Dohlman, R. Langer and D. S. Kohane, A drug-eluting contact lens, Invest. Ophthalmol. Vis. Sci., 2009, 50(7), 3346–3352 CrossRef
.
- J. B. Ciolino, C. F. Stefanescu, A. E. Ross, B. Salvador-Culla, P. Cortez, E. M. Ford, K. A. Wymbs, S. L. Sprague, D. R. Mascoop, S. S. Rudina and S. A. Trauger, In vivo performance of a drug-eluting contact lens to treat glaucoma for a month, Biomaterials, 2014, 35(1), 432–439 CrossRef CAS
.
- Q. Zhu, C. Liu, Z. Sun, X. Zhang, N. Liang and S. Mao, Inner layer-embedded contact lenses for pH-triggered controlled ocular drug delivery, Eur. J. Pharm. Biopharm., 2018, 128, 220–229 CrossRef CAS
.
- Q. Zhu, H. Cheng, Y. Huo and S. Mao, Sustained ophthalmic delivery of highly soluble drug using pH-triggered inner layer-embedded contact lens, Int. J. Pharm., 2018, 544(1), 100–111 CrossRef CAS
.
- Q. Zhu, Y. Wei, C. Li and S. Mao, Inner layer-embedded contact lenses for ion-triggered
controlled drug delivery, Mater. Sci. Eng. C, 2018, 93, 36–48 CrossRef CAS
.
- A. S. Carreira, P. Ferreira, M. P. Ribeiro, T. R. Correia, P. Coutinho, I. J. Correia and M. H. Gil, New drug-eluting lenses to be applied as bandages after keratoprosthesis implantation, Int. J. Pharm., 2014, 477(1–2), 218–226 CrossRef CAS
.
- L. Jones, M. Senchyna, M. A. Glasier, J. Schickler, I. Forbes, D. Louie and C. May, Lysozyme and lipid deposition on silicone hydrogel contact lens materials, Eye Contact Lens, 2003, 29(1), S75–S79 CrossRef
.
- J. Talbot, G. Tarjus, P. R. Van Tassel and P. Viot, From car parking to protein adsorption: an overview of sequential adsorption processes, Colloids Surf., A, 2000, 165(1–3), 287–324 CrossRef CAS
.
- X. F. Xiao, X. Q. Jiang and L. J. Zhou, Surface modification of poly ethylene glycol to resist nonspecific adsorption of proteins, Chin. J. Anal. Chem., 2013, 3(41), 445–453 Search PubMed
.
- K. Lorenz, R. Steffen, K. McCabe and L. Copper inventors Johnson, Johnson Vision Care Incassignee, Method for the mitigation of symptoms of contact lens related dry eye, US Pat. 11/347973, 2007
.
- L. Cheng, S. J. Muller and C. J. Radke, Wettability of silicone-hydrogel contact lenses in the presence of tear-film components, Curr. Eye Res., 2004, 28(2), 93–108 CrossRef
.
- P. L. Valint Jr, G. L. Grobe, D. M. Ammon Jr and M. J. Moorehead inventors Bausch, Lomb Incassignee, Plasma surface treatment of silicone hydrogel contact lenses, US Pat. 6193369, 2001
.
- T. Sato, R. Uchida, H. Tanigawa, K. Uno and A. Murakami, Application of polymer gels containing side-chain phosphate groups to drug-delivery contact lenses, J. Appl. Polym. Sci., 2005, 98(2), 731–735 CrossRef CAS
.
- J. C. Ruiz, C. Alvarez-Lorenzo, P. Taboada, G. Burillo, E. Bucio, K. De Prijck, H. J. Nelis, T. Coenye and A. Concheiro, Polypropylene grafted with smart polymers (PNIPAAm/PAAc) for loading and controlled release of vancomycin, Eur. J. Pharm. Biopharm., 2008, 70(2), 467–477 CrossRef CAS
.
- S. M. Mathews, J. E. Spallholz, M. J. Grimson, R. R. Dubielzig, T. Gray and T. W. Reid, Prevention of bacterial colonization of contact lenses with covalently attached selenium and effects on the rabbit cornea, Cornea, 2006, 25(7), 806–814 CrossRef
.
- M. D. Willcox, E. B. Hume, A. K. Vijay and R. Petcavich, Ability of silver-impregnated contact lenses to control microbial growth and colonisation, J. Optom., 2010, 3(3), 143–148 CrossRef
.
- M. D. Willcox, E. B. Hume, Y. Aliwarga, N. Kumar and N. Cole, A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses, J. Appl. Microbiol., 2008, 105(6), 1817–1825 CrossRef CAS
.
- C. R. Arciola, L. Montanaro, R. Caramazza, V. Sassoli and D. Cavedagna, Inhibition of bacterial adherence to a high-water-content polymer by a water-soluble, nonsteroidal, anti-inflammatory drug, J. Biomed. Mater. Res., 1998, 42(1), 1–5 CrossRef CAS
.
- B. M. Bandara, P. R. Sankaridurg and M. D. Willcox, Non-steroidal anti inflammatory agents decrease bacterial colonisation of contact lenses and prevent adhesion to human corneal epithelial cells, Curr. Eye Res., 2004, 29(4–5), 245–251 CrossRef CAS
.
- L. Selan, S. Palma, G. L. Scoarughi, R. Papa, R. Veeh, D. Di Clemente and M. Artini, Phosphorylcholine impairs susceptibility to biofilm formation of hydrogel contact lenses, Am. J. Ophthalmol., 2009, 147(1), 134–139 CrossRef CAS
.
- H. U. Zhu, A. Kumar, J. Ozkan, R. Bandara, A. Ding, I. Perera, P. Steinberg, N. Kumar, W. Lao, S. S. Griesser and L. Britcher, Fimbrolide-coated antimicrobial lenses: their in vitro and in vivo effects, Optom. Vis. Sci., 2008, 85(5), 292–300 CrossRef
.
- H. Thissen, T. Gengenbach, R. du Toit, D. F. Sweeney, P. Kingshott, H. J. Griesser and L. Meagher, Clinical observations of biofouling on PEO coated silicone hydrogel contact lenses, Biomaterials, 2010, 31(21), 5510–5519 CrossRef CAS
.
- D. Dutta, B. Kamphuis, B. Ozcelik, H. Thissen, R. Pinarbasi, N. Kumar and M. D. Willcox, Development of silicone hydrogel antimicrobial contact lenses with Mel4 peptide coating, Optom. Vis. Sci., 2018, 95(10), 937–946 CrossRef
.
- N. Cole, E. B. Hume, A. K. Vijay, P. Sankaridurg, N. Kumar and M. D. Willcox, In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU, Investig. Ophthalmol. Vis. Sci., 2010, 51(1), 390–395 CrossRef
.
- D. Dutta, N. Cole, N. Kumar and M. D. Willcox, Broad spectrum antimicrobial activity of melimine covalently bound to contact lenses, Investig. Ophthalmol. Vis. Sci., 2013, 54(1), 175–182 CrossRef CAS
.
- R. Tuby, S. Gutfreund, I. Perelshtein, G. Mircus, M. Ehrenberg, M. Mimouni, A. Gedanken and I. Bahar, Fabrication of a Stable and Efficient Antibacterial Nanocoating of Zn-CuO on Contact Lenses, ChemNanoMat, 2016, 2(6), 547–551 CrossRef CAS
.
- F. A. Maulvi, M. A. Mangukiya, P. A. Patel, R. J. Vaidya, A. R. Koli, K. M. Ranch and D. O. Shah, Extended release of ketotifen from silica shell nanoparticle-laden hydrogel contact lenses: in vitro and in vivo evaluation, J. Mater. Sci.: Mater. Med., 2016, 27(6), 113 CrossRef
.
- J. Van Meerloo, G. J. Kaspers and J. Cloos, Cell sensitivity assays: the MTT assay, in Cancer cell culture, Humana Press, 2011, pp. 237–245 Search PubMed
.
- J. B. Ciolino, C. F. Stefanescu, A. E. Ross, B. Salvador-Culla, P. Cortez, E. M. Ford, K. A. Wymbs, S. L. Sprague, D. R. Mascoop, S. S. Rudina and S. A. Trauger, In vivo performance of a drug-eluting contact lens to treat glaucoma for a month, Biomaterials, 2014, 35(1), 432–439 CrossRef CAS
.
- A. Danion, C. J. Doillon, C. J. Giasson, S. Djouahra, P. Sauvageau, R. Paradis and P. Vermette, Biocompatibility and light transmission of liposomal lenses, Optom. Vis. Sci., 2007, 84(10), 954–961 CrossRef
.
- X. Liu, D. P. Rodeheaver, J. C. White, A. M. Wright, L. M. Walker, F. Zhang and S. Shannon, A comparison of in vitro cytotoxicity assays in medical device regulatory studies, Regul. Toxicol. Pharmacol., 2018, 97, 24–32 CrossRef CAS
.
- A. Bruinink and R. Luginbuehl, Evaluation of biocompatibility using in vitro methods: interpretation and limitations, in Tissue Engineering III: Cell-Surface Interactions for Tissue Culture, Springer, Berlin, Heidelberg, 2011, pp. 117–152 Search PubMed
.
- N. P. Singh, M. T. McCoy, R. R. Tice and E. L. Schneider, A simple technique for quantitation of low levels of DNA damage in individual cells, Exp. Cell Res., 1988, 175(1), 184–191 CrossRef CAS
.
- F. Coelho, G. V. do Vale Braido, M. Cavicchioli, L. S. Mendes, S. S. Specian, L. P. Franchi, S. J. Ribeiro, Y. Messaddeq, R. M. Scarel-Caminaga and T. S. Capote, Toxicity of therapeutic contact lenses based on bacterial cellulose with coatings to provide transparency, Contact Lens Anterior Eye, 2019, 42(5), 512–519 CrossRef
.
- C. H. Tsai, P. Y. Wang, I. Lin, H. Huang, G. S. Liu and C. L. Tseng, Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application, Int. J. Mol. Sci., 2018, 19(9), 2830 CrossRef
.
| This journal is © The Royal Society of Chemistry 2020 |