Open Access Article
Open Access ArticleLow-temperature storage after harvest retards the deterioration in the sensory quality, health-promoting compounds, and antioxidant capacity of baby mustard†
Bo Sun‡
,
Pei-Xing Lin‡,
Ping-Xin Xia‡,
Hong-Mei Di,
Jia-Qi Zhang,
Chen-Lu Zhang and
Fen Zhang *
*
College of Horticulture, Sichuan Agricultural University, Chengdu 611130, China. E-mail: zhangf@sicau.edu.cn
First published on 5th October 2020
Abstract
Baby mustard is a perishable vegetable, and thus its distribution and sale as fresh produce face several challenges. However, little effort has been made to identify optimal techniques for postharvest storage of baby mustard. Here, we evaluated the sensory quality, health-promoting compounds, and antioxidant capacity of baby mustard during postharvest storage for 6 days at low temperature (4 °C, LT) and ambient temperature (20 °C). The results showed that visual quality scores, weight, firmness, the contents of most glucosinolates, and the ferric reducing antioxidant power value decreased in the lateral buds of baby mustard during both treatments; however, LT treatment delayed declines in these characteristics. In addition, the contents of glucose, fructose, total soluble sugars, ascorbic acid, and flavonoids, as well as the level of 2,2-azinobis(3-ethyl-benzothiazoline-6-sulfonic acid), decreased considerably throughout the storage period, sucrose content increased throughout the storage period, and the contents of proanthocyanidin and total phenolics first increased and then decreased at 20 °C; however, their contents remained stable throughout the storage period under the LT treatment. These findings indicate that LT provides a promising approach for maintaining the sensory and nutritional quality of baby mustard.
Introduction
Baby mustard (Brassica juncea var. gemmifera), a variant of stem mustard, is primarily distributed in southwest China. The heads of baby mustard are commonly consumed, while the lateral buds of the head are tender with a sweet and fragrant flavor and can be eaten cooked or pickled.1 Epidemiological studies have provided convincing evidence that the consumption of Brassica vegetables is associated with a reduced risk of various cancers and cardiovascular diseases. These health-promoting and anti-carcinogenic properties have been primarily attributed to the high contents of glucosinolates present in Brassica vegetables.2 Aside from glucosinolates, baby mustard contains other health-promoting secondary metabolites, such as carotenoids, ascorbic acid, and various phenolics.1The highly perishable nature of baby mustard poses a challenge for its distribution and sale as fresh produce. For example, extending the shelf life of fresh baby mustard for more than 3 days at ambient temperature (20 °C) postharvest is difficult because of the active metabolism of the lateral buds. Baby mustard, which remains alive after postharvest, shows various signs of deterioration, including the development of browning, dehydration, and the loss of health-promoting compounds. Therefore, the use of storage techniques that extends the postharvest lifespan and retains the health-promoting compounds of baby mustard is important for its postharvest preservation. Various postharvest approaches, including low temperature (LT) treatments,2–5 biochemical treatments,6 and light treatments,7 have been used to delay senescence, extend shelf life, and preserve quality in vegetables. LT treatment is one of the most commonly used strategies to maintain the freshness of vegetables for its ability to effectively retard the metabolic processes and extend the postharvest lifespan of vegetables. For example, Rao et al.4 suggested that the quality and shelf life of sweet pepper were enhanced at LT (10 °C) compared with ambient temperature (25 °C). Rybarczyk-Plonska et al.2 found that glucosinolate contents in broccoli flower buds under LTs (0 °C and 4 °C) were significantly higher relative to broccoli kept under ambient temperature (18 °C).
Baby mustard is usually harvested, stored, and sold at ambient temperature. However, little information exists on the effect of LT treatment on the sensory and nutritional qualities of baby mustard during storage. Here, we studied the effect of LT treatment on the sensory quality, the presence of health-promoting compounds, and the antioxidant capacity in baby mustard lateral buds during postharvest storage. Our findings contribute to the improvement of the postharvest quality of baby mustard.
Experimental
Chemicals and regents
Analytical grade of sodium hypochlorite, oxalic acid, Folin–Ciocalteu, sodium carbonate, gallic acid, sodium salt trihydrate, and 2,2-azinobis(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) were obtained from Sangon Biotech Co., Ltd. (shanghai, China). Analytical grade of ethanol, acetone, acetic acid, hydrochloric acid, aluminum trichloride, ferrous sulfate (FeSO4·7H2O), pyridine, and sodium hydroxide were purchased from Chengdu Kelong Chemical Co., Ltd. (Chengdu, China). The standards of chlorophylls (a and b), carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene), soluble sugars (glucose, fructose, and sucrose), authentic ascorbic acid, and quercetin were obtained from Solarbio Science & Technology Co., Ltd. (Beijing, China). High-performance liquid chromatography (HPLC) grade of p-dimethylaminocinnamaldehyde, 2,4,6-tris(2-pyridyl)-s-triazine, ortho-nitrophenyl β-D-galactopyranoside, sulfatase, and DEAE-sephadex A-25, as well as Procyanidin B2 standards were purchased from Sigma Chemical Co. (Saint Louis, USA). HPLC grade of isopropanol, acetonitrile, and methyl alcohol were purchased from Tedia Company, Inc. (Fairfield, USA).Plant materials
The heads of baby mustard (Brassica juncea var. gemmifera cv. Linjiang-Ercai) that were uniform in size, well-developed, and free of external damages were harvested from a local farm in Chengdu City, China. Healthy lateral buds, the main edible parts of baby mustard, were removed and washed in a sodium hypochlorite solution (50 mg kg−1) for 3 min, rinsed with tap water for 1 min, and then air-dried on botting paper for 30 min at room temperature. The lateral buds were randomly divided into two treatment groups.Storage treatments
The lateral buds of baby mustard were placed into transparent polypropylene containers and were stored under constant darkness (approximately 0.1 μmol m−2 s−1) and a relative humidity (RH) of 75% for 6 days. The lateral buds were subjected to two storage treatments: 20 °C (control) and 4 °C (LT). Samples were taken after 0, 2, 4, and 6 days. Six lateral buds were collected as a replicate, and four replicates in each treatment were performed per sampling period. Several fresh samples were used for analyses of visual quality, weight loss, firmness, and color, while other samples were lyophilized in a freeze dryer and stored at −20 °C for subsequent analyses of health-promoting compounds and antioxidant capacity.Quality assessment
Weight loss and firmness
Weight loss (%) was calculated by the formula (WX − W0)/W0 × 100, where W0 is the weight at 0 day, and WX is the weight at a certain day after storage. The firmness assay was made using a WDGY-4 fruit sclerometer (Beijing Jinyang Wanda Technology Co., Ltd., China) with a probe 3.5 mm in diameter and a penetration depth of 10 mm. Measurements were made on fleshy part of each lateral bud.For total phenolics assay, the supernatant was mixed with Folin–Ciocalteu reagent, after 3 min, saturated sodium carbonate was added. The absorbance was measured at 760 nm with the spectrophotometer as previously described.1 Gallic acid was used as a standard and the results were expressed as mg gallic acid equivalent g−1 dry weight.
Statistical analysis
To measure shelf life and visual quality, 12 replicates were prepared for each treatment. For weight loss and firmness assays, six replicates were prepared. Other assays were performed in quadruplicate. Statistical analysis was performed using the SPSS package program version 18. Data were analyzed using one-way ANOVAs. Principal component analysis (PCA) was performed in SIMCA-P 11.5 Demo software with unit variance scaling to assess relationships among samples.1 A time-related trajectory analysis based on a two-dimensional PCA map was used to visualize temporal changes in postharvest quality at 20 °C and 4 °C.13 The correlations were visualized using Cytoscape v. 3.5.1 software,1 and the data are shown in ESI Table 1.†Results
External aspect
After 2 days, the lateral buds of baby mustard stored at 20 °C showed symptoms of shriveling and browning on the peel, approaching the threshold beyond which they would not be saleable; the shriveling and browning of the control became more severe after 6 days. However, the external aspect of the lateral buds stored under LT showed only slight shriveling, and no browning was observed after 6 days (Fig. 1).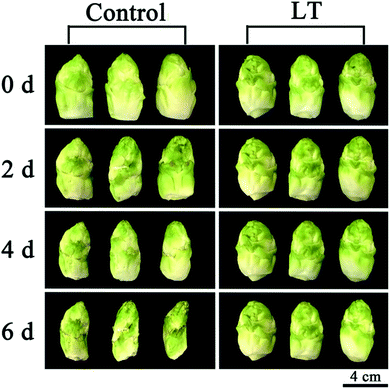 | ||
| Fig. 1 Visual external aspect of baby mustard lateral buds stored at room temperature (20 °C, control) and low temperature (4 °C, LT) under continuous darkness. | ||
Scores, weight loss, and firmness
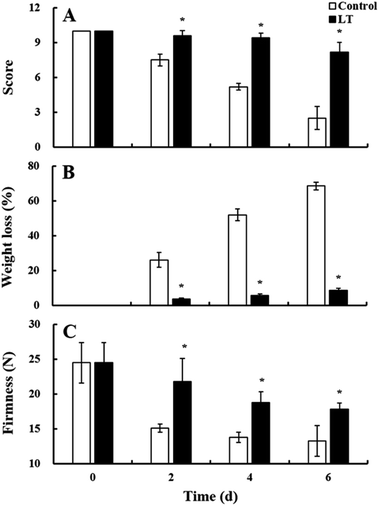
Visual quality scores obtained based on appearance are an important parameter for assessing the sensory quality of baby mustard. Visual quality scores of baby mustard gradually decreased in both treatments during storage, with the control showing lower values (Fig. 2A). LT treatment significantly inhibited the decline in score values. The score of the LT treatment in lateral buds (8.2) was higher than that of the control (2.5) on day 6.Weight loss is closely related to the sensory quality of baby mustard during storage. Weight loss was positively correlated with both temperature and storage time (Fig. 2B). Weight loss under the control was severe, and weight loss exceeded 70% on day 6. However, LT treatment significantly suppressed the increase in weight loss, as weight loss was less than 9% on day 6. Thus, LT treatment was effective in attenuating weight loss.
Firmness is an important parameter that reflects the texture of baby mustard. Firmness values decreased for both treatments during storage, and the rate of change for samples stored at LT was slower than those stored at 20 °C. On day 2, the firmness of the control decreased sharply from 24.48 N at the start of the experiment to 15.08 N, a reduction of nearly 40%. However, the firmness under LT treatment was still 17.85 N at the end of the storage period, which was significantly higher than that of the control (Fig. 2C), indicating that LT treatment can significantly delay the softening of the lateral buds in baby mustard.
Surface color, chlorophylls, and carotenoids
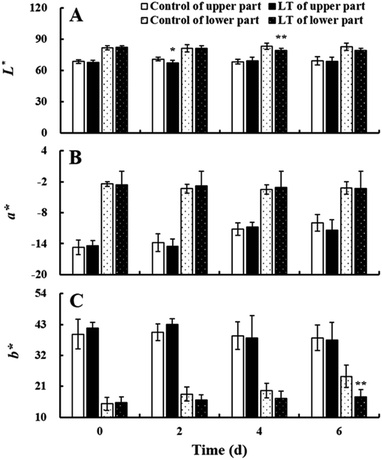
The baby mustard lateral buds can be divided into two parts: the light green upper part and the white lower part. L*, a*, and b* values of the upper and lower parts were basically stable under LT treatment and the control during the entire storage period, and there were no significant differences between the two treatments (Fig. 3). Trends in total and individual chlorophyll and carotenoid contents were similar to color values during storage (ESI Fig. 1†).Soluble sugars
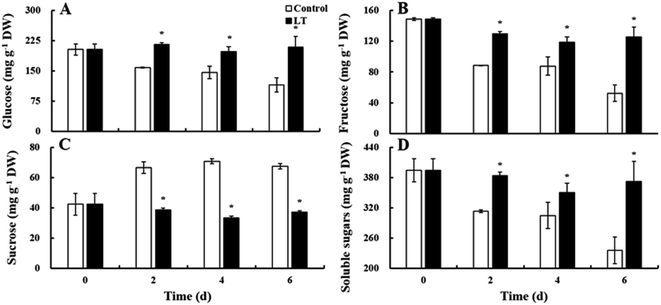
Three kinds of soluble sugars (glucose, fructose, and sucrose) were detected in the lateral buds of baby mustard. Glucose accounted for more than 50% of soluble sugars detected, followed by fructose and then sucrose (Fig. 4). The contents of glucose, fructose, and total soluble sugars gradually decreased in the control during the entire storage period. However, the contents of these sugars showed virtually no change in the LT treatment. Their contents in the LT treatment were significantly higher than those of the control: specifically, the levels of glucose, fructose, and total soluble sugars were 1.81-, 2.40-, and 1.58-fold higher, respectively, in the LT treatment compared with levels of these sugars observed in the control on day 6. Sucrose content gradually increased in the control throughout the storage period, and its overall increase at the end of the storage period was 59.1% higher than levels observed on day 0; in contrast, sucrose content remained relatively stable in the LT treatment (Fig. 4C).Ascorbic acid, proanthocyanidins, flavonoids, and total phenolics
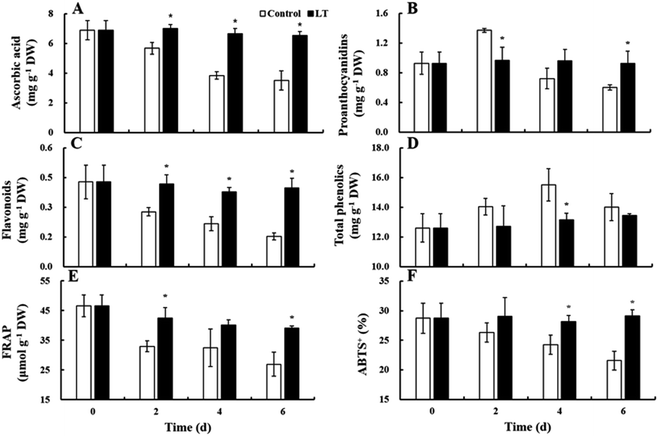
Ascorbic acid and flavonoid contents gradually decreased in the control during storage. However, ascorbic acid and flavonoid contents in the LT treatment remained virtually unchanged. In addition, their contents under the LT treatment were significantly higher than at 20 °C: specifically, the levels of ascorbic acid and flavonoids were 1.72- and 2.60-fold higher, respectively, in the LT treatment compared with levels of ascorbic acid and flavonoids observed in the control on day 6 (Fig. 5A and C). The contents of proanthocyanidin and total phenolics in the control increased during the first 2 and 4 days, respectively, and then decreased. However, the contents of proanthocyanidin and total phenolics remained stable in the LT treatment throughout storage (Fig. 5B and D).Antioxidant activity
The antioxidant capacity was investigated using both FRAP and ABTS. The levels of FRAP and ABTS both decreased continuously at 20 °C: their reductions at the end of storage were 42.26% and 24.98%, respectively, of the levels observed on day 0. However, FRAP levels under LT treatment slightly decreased throughout storage, whereas ABTS levels remained stable. The levels of FRAP and ABTS under LT treatment were 1.45- and 1.35-fold higher, respectively, than levels of FRAP and ABTS observed in the control on day 6 (Fig. 5E and F).Glucosinolates
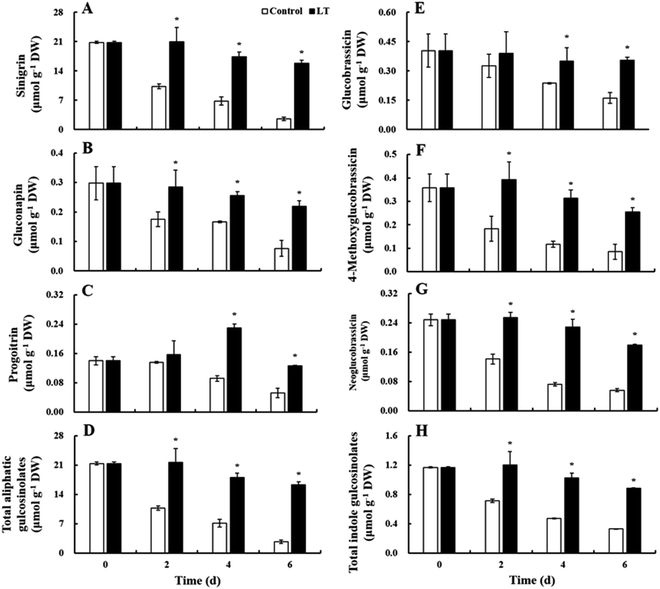
Three aliphatic glucosinolates (sinigrin, gluconapin, and progoitrin) and three indole glucosinolates (glucobrassicin, 4-methoxyglucobrassicin, and neoglucobrassicin) were identified and quantified as the predominant glucosinolates in baby mustard lateral buds; sinigrin content was the highest, accounting for 92.2% of total glucosinolates (Fig. 6). The contents of sinigrin, gluconapin, and total glucosinolates of the control continuously decreased during storage, including a sharp decrease within the first 2 days. In contrast, in the LT treatment, declines in the contents of these compounds were delayed and only slightly decreased throughout the storage period. Their contents under LT treatment were significantly higher than those of the control during storage: specifically, the contents of sinigrin, gluconapin, and total glucosinolates were 6.17-, 2.75-, and 5.96-fold higher, respectively, than the contents of these compounds observed in the control on day 6. The progoitrin content of the control decreased on day 4, whereas progoitrin content in the LT treatment quickly increased to its maximum value on day 4 and then declined thereafter. The individual and total indole glucosinolate contents of the control declined gradually throughout storage, while, in the LT treatment, indole glucosinolate contents remained basically unchanged early on and only slightly decreased on day 6. Total indole glucosinolate contents decreased by 71.8% in the control at the end of storage, whereas they only reduced by 24.8% under the LT treatment.A time-related trajectory analysis
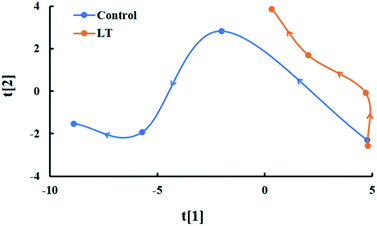
A time-related trajectory analysis was performed to compare the impacts of low temperature on the comprehensive quality of baby mustard during postharvest storage (Fig. 7). The different storage time and temperature of baby mustard were separated, and the greater the distance from the origin (day 0), the higher the degree of postharvest deterioration of lateral buds. The distance from the origin was positively correlated with both temperature and storage time, and the rate of change for samples stored at LT was slower than those stored at 20 °C. The distance from the origin under the LT treatment at day 6 was even shorter than that at 20 °C at day 2, and approximately half of that at 20 °C on day 6. Thus, postharvest deterioration was more pronounced for the control, and the deterioration was significantly retarded by LT treatment.Correlation analysis
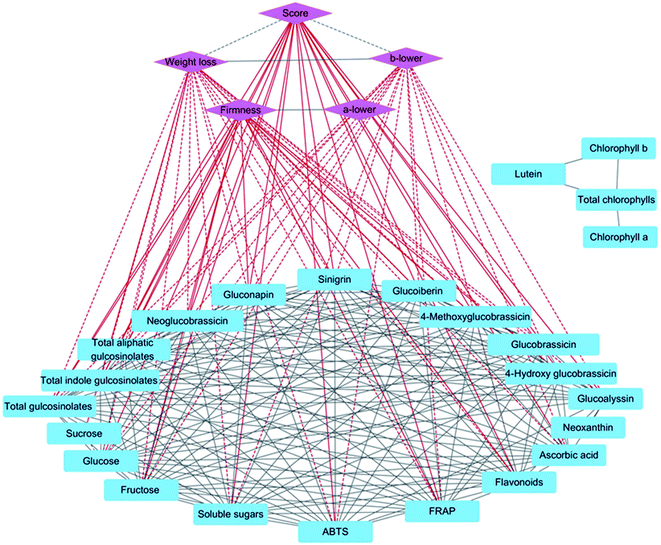
To investigate correlations between sensory and nutritional qualities, Pearson correlation coefficient values were filtered by the threshold R2 > 0.90, and a network analysis was conducted with five sensory quality indices, 24 nutritional quality indices, and 222 edges (Fig. 8). Score and firmness were positively correlated with fructose, flavonoids, FRAP, and most glucosinolates, whereas weight loss and the b* value of the lower part were negatively correlated with fructose, glucose, total soluble sugars, ascorbic acid, flavonoids, antioxidant capacity, and most glucosinolates. Moreover, there were significant positive correlations between fructose, glucose, total soluble sugars, ascorbic acid, flavonoids, antioxidant capacity, and most glucosinolates except for between ascorbic acid and FRAP.Discussion
Temperature plays a vital role in the postharvest storage of fruits and vegetables.14,15 Baby mustard is highly perishable given the rapidity with which it experiences shriveling, browning on the peel, and loss in nutritional quality after harvest. Moreover, the ambient temperature conditions during the storage and sale of baby mustard after harvest hasten the process of deterioration. LT (4 °C) treatment inhibited deterioration in appearance quality, scores and firmness values, and weight loss in the lateral buds of baby mustard, leading to better sensory quality than the control (20 °C) during storage (Fig. 1 and 2). In previous studies, Peng et al.3 found that LT (6 °C) treatment inhibited a decline in firmness values and an increase in weight loss in sweet potato, while Zhan et al.7 suggested that LT (4 and 7 °C) treatments were effective in maintaining the color, texture, odor, and acceptability of fresh-cut broccoli during storage. Similar results were also obtained for radish microgreens16 and sweet pepper.4 The superior sensory quality under LT treatment likely stems from the delay in the senescence of fresh produce as a result of decreased respiration rates, enzymatic processes, and losses in tissue turgidity.7Soluble sugars, especially glucose, fructose, and sucrose, are not only considered to be important indicators of the quality of vegetables but also play critical roles in plant structure as well as the metabolism of the cell and the entire organism.17 Glucose, fructose, and total soluble sugar contents gradually decreased and sucrose contents gradually increased in the control throughout storage. However, glucose, fructose, sucrose, and total soluble sugar contents under LT treatment remained stable throughout storage (Fig. 4), which may be due to LT treatment reduces respiration rates and other metabolic processes that delay the depletion of substrates, such as glucose and fructose.4 In addition, environmental stress during postharvest storage can increase sucrose content.18 Because LT may inhibit the environmental stress and activity of sucrose synthesis-related enzymes, sucrose content increased only in the control but remained stable in the LT treatment.
Brassica vegetables receive much attention for the antioxidants that they contain given that antioxidants can aid the prevention of certain types of cancer and cardiovascular diseases and delay the aging process.2 We studied changes in the contents of ascorbic acid, flavonoids, proanthocyanin, and total phenolics, as well as the antioxidant capacity in the baby mustard lateral buds, under control and LT conditions (Fig. 5). Glucose is a precursor in ascorbic acid synthesis,19 and the glucose content remained unchanged under LT treatment. Therefore, LT treatment effectively maintained the ascorbic acid content during storage (Fig. 5A). Similar results were also obtained for Dendrobium officinale20 and broccoli.21 Fruit and vegetables will produce mores secondary metabolites including phenolics to defend against stress in the postharvest.22 Total phenolics content first increased and then decreased at 20 °C during postharvest storage but remained stable under the LT treatment. Similar results also found that fresh-cut pitaya fruit at 5 °C did not show significant variation in total phenolics content; however, at 15 °C, the total phenolics content increased dramatically during the first 36 hours and then decreased,22 indicating that the stress that baby mustard suffered under LT treatment were less than that at 20 °C. The decrease in total phenolics content in the baby mustard lateral buds stored at 20 °C after 4 days of storage might be attributed to the acceleration of the degradation of total phenolics at 20 °C, as the utilization and degradation rates were likely higher than the rate of biosynthesis of phenolic compounds during later stages of storage.22 In addition, browning is related to the oxidation of phenolics,22,23 and the browning that was observed on the peel of the lateral buds during storage was consistent with this observation (Fig. 1).
Glucosinolates are a group of important health-promoting secondary metabolites in Brassica vegetables that contribute to taste and flavor.2,24 LT treatment retarded the decline in the contents of most individual and total glucosinolates in baby mustard during postharvest storage (Fig. 6). This finding is consistent with that of Rybarczyk-Plonska et al.,2 who found that glucosinolate levels were also preserved under LT treatment in broccoli. Glucosinolate levels in postharvest vegetables are affected by both hydrolysis and biosynthesis.25 LT significantly delayed declines in firmness and increases in weight loss in baby mustard, suggesting that cellular integrity was enhanced and less cell damage occurred under the LT treatment relative to the control. Thus, the burst of vacuoles and contact between glucosinolates and myrosinase were noticeably delayed under LT treatment during storage.24,25 In addition, temperature affects myrosinase activity,26 and LT may inhibit its activity. Therefore, LT treatment can reduce the effects of myrosinase hydrolysis. On the other hand, as a signaling molecule, glucose induces the accumulation of both aliphatic and indole glucosinolates.27,28 We found that LT treatment effectively maintained glucose content in baby mustard, suggesting that LT contributes to glucosinolate biosynthesis via the glucose signaling pathway during postharvest storage. In sum, LT treatment retarded declines in glucosinolate contents in baby mustard during postharvest storage, likely by both inhibiting myrosinase hydrolysis and promoting glucosinolate biosynthesis.
Generally, consumers cannot directly judge the nutrient contents of vegetables. In this study, score and firmness were positively correlated with most glucosinolates, flavonoids, and antioxidant capacity, while weight loss was negatively correlated with most glucosinolates, soluble sugars, several antioxidants, and antioxidant capacity (Fig. 8). Therefore, the close relationship between nutrient contents and sensory indicators of baby mustard can guide consumers in the purchase of products containing higher concentrations of health-promoting compounds.
Besides LT, RH also plays an important role in the postharvest storage of vegetables. Li et al.29 suggested that Low RH (75%) resulted in the visibly shrinkage of straw mushrooms, while 95% RH maintained better sensory quality, whereas Medina et al.30 found that short postharvest storage under low RH (72%) improves quality and shelf life of minimally processed baby spinach compared to those under 85% and 99% RH. In this study, we chose 75% RH to simulate the actual storage environment because the RH of cold storage is 70%∼80% in our local vegetable production companies and supermarkets. However, the effect of RH on postharvest storage of baby mustard is interesting, and it could be included in our future research.
The imperfection of cold chain facilities is the main obstacle to low-temperature storage of baby mustard. China is a developing country with uneven regional development. Baby mustard is mainly produced in Southwest China, which is an underdeveloped area with incomplete cold chain facilities. On the other hand, with the boost of the economy, the cold chain of post-harvested vegetables is gradually being improved, and this obstacle to low-temperature storage of baby mustard is gradually being resolved. In view of the significant effect of low temperature storage on the postharvest quality of baby mustard in this study, it is strongly believed that low temperature storage technology has a good application prospect in the postharvest of baby mustard.
Conclusions
In conclusion, the storage of baby mustard at LT (4 °C) for 6 days was superior for preserving its desirable characteristics compared with storage at 20 °C. LT effectively inhibited deterioration of the appearance of baby mustard and retarded the loss of health-promoting compounds. In detail, compared with storage at 20 °C, storage under LT conditions benefitted baby mustard by reducing weight loss and maintaining the score, firmness, antioxidant capacity, and contents of soluble sugars, ascorbic acid, flavonoids, total phenolics, and glucosinolates. In the future, we plan to study the effects of LT combined with other technologies, such as packaging and light, on the postharvest quality of baby mustard.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by National Natural Science Foundation of China (32072586, 31500247) and Undergraduate Research Interest Cultivation Project of Sichuan Agricultural University (2020296, 2020298).References
- B. Sun, Y. X. Tian, M. Jiang, Q. Yuan, Q. Chen, Y. Zhang, Y. Luo, F. Zhang and H. R. Tang, RSC Adv., 2018, 8, 33845–33854 RSC.
- A. Rybarczyk-Plonska, S. F. Hagenb, G. T. A. Borgeb, G. B. Bengtssonb, M. K. Hansena and A. B. Wolda, Postharvest Biol. Technol., 2016, 116, 16–25 CrossRef CAS.
- Y. Peng, Y. Q. Xu, D. F. Duan and L. C. Mao, Agric. Sci. China, 2009, 8, 244–248 CrossRef CAS.
- T. V. R. Rao, N. B. Gol and K. S. Khilana, Sci. Hortic., 2011, 132, 18–26 CrossRef CAS.
- F. Fratianni, M. Cefola, B. Pace, R. Cozzolino, B. De Giulio, A. Cozzolino, A. d'Acierno, R. Coppola, A. F. Logrieco and F. Nazzaro, Food Chem., 2017, 229, 752–760 CrossRef CAS.
- S. Akan, N. T. Gunes and R. Yanmaz, Food Chem., 2019, 270, 546–553 CrossRef CAS.
- L. J. Zhan, J. Q. Hu, Y. Li and L. Y. Pang, Postharvest Biol. Technol., 2012, 72, 76–81 CrossRef CAS.
- A. Martínez-Sánchez, J. A. Tudela, C. Luna, A. Allende and M. T. Gil, Postharvest Biol. Technol., 2011, 59, 34–42 CrossRef.
- Y. M. Shi, R. Wang, Z. P. Luo, L. F. Jin, P. P. Liu, Q. S. Chen, Z. F. Li, F. Li, C. Y. Wei, M. Z. Wu, P. Wei, H. Xie, L. B. Qu, F. C. Lin and J. Yang, Int. J. Mol. Sci., 2014, 15, 14766–14785 CrossRef CAS.
- D. Li, X. C. Zhang, Y. Q. Xu, L. Li, M. S. Aghdam and Z. S. Luo, Food Chem., 2019, 289, 112–120 CrossRef CAS.
- R. L. Prior, E. Fan, H. Ji, A. Howell, C. Nio, M. J. Payne and J. Reed, J. Sci. Food Agric., 2010, 90, 1473–1478 CrossRef CAS.
- I. F. Benzie and J. J. Strain, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS.
- Y. N. Zhao, L. Zhang, C. X. Zhao, C. X. Hu, Y. L. Li, J. Y. Zhao, J. J. Zhang, L. L. Li, Y. W. Chang, F. Wang, X. Lu, Z. Zhu and G. W. Xu, Metabolomics, 2015, 11, 1802–1814 CrossRef CAS.
- X. M. He, L. Li, J. Sun, C. B. Li, J. F. Sheng, F. J. Zheng, J. M. Li, G. M. Liu, D. N. Ling and Y. Y. Tang, Sci. Hortic., 2017, 219, 118–124 CrossRef CAS.
- J. P. Cao, C. Y. Wang, S. T. Xu, Y. Z. Chen, Y. Wang, X. Li and C. D. Sun, Sci. Hortic., 2019, 247, 42–48 CrossRef.
- Z. L. Xiao, Y. G. Luo, G. E. Lester, L. P. Kou, T. B. Yang and Q. Wang, LWT–Food Sci. Technol., 2014, 55, 551–558 CrossRef CAS.
- L. Wang, S. Shao, M. P. Madebo, Y. Y. Hou, Y. H. Zheng and P. Jin, Food Chem., 2020, 315, 126295 CrossRef CAS.
- A. Itai and T. Tanahashi, Postharvest Biol. Technol., 2008, 48, 355–363 CrossRef CAS.
- Z. L. Xiao, G. E. Lester, Y. G. Luo, Z. H. Xie, L. L. Yu and Q. Wang, Food Chem., 2014, 151, 472–479 CrossRef CAS.
- Z. Yu, Z. Yang, J. A. T. Silva, J. Luo and Y. H. Zheng, Postharvest Biol. Technol., 2019, 148, 97–106 CrossRef CAS.
- A. Rybarczyk-Plonska, M. K. Hansen, A. B. Wold, S. F. Hagen, G. I. Bengtsson and J. Wang, Postharvest Biol. Technol., 2014, 98, 82–89 CrossRef CAS.
- X. A. Li, M. L. Li, C. Han, P. Jin and Y. H. Zheng, Postharvest Biol. Technol., 2017, 129, 90–96 CrossRef CAS.
- D. Q. Li, X. Y. Qin, P. P. Tian and J. Wang, Food Chem., 2015, 196, 1092–1100 CrossRef.
- J. S. Wang, H. F. Yu, Z. Q. Zhao, X. G. Sheng, Y. S. Shen and H. G. Gu, J. Agric. Food Chem., 2019, 67, 12528–12537 CrossRef CAS.
- B. Sun, H. H. Yan, N. Liu, J. Wei and Q. M. Wang, Food Chem., 2012, 131, 519–526 CrossRef CAS.
- C. X. Cai, H. Y. Miao, H. M. Qian, L. S. Yao, B. L. Wang and Q. M. Wang, Food Chem., 2016, 210, 451–456 CrossRef CAS.
- H. Y. Miao, C. X. Cai, J. Wei, J. R. Huang, J. Chang, H. M. Qian, X. Zhang, Y. Zhao, B. Sun, B. L. Wang and Q. M. Wang, Sci. Rep., 2016, 6, 31854 CrossRef CAS.
- H. Y. Miao, J. Wei, Y. T. Zhao, H. Z. Yan, B. Sun, J. R. Huang and Q. M. Wang, J. Exp. Bot., 2013, 64, 1097–1109 CrossRef CAS.
- N. Li, F. M. Chen, F. G. Cui, W. J. Sun, J. S. Zhang, L. S. Qian, Y. Yang, D. Wu, Y. Dong, J. X. Jiang and H. P. Yang, Sci. Hortic., 2017, 225, 56–64 CrossRef.
- M. S. Medina, J. A. Tudela, A. Marín, A. Allende and M. I. Gil, Postharvest Biol. Technol., 2012, 67, 1–9 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra07177c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |