Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Vilsmeier reagent, NaHSe and diclofenac acid chloride: one-pot synthesis of a novel selenoindolinone with potent anticancer activity†
Ana Carolina Ruberte ab,
Carlos Aydilloab,
Arun K. Sharmac,
Carmen Sanmartín
ab,
Carlos Aydilloab,
Arun K. Sharmac,
Carmen Sanmartín *ab and
Daniel Plano
*ab and
Daniel Plano ab
ab
aUniversidad de Navarra, Facultad de Farmacia y Nutrición, Departamento de Tecnología y Química Farmacéuticas, Irunla-rrea 1, E-31008 Pamplona, Spain. E-mail: sanmartin@unav.es; Tel: +34-948425600 ext. 806388
bInstituto de Investigación Sanitaria de Navarra (IdiSNA), Irunlarrea, 3, 31008 Pamplona, Spain
cPenn State College of Medicine, Penn State Cancer Institute, CH72, Department of Pharmacology, 500 University Drive, Hershey, Pennsylvania 17033, USA
First published on 19th October 2020
Abstract
An effective and straightforward synthesis of 3-seleno functionalized indolinone (5) involving Vilsmeier reagent is presented. Likewise, a procedure to achieve lactamization of diclofenac with excellent yields by using hydrides is also ascertained. Compound 5 exhibited impressive growth inhibition in most of the cell lines in an NCI-60 panel, particularly towards resistant breast cancer cells.
Organoselenium compounds have been broadly studied owing to their well-regarded biological activities.1–4 Among the multiple and complex health benefits ascribed to organoselenium compounds, their role as chemotherapy agents is greatly-recognized.3 However, their activity is highly dependent on multiple factors, among which metabolic routes stand out. In this context, methylselenol is one of the main metabolites, and has received great attention as a key executor of organoselenium compounds' anticancer activity.5
A vast number of molecules containing heterocyclic rings possess a great variety of biological applications, including antitumor activity. Accordingly, indole derivatives show potent antitumoral activity against several cancers, such as lung, pancreatic and breast, in preclinical models.6–9 Thus, indole can be considered as a privileged scaffold for designing novel anticancer agents. We hypothesized that the functionalization of indole with a methylseleno group would yield a promising antitumor agent. Therefore, the objective of this work was the synthesis and cytotoxicity evaluation of 1-(2,6-dichlorophenyl)-2-(methylselanyl)-1H-indole (compound 4 in Scheme 1). The synthetic procedure designed to obtain the desired derivative is illustrated in Scheme 1.
The designed synthesis was originally planned in three steps. Firstly, the chlorination of diclofenac (1) to achieve the acid chloride 2 (Scheme 1, step 1). Secondly, the intramolecular cyclization of the acid chloride 2 through the nucleophilic attack of the nitrogen over the acyl chloride (Scheme 1, step 2). Finally, a two-steps reaction to yield the desired compound 4 via formation of the selenoamide and the subsequent methylation of the Se atom (Scheme 1, step 3). Different conditions and reagents have been reported for each of these steps, all of them being optimized. Next, we will discuss some previously reported methods along with our conditions and results for each step.
The chlorination of diclofenac (1) (Scheme 1, step 1) was attempted under three conditions, as previously reported in the literature:10–12 (i) reflux conditions with an excess of thionyl chloride (SOCl2) for 2 h,10 resulting in the degradation of diclofenac (1), observed by 1H-NMR spectra; (ii) reaction with oxalyl chloride [Cl(CO)2Cl] in methylene chloride (DCM) at room temperature,11 that did not yield the acid chloride 2 even at long periods of time (up to 72 h); and (iii) the use of few drops of N,N-dimethylformamide (DMF) as catalyst for the reaction for oxalyl chloride, which turned out to be the optimal conditions with quantitative yields (99%).12
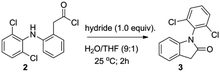
Regarding to molecular cyclization to render the lactam 1-(2,6-dichlorophenyl)indolin-2-one (3) (Scheme 1, step 2), several procedures have been published on the literature: radical photoredox cyclization,13,14 acid catalyzed cyclization15,16 and Mn(III)-based oxidative cyclization methods,17 as well as 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC)-based carboxyl activation cyclization,18 among others. Nevertheless, these methods present limitations, including multiple steps, complex catalysis and use of expensive reagents. Thus, the intramolecular reductive cyclization based on hydrides (e.g. lithium aluminum hydride (LiAlH4); lithium tri-t-butoxy aluminum hydride (LiAlH(OtBu)3);19 sodium cyanoborohydride (NaBH3CN)20 and sodium borohydride (NaBH4)21) caught our attention because of their straightforward and low cost procedure. Hence, we developed novel and efficient method of intramolecular cyclization with hydrides to obtain lactam 3. This method was achieved by reaction of acid chloride 2 with one equivalent of hydride in water and tetrahydrofuran (THF) at room temperature for 2 h. Besides, we optimized the method using five different hydrides, such as LiAlH4, lithium triethylborohydride (LiEt3BH), LiAlH(OtBu)3, NaBH3CN and NaBH4 (Table 1). Luckily, we obtained excellent yields with all hydrides, but the better yield was with LiAlH(OtBu)3 (Table 1, entry 3).
| Entry | Hydride | Yieldb (%) |
|---|---|---|
| a All reactions were carried out with compound 2 (6.4 mmol) and the corresponding hydride (6.4 mmol) in a mixture of water (18 mL) and THF (2 mL) as solvent at RT for 2 h.b Estimated yields of 3 determined by 1H-NMR. | ||
| 1 | AlLiH4 | 46 |
| 2 | LiEt3BH | 53 |
| 3 | LiAlH(OtBu)3 | 82 |
| 4 | NaBH3CN | 80 |
| 5 | NaBH4 | 78 |
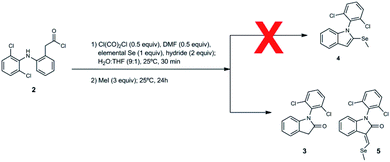
Finally, we focused on formation of the selenoamide and the subsequent methylation of the Se atom to yield the desired compound, 1-(2,6-dichlorophenyl)-2-(methylselanyl)-1H-indole (4) (Scheme 1, step 3). Concerning the selenoamide formation, we considered several reported methods gathered in the literature about the use of hydrogen selenide (H2Se) and hydrogen sulfide (H2S) in the conversion of amides in thioamides;22 thioureas in selenoureas20 and imines in selenones,20 among others. Thus, amide with H2Se should form the selenoamide. However, the toxicity of H2Se prompted us to find safe and effective alternative in alkali metal salt of hydroselenide (MHSe), which may be readily prepared in situ by the reaction of Se and hydride.11,23–25 Another advantage is that hydride was already used in the intramolecular cyclization (Table 1). Hence, the reaction conditions to form the lactam 3 (Scheme 1, step 2) are similar to last step (Scheme 1, step 3), prompting us to perform step 2 and step 3 in one-pot synthesis.
Thus, the formation of desired compound 4 from acid chloride 2 was attempted with oxalyl chloride (0.5 equivalent) and N,N-dimethylformamide (0.5 equivalent), Se (1.0 equivalent), LiAlH(OtBu)3 (2 equivalent to form lactam 3 and subsequently selenamide) and iodomethane (MeI) (3 equivalent) in water and tetrahydrofuran, under two conditions: (i) room temperature, up to 8 days; (ii) reflux, up to 2 days. Unfortunately, these conditions did not yield desired compound 4. In fact, this step did not evolve toward any selenocompound and most of the material recovered was the starting material. This result can be attributed to the lack of formation of lithium hydroselenide (Table S4 and Fig. S12, ESI†) necessary to form selenoamide and subsequently desired compound. Therefore, we studied the formation of MHSe with the rest of hydrides used previously (Table S4 and Fig. S10–S14, ESI†). Remarkably, NaBH4 was the only one that formed MHSe (Table S4 and Fig. S14, ESI†).
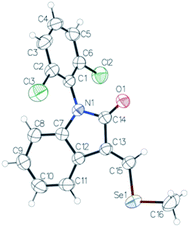
We decided to repeat the previous methods with NaBH4 instead of LiAlH(OtBu)3 (Scheme 2). Surprisingly, desired compound 4 was not detected at all, unlike lactam 3 with 49% of yield. Remarkably, we have discovered that the reaction gives rise to an unexpected 3-alkylidene-2-oxindole with Se ((E)-1-(2,6-dichlorophenyl)-3-((methylselanyl)methylene)indolin-2-one, 5) with 10% of yield (Scheme 2). This unexpected derivative was purified by column chromatography, characterized by NMR and its structure (Fig. 1) unambiguously determined by X-ray diffraction (CCDC 1983076, Tables S8–S15, ESI†).
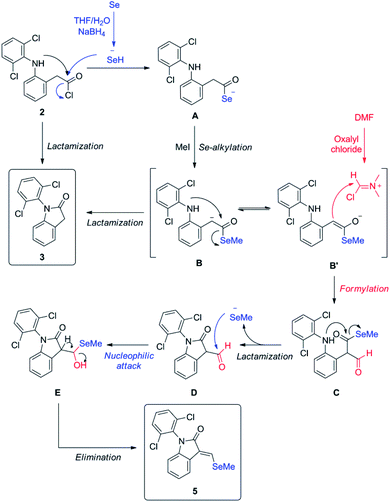
Since isolated diclofenac lactam 3 did not yield selenated product 5 under these conditions, the proposed mechanism (Scheme 3) would need to start from diclofenac carbonyl chloride 2, and involve the formation of selenocarboxylate A by the attack of in situ generated hydroselenide (HSe−), and posterior esterification with MeI to form B. Vilsmeier reagent generated by DMF and oxalyl chloride present in the reaction mixture is attacked by selenomethyl ester enolate, probably mediated by the presence of NaBH4, generating after hydrolysis the formylated intermediate C. Examples of related formylation of esters with alkyl formats in presence of base (hydrides26 and alkoxides27) have been previously reported. The next step would involve the intramolecular amide formation by the attack of secondary amine to the activated selenomethyl ester, generating intermediate D. Leaving methyl selenide can attack nucleophilically at aldehyde carbon, generating E, which could easily undergo water elimination, taking into account the additional stabilization gained by conjugation with aromatic system and the lactam in the final product 5. Low yields of product 5 are justified as lactamization is a favored process, even more with activated species, like acid chlorides and selenoesters, which have been known for acyl activation in peptide synthesis28 and protein chemical ligation.29
Once that Vilsmeier reagent is suggested to be a key part of product 5 generation, we attempted to optimize the method using more equivalents of DMF than in previously used method (Table 2). Nevertheless, the yield of unexpected derivative 5 and lactam 3 decreased probably due to the increased solubility of the organic compounds in the reaction medium and the subsequent additional issues to isolate the desired compounds.
| Entry | DMF (equiv.) | Cl(CO)2Cl (equiv.) | Yield (%) comp. 3b | Yield (%) comp. 5b |
|---|---|---|---|---|
a All reactions were carried out with compound 2 (6.4 mmol), oxalyl chloride (3.2 mmol) and DMF (0, 3.2, 6.4 or 9.6 mmol), elemental selenium (6.4 mmol) and NaBH4 (12.8 mmol) in a mixture of water and THF (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at RT. After 30 min, MeI (19.2 mmol) was added to the reaction and stirred at RT for 24 h.b Estimated yields of 3 and 5 determined by 1H-NMR.c No reaction. 1) at RT. After 30 min, MeI (19.2 mmol) was added to the reaction and stirred at RT for 24 h.b Estimated yields of 3 and 5 determined by 1H-NMR.c No reaction. |
||||
| 1 | 0 | 0 | 78 | NRc |
| 2 | 0.5 | 0.5 | 58 | 14 |
| 3 | 1 | 0.5 | 49 | 2 |
| 4 | 1.5 | 0.5 | 47 | 1< |
Finally, this unexpected new organoselenium derivative 5 with the code NSC: 811012 was submitted to the National Cancer Institute's (NCI) Developmental Therapeutics Program (DTP), to be tested at a single dose of 10 μM in a panel of NCI-60 human tumor cell lines. These cell lines include nine cancer types (leukemia, melanoma, non-small cell lung, colon, CNS, ovarian, renal, prostate and breast cancers).30 The results (Fig. S1, ESI†) were expressed in growth percent (GP), that is the growth of treated cells compared to the growth of an untreated cells. GP (%) values between 0 and 50 means antiproliferative properties and between −100 and 0 stand for cytotoxic properties. Compound 5 demonstrated antitumor activity in most NCI-60 cell lines, with a GP mean value towards all cell lines of 17.48% (Fig. S1, ESI†). In addition, compound 5 showed potent cytotoxic activity with GP lower than −30.80% in 6 out of the 60 tumor cell lines tested, including renal (UO-31 and SN12C), breast (BT-549), CNS (SNB-75), melanoma (MDA-MB-435) and leukemia (HL-60 (TB)) cancers (Fig. 2). In this context, compound 5 exhibited the most cytotoxic activity (GP equal to −96.55%) towards UO-31, a renal cancer cell line. Additionally, this new derivative showed great cytotoxic activity (GP equal to −35.63%) in the most resistant breast cancer cell of NCI panel towards more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds tested (BT-549).30
000 compounds tested (BT-549).30
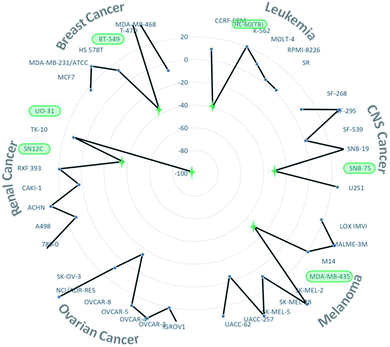 | ||
| Fig. 2 Growth percent values towards several breast, renal, ovarian, melanoma, CNS and leukaemia cancer cell lines. | ||
In conclusion, we developed a novel and efficient method of intramolecular cyclization with hydrides to obtain lactam 3. Besides, we report an unexpected and unprecedented Vilsmeier reagent application to afford a selenated lactam 5 with potent cytotoxic activity in resistant breast cancer cells. Hence, this new synthesis may be an attractive approach for the discovery of new potent antitumoral agents, particularly for therapy of resistant breast cancers.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Hemant Yennawar at the X-ray diffraction facility at Penn State University for the determination of the crystal structure. C. S. and D. P. wish to express their gratitude to PIUNA (refs 2014–26 and 2018–19) and UNED–Pamplona, Fundación Bancaria “La Caixa”, and “Fundación Caja Navarra” for financial support for the project. A. C. R. acknowledges the support of the Asociación de Amigos de la Universidad de Navarra and Caixabank for PhD fellowship.Notes and references
- C. Sanmartin, A. C. Ruberte, E. Ibanez, E. Moreno, S. Espuelas and D. Plano, Curr. Org. Synth., 2017, 14, 1075–1081 CAS.
- Q. Miao, J. Y. Xu, A. J. Lin, X. M. Wu, L. Wu and W. J. Xie, Curr. Med. Chem., 2018, 25, 2009–2033 CrossRef CAS.
- V. Gandin, P. Khalkar, J. Braude and A. P. Fernandes, Free Radical Biol. Med., 2018, 127, 80–97 CrossRef CAS.
- I. Di Leo, F. Messina, V. Nascimento, F. G. Nacca, D. Pietrella, E. J. Lenardao, G. Perin and L. Sancineto, Mini-Rev. Org. Chem., 2019, 16, 589–601 CrossRef CAS.
- J. Lu, J. Zhang, C. Jiang, Y. Deng, N. Ozten and M. C. Bosland, Nutr. Cancer, 2016, 68, 1–17 CrossRef CAS.
- P. S. Bhale, B. P. Bandgar, S. B. Dongare, S. N. Shringare, D. M. Sirsat and H. V. Chavan, Phosphorus Sulfur Relat. Elem., 2019, 194, 843–849 CrossRef CAS.
- F. L. Lu, B. B. Chen, C. H. Wang, C. L. Zhuang, Z. Y. Miao, X. D. Zhang and Y. L. Wu, Monatsh. Chem., 2019, 150, 1545–1552 CrossRef CAS.
- G. L. Petri, S. Cascioferro, B. El Hassouni, D. Carbone, B. Parrino, G. Cirrincione, G. J. Peters, P. Diana and E. Giovannetti, Anticancer Res., 2019, 39, 3615–3620 CrossRef CAS.
- K. Kaur and V. Jaitak, Anti-Cancer Agents Med. Chem., 2019, 19, 962–983 CrossRef.
- R. Cassano, S. Trombino, T. Ferrarelli, E. Barone, V. Arena, C. Mancuso and N. Picci, Biomacromolecules, 2010, 11, 1716–1720 CrossRef CAS.
- D. Plano, D. N. Karelia, M. K. Pandey, J. E. Spallholz, S. Amin and A. K. Sharma, J. Med. Chem., 2016, 59, 1946–1959 CrossRef CAS.
- N. Mekala, S. R. Buddepu, S. K. Dehury, K. M. V. R. Moturu, S. K. V. Indukuri, U. R. Vasireddi and A. R. Parimi, RSC Adv., 2018, 8, 15863–15869 RSC.
- Z. Gonda, F. Beke, O. Tischler, M. Petro, Z. Novak and B. L. Toth, Eur. J. Org. Chem., 2017, 15, 2112–2117 CrossRef.
- G. Bergonzini, C. Cassani, H. Lorimer-Olsson, J. Horberg and C. J. Wallentin, Chem. – Eur. J., 2016, 22, 3292–3295 CrossRef CAS.
- L. Kukuljan, K. Kranjc and F. Perdih, Acta Chim. Slov., 2016, 63, 905–913 CrossRef CAS.
- M. Hanif, M. Rafiq, M. Saleem, G. Qadeer and W. Y. Wong, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o583 CrossRef CAS.
- N. Kikue, T. Takahashi and H. Nishino, Heterocycles, 2015, 90, 540–562 CrossRef CAS.
- M. C. Chung, J. L. dos Santos, E. V. Oliveira, L. Blau, R. F. Menegon and R. G. Peccinini, Molecules, 2009, 14, 3187–3197 CrossRef CAS.
- P. Kocovsky, V. Dunn, A. Gogoll and V. Langer, J. Org. Chem., 1999, 64, 101–119 CrossRef CAS.
- G. L. Sommen and D. Thomae, Curr. Org. Synth., 2010, 7, 44–61 CrossRef CAS.
- G. W. Gribble, Chem. Soc. Rev., 1998, 27, 395–404 RSC.
- A. B. Charette and M. Grenon, J. Org. Chem., 2003, 68, 5792–5794 CrossRef CAS.
- H. Ishihara, M. Koketsu, Y. Fukuta and F. Nada, J. Am. Chem. Soc., 2001, 123, 8408–8409 CrossRef CAS.
- C. Sanmartín, D. Plano, E. Domínguez, M. Font, A. Calvo, C. Prior, I. Encío and J. A. Palop, Molecules, 2009, 14, 3313–3338 CrossRef.
- G. Ming Li and R. A. Zingaro, J. Chem. Soc., Perkin Trans. 1, 1998, 647–650, 10.1039/A707792K.
- J. P. Spengler and W. Schunack, Arch. Pharm., 1984, 317, 425–430 CrossRef CAS.
- S. J. Davies, A. P. Ayscough, R. P. Beckett, R. A. Bragg, J. M. Clements, S. Doel, C. Grew, S. B. Launchbury, G. M. Perkins, L. M. Pratt, H. K. Smith, Z. M. Spavold, S. W. Thomas, R. S. Todd and M. Whittaker, Bioorg. Med. Chem. Lett., 2003, 13, 2709–2713 CrossRef CAS.
- A. Temperini, F. Piazzolla, L. Minuti, M. Curini and C. Siciliano, J. Org. Chem., 2017, 82, 4588–4603 CrossRef CAS.
- S. S. Kulkarni, E. E. Watson, B. Premdjee, K. W. Conde-Frieboes and R. J. Payne, Nat. Protoc., 2019, 14, 2229–2257 CrossRef CAS.
- W. C. Reinhold, S. Varma, F. Sousa, M. Sunshine, O. D. Abaan, S. R. Davis, S. W. Reinhold, K. W. Kohn, J. Morris, P. S. Meltzer, J. H. Doroshow and Y. Pommier, PLoS One, 2014, 9, e101670 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, compound characterization, NMR spectra. CCDC 1983076. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra07332f |
| This journal is © The Royal Society of Chemistry 2020 |