Open Access Article
Open Access ArticleChanges of physicochemical properties, oxidative stability and cellular anti-inflammatory potentials for sea-buckthorn pulp oils during refining
Xiaofei Jianga,
Wei Liab,
Shengmin Zhou *a and
Yuanrong Jianga
*a and
Yuanrong Jianga
aWilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd, No. 118 Gaodong Road, Shanghai 200137, P. R. China. E-mail: zhoushengmin@cn.wilmar-intl.com; Fax: +86 21 58481079; Tel: +86 21 31153015
bUniversity of Shanghai for Science and Technology, School of Medical Instrument & Food Engineering, Shanghai 200093, P. R. China
First published on 6th October 2020
Abstract
The impact of the refining process on physicochemical properties, oxidative stability and cellular anti-inflammatory potentials of sea-buckthorn pulp oil (SBO) was investigated in this study. The results showed that acid and peroxide values of the tested SBOs decreased significantly after the refining process, while oxidative stability index (OSI) and anti-inflammatory potentials, measured as reduction in cellular inflammatory cytokine production, increased significantly. Interestingly, bleaching caused an unexpected increase in tocopherols as well as the greatest reduction in polycyclic aromatic hydrocarbons (PAHs). According to correlation analyses, tocopherol concentrations were significantly and positively correlated with OSI values and cellular anti-inflammatory potentials, while PHAs were negatively correlated with these factors. In general, refining is an effective way to improve the oxidative stability and anti-inflammatory capacity of SBO.
1. Introduction
Sea buckthorn (Hippophaë rhamnoides L., SB) is a hardy, deciduous, fast-growing spiny shrub with orange or yellow fleshy, juicy berries.1 It can grow in extreme conditions like droughts, high salinity and low or high temperatures. Therefore, SB is cultivated in Asia and Northern Europe because of its excellent abilities for water and soil conservation, and for land reclamation based on its nitrogen-fixing root nodules.2Sea buckthorn pulp oil (SBO), a nutritive oil product extracted from the pulp of SB berries, has been reported to contain a broad range of functional fatty acids such as palmitoleic acid (C16:1 ω-7), oleic acid (C18:1 ω-9), linoleic acid (C18:2 ω-6) and linolenic acid (C18:3 ω-3).3 More importantly, SBO is an edible oil that is remarkedly rich in natural micronutrients such as tocopherols, phytosterols and carotenoids.4 Previous studies have been reported that these micronutrients have anti-oxidative, antimicrobial, anti-aging, anti-cancer and anti-inflammatory properties benefiting human health.5–7 In addition, SBO or individual substances seperated from SB berries have also shown positive effects on symptoms and diseases such as acute alcohol intoxication,8 atherosclerosis,9 depression,10 burn wounds,11 and dry eyes.12
Due to these outstanding characteristics, SBO has attracted more and more attention in recent years. Most researchers focused on the effects of different oil extraction methods on the quality and micronutrient content of SBO.13 Compared with conventional (solvent, pressing and expelling) extraction methods, supercritical and subcritical extraction technologies can be considered as alternative ways to enhance the quality and micronutrients of SBO.2,14 However, few studies have focused on the changes of physicochemical properties in SBO during the subsequent processing steps after its extraction from the berry pulp.
In fact, crude SBO also contains certain amounts of undesirable minor components, i.e., free fatty acid (FFAs) and polycyclic aromatic hydrocarbons (PAHs), which can adversely affect oil quality and physicochemical properties. Refining is an effective way to eliminate these undesirable components in crude oils. However, micronutrients such as phytosterols and tocopherols can also be destroyed or removed due to the high temperature and/or chemical reagents used in the refining process.15
Considering the effect of refining on the quality and safety of oil products, the present work is aimed (i) to make a comprehensive comparison of physicochemical properties, oxidative stability and cellular anti-inflammatory potentials of SBOs obtained during the refining steps; (ii) to evaluate the relationship between physicochemical properties, oxidative stability and anti-inflammatory potentials of these different SBO samples. In brief, this study provides a quality assessment and comparison of important characteristics of SBOs obtained from different steps in refining. It can provide the academic foundation for the usage of SBO as a food ingredient.
2. Materials and methods
2.1 Materials and reagents
Crude SBO was extracted from frozen dried sea-buckthorn pulp by cold pressing. It was provided by Aikang Sea Buckthorn Pharmaceutical Co., Ltd (Xian, Shanxi, China). The neutralized, bleached and deodorized SBOs were prepared in laboratory, and the detailed procedures are listed in Section 2.2.Standards of 5α-cholestan-3β-ol, tocopherols (α-, β-, γ- and δ-tocopherols), phytosterols (campesterol, β-sitosterol, and 7-stigmasterol), and β-carotene were purchased from Sigma-Aldrich (Shanghai, China). 3-Monochloropropane-1,2-diol (3-MCPD) and 3-MCPD-d5 were purchased from CDN Isotopes Inc (Pointe-Claire, Canada). A standard mixture of PAHs was purchased from AccuStandard (New Haven, USA). BSTFA + TMCS (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for derivatization was obtained from Sigma-Aldrich (Shanghai, China). The HPLC grade solvents, such as tetrahydrofuran, acetone, n-hexane, toluene, isopropanol, dichloromethane, acetonitrile, chloroform, were provided by CNW (Darmstadt, Germany). All other reagents and solvents were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
1) for derivatization was obtained from Sigma-Aldrich (Shanghai, China). The HPLC grade solvents, such as tetrahydrofuran, acetone, n-hexane, toluene, isopropanol, dichloromethane, acetonitrile, chloroform, were provided by CNW (Darmstadt, Germany). All other reagents and solvents were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China).
2.2 Oil refining process
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 1 min. After that, the mixture was stirred (500 rpm) at 70 °C for acid treatment for 60 min. Then the mixture was centrifuged at 10
000 rpm) for 1 min. After that, the mixture was stirred (500 rpm) at 70 °C for acid treatment for 60 min. Then the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min (HITACHI, model CR21G). The supernatant was collected and named degummed SBO.
000 rpm for 10 min (HITACHI, model CR21G). The supernatant was collected and named degummed SBO.The degummed SBO was added with sodium hydroxide solution (15% concentration) and mixed at 88 °C. After being stirred (250 rpm) at this temperature for 10 min, the mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Then the supernatant was collected, and it was washed three times with hot distilled water, and dried at 105 °C under vacuum to obtain neutralized SBO.
000 rpm for 10 min. Then the supernatant was collected, and it was washed three times with hot distilled water, and dried at 105 °C under vacuum to obtain neutralized SBO.
2.3 Determination of oil quality indices
The acid value (AV) and peroxide value (PV) of the oil samples were determined according to the Chinese National Standard method GB 5009.229-2016 and GB 5009.227-2016, respectively.2.4 Determination of micronutrients
The tocopherol contents (α,β-, γ-, δ-tocopherols) of the oil samples were determined based on the AOCS Official Method Ce 8-89. They were analyzed using a Agilent high-performance liquid chromatography (HPLC) system with a UV detector (292 nm) and a silica column (4.6 mm × 250 mm, 5 μm, Waters Sepherisorb Silica). The tocopherols were identified by comparing with the corresponding standards and quantified using the standard curves of corresponding tocopherols.The phytosterols contents were determined based on the method of Xie et al.15 The oil samples were added with internal standard (1 mg mL−1 5α-cholestan-3β-ol in hexane). Then, 5 mL of KOH–ethanol (2 mol L−1) was added for saponification at 60 °C for 1 h. After that, 4 mL of H2O was added and then 5 mL hexane was added to extract the unsaponifiable matter for three times. Afterwards, 1.5 g of sodium sulfate anhydrous was added. All the organic phases were combined and evaporated. Afterwards, 0.2 mL (BSTFA + TMCS) was added and silylated at 105 °C for 20 min, and the resulting product was redissolved in 1 mL hexane before analysis. The phytosterols were analyzed using a Thermo Scientific GC spectrometry (GC) equipped with a DB-5 capillary column (0.32 mm × 30 m, 0.25 μm, Agilent Corp.). The phytosterols (campesterol, β-sitosterol, and 7-stigmasterol) were identified with comparison of corresponding standards, and quantified based on the internal standard.
The squalene content was determined based on the method of Chiou et al.17 After the sample was saponified and derivatized. The squalene content was analyzed using a Thermo Scientific GC spectrometry (GC) equipped with a HP-5 capillary column (0.32 mm × 30 m, 0.25 μm, Agilent Corp.). The squalene was identified by comparing with the squalene standard (Merk, Life Science) and quantified using the standard curves of squalene.
The β-carotene content in the oil samples was determined based on the AOCS Recommended Method Ce 9-01 using a Agilent high-performance liquid chromatography (HPLC) system with a UV detector (45 nm) and a C30 column (4.6 mm × 150 mm, 5 μm, YMC-Carotenoid, Japan). The oil is saponified and then extracted with petroleum ether. The extract is washed, concentrated, and then dissolved in methylene chloride for HPLC analysis. The β-carotene was identified by comparing with the β-carotene standard and quantified using the standard curves of β-carotene.
2.5 Determination of hazardous substances
Four different kinds of PAHs including benzo(a)anthracene (B(a)A), chrysene (CHR), benzo(b)fluoranthene (B(b)F) and benzo(a)pyrene (B(a)P) were determined based on ISO 15753-2006 Method.The 3-chloropropane-1,2-diol (3-MCPD) esters and 2,3-epoxy-propane-1-ol (glycidol) esters in the oils were determined according to the AOCS Official Method Cd 29a-13 and Cd 29c-13.
2.6 Determination of oxidative stability
The oxidative stability index (OSI) of the SBO samples were analyzed with a Metrohm 743 Rancimat equipment (Herisau, Switzerland). Samples of 3.0 g were placed in a heating block with a constant airflow of 20 L h−1 at 120 °C.2.7 Evaluation of anti-inflammatory potential
2.8 Statistical analysis
Data are reported as mean ± standard deviation of at least two replicates. Statistical analysis was done using software SPSS (Version 19.0). Significant differences (P ≤ 0.05) were evaluated by One-way Variance (ANOVA) combined with Tukey's adjustment test. The correlations between the physicochemical properties, oxidative stability and cellular anti-inflammatory capacity of the SBO samples obtained from different refining steps were evaluated using a Pearson correlation test.3. Results and discussion
3.1 Tocopherols
Table 1 shows the concentrations of individual and total tocopherols in SBOs obtained from each refining step. The total tocopherols in crude, neutralized, bleached and deodorized SBOs were 889.80 ± 62.37, 982.38 ± 14.87, 1228.30 ± 34.51, and 1198.79 ± 63.64 mg kg−1, respectively. These concentrations of total tocopherols were in the range reported by other researchers.19 It has been reported that the compositions and concentrations of tocopherols contained in SBOs can be quite different based on the difference in cultivars, harvest time, geographic location, and oil extraction methods.2,19,20 In general, α-tocopherol was the predominant tocopherol detected in the SBO, which was consistent with our findings. However, the compositions and concentrations of other tocopherols could vary greatly.| Crude | Neutralized | Bleached | Deodorized | |
|---|---|---|---|---|
| a The letters a, b, and c represent the differences among crude, neutralized, bleached and deodorized oils: the same letter indicates no significant difference (P > 0.05), different letters indicate a significant difference (P < 0.05). ND: not detected. | ||||
| Total tocopherols (mg kg−1) | 889.80 ± 62.37a | 982.38 ± 14.87a | 1228.30 ± 34.51b | 1198.79 ± 63.64b |
| α-Tocopherol | 848.05 ± 67.67a | 868.42 ± 11.27a | 1113.67 ± 43.22b | 1112.08 ± 71.29b |
| β-Tocopherol | NDa | 44.95 ± 3.88b | 55.70 ± 7.34b | 40.66 ± 5.46b |
| γ-Tocopherol | ND | ND | ND | ND |
| δ-Tocopherol | 41.75 ± 5.30a | 69.01 ± 7.48b | 51.74 ± 8.71b | 46.05 ± 2.19b |
| Total phytosterols (mg kg−1) | 8560.87 ± 67.39a | 9981.95 ± 115.05c | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258.89 ± 96.23c 258.89 ± 96.23c |
9493.97 ± 79.23b |
| Campesterol | 206.99 ± 9.34b | 227.86 ± 11.10b | 230.43 ± 28.89b | 175.82 ± 19.54a |
| β-Sitosterol | 6597.78 ± 31.42a | 7211.66 ± 69.39c | 7311.58 ± 44.66c | 6783.84 ± 12.49b |
| 7-Stigmasterol | 1756.09 ± 26.64a | 2542.43 ± 34.54b | 2716.87 ± 65.49b | 2534.32 ± 47.19b |
| Squalene (mg kg−1) | 18.95 ± 0.53ab | 21.01 ± 1.14ab | 23.57 ± 1.99b | 17.00 ± 1.10a |
| β-Carotene (mg kg−1) | 153.99 ± 4.97a | 144.15 ± 6.43a | 43.08 ± 1.81b | NDc |
Compared with crude SBO, β-tocopherol was newly generated in the neutralized SBO (Table 1). We speculate that the alkaline solution used in the neutralization step might cause the release of β-tocopherol from the dimeric or other esterified compounds. After the bleaching step, the total tocopherols increased significantly from 982.38 ± 14.87 mg kg−1 (in neutralized SBO) to 1228.30 ± 34.51 mg kg−1 (in bleached SBO) (P < 0.05), while insignificant reduction (P > 0.05) was found after the deodorization step (1198.79 ± 63.64 mg kg−1 in deodorized SBO). These findings were quite different from previous studies of different oils, showing that total tocopherols were mainly reduced in the neutralization and deodorization steps. Some researchers revealed that the concentration of total tocopherols in refined rice bran oil was lower than or similar to that in crude rice bran oil.21 Other researchers reported that a 31% loss of tocopherols was observed in soybean oil during the whole refining process.22 Similar observations on total loss of tocopherols during refining have also been reported in peanut oil.23
However, this result was in agreement with the result of Chew et al.24 who found that tocopherol contents were increased in the degummed and bleached kenaf seed oils. Rossi et al.25 also reported that the tocopherol contents were increased in bleached palm oils. They explained that the acidity of the bleaching earth used in the bleaching step might cause the release of tocopherols from the linked forms, and tocopherols in free form were regenerated from the dimeric or other esterified compounds. The difference in the tocopherol behaviors during the refining process might be related to both the natural characteristics of the crude oils and the different experimental conditions used in the refining process.26
3.2 Phytosterols
Table 1 shows the concentrations of individual and total phytosterols in SBOs obtained from each refining step. The main phytosterol present in SBO was β-sitosterol. Compared with crude SBO, phytosterols increased by 10.90% after the whole refining process (Table 1). The dynamic of changes in phytosterols concentrations in different SBOs obtained during refining was quite different from the dynamic observed in other edible oils during refining. Previous studies reported that depending on different refining conditions, a range of 10–70% decrease of total phytosterols was found in different vegetable oils during refining.22 The highest loss of phytosterols was observed in the neutralization step.15,24In this study, increase of phytosterols was observed in SBO after the neutralization step. At present, we can not fully explain why total phytosterols in neutralized SBO were significantly increased. However, Verleyen et al.27 also observed an increase of total phytosterols in acid-degummed palm oil compared to crude palm oil. We speculate that the increase of total phytosterols in neutralized SBO might be related to the natural characteristics of crude SBO, the citric acid used in the degumming process, and the reduction of free fatty acids in SBO (the proportion of phytosterols adsorbed by the soap is less than the proportion of free fatty acids removed from crude SBO). However, more research needs to be done in this field.
In our study, phytosterols contents were not significantly changed after the bleaching step (P > 0.05), while a significant decrease of total phytosterols (P < 0.05) was found after the deodorization step.
3.3 Squalene
The squalene content in SBO (Table 1) was much lower than reported for other edible oils such as olive, sunflower, soybean and rapeseed oils.28 In our study, compared with crude SBO, insignificant reductions (P > 0.05) of squalene were found after the neutralization and bleaching steps. Compared with bleached SBO, a significant reduction of squalene (P < 0.05) was found after the deodorization step. This result is in agreement with other studies where squalene in edible oils was lost the most during deodorization, probably due to the high temperature used in deodorization resulting in the degradation and/or evaporation of squalene.293.4 β-Carotene
Previous studies revealed that carotenoids mainly present in the soft parts of sea-buckthorn berries, giving them beautiful yellow-orange color.30 It has been reported that total carotenoids in the sea-buckthorn pulp oils were 692–3420 mg kg−1.19Table 1 shows the changes in β-carotene contents during refining. Most of the β-carotene (72.02%) was removed during the bleaching step, and the deodorization step further reduced β-carotene levels. Rossi et al.25 reported the impact of bleaching on the concentraion of carotenoids in palm oil, and the results showed that the removal efficiency of carotenoids was affected by the concentration, activity and types of clays and synthetic silica mixtures used in the bleaching step. Also, the high temperature and long-term reaction applied in the deodorization step could cause the degradation of β-carotene.
3.5 Polycyclic aromatic hydrocarbons (PAHs)
PAHs with four or more rings are called heavy PAHs. Compared to light PAHs, heavy PAHs are more toxic and stable. Table 2 exhibits the changes of four types of heavy PAHs: (B(a)A, CHR, B(b)F, and B(a)P) in SBOs during each step of refining. Compared with crude SBO, significant reduction of total PAHs (82.7%) was observed in deodorized SBO.| Crude | Neutralized | Bleached | Deodorized | |
|---|---|---|---|---|
| a The letters a and b represent the differences among crude, neutralized, bleached and deodorized oils: the same letter indicates no significant difference (P > 0.05), different letters indicate a significant difference (P < 0.05). | ||||
| Total PAHs (μg kg−1) | 260.90 ± 24.82a | 265.91 ± 32.53a | 30.78 ± 10.54b | 45.13 ± 5.90b |
| B(a)A | 59.43 ± 11.35a | 68.23 ± 10.99a | 10.26 ± 2.60b | 12.44 ± 1.24b |
| CHR | 78.48 ± 2.86a | 81.99 ± 5.97a | 14.96 ± 4.80b | 22.62 ± 2.09b |
| B(b)F | 85.25 ± 8.83a | 78.15 ± 9.97a | 3.95 ± 2.61b | 8.47 ± 2.02b |
| B(a)P | 37.75 ± 1.17a | 37.54 ± 5.59a | 1.63 ± 0.52b | 2.61 ± 0.52b |
| 3-MCPD esters (mg kg−1) | <0.10a | <0.10a | <0.10a | 21.56 ± 2.31b |
| Glycidol esters (mg kg−1) | <0.10a | <0.10a | <0.10a | 8.24 ± 1.05b |
It has been reported that the concentrations and types of PAHs contained in vegetable oils could vary greatly.31 The contamination of PHAs in vegetable oils was affected by solvent extraction, seed pretreatment, mineral oil residues, package material, and migration from contaminated soils or water. In our study, PHAs were mainly removed in the bleaching step, indicating that absorbents such as acid-activated bleaching earth and activated carbon used in this step could remove the PHAs efficiently. The neutralization and deodorization steps did not affect the contents of PAHs in SBOs significantly (P > 0.05).
3.6 3-MCPD and glycidol esters
Table 2 shows the contents of 3-MCPD and glycidol esters in SBOs during refining. Very low amounts of 3-MCPD and glycidol esters were found in the crude, neutralized and bleached SBOs. However, significant increases of 3-MCPD and glycidol esters (P < 0.05) were found after the deodorization step. These results indicated that the deodorization step played a leading role in the formation of 3-MCPD and glycidyl esters, while the influences of other refining steps could be neglected. The high temperature used in the deodorization step could cause the formation of 3-MCPD and glycidol esters in the deodorized SBO.323.7 Acid value and peroxide value
Acid value (AV) and peroxide value (PV) are the most basic and important parameters to assess the quality of edible oil. As shown in Table 3, after the whole refining process, the AV and PV of the SBOs decreased from 22.16 to 0.30 mg KOH/g, and from 3.04 to 0.71 mmol kg−1, respectively. The AV and PV of the deodorized SBO were both within the permitted levels according to the Codex Stan 210-1999 Codex Standard for named vegetable oils (≤0.6 mg KOH/g; ≤10.0 mmol kg−1).| Crude | Neutralized | Bleached | Deodorized | |
|---|---|---|---|---|
| a The letters a, b, c and d represent the differences among crude, neutralized, bleached and deodorized oils: the same letter indicates no significant difference (P > 0.05), different letters indicate a significant difference (P < 0.05). | ||||
| AV (mg KOH/g) | 22.16 ± 0.05a | 0.47 ± 0.03b | 0.76 ± 0.04c | 0.30 ± 0.01d |
| PV (mmol kg−1) | 3.04 ± 0.04a | 4.21 ± 0.01b | 0.96 ± 0.05c | 0.71 ± 0.03d |
| OSI (h) | 2.06 ± 0.13a | 5.94 ± 0.81b | 10.65 ± 1.20c | 9.60 ± 0.99c |
It has been reported that compared with other edible oils, the AV of crude SBO was quite high.33 Therefore, refining could be an effective way to decrease the AV of crude SBO.
3.8 Oxidative stability
The oxidative stability index (OSI) of SBOs during refining is shown in Table 3. Compared with crude SBO, the OSI values of the neutralized, bleached and deodorized SBOs were significantly higher (P < 0.05). These results are different from previous researchers who reported that other crude oils had a higher oxidative stability than refined oils, because more natural antioxidants (such as tocopherols and phospholipids) were contained in crude oils.15,20The difference in the oxidative stability behavior among SBO and other vegetable oils may be related to the natural characteristics of SBO. As shown in Table 3, the AV of crude SBO was relatively high (22.16 mg KOH/g), which could have a negative effect (r = −0.858; P < 0.05) on its oxidative stability (Table 4). In our study, after the whole refining process, more natural antioxidants (such as tocopherols and sterols), and fewer PAHs were found in the bleached and deodorized SBOs (Tables 1 and 2), which might be attributed to the improvement of the oxidative stability of SBO.
| Tocopherols | Phytosterols | Squalene | β-Carotene | PAHs | AV | OSI | TNF-α | IL-8 | |
|---|---|---|---|---|---|---|---|---|---|
| a *P < 0.05, **P < 0.01. | |||||||||
| Phytosterols | 0.598 | ||||||||
| Squalene | −0.023 | 0.576 | |||||||
| β-Carotene | −0.947** | −0.339 | 0.334 | ||||||
| PAHs | −0.963** | −0.374 | 0.146 | 0.970** | |||||
| AV | −0.738 | −0.910** | −0.185 | 0.585 | 0.533 | ||||
| OSI | 0.978** | 0.746 | 0.089 | −0.877** | −0.886** | −0.858* | |||
| TNF-α | −0.998** | −0.403 | 0.714 | 0.984** | 0.967** | 0.694 | −0.954** | ||
| IL-8 | −0.995** | −0.548 | 0.590 | 0.941** | 0.913** | 0.802 | −0.990** | 0.986** | |
| IL-1β | −0.999** | −0.421 | 0.700 | 0.980** | 0.962** | 0.708 | −0.960** | 1** | 0.989** |
3.9 Cell viability
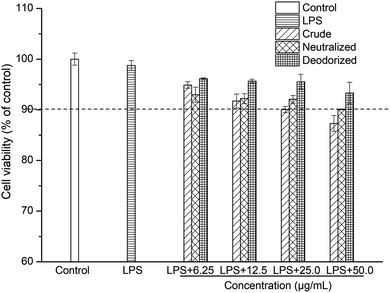
Caco-2 cells were exposed to LPS at a final concentration of 100 μg mL−1 for a 24 h incubation period to stimulate inflammation. At this concentration, LPS can induce Caco-2 cells to produce a pronounced inflammatory response, and at the same time the cell viability can be maintained above 98% (Fig. 1 and 2).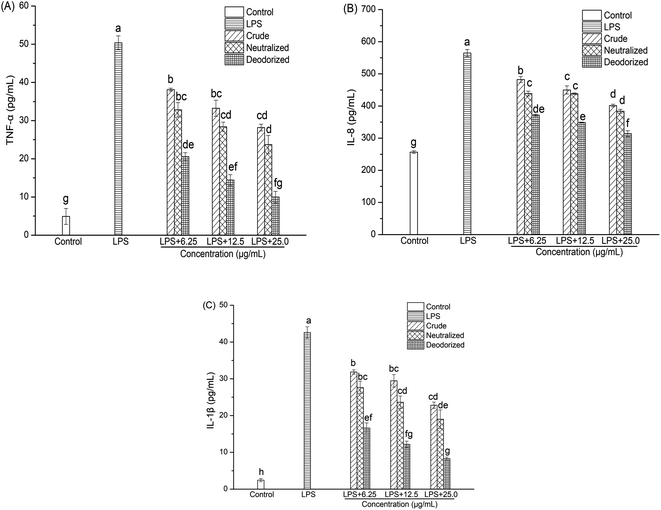 | ||
| Fig. 2 Effect of sea-buckthorn pulp oils on the LPS-induced production of proinflammatory cytokines in Caco-2 cells ((A): TNF-α; (B): IL-8; (C): IL-1β). | ||
The viability of the Caco-2 cells in the presence of crude, neutralized and deodorized SBOs at different concentrations are shown in Fig. 1. The viability of Caco-2 cells remained above 90% when treated with crude, neutralized and deodorized SBOs (up to a concentration of 25.0 μg mL−1). According to this result, concentrations of up to 25.0 μg mL−1 of SBOs were chosen for subsequent experiments.
3.10 Anti-inflammatory potentials
Productions of cytokines (TNF-α, IL-8 and IL-1β) in the culture medium of the Caco-2 cells were determined using ELISA kits. As shown in Fig. 2, the addition of LPS resulted in remarked increases in TNF-α, IL-8 and IL-1β levels. However, crude, neutralized, and deodorized SBOs could inhibit LPS-induced cytokine production in a dose-dependent manner after treatment for 24 h. As the concentrations of SBOs increased, the ability to inhibit the productions of TNF-α, IL-8 and IL-1β also improved. It should be noted that inflammatory cytokines in the deodorized SBO were significantly lower than those in the crude oil (P < 0.05), which indicated that refining could be an effective way to enhance the anti-inflammatory potential of SBO.3.11 Correlations between physicochemical properties, oxidative stability, and cellular anti-inflammatory capacity
Correlations between cytokine (TNF-α, IL-8, and IL-1β) concentrations, OSI, tocopherols, phytosterols, squalene, β-carotene, PAHs and AV in SBOs obtained at different steps of the refining was studied by regression analysis (Table 4).Noticeably, tocopherols had the highest degree of positive correlation with OSI (r = 0.978, P < 0.01), while β-carotene (r = −0.877, P < 0.01), PAHs (r = −0.886, P < 0.01) and AV (r = −0.858, P < 0.05) had negative correlations with OSI, suggesting that the increase of tocopherols and the reductions of β-carotene, PAHs and AV during refining directly affected the oxidative stability of SBO. Though β-carotene has been widely considered as a natural antioxidant in food industry, our study found that the reduction of β-carotene in the bleached and deodorized SBOs would not cause a decrease in oxidative stability, which indicated that the oxidative stability is affected by a comprehensive effect of multiple components contained in SBO.
All three cytokines (TNF-α, IL-8, and IL-1β) had significantly negative correlations with tocopherols (r = −0.999 to −0.995), and positive correlations with PHAs (r = 0.913–0.967), suggesting the increase of tocopherols and the reduction of PHAs during SBO refining could improve the anti-inflammatory capacity of SBO. It should be noted that very high negative correlations (r = −0.990 to −0.954) were found between OSI and the productions of cytokines, which indicates that improving the oxidative stability of SBO could be an effective way to improve the anti-inflammatory abilities of SBO.
In general, the oxidative stability and cellular anti-inflammatory capacity of SBO is affected by a wide-ranging effect of multiple components contained in SBO, and refining is an effective way to enhance the oxidative stability and anti-inflammatory potentials of SBO.
4. Conclusions
Compared with crude SBO, improvement of oxidative stability and cellular anti-inflammatory potentials were found in neutralized and deodorized SBOs obtained during the refining process. The data shows that OSI was highly related to the increase of tocopherols, as well as the reductions of PAHs and AV during the refining steps. Besides, the data also demonstrates that there was a high positive correlation between OSI and the cellular anti-inflammatory potentials of SBO.Moreover, it is noteworthy that bleaching caused an unexpected increase in tocopherols, which might be attributed to the acidity of the bleaching earth. It could cause the release of free tocopherol from the dimeric or esterified compounds. Meanwhile, bleaching caused the highest reduction of PHAs. The neutralization condition used in this study did not cause losses of tocopherols or phytosterols in SBO. On the other hand, the neutralization step could effectively reduce the AV of SBO, which had a positive effect on the oxidative stability of SBO. In general, appropriate refining is an effective way to enhance the oxidative stability and anti-inflammatory potentials of SBO.
Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This study was supported by the Shanghai Talent Development Foundation (No. 2018126) and Shanghai Rising-Star Program (No. 19QB1400200). The authors wish to thank Dr Joerg Jacoby for assistance in manuscript revision.References
- R. M. Pop, Y. Weesepoel, C. Socaciu, A. Pintea, J. P. Vincken and H. Gruppen, Food Chem., 2014, 147, 1–9 CrossRef CAS.
- L. Zheng, L. K. Shi, C. W. Zhao, Q. Z. Jin and X. G. Wang, LWT–Food Sci. Technol., 2017, 86, 507–513 CrossRef CAS.
- S. Czaplicki, D. Ogrodowska, R. Zadernowski and I. Konopka, Plant Foods Hum. Nutr., 2017, 72, 198–204 CrossRef CAS.
- S. Czaplicki, M. Tańska and I. Konopka, Ital. J. Food Sci., 2016, 28, 412–425 CAS.
- B. Olas, Food Chem. Toxicol., 2016, 97, 199–204 CrossRef CAS.
- L. M. Bal, V. Meda, S. N. Naik and S. Satya, Food Res. Int., 2011, 44, 1718–1727 CrossRef CAS.
- A. Zielińska and I. Nowak, Lipids Health Dis., 2017, 16, 95–105 CrossRef.
- D. C. Wen, X. Y. Hu, Y. Y. Wang, J. X. Luo, W. Lin, L. Y. Jia and X. Y. Gong, J. Ethnopharmacol., 2016, 192, 67–73 CrossRef CAS.
- M. Basu, R. Prasad, P. Jayamurthy, K. Pal, C. Arumughan and R. C. Sawhney, Phytomedicine, 2007, 14, 770–777 CrossRef CAS.
- J. S. Tian, C. C. Liu, H. Xiang, X. F. Zheng, G. J. Peng, X. Zhang, G. H. Du and X. M. Qin, Food Funct., 2015, 6, 3585–3592 RSC.
- H. Ito, S. Asmussen, D. L. Traber, R. A. Cox, H. K. Hawkins, R. Connelly, L. D. Traber, T. W. Walker, E. Malgerud, H. Sakurai and P. Enkhbaatar, Burns, 2014, 40, 511–519 CrossRef.
- P. S. Larmo, R. L. Järvinen, B. Yang and H. P. Kallio, in Handbook of Nutrition Diet & the Eye, 2014 Search PubMed.
- L. F. Gutiérrez, C. Ratti and K. Belkacemi, Food Chem., 2008, 106, 896–904 CrossRef.
- B. Yang, M. Ahotupa, P. Määttä and H. Kallio, Food Res. Int., 2011, 44, 2009–2017 CrossRef CAS.
- D. Xie, H. Zhou and X. Jiang, J. Food Meas. Charact., 2019 DOI:10.1007/s11694-019-00058-y.
- X. Jiang, M. Chang, Q. Jin and X. Wang, Process Biochem., 2015, 50, 432–437 CrossRef CAS.
- A. Chiou, N. Kalogeropoulos, F. N. Salta, P. Efstathiou and N. K. Andrikopoulos, LWT–Food Sci. Technol., 2009, 42, 1090–1097 CrossRef CAS.
- Y. Yan, Z. Wang, J. Greenwald, K. S. D. Kothapalli, H. G. Park, R. Liu, E. Mendralla, P. Lawrence, X. Wang and J. T. Brenna, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2017, 116, 27–31 CrossRef CAS.
- A. Ranjith, K. Sarin Kumar, V. V. Venugopalan, C. Arumughan, R. C. Sawhney and V. Singh, J. Am. Oil Chem. Soc., 2006, 83, 359–364 CrossRef CAS.
- R. Zadernowski, M. Naczk and R. Amarowicz, J. Am. Oil Chem. Soc., 2003, 80, 55–58 CrossRef CAS.
- V. R. Pestana-Bauer, R. C. Zambiazi, C. R. B. Mendonça, M. Beneito-Cambra and G. Ramis-Ramos, Food Chem., 2012, 134, 1479–1483 CrossRef CAS.
- M. Y. Jung, S. H. Yoon and D. B. Min, J. Am. Oil Chem. Soc., 1989, 66, 118–120 CrossRef.
- M. Zhu, X. Wen, J. Zhao, F. Liu, Y. Ni, L. Ma and J. Li, J. Am. Oil Chem. Soc., 2016, 93, 285–294 CrossRef CAS.
- S. C. Chew, C. P. Tan, K. Long and K. L. Nyam, Ind. Crops Prod., 2016, 89, 59–65 CrossRef CAS.
- M. Rossi, M. Gianazza, C. Alamprese and F. Stanga, J. Am. Oil Chem. Soc., 2001, 78, 1051–1055 CrossRef CAS.
- R. Liu, R. Liu, L. Shi, Z. Zhang, T. Zhang, M. Lu, M. Chang, Q. Jin and X. Wang, LWT–Food Sci. Technol., 2019, 109, 26–32 CrossRef CAS.
- T. Verleyen, U. Sosinska, S. Ioannidou, R. Verhe, K. Dewettinck, A. Huyghebaert and W. De Greyt, J. Am. Oil Chem. Soc., 2002, 79, 947–953 CrossRef CAS.
- C. Nergiz and D. Çelikkale, J. Food Sci. Technol., 2011, 48, 382–385 CrossRef CAS.
- F. Shahidi and U. N. Wanasundara, J. Food Lipids, 1999, 6, 159–172 CrossRef CAS.
- B. Yang and H. Kallio, Trends Food Sci. Technol., 2002, 160–167 CrossRef CAS.
- S. Moret and L. S. Conte, J. Chromatogr. A, 2000, 882, 245–253 CrossRef CAS.
- K. Hrncirik and G. van Duijn, Eur. J. Lipid Sci. Technol., 2011, 113, 374–379 CrossRef CAS.
- R. Kumar, G. Phani Kumar, O. P. Chaurasia and S. B. Singh, Res. J. Med. Plant, 2011, 5, 491–499 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |